Abstract
Usually, physicians use an acoustic stethoscope to detect abnormalities in the heart sound and predict abnormal conditions of the human heart. As the frequency range and intensity of heart sound is very low, doctors are facing problems while detecting the cardiac sound and its abnormalities. To eradicate these severe problems, it is required to design and develop an electronic stethoscope which would assist the doctor to analyze heart sound and to detect disease of the heart. Here an acoustic stethoscope along with microphone and preamplifier module is used to increase the amplitude of the input audio signal received by the stethoscope. The soft scope of MATLAB program has also been used for analyzing the continuous set of cardiac sound and to detect its various characteristics like frequency, amplitude, etc. It is aimed to design an electronic stethoscope which would assist the doctors to analyze heart sound and identify a disease condition of the heart, but preliminarily we have achieved to detect different components of it which are lub (s1), dub (s2), s3, s4, etc. Finally, the sound signal received from the heart in the MATLAB program after filtering the noise out of it also has been plotted and analyzed in the frequency domain. As the heart sound is a complex waveform signal, harmonic distribution is used. Amplitude and phase are the two essential parameters. Thus the harmonic distribution of Amplitude and Phase are carried out. Amplitude Distribution of harmonics leads to some crucial characteristics features like RMS Value, Mean Value, Average Energy, Average Power, Mean Squared Error, Spectrogram Analysis, Periodogram Analysis, and Kalman Filtered Response. These features will readily identify and distinguish between Normal heart sound, abnormal heart sound and cardiac murmurs in Matlab programming.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Rouanet, around 140 years ago, attributed the second heart sound to the closure of the aortic and pulmonic valves. Although this explanation has generally been accepted to the present time, several theories have been explained to examine the genesis of the second heart sound. The most tenable to date suggests that closure of the aortic and pulmonic valves initiates the series of events that generate the second heart sound. The main audible components, however, originate from vibrations of the cardiac structures after valve closure. By using high-fidelity, catheter-tipped micro-manometers and echo phono-cardiography, it has been shown that the aortic and pulmonic valves close silently and that coaptation of the aortic valve cusps precedes the onset of the second sound by a few milliseconds. The second heart sound, therefore, originates from after vibrations in the cusps and in the walls, blood columns of the large vessels and their respective ventricles. The energy from these oscillations comes from the fact that sudden deceleration of retrograde blood flow of the blood in the aorta and pulmonary artery when the elastic limits of the tensed valve leaflets are met. This abrupt deceleration sets the whole cardio hemic system into a system of vibratory motion.
To understand the splitting of the second heart sound, knowledge of its relationship to the cardiac cycle is crucial. A2 and P2 are the actual sound coincident with the incisurac of the aorta and pulmonary artery pressure curves, respectively, and terminate left and right ventricular ejection periods. Right ventricular ejection begins before left ventricular ejection, has a slightly longer duration, and ends after left ventricular ejection, resulting in P2 usually occurring after the sound A2.
The differences between the aortic and pulmonary artery vascular impedance characteristics are also [1] crucial to understand the effects of respiration on the splitting of sound S2. When the pressure curves of the pulmonary artery and right ventricle are recorded concurrently, the pulmonary arterial plot at the level of the incisura lags behind the right ventricular curve, or “hangs out” after it. The duration of the “hangout interval” is the measure of impedance in the pulmonary artery system. In the highly compliant (low-resistance, high-capacitance) pulmonary vascular bed, the hangout interval may vary from 30 to 120 ms, contributing significantly to the duration of the right ventricular ejection. In the left side of the heart, as impedance is much higher, the hangout interval between the aorta and left ventricular pressure curves is negligible which is less than or equal to 5 ms. The hangout interval, therefore, correlates closely with the impedance of the vascular bed into which blood is being injected. Its duration appears to be inversely proportional to vascular impedance.
Alterations in the impedance characteristics of the pulmonary vascular bed and the right-sided hangout interval are responsible for observed abnormalities in S2. In a general physiologic setting, inspiration lowers the impedance in the pulmonary circuit, prolongs the hangout interval and delays pulmonic valve closure, resulting in the audible splitting of the sound A2 and P2. On expiration, the reverse occurs: pulmonic valve closure happens [2] earlier, and the A2–P2 interval is separated by less than 30 ms and may sound only once. The pulmonary circulation has a much lower impedance than the systemic circulation; therefore, the blood flow through the pulmonic valve takes longer time than blood flow through the aortic valve. The inspiratory split widens mainly because of delay in the pulmonic component (Fig. 1).
It was believed traditionally that an inspiratory drop in intrathoracic pressure favored the greater venous return to the right ventricle, pooling of blood in the lungs, and decreased return to the left ventricle. The increase in right ventricular blood volume prolonged right-sided ejection time and delayed P2; the decrease in left ventricular volume reduced left-sided ejection time and caused A2 to occur earlier than usual. The delayed occurrence of P2 and early presence of A2 associated with the inspiration, however, are best understood as an interplay between the [3] changes in the pulmonary vascular impedance and changes in systemic and pulmonary venous return. The net effect is that right ventricular ejection is prolonged, left ventricular ejection is shortened, and the A2–P2 interval widens during inspiration.
Clinical Significance
The clinical evaluation of the second heart sound has been called the “key to auscultation of the heart” [4]. It involves an assessment of splitting and a determination of the relative intensities of A2 and P2. Usually, the aortic closure sound (A2) occurs before the pulmonic closure sound (P2), and the interval between the two (splitting) widens on inspiration and narrows on expiration. With quiet respiration, A2 will generally precede P2 by 0.02 to 0.08 s (mean, 0.03 to 0.04 s) with inspiration. In younger subjects, inspiratory splitting averages 0.04 to 0.05 s during quiet respiration. With expiration, A2 and P2 may be superimposed and are rarely split as much as 0.04 s. If greater than 0.04 s on expiration splits the second sound, it is usually abnormal. Therefore, the presence of audible splitting during expiration (i.e., the ability to hear two distinct sounds during expiration) is of greater significance at the bedside in identifying underlying cardiac pathology that is the absolute inspiratory increase in the A2–P2 interval.
The respiratory variation of the second heart sound can be categorized as follows:
-
1.
Regular (physiologic) splitting.
-
2.
Persistent (audible expiratory) splitting, with normal respiratory variation.
-
3.
Persistent splitting without respiratory modification (fixed splitting).
-
4.
Reversed (paradoxical) splitting.
Physiologic splitting is demonstrated during inspiration in healthy individuals since the splitting interval widens primarily due to the delayed P2. During expiration, the A2–P2 range is so narrow that only a single sound is usually heard.
Persistent (audible) expiratory splitting suggests an audible expiratory interval of at least 30–40 ms between the two sounds. Persistent splitting that is audible during both respiratory phases with appropriate inspirational and aspirational changes (i.e., further increase of the A2–P2 interval with inspiration) may occur in the recumbent position in healthy children, teenagers, and young adults. If these individuals sit, stand, or perform a Valsalva maneuver, however, the second sound often becomes single on expiration. In almost all the patients with heart disease and audible expiratory splitting in the recumbent position, expiratory [5] splitting persists when the patient is examined in the sitting or standing position. Thus, the finding of distinct expiratory splitting in both the supine and upright positions is a susceptible screening test for heart disease. Right bundle branch block (RBBB) is the most common cause of the persistence of audible expiratory splitting on standing.
Other causes of persistent expiratory splitting on standing may be due to either a delay in pulmonic valve closure or to the early closing of the aortic valve. A delay in the occurrence of P2 is mainly attributed to the following events:
-
1.
Delayed electrical activation of the right ventricle (e.g., left ventricular ectopic or paced beats, Wolff-Parkinson-White syndrome, and RBBB).
-
2.
The decreased impedance [6] of the pulmonary vascular bed (e.g., atrial septal defect, partial anomalous pulmonary venous return, and idiopathic dilatation of the pulmonary artery).
-
3.
Right ventricular pressure overload lesions (e.g., pulmonary hypertension with right heart failure, moderate to severe valvular pulmonic stenosis, and acute massive pulmonary embolus).
An early A2 may occur in patients with decreased resistance to left ventricular outflow (e.g., mitral regurgitation or constrictive pericarditis). Moderately large ventricular septal defects may also cause extensive splitting of the second sound, but the aortic component is usually difficult to hear because of the loud holosystolic murmur.
Expiratory splitting of S2 may occur in patients with severe congestive heart failure. The expiratory splitting usually disappears after satisfactory therapy of the heart failure. The high prevalence of expiratory splitting of S2 in cardiomyopathy may be explained by a combination of low cardiac output, mitral regurgitation, pulmonary hypertension, right heart failure, and bundle branch block.
Fixed splitting denotes the absence of significant variation of the splitting interval with respiration, such that the separation of A2, and P2, remains unchanged during inspiration and expiration. An atrial septal defect, with either standard or high pulmonary vascular resistance, is the classic example of the fixed splitting of the second sound. The audible expiratory splitting in these patients is primarily a reflection of changes in the pulmonary vascular bed rather than selective volume overload of the right ventricle prolonging right ventricular systole [7]. The fixed nature of the split is due to the approximately equal inspiratory delay of the aortic and pulmonic components, indicating that the two ventricles share a common venous reservoir. Respiratory splitting of the second sound immediately returns to standard following surgical repair of an atrial septal defect, [8] although the pulmonic closure sound may remain delayed for weeks or months.
