Abstract
This chapter will summarize key data about glutamatergic transmission in the hippocampus. Glutamate is the major excitatory neurotransmitter similar to other CNS regions. Biophysical properties of various receptors and channels will be described and functional relevance of these parameters discussed.
The major components of the excitatory synaptic network in the hippocampus form the so-called tri-synaptic circuit. This circuit consists of the perforant pathway input from the entorhinal cortex to the dentate gyrus, mossy fibers projecting from the dentate gyrus to the CA3 area, and Schaffer collaterals, axons of CA3 pyramidal cells innervating the CA1 area. This chapter will focus on the properties of these glutamatergic synapses, highlighting the most distinct features these inputs possess.
Glutamatergic transmission in the hippocampus is known to play a crucial role in learning and memory due to activity-dependent changes in synaptic efficacy. However, this chapter will focus on the basic properties of glutamatergic synapses, and “Synaptic Plasticity at Hippocampal Synapses” chapter will discuss synaptic plasticity in detail.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The main excitatory transmitter in the hippocampus is glutamate. Its action is mediated via two main classes of glutamate receptors: ionotropic and metabotropic receptors (Fig. 1).
Major excitatory pathways in the hippocampus. Layer II neurons in the entorhinal cortex project to the dentate gyrus and the CA3 via the perforant pathways (1, blue). Neurons in layer III of the entorhinal cortex send their axons to the CA1 subfield and the subiculum (1, purple). Dentate granule cells innervate the CA3 area via mossy fibers (2, green). Pyramidal cells of the CA3 subregion project to the CA1 area via Schaffer collaterals (3, red)
The ionotropic glutamate receptors are ligand-gated ion channels; they are responsible for the vast majority of fast excitatory neurotransmission in the CNS. In these receptors, glutamate binding causes channel opening, with the resulting predominant Na+ influx leading to membrane depolarization. Based on their particular pharmacology, ionotropic glutamate receptors fall into three major classes which are named after their selective agonists: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), NMDA (N-methyl-D-aspartate), and kainate receptors . All three receptors types form heteromeric structures consisting of four subunits.
AMPA receptors are composed of a combination of four subunits: GluA1 , GluA2, GluA3, and GluA4 (Dingledine et al. 1999). The presence or absence of the GluA2 subunit in the complex will determine several biophysical properties of the receptor. This subunit undergoes posttranscriptional RNA editing at the “Q/R site ”; insertion of the edited form into AMPA receptors will result in low-conductance, Ca2+-impermeable channels with linear I-V relationship. In contrast, GluA2-lacking AMPA receptors have higher conductance, are Ca2+-permeable, and show inwardly rectifying I-V relationships due to the block by endogenous polyamine at positive membrane potentials (Bowie and Mayer 1995; Kamboj et al. 1995; Koh et al. 1995). Principal cells in the hippocampus express high levels of GluA2; hence Ca2+-impermeable AMPA receptors dominate synaptic transmission in these cells. Ca2+-permeable AMPA receptors are present on some hippocampal interneurons , and during development, some fetal GluA2 subunits remain unedited. Inward rectification of GluA2-lacking receptors is caused by voltage-dependent block by intracellular polyamines (Bowie and Mayer 1995; Donevan and Rogawski 1995; Kamboj et al. 1995; Koh et al. 1995). Therefore, polyamine toxins, such as philanthotoxin (PhTx), a high-affinity agonist of currents mediated by receptors lacking the GluA2 subunit, can be used to pharmacologically differentiate between Ca2+-permeable and Ca2+-impermeable AMPA receptors (Blaschke et al. 1993; Washburn and Dingledine 1996).
In the hippocampus, NMDA receptors are heteromultimers of GluN1 and GluN2A-D subunits. NMDA receptors have very slow kinetics compared to AMPA or kainate receptors , which is explained by the slow dissociation rate of glutamate. While glutamate is bound, NMDA channels can undergo repeated opening. NMDA receptors activation requires two agonists, glycine and glutamate. The glycine site must be occupied first, followed by glutamate binding. When this occurs, the channel can open and Na+ and Ca2+ enter the cell. However, channel opening can only occur when Mg2+ block is removed from NMDA receptors while the membrane is depolarized. At resting membrane potentials, glutamate binding does not lead to channel opening. Ca2+ entering the cell via NMDA receptors plays important role in synaptic plasticity.
Kainate receptors are also heteromultimers composed of the combination of GluK1 ,2,3 and GluK4,5 subunits. GluK4 and GluK5 subunits alone are nonfunctional and are retained in the endoplasmic reticulum but can combine with GluK1–3 to form surface-localized functional receptors. GluK1 and GluK6 subunits undergo alternative splicing similar to the GluA2 subunit of AMPA receptors at the Q/R site . Receptors incorporating edited GluK1 and GluK6 subunits have linear I-V relationship, while insertion of the unedited form of GluK1 or GluK6 results in inward rectification due to intracellular polyamine block at depolarized membrane potentials. Receptors containing solely unedited GluK1 and GluK2 are also weakly permeable to Ca2+. Relationship between rectification, Ca2+-permeability, and subunit composition is more complex than in AMPA receptors because only a portion of GluK1 and GluK6 subunits are edited at the Q/R site. Recombinant kainate receptors have fast kinetics; they rapidly activate and deactivate in the submillisecond range. Interestingly these parameters are markedly different from the relatively slow kinetics of synaptically evoked kainate responses.