Severe right heart failure can lead to a relatively fixed split. It occurs because the right ventricle fails to respond to the increased blood volume produced by inspiration and because the lungs are so congested that impedance to forward blood flow from the right ventricle barely falls during inspiration. In anomalous pulmonary venous return without atrial septal defect, fixed splitting is not usually seen despite the simultaneous inspiratory delay in aortic and pulmonic closure.
The Valsalva maneuver [9] may be used to exaggerate the effect of respiration and obtain more explicit separation of the two components of the second sound. Patients with atrial septal defects show continuous splitting during the strain phase, and upon release, the interval between the components increases by less than 0.02 s. In healthy patients, however, splitting is exaggerated during the release phase of the Valsalva maneuver. Variation of the cardiac cycle length may also be used to evaluate splitting of S2. During the longer cardiac cycle, patients with the atrial septal defect may show greater splitting as a result of increased atrial shunting and greater disparity between stroke volume of the two ventricles. In healthy subjects, there is no tendency to widen the splitting with longer cardiac cycles.
Pulmonary artery hypertension causes variable effects on the splitting of the second sound. Patients with ventricular septal defect who develop pulmonary hypertension may no longer have the splitting of S2. Patients with an atrial septal defect and associated pulmonary hypertension maintain an extensive and fixed split of S2. Splitting is shortened (less than 30 ms), but remains physiologic, in patients with patent ductus arteriosus who develop pulmonary hypertension.
Paradoxical or reversed splitting [10] is the result of a delay in the aortic closure sound. Therefore P2 precedes A2, and splitting is maximal on expiration and minimal or absent on inspiration. Identification of the reversed order of valve closure may be possible by judging the intensity and transmission of each component of the second sound. Often, however, the pulmonic component [11] is as loud as the aortic component because of pulmonary artery hypertension secondary to left ventricular failure. The paradoxical narrowing or disappearance of the split on inspiration is a necessary criterion for diagnosing reversed splitting by auscultation.
Paradoxical splitting [12] always indicates significant underlying cardiovascular disease and is usually due to prolongation of left ventricular activation or prolonged left ventricular emptying. The most common cause of paradoxical splitting of the second sound is left bundle branch block. Obstruction to left ventricular outflow of sufficient severity to delay aortic valve closure may also cause paradoxical splitting. In the context of aortic stenosis, such an auscultatory finding implies severe obstruction. Paradoxical splitting [13], however, occurs more commonly with hypertrophic cardiomyopathy than with aortic stenosis. Paradoxical splitting of the second sound may occur during the first few days after an acute myocardial infarction or secondary to severe left ventricular dysfunction.
A mistaken diagnosis of the abnormal splitting of the second sound must be avoided. A late systolic click of mitral valve prolapse, the opening snap (OS) of mitral stenosis, a third heart sound (S3), or a pericardial knock may be incorrectly thought to represent the fixed splitting of the second sound. A systolic knock may vary its location in systole with specific maneuvers that change the shape of the left ventricle. The best way to differentiate between an A2–P2 from an A2–OS is to have the patient stand up. The A2–P2 interval remains the same or narrows, whereas the A2–OS interval widens. The third heart sound, which forms the S2–S3 complex, is lower in frequency range than S2, is best [14] heard at the apex, is usually not understood at the basal auscultatory area, and occurs 0.12–0.16 s after A2. The pericardial knock is a third heart sound that is slightly higher pitched and earlier than the usual S3 and is also best heard at the apex.
The second heart sound can remain single throughout the respiratory cycle due to either absence of one component or to the simultaneous occurrence of the two components. Since the pulmonary vascular impedance increases with age, many normal patients over age 50 have a single S2 or at most a narrow physiologic split on inspiration because P2 occurs early. A unique second sound, however, is usually due to inability to auscultate a relatively soft pulmonic component. Such failure is rare in healthy infants, children, and young adults and is uncommon even in older persons under good auscultatory conditions using a rigid stethoscopic diaphragm [15].
Hyperinflation of the lungs is perhaps the most common cause of inability to hear the pulmonic closure sound. All the conditions are causing paradoxical splitting that delay A2 may produce a single second sound when the splitting interval becomes less than 0.02 s. Inaudibility of P2 due to an actual decrease in its intensity is relatively rare, however, and suggests tetralogy of Fallot or pulmonary atresia. The pulmonic component may be inaudible in chronic right ventricular failure, or the aortic component may be masked by the systolic murmur in patients with aortic stenosis.
The pulmonary closure [16] is completely fused with aortic closure throughout the respiratory cycle only in Eisenmenger’s syndrome with a large ventricular septal defect or in cases of single ventricle, where the duration of right and left ventricular systole are virtually equal. The second sound may also be unique in a variety of congenital heart defects (e.g., truncus arteriosus, tricuspid atresia, hypoplastic left heart syndrome, transposition of the great arteries, and, occasionally, corrected transposition of the great arteries).
The loudness of each component [17] of the second heart sound is proportional to the respective pressures in the aorta and pulmonary artery at the onset of diastole. Dilatation of the aorta or pulmonary artery may also cause accentuation of the aortic and pulmonic components, respectively. The aortic component usually is of higher intensity than the pulmonic component. The aortic component, therefore, radiates widely over the chest, whereas the pulmonic component is heard mainly in the second left ICS with some radiation down the left sternal border. The higher radiation of the aortic component is probably due to the higher pressure in the aorta compared to that in the pulmonary artery. At any given level of pressure, however, the pulmonic component will be proportionately louder than the aortic component because of the close proximity of the pulmonic valve and the pulmonary artery to the chest wall. These considerations account for the relative loudness of P2 in young patients in whom the pulmonary arteries are quite close to the chest wall. They also account for the decreased intensity of both components of the second sound in patients with emphysema in whom both arteries are displaced from the chest wall.
The pulmonic component is considered to be abnormally loud in a subject over age 20 if it is higher than the aortic component in the second left ICS or if it is audible at the cardiac apex. It may be due either to pulmonary artery hypertension or right ventricular dilatation, with part of the right ventricle assuming the position usually occupied by the left ventricle. A split second sound at the apex is, therefore, definitely abnormal. The loud P2 commonly heard at the apex in patients with an atrial septal defect is probably due to a dilated right ventricle encroaching upon the cardiac apex.
Decreased intensity of either component of the second sound may be due to a stiff semilunar valve, reduced pressure beyond the semilunar valve [18], or deformity of the chest wall or lung. The reduced intensity of P2 is most common in patients with chronic obstructive lung disease or valvular pulmonic stenosis. A decreased intensity [10] of A2 is most common in patients with valvular aortic stenosis (Fig. 2).
Detection of Heart Sound
Stethoscope plays a vital role in the detection of the heart sound. Here we are going to know the history and evolution of stethoscope through ages to see the story behind detection of cardiac sounds.
History and Evolution of Stethoscope
The word stethoscope is derived from the two Greek words, stethos (chest) and scopos (examination). Apart from listening to the heart and chest sounds, it is also used to hear bowel sounds and blood flow noises in arteries and veins.
In the early 1800s, and before the development of the stethoscope, physicians would often perform physical examinations using techniques such as percussion and immediate auscultation. In immediate auscultation, physicians placed their ear directly on the patient to observe internal sounds.
This technique suffered from several drawbacks, the foremost being that it required physical contact between the physician and the patient and proper placement of the ear. Besides, the sounds observed by the physician were not amplified in any way, creating the possibility of missing key sounds that might indicate potential illness. Finally, the act of performing immediate auscultation could be awkward for both the physician and patient.
The invention of the first stethoscope: The stethoscope was invented in France in 1816 by René Laennec at the Necker-Enfants Malades Hospital in Paris. It comprised a wooden tube and was monaural. Laennec invented the stethoscope because he was uncomfortable placing his ear on women’s chests to hear heart sounds. His device was similar to the common ear trumpet, a historical form of hearing aid; indeed, his invention was almost indistinguishable in structure and function from the trumpet, which was commonly called a “microphone”. It was named as a stethoscope (Fig. 3).
Evolution of Stethoscope
In 1840, Golding Bird described a stethoscope he had been using with a flexible tube. The bird [19] was the first to publish a description of such a stethoscope, but he noted in his paper the prior existence of an earlier design (which he thought was of little utility) which he described as the snake ear trumpet. Bird’s stethoscope had a single earpiece.
In 1851, Irish physician Arthur Leared invented a binaural stethoscope, and in 1852 George Philip Cammann perfected the design of the stethoscope instrument (that used both ears) for commercial production, which has become the standard ever since. Cammann also wrote a significant treatise on diagnosis by auscultation, which the refined binaural stethoscope made possible. By 1873, there were descriptions of a differential stethoscope that could connect to slightly different locations to create a slight stereo effect, though this did not become a standard tool in clinical practice.
Rappaport and Sprague designed a new stethoscope in the 1940s, which became the standard by which other stethoscopes are measured, consisting of two sides, one of which is used for the respiratory system, the other for the cardiovascular system. Hewlett-Packard later made the Rappaport-Sprague.
The original Rappaport-Sprague stethoscope was finally abandoned ca. 2004, along with Philips’ brand (manufactured by Andromed, of Montreal, Canada) electronic stethoscope model. The Rappaport-Sprague model stethoscope was heavy and short [18–24 in (46–61 cm)] with an antiquated appearance recognizable by their two large independent latex rubber tubes connecting an exposed-leaf-spring-joined-pair of opposing “f”-shaped chrome-plated brass binaural ear tubes with a dual-head chest piece.
Several other minor refinements were made to stethoscopes, until in the early 1960s David Littmann, a Harvard Medical School professor, created a new stethoscope that was lighter than previous models and had improved acoustics. In the late 1970s, 3 M-Littmann introduced the tunable diaphragm: a very hard (G-10) glass-epoxy resin diaphragm member with an over-molded silicone flexible acoustic surround which permitted increased excursion of the diaphragm member in a “z”-axis concerning the plane of the sound collecting area. The left shift to a lower resonant frequency increases the volume of some low-frequency sounds due to the longer waves propagated by the increased excursion of the hard diaphragm member suspended in the concentric acoustic surround [20]. Conversely, restricting excursion of the diaphragm by pressing the stethoscope diaphragm surface firmly against the anatomical area overlying the physiological sounds of interest, the acoustic surround could also be used to dampen excursion of the diaphragm in response to “z”-axis pressure against a concentric fret. It raises the frequency bias by narrowing the wavelength to auscultate a higher range of physiological sounds.
In 1999, Richard Deslauriers patented the first external noise-reducing stethoscope, the DRG Puretone. It featured two parallel lumens containing two steel coils which dissipated infiltrating sound as inaudible heat energy. The steel coil “insulation” added.30 lb to each stethoscope. In 2005, DRG’s diagnostics division was acquired by TRIMLINE Medical Products.
In 2015, Dr. Tarek Loubani announced an open-source 3D-printed stethoscope based on the 1960s-era Littmann Cardiology 3 stethoscope, which is out of patent. The 3D-printed equivalent is nearly an order of magnitude more affordable than the aforementioned non-3D-printed stethoscope and is intended to make the medical device more accessible to obtain, particularly in developing countries [21] (Fig. 4).
Golding Bird’s instrument is on the left. The device on the right was current around 1855 when this image was first published [22].
Electronic Stethoscope: An electronic stethoscope (or stethophone) overcomes the low sound levels by electronically amplifying body sounds. However, it suffers from stethoscope contact artifacts and frequency response of the microphone, pre-amplifier, power amplifier, and speakers, which limit the overall utility. The Electronic stethoscopes require the conversion of acoustic sound waves to electrical signals. After that, the amplified signal is processed for optimal listening. Another method used in Welch-Allyn’s Meditron stethoscope, made by placement of a piezoelectric crystal at the head of a metal shaft. The bottom of the metal shaft makes contact with a diaphragm. 3 M also uses a piezo-electric crystal placed within foam behind a thick rubber-like diaphragm. The Thinklabs’ Rhythm 32 uses an Electromagnetic Diaphragm with a conductive inner surface to form a capacitive sensor. This diaphragm responds to sound waves, with changes in an electric field replacing changes in air pressure. The Eko Core enables wireless transmission of heart seems to a smartphone or tablet.
Because the generated sounds are transmitted electronically, an electronic stethoscope can be viewed as a wireless device, a recording device, and can provide noise reduction, signal enhancement, and both visual and audio output. Around 2001, Stethographics introduced PC-based software which enabled a phonocardiograph, graphic representation of cardiologic and pulmonologic sounds to be generated, and interpreted according to related algorithms. All of these characteristics are helpful for telemedicine (remote diagnosis) and teaching [23].
3-D Printed Stethoscope: A 3D-printed stethoscope is an open-source medical device meant for auscultation and manufactured via means of 3D printing. The 3D stethoscope was developed by Dr. Tarek Loubani and a team of medical and technology specialists. The 3D-stethoscope was developed as part of the Glia project, and its design is open source from the outset. The stethoscope gained widespread media coverage in summer 2015.
The need for a 3D-stethoscope was borne out of a lack of stethoscopes and other vital medical equipment because of the blockade of the Gaza Strip, where Loubani, a Palestinian-Canadian, worked as an emergency physician during the 2012 conflict in Gaza. The 1960s-era Littmann Cardiology 3 stethoscope became the basis for the 3D-printed stethoscope developed by Loubani [23] (Figs. 5 and 6).
Introduction to Digital Stethoscope
A stethoscope, whether acoustic or digital, is used mainly to listen to heart and lung sounds in the body as an aid for diagnosis. Listening or auscultation has been done with acoustic [3] stethoscopes for almost two hundred years; recently, electronic digital stethoscopes have been developed over the years to withstand the high chance of dealing with cardiac related problems.
The goal of a necessary digital stethoscope is to have it retain the look and feel of an acoustic stethoscope but to improve listening performance. In addition to that, high-end digital stethoscopes offer sophisticated capabilities such as audio recording and playback. They also provide data to visually chart results by connecting to an off-instrument display such as a computer monitor. This advanced functionality increases the physician’s diagnostic capability maintaining the existing acoustic stethoscope form.
Audio Signal Path
The essential components of a digital stethoscope are the sound transducer, the audio codec electronics, and the speakers. The sound transducer, which converts sound into an analog voltage, is the most critical piece in the chain. It determines the diagnostic quality of the digital stethoscope and ensures a familiar user experience to those accustomed to acoustic stethoscopes.
The analog voltage needs to be conditioned and then converted into a digital form by using a sound analog-to-digital converter (ADC) or audio codec. Some digital stethoscopes have inbuilt noise cancellation device that requires a secondary sound transducer or microphone to record the ambient noise so that it can be removed digitally. In this approach, two audio ADCs are needed (Fig. 7).
Once in the digital domain, a microcontroller unit (MCU) or digital signal processor (DSP) performs signal processing, including ambient noise reduction and filtering, to limit the bandwidth to the range for cardiac or pulmonary listening. The processed digital signal is then converted back to analog by an audio digital-to-analog converter (DAC) or audio codec [24].
A headphone or speaker amplifier conditions the audio signal before outputting to a speaker. A single speaker can be used below where the stethoscope tube bifurcates, with the amplified sound traveling through the binaural tubes to the ears. Alternatively, two speakers can be used, with one speaker at the end of each earpiece. The frequency response [25] of the speaker is similar to that of a bass speaker because of the low-frequency sound production needed. Depending upon the implementation, one or two speaker amplifiers are used.
A stethoscope must be most sensitive to cardiac sound in the 20–400 Hz range and pulmonary sound in the 100–1200 Hz range. Note that the frequency ranges vary by manufacturer, and the DSP algorithms filter out sound beyond these optimal ranges.
Data Storage and Transfer
Once the captured sound is converted to an analog voltage, it can be sent out through an audio jack and played back on either a computer or through the digital stethoscope. The captured sound can also be manipulated digitally. It can be stored in the stethoscope using internal or removable nonvolatile (NV) memory like EEPROM or flash and then played back through the stethoscope’s speakers, or it can be transferred to a computer for further analysis. Adding a real-time clock (RTC) facilitates tagging the recording with time and date. The sound is commonly assigned with a wired interface, such as USB, or with a wireless interface like Bluetooth or other proprietary wireless interfaces which are widely used these days.
Display and Backlighting
Some digital stethoscopes [23] have a small, simple display due to the limited space available; others have only buttons and LED indicators. Backlighting for the display is required because the ambient lighting during the procedure is often at a low level. The small display requires just one or two white light-emitting diodes (WLEDs) controlled by an LED driver or an electroluminescent (EL) panel controlled by an EL driver. Most of the user-interface buttons can be eliminated by adding a touch-screen display and controller.
Power Management
Most digital stethoscopes use either one or two AAA 1.5 V primary batteries. This design requires a step-up, or boost, switching regulator to increase the voltage to 3.0 V or 5.0 V, depending on the circuitry utilized these days for cardiac-related problems.
If a single 1.5 V battery is installed, the switching regulator will probably be on all the time, making low quiescent current a vital factor for long battery life. The longer the battery life, the more convenient the digital stethoscope is to use and the closer the experience will be to use an acoustic stethoscope [17].
When using two 1.5 V batteries in series, the switching regulator can be left on all the time or shut down when not in use. If the circuit operates from 3.6 V down to 1.8 V, then a switching regulator may not be needed. The cost will be reduced and space saved. A low-battery warning is required so that a patient’s examination need not be interrupted to replace the battery.
Battery Management
Rechargeable batteries can be used; the best choice is a single-cell Li+ battery. If a rechargeable battery is used, a battery charger is required either in the digital stethoscope or a charging cradle. A fuel gauge is the best solution to determine the remaining battery life accurately. If the battery is removable, then authentication is also required for safety and aftermarket management.