The metabotropic glutamate receptors contain seven transmembrane domains, and their actions are mediated via G proteins. Metabotropic receptors fall into three groups based on amino acid homology, signal transduction pathway, and their pharmacological profile.
Group I metabotropic receptors are generally localized postsynaptically, coupled to the Gq signaling pathway , and their activation increases cell excitability. Group II and III receptors are localized on the presynaptic membrane and coupled to adenylyl cyclase via G proteins; in general they are involved in the control of neurotransmitter release. Presynaptically located metabotropic glutamate receptors are generally involved in plastic changes leading to modifications in synaptic strength, while glutamate binding to postsynaptic metabotropic receptors can lead to ion channel opening and closing and the generation of various intracellular messengers (Table 1).
The Perforant Pathway
The hippocampus receives its major cortical input from the entorhinal cortex via the perforant pathway . This pathway originates from layer II and III of the entorhinal cortex and provides direct input to all three major areas of the hippocampus. Distal dendrites of dentate granule cells receive input from the lateral entorhinal cortex, while the medial entorhinal cortex innervates the middle third of the molecular layer of the dentate gyrus (Amaral and Witter 1989). In tCA1 region, entorhinal terminals are scattered throughout the stratum lacunosum-moleculare. Projections from the lateral entorhinal cortex innervate the superficial layers of the stratum lacunosum-moleculare, and input from the medial entorhinal area projects to the deep half of this layer (Witter 1993). Medial entorhinal cortical inputs preferentially excite pyramidal cells in the deep pyramidal layer toward the CA2 area, while lateral entorhinal input favors superficial pyramidal cells closer to the subiculum (Masurkar et al. 2017). Direct input to the dentate gyrus and the CA3 originates from layer II of the entorhinal cortex; in contrast distal dendrites in the CA1 area are innervated by axons from layer III. Input from the lateral entorhinal cortex has been shown to play a critical role in episodic memory both in rodents and humans (Wilson et al. 2013; Reagh and Yassa 2014).
Salient Features of Perforant Pathway Synapses:
-
Distinct features of medial and lateral perforant pathway inputs.
-
Direct input to CA1 plays important role in feed-forward inhibition.
-
Input-specific subunit composition of NMDA receptors.
-
Complimentary distribution of metabotropic receptors at the medial and lateral perforant pathway.
AMPA Receptors
Stimulation of the perforant pathway from the entorhinal cortex evokes monosynaptic responses in the dentate granule cells. Both the medial and lateral perforant pathways use glutamate as principal transmitter, and accordingly CNQX blocks 80–90% of the synaptic events at resting membrane potential (Lambert and Jones 1990). However, the physiological and pharmacological properties of these inputs are distinct. Topographical separation of the medial and lateral pathway in the dentate gyrus allows their investigation in isolation. In response to repeated stimuli, lateral perforant pathway synapses exhibit marked facilitation, while medial perforant path synapses show less facilitation or even depression using a paired-pulse paradigm (McNaughton 1980). During the course of a longer train of stimulus, the medial perforant path input shows significant depression, while the lateral pathway shows minimal change (McNaughton 1980; Rush et al. 2002). Since the ratio of the EPSP to fiber response is greater in the medial pathway and the observed short-term depression converts to facilitation in lower extracellular [Ca2+], it is very likely that the initial release probability is lower at lateral pathway synapses than at medial perforant input. Discrepancy between the synaptically released quanta sensed by NMDA and AMPA receptors is observed in the lateral, but not in the medial perforant path, indicating that silent synapses are present only at lateral pathway synapses. Consequently, NMDA receptor-mediated recruitment of AMPA receptors to the active zone could play an important role in plastic changes at this synapse (Min et al. 1998). Several presynaptic receptors have different modulatory effects on these two inputs. Carbachol selectively depresses synaptic potentials evoked with the stimulation of the medial perforant pathway, indicating that acetylcholine receptors are selectively involved in the regulation of the glutamatergic responses at the medial but not at the lateral perforant pathway (Kahle and Cotman 1989). Noradrenalin has opposing effects on the long-term plasticity of medial and lateral perforant path inputs (Dahl and Sarvey 1989; Pelletier et al. 1994; Dahl and Sarvey 1989; Pelletier et al. 1994). Glutamatergic input from hilar mossy cells onto granule cells is potently and transiently suppressed by endocannabinoids ; similarly the lateral perforant path input is also affected, while the medial performant path does not show similar modulation (Chiu and Castillo 2008; Wang et al. 2016).
Pharmacological and electrophysiological differences between lateral and medial perforant inputs terminating on CA3 pyramidal cells show similar distinct pattern even though synaptic inputs are not spatially segregated here (Berzhanskaya et al. 1998).