The Bluetooth word mark and logos are registered trademarks owned by Bluetooth SIG, Inc., and any use of such marks by Maxim is under license.
Importance of Heart Sound Analysis
Physicians use the stethoscope for hearing heart sounds. They generally try to analyze and understand congenital heart disease by analyzing the heart sound and abnormalities in it. Naturally, the frequency and repetition of sound are significant in detecting the problems of heart disease. In case of an ordinary stethoscope, there is another problem of detecting heart sound that is the limitation of hearing music by human ears. So we need to digitalize the sound signal and analyze the sound correctly using a modern tool. Then anyone can detect precisely the wellness of heart and its various components.
Process: To develop a digital algorithm that detects first and second heart sounds, defines the systole and diastole, and characterizes the systolic murmur. Heart sounds [26] were recorded in 300 children with a cardiac murmur, using an electronic stethoscope. A Digital algorithm was developed for detection of first and second heart sounds. R-waves and T-waves in the electrocardiography were used as references for detection. The sound signal analysis was carried out using the short-time Fourier transform. The first heart sound detection rate, with reference to the R-wave, was 100% within 0.05–0.2R-R interval. The second heart sound detection rate between the end of the T-wave and the 0.6R-R interval was 97%. The systolic and diastolic phases of the cardiac cycle could be identified. Because of the overlap between heart sounds and murmur a systolic segment between the first and second heart sounds (20–70%) was selected for murmur analysis. The maximum intensity of the systolic murmur, its average frequency, and the mean spectral power was quantified. The frequency at the point with the highest sound intensity in the spectrum and its time from the first heart sound, the highest frequency, and frequency range were also determined. This method will serve as the foundation for computer-based detection of heart sounds and the characterization of cardiac murmurs [24].
Different Work Done
Renowned researchers have done previously many research works on advancement and development of stethoscope. While working on our project, we have gone through several papers and practices to gain and utilize the knowledge in the project.
-
1.
Department of Electro-medical Engineering, Health Polytechnic Ministry of Health Surabaya, Indonesia has done a detailed research on analysis and detection of heart sound using wavelet decomposition technique. A paper in the International Journal of computer networks and communications security was published on their work. The paper was published in the year 2013, December [17].
-
2.
In 2013 another renowned work was done by Kuldeep Singh and Preeti Abrol on Design and Development of a Digital Stethoscope for detection of Cardiac Murmur. They developed an A.V.R. based digital stethoscope to capture heart sounds and diagnose them with the help of display and analytic tools. The proposed design of the system includes an ATmega16 microcontroller, graphic LCD, memory, and MATLAB interface [25].
-
3.
In September 2013, Mandeep Singh and Amandeep Cheema published a paper on Heart Sounds Classification using Feature Extraction of Phonocardiography Signal. They have analyzed a set of P.C.G. signal using MATLAB. They have taken the heart sound signal as the sample and analyzed in both frequency and time domain [27].
-
4.
Anju and Sanjay Kumar have also done research work on Detection of Cardiac Murmur. They have used FPGA system to detect cardiac murmur and Artificial Neural Network to differentiate between normal and pathogenic cardiac murmur [28].
-
5.
S. M. Debbal’s work was on computerized heart sound analysis. He analyzed the sound signal using digital signal processing and Fourier transform method [11].
-
6.
Ms. D. D. Kadam Patil and Mr. R. K. Shastri have researched and developed wireless electronic stethoscope using based on ZIGBEE transmission method. They have transmitted analyzed data from one place to another using ZIGBEE protocol [6].
-
7.
L. B. Dahl, P. Hasvold, E. Arild and T. Hasvold have done their work on, recording of heart murmur by using a sensor based electronic stethoscope and e-mail it for remote assessment [29].
-
8.
Bill Dillard and Wah W. Myint designed an electronic stethoscope with diagnosis capability. They have performed time-frequency analysis in every systolic and diastolic murmur [9].
Disease Categories
Electrical: Abnormal heart sounds are caused by problems with the electrical system that regulates the steady heartbeat. The heart rate may be too slow or too fast; it may stay steady or become chaotic (irregular and disorganized). Some arrhythmias are very dangerous and cause sudden cardiac death, while others may be bothersome but not life-threatening.
Circulatory: High Blood Pressure [15] and coronary artery disease (blockage in the pipes of the heart) are the main causes of blood vessel disorders. The results, such as stroke or heart attack, can be devastating. Fortunately, there are many treatment options.
Structural: Heart muscle disease (cardiomyopathy) and congenital abnormalities (problems present from birth) are two problems that can damage the heart muscle or valves.
The third heart sound (S3) is a low-pitched, early diastolic sound audible during the rapid entry of blood flow from the atrium to the ventricle. S3 can be physiologically present in patients younger than 40 years. These patients often have a thin chest wall to permit the easy transmission of S3. In the presence of heart failure, S3 is a bad prognostic sign. Conditions associated with pathological S3 include the following:
-
Systolic and/or diastolic ventricular dysfunction
-
Ischemic heart disease
-
Hyperkinetic states—Anemia, fever, pregnancy, thyrotoxicosis, AV fistula
-
MR or TR
-
Chronic AR with systolic dysfunction
-
Systemic and pulmonary hypertension
-
Acute aortic regurgitation
-
Volume overload—Renal failure (Fig. 8).
The fourth heart sound (S4) is a late diastolic sound that corresponds to late ventricular filling through the active atrial contraction. It is a low-intensity sound heard best with the bell of the stethoscope. Some of the conditions associated with S4 include the following:
-
Ventricular hypertrophy—LV hypertrophy (systemic hypertension, hypertrophic cardiomyopathy, AS); RV hypertrophy (pulmonary hypertension, pulmonary stenosis [PS])
-
Ischemic heart disease—Acute MI, angina
-
A ventricular aneurysm
-
Hyperkinetic states that cause forceful atrial contraction (Fig. 9).
S4 is a low-frequency gallop sound that results from a forceful atrial contraction during presystole that ejects blood into a ventricle which cannot expand further. S4 occurs ~90 ms before S1. The fourth heart sound or S4 is an extra heart sound that occurs during late diastole, immediately before the usual two “lub-dub” heart sounds (S1 and S2). It occurs just after atrial contraction and immediately before the systolic S1 and is caused by the atria contracting forcefully to overcome an abnormally stiff or hypertrophic ventricle.
In abnormal heart, the additional sounds, called Murmurs are heard between the normal heart sounds. Murmurs are generally caused either by improper opening of the valves or by regurgitation. Heart murmurs are generated by the turbulent flow [30] of blood, which may occur inside or outside the heart. Murmurs may be physiological (benign) or pathological (abnormal). Abnormal murmurs can be caused by stenosis restricting the opening of a heart valve, resulting in turbulence as blood flows through it. Abnormal murmurs may also occur with valvular insufficiency (regurgitation), which allows backflow of blood when the incompetent valve closes with only partial effectiveness. Different murmurs are audible in different parts of the cardiac cycle, depending on the cause of the murmur.
Regurgitation through the mitral valve and Aortic valve is by far the most commonly heard murmur, producing a pansystolic/holosystolic murmur which is sometimes reasonably loud to a practiced ear, even though the volume of regurgitant blood flow may be quite small.
Stenosis of the Aortic valve and Mitral valve is typically the next most common heart murmur, a systolic ejection murmur (Fig. 10).
Multi-Signal Correlations: These are the parameters of the cardiac cycle associated with a healthy human considered in Phonocardiogram signal analysis.
-
Ventricular pressure
-
Aortic pressure
-
Atrial pressure
-
Aortic blood flow
-
Venous pulse
-
Electrocardiogram
-
Phonocardiogram
Heart Diseases: The abnormal heart sounds in a Phonocardiogram generated due to following heart problems.
-
Heart failure
-
Coronary artery disease
-
Hypertension
-
Cardiomyopathy
-
Valve defects
-
Arrhythmia
Some Literature Review Related to Heart Sound Detection and Analysis
Computer-Based Detection and Analysis of Heart Sound and Murmur
A Digital algorithm [31] was developed for detection of first and second heart sounds. R-waves and T-waves in the electrocardiography were used as references for identification. The sound signal analysis was carried out using the short-time Fourier transform. The first heart sound detection rate, with reference to the R-wave, was 100% within 0.05–0.2R-R interval. The second heart sound detection rate between the end of the T-wave and the 0.6R-R interval was 97%. The systolic and diastolic phases of the cardiac cycle could be identified. Because of the overlap between heart sounds and murmur a systolic segment between the first and second heart sounds (20–70%) was selected for murmur analysis. The maximum intensity of the systolic murmur, its average frequency, and the mean spectral power was quantified. The frequency at the point with the highest sound intensity in the spectrum and its time from the first heart sound, the highest frequency, and frequency range were also determined. This method will serve as the foundation for computer-based [31] detection of heart sounds and the characterization of cardiac murmurs.
A Signal Processing Module for the Analysis of Heart Sounds and Heart Murmurs
A Signal Processing Module (SPM) for the computer-aided analysis of heart sounds has been developed. The module reveals essential information of cardiovascular disorders and can assist the general physician to come up with more accurate and reliable diagnosis at early stages. It can overcome the deficiency of expert doctors in rural as well as urban clinics and hospitals. The module has five main blocks: Data Acquisition and Pre-processing, Segmentation, Feature Extraction, Murmur Detection and Murmur Classification. The heart sounds are first acquired using an electronic stethoscope which has the capability of transferring these signals to the nearby workstation using wireless media. Then the signals are segmented into individual cycles as well as individual components using the spectral analysis of heart without using any reference signal like ECG. Then the features are extracted from the individual components using Spectrogram and are used as an input to a MLP (Multiple Layer Perceptron) Neural Network that is trained to detect the presence of heart murmurs. Once the murmur is discovered, they are classified into seven classes depending on their timing within the cardiac cycle using Smoothed Pseudo Wigner-Ville distribution. The module has been tested with real heart sounds from 40 patients and has proved to be quite efficient and robust while dealing with a large variety of pathological conditions.
Heart Sounds Classification Using Feature Extraction of Phonocardiography Signal
The Phonocardiogram (PCG) signals contain beneficial information about the condition of the heart. By analyzing these signals, early detection and diagnosis of heart diseases can be done. It is also instrumental in the case of infants, where ECG recording and other techniques are challenging to implement. In this paper, [32] a classification method is proposed to classify normal and abnormal heart sound signals having murmurs without getting into the cumbersome process of segmenting fundamental heart sounds (FHS) using Electrocardiogram (ECG) gating. The proposed algorithm can be easily implemented on latest electronic stethoscopes, and therefore the unnecessary ECG can be avoided.
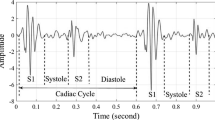
Heart disease is a significant health problem and a leading cause of fatality throughout the world. The treatment can be more comfortable, efficient and economical if the condition is detected early. So it would be very beneficial to detect heart diseases at an early stage. Cardiac disorders can be detected efficiently and economically using auscultation as it requires minimal equipment. Sometimes this is the only available option for diagnosis as in case of primary health care centers, where other high-end instruments for diagnosis are unavailable and also in case of infants where other techniques like ECG are challenging to implement. Conventional auscultation requires extensive training and experience and storage of records for follow-ups and future references are not possible [3]. It is the driving force for this study to move towards automatic auscultation using electronic stethoscopes. Heart sounds comprise four components among which the dominant two S1 and S2 (see Fig. 1) are commonly known as FHS and are caused by the closing of valves. After the vibration of ventricular valves causes that S3 as a result of first rapid filling, S4 occurs when atria contract during the second phase of ventricular filling. The low-pitched S1 is best heard at mitral auscultation site as it is caused by the closure of mitral and tricuspid valves. The shorter duration but louder sound S2 is generated by aortic and pulmonary valve closure and is best heard over aortic auscultation area.
The authors aims to develop a method for classification of heart sounds into normal and abnormal sounds so that state of heart could be periodically checked at home and everybody doesn’t have to wait for symptoms of the disease to appear and then approach a cardiologist. The PCG signals can be an early indicator of heart problems so before worsening of the problem; a proper diagnosis can be made. The other techniques like Echocardiography could be implemented to get a better view of the problem. The proposed method is not replacing other technologies but an early indicator of the problem to prevent worst health condition. During the study, the PCG signals were analyzed for harmonic amplitude for normal, abnormal and murmur heart sounds. The result shows that the amplitude of the murmur signal is higher than normal signal between the fundamental heart sounds S1 and S2. In the experimental procedure, the segments between S1 and S2 of the cardiac cycle and consecutive [1] same segments of the sample wave form were taken for consecutive analysis. The features were extracted to differentiate between normal and murmur signals.
Heart Sound Analysis Technique
The Heart auscultation [32] is the interpretation of heart activities by the physician during cardiac diagnosis. It requires difficult skill to acquire. It is convenient to diagnosis the failure using some monitoring techniques. This paper reviews different signal processing technique for analyzing Heart Sound (HS) Vibration signals which (the interpretation by a physician of heart sounds) are used primarily to diagnose these diseases. Conventional methods for fault diagnosis are mainly based on observing the amplitude differences in time or frequency domain such as Fourier Transform (FT), Short Time Fourier Transform (STFT), and Wavelet transform. This paper includes Spectral analysis method of heart sound by using autoregressive power spectral density (AR-PSD) for discriminating normal and abnormal HS, another approach to diagnosing heart sounds such as Wavelet packet analysis and classifiers like Hidden Markov Model (HMM), Artificial Neural Network (ANN).
Signal Processing Techniques for Heart Sound Analysis in Clinical Diagnosis
It presents an overview of approaches to the analysis of heart sound signals. The paper reviews the milestones in the development of phonocardiogram (PCG) signal analysis. It describes the various stages involved in the analysis of heart sounds and discrete wavelet transform as a preferred method for bio-signal processing. Besides, the gaps that still exist between contemporary methods of signal analysis of heart sounds and their applications for clinical diagnosis is reviewed. A lot of progress has been made, but critical gaps still exist. It is observed that there is a lack of consensus in research outputs. The inter-patient adaptability of signal processing algorithm is always problematic. The process of clinical validation of analysis techniques was not sufficiently rigorous in most of the reviewed literature and as such data integrity and measurement are still in doubt, which most of the time led to an inaccurate interpretation of results. Besides, the existing diagnostic systems are too complicated and expensive. The paper concluded that the ability to correctly acquire, analyze and interpret heart sound signals for improved clinical diagnostic processes had become a priority.
Automatic Detection of Sounds and Murmurs in Patients with Lonescu-Shiley Aortic Bioprostheses
The problems faced in the automatic identification of cardiac sounds and murmurs are numerous. The phonocardiogram (PCG) is a complex sound signal produced by deterministic [32] events such as the opening and closing of the heart valves, and by random phenomena such as blood flow turbulence. In addition, background noise and the dependence of the PCG on the recording sites render automatic detection a difficult task. In the paper, we present an iterative automatic detection algorithm based on the a priori knowledge of spectral and temporal characteristics of the first and second heart sounds, the valve opening clicks, and the systolic and diastolic murmurs. The algorithm uses estimates of the PCG envelope and noise level to identify iteratively the position and duration of the significant acoustic events contained in the PCG. The results indicate that it is particularly useful in detecting the second heart sound and the aortic component of the second heart sound in patients with lonescu-Shiley aortic valve bioprostheses. It also has some potential for the detection of the first heart sound, the systolic murmur, and the diastolic murmur.
Recent Advances in Heart Sound Analysis
Cardiovascular disease (CVD) continues to be the leading cause of morbidity and mortality worldwide with an estimated 17.5 million people [33] have died from CVD-related conditions in 2012, representing 31% of all global deaths. The burden on low to middle-income countries (LMICs) is particularly worrisome, and yet high-quality diagnostics can often be difficult to obtain in much of these resources constrained regions. Although ultrasound and magnetic resonance imaging have displaced auscultation in the more vibrant economies, heart sound auscultation remains a stalwart diagnostic of the ambulatory doctor. However, with the patient to doctor ratios as high as 50,000:1 in some regions of the world, access to expert diagnosis is often impeded. A potential solution to this is to provide an automated diagnosis on the mobile phone or in the cloud [33].
In the past few decades, automated heart sound signal (i.e., phonocardiogram or PCG) analysis has been widely studied. Research shows that automated heart sound segmentation and classification techniques have the potential to screen for pathologies in a variety of clinical applications at a relatively low cost. However, comparative analyses of algorithms in the literature have been hindered by the lack of a large and open database of heart sound recordings. The 2016 PhysioNet/Computing in Cardiology (CinC) Challenge addressed this issue [33] by assembling the largest public heart sound database, aggregated from eight sources obtained by seven independent research groups around the world. The database includes 4,430 recordings taken from 1,072 subjects, totaling 233,512 heart sounds [33] collected from both healthy subjects and patients with a variety of conditions such as heart valve disease and coronary artery disease. These recordings were collected using heterogeneous equipment in both clinical and nonclinical (such as in-home visits). A total of 48 teams submitted 348 open source entries. This special issue provides an extended forum to document and discuss this challenge, and to provide follow-up and improvements.
Heart Sound Detection and Analysis in Real-Time [34]
Usually, physicians use an acoustic stethoscope to detect abnormalities in the heart sound and to detect abnormal conditions of the human heart. As the frequency range and intensity of heart sound is very low, doctors face problems while detecting the cardiac sound and its abnormalities. To eradicate these severe problems, it is required to design and develop an electronic stethoscope which would assist the doctor to analyze heart sound and to detect a diseased heart. Here an acoustic stethoscope along with microphone and preamplifier module is used to increase the amplitude of the input sound signal received by the stethoscope. The soft scope of MATLAB program has also been used to analyze the continuous set of cardiac sound and to detect its various characteristics like frequency, amplitude, etc. It is aimed to design an electronic stethoscope which would assist the doctors to analyze heart sound and detect a diseased heart, but preliminarily we have achieved to detect different components of it which are lub (s1), dub (s2), s3, s4, etc. Finally, the sound signal received from the heart in the MATLAB program after filtering the noise out of it also has been plotted (Fig. 11).