The dendritic arborization of certain types of inhibitory cells located in the dentate gyrus indicate that they are receiving the vast majority of their inputs from the perforant pathway (MOPP, molecular layer perforant path-associated cells); the functional role of these cells in the modulation of hippocampal activity still needs to be determined (Han et al. 1993). In the CA3 region, interneurons receiving inputs from the perforant pathway and mossy fibers were suggested to act as coincidence detectors manifesting supralinear EPSP summation (Calixto et al. 2008).
Stimulation of the direct perforant path input to the CA1 area evokes a small glutamatergic current in CA1 pyramidal cells. This input is shown to have very little effects on the firing pattern of the postsynaptic cells (Colbert and Levy 1992; Empson and Heinemann 1995b). However, it initiates a powerful feed-forward inhibition and is capable of regulating the probability of Schaffer collateral -evoked CA1 spikes (Empson and Heinemann 1995a; Jarsky et al. 2005; Remondes and Schuman 2002).
Properties of individual perforant pathway inputs onto granule cells and inhibitory cells were investigated using minimal stimulation. While the kinetics of synaptic inputs terminating on inhibitory and excitatory cells are similar, the amplitude of perforant pathway EPSC/Ps is significantly bigger on identified PV basket cells. This difference could stem from larger number of active zones or higher number of AMPA receptors in synapses innervating basket cells (Sambandan et al. 2010) (Table 2).
NMDA Receptors
NMDA receptors significantly contribute to EPSPs evoked by perforant pathway stimulation in dentate granule cells, CNQX blocks 80–90% of the synaptic events at resting membrane potential, and further addition of APV completely abolishes the residual component (Lambert and Jones 1989, 1990). Complete and selective deletion of the GluN1 subunit in granule cells lead to impaired context discrimination in the incremental fear-conditioning paradigm and context-modulated place cell activity in the CA3. However, both of these deficits only manifested in the initial phases of the experiments and were overcome by experience. This indicates that NMDA receptors on granule cells play an important role in the animals’ ability to rapidly discriminate between similar contexts (McHugh et al. 2007). Pharmacological blockade of the GluN2B-containing NMDA receptors also leads to learning difficulties and diminished activity-dependent synaptic plasticity at the medial perforant pathway-granule cell synapses (Valenzuela-Harrington et al. 2007).
In CA1 pyramidal cells, perforant path input forms synapses on distal dendrites in the stratum lacunosum-moleculare; NMDA /AMPA charge ratio of this input is significantly larger than those of the Schaffer collateral inputs. The properties of the NMDA component were also quite different, as the NMDA-mediated current at +60 mV in the perforant pathway input is six times smaller than in the Schaffer collateral input after scaling by the maximal inward current at −20 mV (Otmakhova et al. 2002). Different NMDA receptor properties could contribute to different subunit compositions of the receptors facing the two different inputs. GluN2B subunit contribution to NMDA responses at Schaffer collateral inputs is larger than at perforant pathway inputs on a single CA1 pyramidal cell, indicating that NMDA receptors with distinct subunit composition are segregated in an input-specific manner along the dendritic tree (Arrigoni and Greene 2004). Synaptic plasticity is also expressed in an input-specific manner; performant pathway LTP in the CA1 area of the hippocampus in vivo is only partially affected by NMDAR antagonists and can be sensitive to VGCC antagonists. In contrast, perforant pathways LTP in the CA3 area is NMDAR dependent (Aksoy-Aksel and Manahan-Vaughan 2015).
Metabotropic Glutamate Receptors
In the perforant pathway terminating in the CA3 area and the dentate gyrus, the localization of presynaptic mGluRs, mGluR2, and mGluR8 is complimentary; mGluR2 is present at the medial and mGluR8 at the lateral perforant input (Shigemoto et al. 1997). Differential regulation of the medial and lateral perforant path by different metabotropic receptors has been demonstrated by selective group II and group III agonists and antagonists, indicating that group III metabotropic receptors regulate glutamate release at the lateral perforant pathway , while group II mGluRs serve as autoreceptors at the medial perforant path (Macek et al. 1996). Activation of presynaptic group II mGluRs at the medial perforant pathway reduced synaptic transmission and resulted in a reduction of short-term depression (Kilbride et al. 2001). While short-term depression is not prominent at the lateral perforant input at lower frequencies, it increases with higher stimulus frequencies, and L-AP4, a selective agonist of group III mGluRs, reduced this depression (Rush et al. 2002). In the CA1 area, perforant pathway axons display both group II and group III mGluRs; while mGluR7 a and mGluR4 are detected in active zones, mGluR2 can be found in preterminal zones (Shigemoto et al. 1997). Segregation of these mGluRs to different zones of the presynaptic terminal and their different signaling suggest that they could be involved in distinct regulatory roles (Capogna 2004). Similar pattern of mGluR distribution and regulatory function was observed on perforant path inputs terminating on CA1 interneurons located in the stratum lacunosum-moleculare (Price et al. 2005). II mGluR activation can prevent LTP at perforant pathway synapses in the CA3 area but not in the CA1 (Aksoy-Aksel and Manahan-Vaughan 2015) (Fig. 2).
Properties of the perforant path input. Different properties of short-term plasticity of the medial (filled circles) and lateral (open circles) pathway, demonstrated in vivo (Aa) and in vitro (Ab). Graphs illustrating paired-pulse ratios at various interstimulus intervals (McNaughton 1980). Comparison of current-voltage relationship of AMPA (Ba-Bc) and NMDA (Bd-Bh) responses at Schaffer collateral and perforant path inputs (Otmakhova et al. 2002). Simultaneous activation of Schaffer collaterals and the perforant path (Ca) decreases both EPSP duration (Cb) and amplitude (Cc) by activating strong feed-forward inhibition (Empson and Heinemann 1995b)
Mossy Fibers
Dentate granule cells send their axons to the hilus and the CA3 area of the hippocampus. Mossy fibers are unmyelinated axons arborizing in the hilar area and forming a distinctive axon bundle in the stratum lucidum of the CA3 area. They form three distinct types of presynaptic terminals: complex en passant presynaptic terminals called mossy fiber expansions (Amaral and Dent 1981), filopodial extensions of large mossy boutons, and small en passant terminals (Acsády et al. 1998). Large mossy terminals innervate exclusively excitatory cells, mossy cells in the hilus, and pyramidal cells in the CA3 area. In contrast, small filopodial extensions originating from the large terminals and small en passant terminals specifically terminate on GABAergic cells both in the hilus and in the CA3 (Acsády et al. 1998). Complex mossy terminals are large (4–10 μm) and form several (30–40) synaptic contacts (Chicurel and Harris 1992) with a single thorny excrescence on each CA3 pyramidal neuron or mossy cell. A single granule cell gives rise to 10–18 large mossy terminals (Amaral et al. 1990) innervating 11–15 CA3 pyramidal cells and 7–12 hilar mossy cells. Acsády et al. (1998) elegantly demonstrated that the number of GABAergic targets innervated via small filopodial extensions and en passant terminals is ten times larger than the number of excitatory targets.
Salient Features of Mossy Fiber Synapses:
-
Large presynaptic terminal with several release sites.
-
Inputs to pyramidal cells and interneurons have distinct features.
-
Robust short-term and frequency facilitation.
-
NMDA-independent LTP.
-
Small amplitude, slow postsynaptic kainate responses.
AMPA Receptors
Mossy Fiber: CA3 Pyramidal Cell Synapses
Investigation of the I-V relationship of mossy fiber inputs onto CA3 pyramidal cells showed that the excitatory connection between granule cells and CA3 pyramidal cells has linear I-V relationship indicating that these receptors are Ca2+-impermeable (Jonas et al. 1993; Koh et al. 1995). Mossy fiber evoked EPSCs were insensitive to PhTx, further supporting the idea that mossy fiber input onto CA3 pyramidal cells is exclusively mediated by GluA2-containing, Ca2+-impermeable AMPA receptors (Toth et al. 2000). However, in a recent study Ho et al. (Ho et al. 2007) demonstrated that while AMPA receptors at mature mossy fiber synapses are Ca2+-impermeable, during the first 3 weeks of postnatal development Ca2+-permeable AMPA receptors contribute to mossy fiber transmission. This transient, developmentally regulated expression of Ca2+-permeable AMPA receptors could play an important role in synapse maturation and various forms of synaptic plasticity.
Unitary EPSCs have fast kinetics with a latency of 2.3–4.2 ms, a 20–80% rise time of 0.6–1.7 ms, a decay time constant of 6.2–9.6 ms, and a maximal peak conductance of 1 nS (Jonas et al. 1993; Tóth and McBain 2000). In these studies, fast kinetics were used as a criterion to ensure that evoked events are purely originating from mossy fibers ; hence potential events with slower kinetics were excluded. However, in a study by Henze et al. (Henze et al. 1997), the authors found large presumptive mEPSCs with significantly slower kinetics. Later they also postulated that these events are monoquantal (Henze et al. 2002a). The amplitude of mossy fiber EPSPs can be 2–10 mV, and unitary EPSCs show amplitudes up to 1 nA; these values are severalfold larger than synaptic events evoked with the stimulation of small glutamatergic synapses. Large unitary EPSCs are the result of a highly synchronized release from multiple release sites. The number of release sites was estimated to be between 8 and 21 in the study by Lawrence et al. (Lawrence et al. 2004); in the same study using variance-mean analysis, the quantal amplitude of mossy fiber events was calculated to be ∼30pA. This value is quite different from earlier estimates deriving quantal parameters from amplitude histograms (8 pA) (Jonas et al. 1993; von Kitzing et al. 1994). Interestingly, in the recordings used for variance-mean analysis , in low extracellular Ca2+ conditions, smaller (7–12 pA) events could also be resolved. This indicates that quantal size might show high degree of variability among various release sites and potentially help to reconcile findings of these two studies. This possibility is further supported by recent morphological data finding large variability in the size of active zones within the mossy fiber terminal (from 0.07 to 0.17 μm2) (Rollenhagen et al. 2007). The initial release probability at mossy fiber-pyramidal cell synapses is estimated to be between 0.20 and 0.28 (Lawrence et al. 2004; von Kitzing et al. 1994). However, this low release probability is increased dramatically after repeated activation of mossy fibers; frequency-dependent facilitation can lead to up to 600% increase in EPSC amplitude (Salin et al. 1996; Toth et al. 2000). Short-term facilitation can be observed at frequencies as low as 0.1 Hz. The combination of low initial probability and pronounced short-term facilitation leads to increased spike transmission following short trains. Single action potentials initiated in the dentate granule cells in vivo rarely drive their postsynaptic targets, whereas high-frequency trains with short interspike intervals robustly increased spike transmission probability (Henze et al. 2002b). In in vitro experiments, the probability that the initial EPSP in a train elicited action potentials in CA3 pyramidal cells is only 0.28; however this value rapidly increases to 0.76 over the course of 40 Hz stimulation (Lawrence et al. 2004).