Problem Definition and Objective
Problem Definition:
-
The electronic stethoscopes available in the market are very costly with an average cost of €300–€399 [5]. This high cost makes them less than ideal for those fields where the additional functionality of the electronic stethoscopes are not required [35].
-
Moreover, their battery life is also very poor with an average of 60 h of use [5]. In most of the cases, the batteries are irreplaceable, and thus the stethoscope becomes of no use once the battery life is over. In some cases, though, the batteries can be replaced which leads to additional cost after paying a heavy initial price for the electronic stethoscope.
-
India has not been able to make any significant contribution to the market of the electronic stethoscope. Most of the electronic stethoscopes available in the Indian market are imported.
-
Most importantly, the electronic stethoscopes available in the market are unable to diagnose heart disease by itself. It only assists the doctors in identifying heart problems quickly and efficiently. Hence these stethoscopes are not for the laymen to use. The most commonly available electronic stethoscopes in the market are 3MTHLittman Electronic Stethoscopes, E-scope, AMD-3150 and ADSCOPETH 657. All of these stethoscopes are unable to analyze heart disease by themselves and require a medical specialist for it.
Objective:
The primary aim of the research work is to develop an electronic stethoscope which will be devoid of the problems of the currently available electronic stethoscopes in the market as mentioned above. The intention is to create a stethoscope that will be able to diagnose heart disease by itself and hence can be used by the laymen. The main structure of the electronic stethoscope can be constructed by using an acoustic stethoscope along with a microphone and preamplifier module. Moreover, the aim is to evaluate the performance of the electronic stethoscope without the help of an external analog-to-digital converter (ADC). This research work also depicts the use of feature analysis to distinguish between Normal heart sound, abnormal heart sound, and Cardiac.
Methods and Materials
The entire work is a materialization of a prolonged study and thoughts. Many articles and research papers have been studied on detection of cardiac murmur, analysis of heart sound, detection of a diseased heart sound, development of electronic stethoscope, etc. In every case it has been seen through detailed and advanced work is done but the expense of developing a digitalized stethoscope is very high. It influenced us to develop an electronic stethoscope at low cost. It has been aimed to design an electronic stethoscope which would assist the doctor to analyze heart sound and detect a diseased heart.
Figure 12 shows the image of the hardware that has been implemented in the research work. It shows an acoustic stethoscope attached to condenser microphone of the preamplifier module with the help of a hollow rubber-like tube. The battery along with the required circuit, made using a Vero-board, is placed on a thin Bakelite board. Aluminum strip is used to hold the battery and the microphone to the stetho-tube connector to the bakelite board.
As seen from the circuit diagram, the hardware part of the circuit consists of the following components:
The acoustic sensor and the amplifier module are discussed in details in the later section. The battery used is a rechargeable battery which can be charged by connecting the USB port to a laptop or a similar device. When the battery is charged, the LED-2 glows. A resistance of 330 Ω is applied before each LED to prevent the LEDs from getting damaged. The switch used is a dual pole dual throw (DPDT) switch. When the switch is connected to point A then the power from the battery reaches the amplifier module and it starts working. At that time the LED-1 glows. The switch is connected to point B in order to charge the battery. The audio jack, when connected to the microphone plug of the laptop or CPU, transmits sound signal from the amplifier module to the laptop/computer (Figs. 13 and 14).
Testing of the stethoscope to find its frequency response
Step 1: To find out the frequency response of the developed electronic stethoscope, it needs a sound source with variable frequency. One small speaker unit of diameter same as that of the chest piece of the stethoscope and amplifier module is used to excite the speaker unit. In this testing, a smartphone app named Hz Generator is used which served as a function generator for the research work [36]. A 3.5 mm double-sided audio jack cable is used to connect the amplifier to the smartphone.
Step 2: The chest piece of the acoustic stethoscope is then attached to the speaker of the speaker and amplifier module with the help of cello tape.
Step 3: The setup shown in Fig. 5 is then covered with thermocol to make it soundproof. It is because thermocol is a good absorber of sound and thus prevent the ambient noise from reaching the setup. The sine wave signals of particular frequencies are fed to the amplifier module which then reaches the chest piece of the stethoscope. In the research work, the sound signals are transmitted from the mobile, through a cable, using cellphone application software named as Hz Generator. This software can generate sound signals of any frequency and shape as per the requirement.
Step 4: The below image shows the entire experimental setup required for finding the frequency response characteristic of the stethoscope used in the research work. The stethoscope amplifier output signal is fed to the audio input of the computer system for analysis. Then sine wave sound signals of fixed amplitude and different frequencies are sent successively, and the corresponding amplitudes are measured in the soft scope of MATLAB through the computer system.
Frequency versus amplitude data is plotted as obtained experimentally and shown in Fig. 6. It is observed from the plot, the frequency response of the developed electronic stethoscope is flat from 20 to 3100 Hz. After that, a sharp cutoff frequency has been found (Fig. 15).
Software Development
Software development is the process of computer programming, documenting, testing and bug fixing involved in creating, maintaining applications and frame works involved in software released life cycle and resulting in a software product.
For this work two different approaches have been used of Software Development: (a) Matlab Scripts, (b) Simulink
It has been experimented to detect heart sound by the developed electronic stethoscope in actual human subject. Initially, it has tested individual filters by calibrated signal and then finally used to tune the measurement system.
Since the electronic stethoscope has many disadvantages like it is very costly and the sound which is heard in the earpiece of stethoscope is of very low strength and also has a lot of noise incorporated in it and hence it is very difficult to analyze.
For this reason the heart sound wave can be plotted in Matlab using Matlab scripts or Simulink and can be analyzed better by observing the spectrum. Also real time data acquisition can be done with the help of Simulink.
The filters are also designed using Matlab scripts and the Digital Signal Processing toolbox and hence it becomes cost effective and is efficient in function and gives us a noise free signal.
The Simulink Model is the second approach of the Software Development. The Simulink Model is created for a better analysis of the heart sound waveform than the Matlab Scripts. The Simulink Model can also be used for the real time data acquisition of the system. In the proposed system the developed electronic stethoscope output is fed to the microphone input of the laptop which is called legacy input of the laptop computer system. The laptop is normally have built in A/D converter with DSP (Legacy input circuit) is being exploited by MATLAB environment for heart sound data acquisition, storage and analysis (Fig. 16).
Filter Design for Removal of Noise
Filter design part is needed as it plays a significant role in eliminating noise from heart sounds. It is a pretty perfect solution, since the background noise is embedded in the low-frequency range of the spectrum, whereas the heart sound has a higher frequency. As such, a band pass filter can be applied to get rid of the low noise, capture most of the Heart sound, and any noisy frequencies on the higher side will get canceled as well.
Here are the steps that are implemented
-
1.
Read in the audio file using wavread function in Matlab.
-
2.
Plot the sound signal in time domain, for time domain analysis.
-
3.
Take the Fast Fourier Transform (FFT) and see the frequency distribution.
-
4.
Using (3), figure out the rough approximation of where should the cut-off frequencies be selected.
-
5.
Design a Butterworth bandpass filter that cuts off these frequencies.
Here some standard Normal Heart Sounds have been collected from Heart-Sound Library source, and those sound files have been read in mat lab and then time domain analysis, frequency domain analysis have been performed. Finally, the filtering operation has been done of low-frequency background noise from the standard Normal heart sound using Butterworth Band Pass Filter of order-1 after selecting the proper cutoff frequencies, and then the amplitude spectrum of that signal has been plotted.
Step-1:
%% Read in the audio file
clearvars;
close all;
[f,fs] = wavread('PCG.wav');
“PCG.wav” file is the normal heart sound in wav format contains only lub dub.
f would be the signal read into MATLAB & f here is a 2D matrix.
fs is the sampling frequency of the audio signal.
Step -2:
%% Plot audio channel in Time Domain
N = size(f,1); % Determine total number of samples in audio file
figure;
stem(1:N, f(:,1));
title('Time Domain Analysis');
xlabel('Time');
ylabel('Amplitude');
The output of Step-2 of the above written MatLab scripts is given in Fig. 17
Step -3:
FFT is used to take a look at the frequency spectrum of the signal. FFT can be called by specifying the input signal you want as the first parameter, followed by how many points required to evaluate at with the second parameter. It is customary that specify the number of points in the FFT to be the length of the signal. As the Frequency spectrum obtained after doing FFT is un-centered so take the fftshift to make it a centered one. When the frequency spectrum is plotted, it indicates how much contribution that frequency has with the output. That is defined with the magnitude of the signal. It has been done by taking the abs function.