The efficacy and timing of transmitter release is largely dependent on the spatiotemporal profile of presynaptic Ca2+ transients . Presynaptic Ca2+ channels have fast activation and deactivation kinetics, with time constants in the millisecond range; gating of these channels appears to be optimized to generate maximal Ca2+ influx during a minimal period of time (Bischofberger et al. 2002; Geiger and Jonas 2000). Presynaptic Ca2+ influx is triggered by presynaptic action potentials. The duration of these action potentials is not constant, but they broaden with increased presynaptic stimuli (Geiger and Jonas 2000).
Mossy fibers can follow high-frequency stimuli with high precision and efficacy; this is only possible if the terminal has large enough releasable vesicle pool. Capacitance measurements indicated that sustained Ca2+ inflow (30 ms, 0 V) will lead to the release of ∼1400 vesicles; this corresponds to ∼40 vesicles per active zone (Hallermann et al. 2003). These measurements were closely matched with data stemming from detailed electron microscopic investigation of the mossy fiber terminal (Rollenhagen et al. 2007)103]. During high-frequency stimulation, short-term facilitation is supported by a switch from univesicular to multivesicular release and the subsequent recruitment of additional release sites (Chamberland et al. 2014). P/Q and N-type calcium channels contribute to short-term facilitation in a distinct fashion. While N-type calcium channels are responsible for calcium increase in the close vicinity of active zones, P/Q-type calcium channels are contributing to increased calcium levels at large segments of the terminal (Chamberland et al. 2017).
Mossy Fiber: Interneuron Synapse
While principal cells express high levels of GluA2, some GABAergic inhibitory interneurons in the hippocampus have inwardly rectifying I-V relationships and are Ca2+-permeable (Geiger et al. 1995; Jonas et al. 1994; Koh et al. 1995; McBain and Dingledine 1993). Mossy fibers innervate GABAergic interneurons via synapses comprised of either Ca2+-permeable or Ca2+-impermeable AMPA receptors. The two different types of AMPA receptors differ in the plastic properties and degree by which they colocalize with NMDA receptors.
The kinetics of mossy fiber-interneuron transmission is significantly faster than the input onto pyramidal cells. The mean 10–90% rise time of EPSCs at both types of synapses was found to be in the submillisecond range, with the time constant for decay between 1 and 4 ms (Geiger et al. 1997). Geiger et al. (1997) have suggested that the kinetics at mossy fiber-interneuron synapses are fast due to the precise timing of glutamate release and the rapid deactivation of AMPA receptors.
Anatomical and physiological data equally suggest that the mossy fiber-interneuron synapse comprises of a small number of release sites (1–2). Variance-mean analysis indicated that the initial release probability at these synapses is significantly higher (0.1–0.5) than at pyramidal cell synapses (Lawrence et al. 2004). High initial release probability contributes to the mild facilitation or depression observed at these synapses during brief stimulus trains.
The unitary quantal amplitude at this synapse was found to be 27 pA (Lawrence et al. 2004); this value was calculated using variance-mean analysis and confirmed with recorded unitary events in the presence of strontium. The average size of the EPSCs evoked in CA3 interneurons is approximately three times smaller than EPSCs recorded from pyramidal cells (88 pA vs. 20 pA) (Lawrence et al. 2004). However, the probability that an EPSC would evoke an action potential in the postsynaptic cell is not significantly different between these cells when only a single stimulus was used. After brief trains of stimulation however, the probability of spike transmission is greater in pyramidal cell synapses. This difference could be explained by the distinct short-term plastic properties expressed by these synapses.
Properties of individual contacts between mossy fiber terminals and GABAergic cells have been elegantly investigated using mossy fiber bouton to interneuron paired recordings (Szabadics and Soltesz 2009). This study demonstrated that the amplitude and transmission probability of these synaptic interactions were largely target cell dependent. Inputs onto fast-spiking basket cells and spiny lucidum cells were small in amplitude and had low transmission probabilities. In contrast, regular-spiking basket cells and ivy cells received inputs with larger amplitude and transmission probability (Szabadics and Soltesz 2009) (Fig. 3).