%% Plot the spectrum
df = fs/ N;
w = (−(N/2):(N/2) − 1)*df;
y = fft(f(:,1), N)/ N; % For normalizing, but not needed for our analysis
y2 = fftshift(y);
figure;
plot(w,abs(y2));
xlabel('Frequency');
ylabel('Amplitude Distribution');
title('Amplitude Spectrum of Normal Heart Sound');
The output of Step-3 of the above written MatLab scripts is given in Fig. 18.
Step -4:
The bandpass flag means you want to design a bandpass filter, beginFreq and endFreq map to the normalized beginning and ending frequency you want to for the bandpass filter. In this work, that’s beginFreq = 70/Nyquist and endFreq = 400/Nyquist. b, a is the coefficients used for a filter that will help you perform this task
%% Design a bandpass-filter that filters out between 800 and 1200 Hz
n = 1;
beginFreq = 70/(fs/2);
endFreq = 400/(fs/2);
[b,a] = butter(n, [beginFreq, endFreq], ‘Bandpass’);
Step -5:
“filter” is used to filter the signal using what has been obtained from Step 4. fOut will be the filtered signal. Finally to obtain the amplitude spectrum of the filtered signal again use fft and fftshift functions respectively. Amplitude Spectrum of the Noise Free Normal Heart Sound is shown below.
%% Filter the signal
fOut = filter(b, a, f);
g=fft(fOut(:,1),N)/N;
g2=fftshift(g);
figure;
plot(w,abs(g2));
xlabel('Frequency');
ylabel('Amplitude Distribution');
title('Amplitude Spectrum of Noise Free Normal Heart Sound');
The output of Step-5 of the above written MatLab scripts is given in Fig. 19.
Feature Analysis
After removal of noise from actual heart sound signal following features are selected for analysis
-
1.
Mean value of Amplitude
-
2.
Mean value of Phase
-
3.
Kalman Filter Approach
During the analysis of Harmonic-Distribution of Amplitude and Phase, the contribution of higher order harmonics being neglected. The Harmonic Distribution can be obtained first by conversion of the time domain sound signal into frequency domain signal and then separate out the real components with the imaginary components [37].
For Simplicity, harmonics order up to 40 is considered. Following distributions depict the Harmonic-Distribution of Amplitude and Phase for Normal Heart Sound, Abnormal Heart Sound and Cardiac Murmurs. From the three Harmonic amplitude distributions as obtained from real-time analysis, it can be concluded that amplitudes of harmonics in Normal Heart Sound are higher compared to Abnormal Heart Sound and Cardiac Murmurs. Higher Order harmonics are neglected as their amplitudes are very small. Figures 20, 21 and 22 indicate the analysis to support the findings.
Kalman Filter Approach in Heart Sound Diagnosis
Rudolf Emil Kalman, a Professor at Standford University developed a mathematical technique called Kalman Filter widely used in digital computers of control systems, navigation, avionic and outer space vehicles to extract signal from noisy and incomplete measurements. Kalman filtering [38] is a useful tool for a variety of different applications. Kalman filtering is also sometimes called “linear quadratic estimation.” Now let us think about the “filter” part. All filters share a common goal: to let something pass through while something else does not. An example that many people can relate to is a coffee filter. The coffee filter [20] will allow the liquid to pass through while leaving the solid coffee grounds behind. You can also think about a low-pass filter, which lets low frequencies pass through while attenuating high frequencies. A Kalman filter also acts as a filter, but its operation is a bit more complicated and harder to understand. A Kalman filter takes in information [39] which is known to have some error, uncertainty, or noise. The goal of the filter is to take in this imperfect information, sort out the useful parts of interest, and to reduce the uncertainty or noise [38].
The Kalman Filter is a state estimator filter which gives the result as an optimal estimator in the sense that the mean value of the sum of the estimation errors gets a minimal value [38]. The Kalman filter estimated the sum of squared errors. The filter contains a model of dynamic state process that performs a function, and as a feedback correction function method there is no requirement of the store a data and other all associate matrices like measurement system, dynamics and noise are considered to be known. Kalman filter has two steps (Fig. 23)
-
1.
Prediction
-
2.
Correction
In this work, Kalman filter approach has been applied to different heart sounds, and gathered information about the estimated value of the heart sound with an increase in harmonics. As the harmonics of complex heart sound gets increased, it has been found that estimated value comes closer to measured value with the decrease in gain from 1 to 0 value (Fig. 24).
Also, it has been observed that in case of Normal Heart Sound, the difference between Estimated value [40] and Measured value is more compared to Abnormal heart sound and Cardiac Murmurs (Figs. 25, 26, 27, 28 and 29).
In this chart (Fig. 30), 20 odd samples of a Normal heart sound, Abnormal Heart sound, and cardiac murmurs have been considered and compared after computation of Average of Amplitude Harmonic Distribution. It can be seen that from this comparative study of Harmonic distributions, Cardiac Murmurs can be easily distinguished and identified. It has been found that the average value of the amplitude harmonic distribution is higher in case of Cardiac murmurs compared to Normal heart sound and abnormal heart sound.
Conclusion
This electronic stethoscope can be used to detect normal heart sounds, abnormal heart sounds, and cardiac murmurs. Thus it can be used to diagnose a normal and abnormal heart sound accurately. Also with the help of filter design part, the background noise mostly gets eliminated. The instruments developed by us are working satisfactorily and detecting heart sound including murmur as well as we can use this detected signals for feature analysis [41]. The feature analysis part is in our present scope of work, but in next phase, we will carry out experimentation with our system for diseased heart sound.
This Book chapter focused on the problem phonocardiogram analysis as an aid tool to detect and diagnose any abnormalities of a heart condition. With the above-mentioned goal, the investigation can be made in a wide variety of fields ranging from cardiac auscultation, wavelet threshold de-noising, heart sound segmentation, Hilbert–Huang transform, feature extraction and classification by support vector machine. We developed a wavelet-based de-noising scheme that achieves a cleaner signal. We developed an energy-based segmentation method which is validated to perform well for both normal and typical abnormal heart sounds. We can apply Hilbert–Huang Transform to analyze the heart sounds and calculate the energy of selected IMF components that enabled us to construct the feature vector. Finally, the classification goals were accomplished using Support Vector Machine. Simulation results indicated that the proposed algorithm achieves the sought goal with a classification accuracy of 90.48% composed of 100% sensitivity and 81.8% specificity. Overall, a framework to classify normal and abnormal heart sounds is proposed using PCG. This system can be implemented in real time using PCG obtained from the new digital stethoscope technology and by the present state of the art embedded technology. Fourty one other future suggestions can be to aim to further analyze the abnormal into the different classes of heart diseases. Indeed, this goal opens up several avenues for the further research to be more accurate. First, we need to have a deep explore in Cardiac physiology, which is the foundation of all research of heart auscultation to allow for better feature recognition. Second, with the current framework, one can add more features that could present a potential to classify the different murmur signals. We need to extract the features from time domain and frequency domain and combine it with other statistical characteristics to produce a thorough analysis of the framework performance versus the different features. Third, we suggest a multi-classifier such as Multiclass SVM can be very valuable in solving such a classification problem, with the key point is the ability to create a suitable decision tree of SVM.