Anatomical and physiological properties of mossy fiber inputs. Morphological characteristics of mossy fiber terminals: large filopodial extensions originate from the main terminal (Aa) (Acsády et al. 1998); boutons have several (20–30) active zones (Ab) (Amaral and Dent 1981). AMPA /kainate and NMDA receptor-mediated components of mossy fiber inputs (Ba-Be), current-voltage relationship of the peak current (filled circles) and the current measured 50 ms after the peak (open circles) under control conditions (Ba and Bd), in the presence of CNQX alone (Bb and Be) or in the presence of CNQX and APV (Bc) (Jonas et al. 1993). Variance-mean analysis is used to determine quantal parameters at the mossy fiber-CA3 pyramidal cell synapse (Bf-Bi) (Lawrence et al. 2004). Current-voltage relationship of mossy fiber-interneuron synapses, rectification index, and philanthotoxin sensitivity of inputs show high degree of variability (Ca-Cc) (Tóth and McBain 1998). Kinetic properties of mossy fiber inputs terminating on pyramidal cells and on interneurons mediated by calcium-permeable and calcium-impermeable AMPA receptors (Cd-Cf) (Toth et al. 2000)
NMDA Receptors
NMDA receptors are present at mossy fiber synapses; however mossy fiber synapses are showing lower immunostaining intensity than the Schaffer collateral inputs (Petralia et al. 1994; Takumi et al. 1999; Watanabe et al. 1998). Electrophysiological examination showed that mossy fiber synaptic inputs onto pyramidal cells are partially mediated by NMDA receptors; this component has significantly slower kinetics than those of the AMPA component (Jonas et al. 1993; Spruston et al. 1995). In a recent study, the activation of postsynaptic NMDA receptors at mossy fiber synapses was shown to influence short-term plasticity of kainate-mediated transmission (Rebola et al. 2007). NMDA receptor-mediated depression of kainate EPSC (EPSCKA) was observed, while the AMPA component of the event was not modified, this effect was expressed postsynaptically and was homosynaptic. Unlike in the CA1 area of the hippocampus, mossy fiber LTP is independent of NMDA receptor activation. However, NMDA receptor-mediated synaptic currents at this site are potentiated by high-frequency stimuli (Kwon and Castillo 2008b; Rebola et al. 2007).
At mossy fiber-interneuron synapses, the distribution of different subtypes of NMDA receptors and different subtypes of AMPA receptors is highly correlated. Ca2+-permeable AMPA receptors occur at synapses where NMDA receptors contain the GluN2B subunit, while Ca2+-impermeable AMPA receptors are associated with GluN2B-lacking NMDA receptors (Lei and McBain 2002). This particular distribution pattern leads to Ca2+-permeable synapses possessing smaller NMDA components with slower decay kinetics (Lei and McBain 2002).
Kainate Receptor
Kainate receptors are located on both the pre- and postsynaptic sites at hippocampal mossy fibers . While on the postsynaptic site they generate small but prolonged depolarization, the presynaptic receptors modulate excitatory and inhibitory synaptic transmission.
Postsynaptic Receptors
Kainate-mediated postsynaptic responses were first demonstrated at mossy fiber synapses. Kainate-mediated components of the mossy fiber responses (EPSCKA) to a single stimulus are very small, with an amplitude ∼10 times smaller than the AMPA component. However, the amplitude largely increases on repetitive stimulation of the mossy fibers . Short high-frequency trains lead to a severalfold increase in kainate responses, but even a moderate increase in presynaptic stimuli (from 0.05 Hz to 0.2 Hz) could almost double the amplitude of the EPSCKA. Postsynaptic kainate responses are selectively present at mossy fiber inputs in CA3 pyramidal cells and are absent from the commissural/associational inputs. The mossy fiber EPSCKA has very slow decay kinetics with the time constant approximately ten times slower than that of the AMPA component (∼100 ms v. ∼10 ms); the rise time (10–90%) of the kainate response is also significantly slower than the AMPA EPSC (7 ms vs. 3 ms). The slow kinetics of the kainate responses could potentially indicate that these receptors are located extrasynaptically; in this case they would not be able to respond to quantal release of glutamate. This question was addressed by Cossart et al. (Cossart et al. 2002), and their data indicated that kainate receptors are activated by quantal release of glutamate at mossy fiber synapses, as they were able to record pure kainate and mixed AMPA/kainate miniature responses from CA3 pyramidal cells. Frequency analysis showed that 45% of the miniature events involved kainate receptor activation. Morphological data indicates that kainate receptors are localized in postsynaptic densities (Darstein et al. 2003; Petralia et al. 1994a) and the lack of effect of glutamate uptake blockers on the kinetics of kainate responses further strengthen the conclusion that the slow kinetics of EPSCKA are not caused by extrasynaptic localization of these receptors. A recent study by Barberis et al. (2008) rather indicates that slow decay kinetics can be explained by the intrinsic gating properties of GluK2 /GluK4 heteromeric receptors. In fact, studies using knockout animals suggest that kainate receptors on CA3 pyramidal cells are composed of these two subunits (GluK2 and GluK4) (Contractor et al. 2003; Mulle et al. 1998).
The slow kinetics and small amplitude of kainate reposes suggest that they might play a role in frequency-dependent synaptic integration (Frerking and Ohliger-Frerking 2002). However short-term and long-term plasticity of EPSCKA is attenuated compared to the AMPA component, hence endowing the AMPA component with a wider dynamic range and limiting the contribution of kainate receptors in the presence of profound increase in presynaptic strength (Ito et al. 2004). Neto1/Neto 2 have been recently identified as auxiliary proteins regulating several functional parameters of kainate receptor function, including binding affinity, kinetics, and synaptic targeting of GluK2/3-containing postsynaptic KARs (Straub et al. 2011; Tang et al. 2011; Wyeth et al. 2014). Neto1 is expressed at high levels and have been shown to contribute the slow kinetics of kainate responses on mossy fiber synapses (Tang et al. 2011).
In addition to the ionotropic function, postsynaptic kainate receptors also show metabotropic activity via the inhibition of the slow Ca2+-activated K+ current IsAHP, which in turn increases excitability through a G-protein-coupled mechanism (Ruiz et al. 2005; Chamberlain et al. 2013) (Fig. 4).
Properties of kainate neurotransmission at the hippocampal mossy fibers . Kainate-mediated responses (not blocked by GYKI 53655) evoked with repetitive stimulation of the mossy fibers (MF) and are not present at associational/commissional (AC) inputs (Aa) (Castillo et al. 1997). Concentration-dependent, bidirectional modulation of mossy fiber EPSCs by kainate, GluK4 and GluK2 −/− mice shows altered kainate modulation (Ab) (Contractor et al. 2003). Controversy surrounding the role of presynaptic kainate receptors in short-term plasticity of mossy fiber inputs; AMPA receptor-mediated EPSCs from GluK3 −/− mice were shown to be less facilitated by high-frequency stimuli (Ba, Bb) (Pinheiro et al. 2007), having similar properties than their wild-type counterparts (Bc-Be) (Kwon and Castillo 2008a, b)
Presynaptic Receptors
Endogenously applied kainate has a biphasic effect; low doses facilitate mossy fiber transmission, while higher doses depress EPSCs. Endogenous kainate released following a repetitive stimulation of mossy fibers activates presynaptic receptors and facilitates synaptic release (Contractor et al. 2001; Schmitz et al. 2000; Lauri et al. 2001). Enhancement of release via presynaptic kainate receptors was shown to contribute to the robust frequency facilitation observed in mossy fibers (Contractor et al. 2001, 2003; Lauri et al. 2001; Pinheiro et al. 2007; Schmitz et al. 2001, 2003). Presynaptic kainate receptors contributing to frequency facilitation are thought to be calcium-permeable as indicated by their sensitivity to philanthotoxin . Ca2+ influx through these receptors is believed to play a crucial role in frequency facilitation (Lauri et al. 2001). The apparent calcium permeability of the receptors can only be explained by the presence of unedited forms of kainate receptor subunits; however the mechanism by which these subunits are preferentially inserted into mossy fibers is still unknown. The subunit composition of presynaptic kainate receptors has been a matter of debate; initially the GluK1 subunit was believed to be involved in the presynaptic kainate effect (Lauri et al. 2001). However, these data obtained with pharmacological tools could not be confirmed with the genetic ablation of the GluK1 subunit (Contractor et al. 2001) or in subsequent experiments (Breustedt and Schmitz 2004). In GluK4 and GluK3 knockout animals, frequency facilitation was compromised, pointing to the critical role these subunits play in the short-term plasticity (Contractor et al. 2003; Pinheiro et al. 2007).
Even though a large body of literature is dedicated to the existence and exact functional role of presynaptic kainate receptors at the mossy fibers , Kwon and Castillo (2008a) question the existence of presynaptic receptors. The authors used GluK1 specific agonist and GluK2 and GluK3 knockout animals and found that presynaptic kainate receptors do not play a significant role in short-term plasticity. They further postulate that the effects generally attributed to presynaptic kainate receptors are mediated by postsynaptic receptors and the activation of recurrent CA3 network activity.
Functionally, both pre- and postsynaptic kainite receptors amplify unitary mossy fiber inputs and act as conditional amplifiers of spike transmission (Sachidhanandam et al. 2009). While presynaptic receptors can effectively modulate the dynamic range of short-term plasticity, activation of postsynaptic kainate receptors during sustained stimulation leads to prolonged depolarization (Sachidhanandam et al. 2009; Pinheiro et al. 2013)(Table 3).