References
https://www.ukessays.com/essays/engineering/pest-detection-leafs-using-robot-processing-4110.php
L.-G. Durand, Y.-E. Langlois, T. Lanthier, R. Chiarella, P. Coppens, S. Carioto, S. Bertrand-Bradley, Spectral analysis and acoustic transmission of mitral and aortic valve closure sounds in dogs. Med. Biol. Eng. Comput. 28(5), 439–445 (1990)
World Health Organization, Global status report on noncommunicable diseases 2014 (2014)
A. Leatham, Auscultation and phonocardiography: a personal view of the past 40 years. Br. Heart J. 57, 397–403 (1987)
J.R. Kindig, et al., Acoustical performance of the stethoscope: a comparative analysis. Am. Heart J. 104(2 Pt 1), 269–275 (1982); S. Lukkarinen, et al., A new phonocardiographic recording system. Comput. Cardiol. 24, 117–120 (1997)
D.A. Balster et al., Digital acoustic analysis of precordial innocent versus ventricular septal defect murmurs in children. Am. J. Cardiol. 79(11), 1552–1555 (1997)
R.M. Rangayyan, R.J. Lehner, Phonocardiogram signal analysis: a review. Crit. Rev. Biomed. Eng. 15(3), 211–236 (1987)
J.R. Bulgrin et al., Comparison of short-time Fourier, wavelet and time-domain analyses of intracardiac sounds. Biomed. Sci. Instrum. 29, 465–472 (1993)
P. Bentley, G. Nordehn, M. Coimbra, S. Mannor, R. Getz, Classifying heart sounds challenge. http://www.peterjbentley.com/heartchallenge/#downloads
M. Abella, J. Formolo, D.G. Penney, Comparison of the acoustic properties of six popular stethoscopes. J. Acoust. Soc. Am. 91(4 Pt 1), 2224–2228 (1992)
Y. Wapcaplet, Diagram of the human heart (2003) (Online). http://commons.wikimedia.org/wiki/File:Diagram_of_the_human_heart_(cropped).svg
Anju, S. Kumar, Detection of cardiac murmur. Int. J. Comput. Sci. Mob. Comput. 3(7), 81–87 (2014). ISSN 2320–088X
B. Popov, G. Sierra, L.G. Durand, J. Xu, P. Pibarot, R. Agarwal, V. Lanzo, Automated extraction of aortic and pulmonary components of the second heart sound for the estimation of pulmonary artery pressure, in Proceedings of IEEE Engineering in Medicine and Biology Society, vol. 2, pp. 921–924 (2004)
Cardiac cycle, https://en.wikipedia.org/wiki/Cardiac_cycle
M. Tavel, Classification of systolic murmurs: still in search of a consensus. Am. Heart J. 134(2), 330–336 (1997)
C. Cortes, V. Vapnik, Support-vector networks. Mach. Learn. 20(3), 273–297 (1995)
Heart murmur, National Heart & Blood Institute, https://www.nhlbi.nih.gov/health/health-topics/topics/holes/types
Z. Syed, D. Leeds, D. Curtis, F. Nesta, R.A. Levine, J. Guttag, A framework for the analysis of acoustical cardiac signals. IEEE Trans. Biomed. Eng. 54(4), 651–662 (2007)
H. Liang, I. Hartimo, A heart sound feature extraction algorithm based on wavelet decomposition and reconstruction, in Proceedings of 20th Annual International Conference IEEE Engineering in Medicine and Biology Society, vol. 20; Biomed. Eng. 3(3), 1539–1542 (1998), Towards Year 2000 Beyond (Cat. No. 98CH36286)
Z. Xiu-min, C. Gui-tao, A novel de-noising method for heart sound signal using improved thresholding function in wavelet domain, in 2009 International Conference on Future BioMedical Information Engineering (FBIE), pp. 65–68 (2009)
D.L. Donoho, De-noising by soft-thresholding. IEEE Trans. Inf. Theory 41(3), 613–627 (1995)
A. Zaeemzadeh, Z. Nafar, S.-K. Setarehdan, Heart sound segmentation based on recurrence time statistics, in 2013 20th Iranian Conference on Biomedical Engineering (ICBME), no. Icbme, pp. 215–218 (2013)
T.-H. Hung, C.-C. Chou, W.-C. Fang, A.H.-T. Li, Y.-C. Chang, B.-K. Hwang, Y.-W. Shau, Time-frequency analysis of heart sound signals based on Hilbert-Huang transformation, in 2012 IEEE 16th International Symposium on Consumer Electronics, pp. 1–3 (2012)
M. Singh, A. Cheema, Heart sounds classification using feature extraction of phonocardiography signal. Int. J. Comput. Appl. 77(4), 975–8887 (2013)
H. Nygaard et al., Assessing the severity of aortic valve stenosis by spectral analysis of cardiac murmurs (spectral vibrocardiography). Part II: Clinical aspects. J. Heart Valve Dis. 2(4), 468–475 (1993)
R.-E. Fan, P.-H. Chen, C.-J. Lin, Working set selection using second order information for training support vector machines. J. Mach. Learn. Res. 6, 1889–1918 (2005)
D.B. Springer, L. Tarassenko, G.D. Clifford, Support vector machine hidden semi-Markov model-based heart sound segmentation time, in Computing in Cardiology (2014)
H.L. Baranek et al., Automatic detection of sounds and murmurs in patients with Ionescu-Shiley aortic bioprostheses. Med. Biol. Eng. Comput. 27(5), 449–455 (1989)
Review on heart sound analysis technique. SpringerLink, https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-81-322-1299-7_9
Heart sounds—Wikipedia, https://en.wikipedia.org/wiki/Heart_sounds
Heart sounds classification using feature extraction of …, https://pdfs.semanticscholar.org/73a7/3eb140b1f7b496f801d697174392c172c55c.pdf
Recent advances in heart sound analysis—Physiological …, http://iopscience.iop.org/journal/0967-3334/page/Recent-advances-in-heart-sound-analysis
J.K. Roy, T.S. Roy, A simple technique for heart sound detection and real time analysis, in Proceedings of ICST 2017, 2017 Eleventh International Conference on Sensing Technology (ICST) held at Maquare University Sidney, 4–6 Dec 2017, https://doi.org/10.1109/icsenst.2017.8304502
A. Iwata et al., Algorithm for detecting the first and the second heart sounds by spectral tracking. Med. Biol. Eng. Comput. 18(1), 19–26 (1980)
The Second Heart Sound—Clinical Methods—NCBI Bookshelf, https://www.ncbi.nlm.nih.gov/books/NBK341/
Harvard Health Publishing, https://www.health.harvard.edu/heart-health/heart-valve-problems
A Kalman Filtering Tutorial For Undergraduate Students, http://aircconline.com/ijcses/V8N1/8117ijcses01.pdf
Heart murmur causes, https://www.nhlbi.nih.gov/health/health-topics/topics/heartmurmur/causes
M. El-Segaier, O. Lilja, S. Lukkarinen, L. Sörnmo, R. Sepponen, E. Pesonen, Computer-based detection and analysis of heart sound and murmur, Ann. Biomed. Eng. 33(7), 937–942 (2005), http://www.ncbi.nlm.nih.gov/pubmed/16060534
S.M. Debbal, Computerized heart sound analysis. Genie—Biomedical Laboratory (GBM), Department of electronic, Faculty of science engineering, University of Abou Bekr Belkaid, Algeria
R.L. Donnerstein, Continuous spectral analysis of heart murmurs for evaluating stenotic cardiac lesions. Am. J. Cardiol. 64(10), 625–630 (1989)
B. Maurice, E.E. Rappaport, B. Haward, M.D. Sprague, The acoustic stethoscope and the electrical amplifying stethoscope and stethograph. Am. Heart J. 21(3), 257–318 (1940)
M. Nygards, L. Sornmo, Delineation of the QRS complex using the envelope of the E.C.G. Med. Biol. Eng. Comput. 21(5), 538–547 (1983)
H. Nygaard et al., Assessing the severity of aortic valve stenosis by spectral analysis of cardiac murmurs (spectral vibrocardiography). Part I: Technical aspects. J. Heart Valve Dis. 2(4), 454–467 (1993)
J.D. O’Toole et al., The mechanism of splitting of the secondheart sound in atrial septal defect. Circulation 56(6), 1047–1053 (1977)
Gallop rhythm, https://en.wikipedia.org/wiki/Heart_sounds
Cardiac sound generation, http://www.wisegeek.com/what-are-different-types-of-heart-sounds.htm#
The circulatory system, http://lsa.colorado.edu/essence/texts/heart.html#Structure
Pinterest, https://in.pinterest.com/pin/407505466254590427/?autologin=true
Stethoscope, https://en.wikipedia.org/wiki/Stethoscope
D. Gede, H. Wisana, Design electronic stethoscope for cardiac auscultation analyzed using wavelet decomposition. Int. J. Comput. Netw. Commun. Secur. 1(7), 310–315 (2013), www.ijcncs.org. ISSN 2308-9830
K. Singh, P. Abrol, Design and development of a digital stethoscope for cardiac murmur. Int. J. Comput. Appl. (0975–8887) 73(22) (2013)
M. Singh, A. Cheema, Heart sounds classification using feature extraction of phonocardiography signal. Int. J. Comput. Appl. (0975–8887) 77(4) (2013)
D.D. Kadam Patil, R.K. Shastri, Design of wireless electronic stethoscope based on ZIGBEE, Cornell University Library, https://arxiv.org/abs/1202.1680
L.B. Dahl, P. Hasvold, E.Arild, T. Hasvold, Heart murmurs recorded by a sensor based electronic stethoscope and e-mailed for remote assessment, Arch. Dis. Child 87, 297–301, 19 March 2002
W.W. Myint, B. Dillard, An electronic stethoscope with diagnosis capability. Department of Electrical & Computer Eng., Auburn University, College of Engineering, Auburn, AL 36849 USA
https://datasheets.maximintegrated.com/en/ds/MAX9812-MAX9813L.pdf
J. Peter Robinson, Digital electronic stethoscopes. 3 M Healthcare Ltd, Loughborough, UK
History of the Stethoscope—American Diagnostic Corporation, https://www.adctoday.com/learning-center/about-stethoscopes/history-stethoscope
Introduction to Digital Stethoscopes and Electrical …, https://pdfserv.maximintegrated.com/en/an/AN4694.pdf
Automatic detection of sounds and murmurs …. SpringerLink, https://springerlink.bibliotecabuap.elogim.com/article/10.1007/BF02441460
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Roy, J.K., Roy, T.S., Mukhopadhyay, S.C. (2019). Heart Sound: Detection and Analytical Approach Towards Diseases. In: Mukhopadhyay, S., Jayasundera, K., Postolache, O. (eds) Modern Sensing Technologies . Smart Sensors, Measurement and Instrumentation, vol 29. Springer, Cham. https://doi.org/10.1007/978-3-319-99540-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-99540-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99539-7
Online ISBN: 978-3-319-99540-3
eBook Packages: EngineeringEngineering (R0)