Metabotropic Glutamate Receptors
Presynaptic mossy fiber terminals contain two types of metabotropic glutamate receptors . While group II mGluRs are equally present on synapses opposing pyramidal cells and interneurons (Kamiya et al. 1996; Tóth and McBain 2000), mGluR7 is selectively present in synapses terminating on interneurons. Group II mGluRs depress excitatory transmission at both types of synapses. Interestingly mossy fiber-CA3 pyramidal cell synapses can be blocked completely with the group II mGluR agonist DCG-IV; synaptic inputs onto interneurons are only partially depressed. mGluRs decrease the degree of frequency facilitation observed at mossy fiber-pyramidal cell and mossy fiber-interneuron synapses (Scanziani et al. 1997; Toth et al. 2000). Group III metabotropic glutamate receptor7 (mGluR7 ) has low affinity for glutamate, and its activation depresses glutamatergic synaptic responses (O’Connor et al. 1999). In the mossy fiber-interneuron synapses, mGluR7 antagonist MSOP did not influence baseline transmission but prevented high-frequency-induced long-term depression, while application of an mGluR7 agonist leads to the development of a chemical LTD at this synapse (Pelkey et al. 2005). Detailed investigation of the plastic properties of this synapse leads to the discovery that mGluR7 goes through activity-dependent internalization and surface expression (Pelkey et al. 2005, 2007), contributing to state-dependent plasticity.
On the postsynaptic site, activation of Group I receptors can evoke a postsynaptic potential which is independent of the G-protein function (Heuss et al. 1999) while inhibiting IAHP through a G-protein-coupled mechanism. Their activation also leads to Ca2+ release from intracellular stores, which plays a role in plastic changes (Yeckel et al. 1999).
Schaffer Collaterals
The major input to the CA1 area of the hippocampus arrives from CA3 pyramidal cells via the Schaffer collaterals. Axons of CA3 pyramidal cells heavily innervate both the stratum radiatum and the stratum oriens of the CA1 area; proximal postsynaptic dendrites in these layers contain relatively few spines, while distal dendrites are densely spiny, excitatory inputs terminate exclusively on dendritic spines (Megías et al. 2001). A single CA3 pyramidal cell has an extensive axonal arbor and can extend to as much as two-third of the hippocampus and form 30,000–60,000 synapses (Li et al. 1994), while a single CA1 pyramidal cell receives approximately 30,000 excitatory synapses.
Salient Features of Schaffer Collateral Synapses:
-
Small terminals with single release site, variable release probability.
-
Increased synaptic AMPA, but not NMDA conductances at distal synapses.
-
Silent synapses.
-
Kainate receptor activation depresses glutamate transmission.
-
Wide variety of auxiliary proteins modify receptor function.
AMPA Receptors
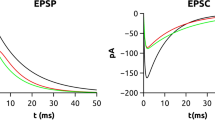
Excitatory synaptic inputs are broadly distributed along the dendritic tree of CA1 pyramidal cells. These inputs are integrated at the level of the axon initial segment to determine the output signal of the cell. The distance between the location of the final integration of the cellular outputs and synaptic inputs shows high degree of variety. How does the distance between the soma and the synapse influence the characteristics of synaptic inputs at the level of the soma? In principle, the further the synapse is, their impact on the final inputs should be more and more diminished, due to cable filtering. Interestingly, the local EPSP amplitude increases with distance from the soma, which leads to proximal and distal synaptic inputs producing very similar amplitudes detected at the soma (Magee and Cook 2000) (Fig. 5). Increased synaptic conductance in distal synapses was attributed to an increased AMPA receptor number on the postsynaptic sites, whereas AMPA receptor subunit composition and channel modulation was indistinguishable at proximal and distal synapses (Andrasfalvy and Magee 2001). Presynaptic properties, such as release probability , paired-pulse facilitation, and the size of the readily releasable pool, were also identical at these sites (Smith et al. 2003).
The amplitude of synaptically evoked EPSP at the soma does not depend on synapse location. Spontaneously occurring EPSPs in distal and proximal dendrites (d) and at the soma (s) in response to high osmolar external solution. (A) Scatterplot of dendritic and simultaneously recorded somatic EPSP amplitude from the cell shown in (A). (B) Averaged EPSPs simultaneously recorded both at the dendrite and at the soma for the neuron receiving distal and proximal inputs. (C) Mean EPSP amplitude for all cells plotted as a function of input distance from the soma. (D) Cumulative amplitude histograms showing that the distribution of distal dendritic EPSPs (light solid line) is skewed to the right compared to more proximal dendritic EPSPs (dark solid line). (E) (Magee and Cook 2000)
Excitatory synaptic potentials evoked by the stimulation of the Schaffer collaterals are composed of AMPA and NMDA components. However, under certain circumstances, EPSCs lack the AMPA component (Isaac et al. 2007; Kullmann 1994; Liao et al. 1995). The subpopulation of glutamatergic synapses lacking functional AMPA receptors are referred to as “silent synapses”; they have been identified in several brain regions including the CA1 area of the hippocampus. These synapses are not conducting currents at resting membrane potentials. The presence of these receptors is developmentally regulated, and during the first few postnatal days, almost all Schaffer collateral synapses are silent, but by the end of the third week, approximately 50% of them are containing functional AMPA receptors (Durand et al. 1996). Activity-dependent modification at these synapses, such as the expression of LTP , leads to the changes which “unsilence” these synapses.
How do these synapses become functional? Incorporation of AMPA receptors into silent synapse following high-frequency stimulation has been observed either through lateral diffusion (Adesnik et al. 2005) or from AMPA receptor-containing endosomes (Hayashi et al. 2000). However, recent data using optical imaging suggest that AMPA receptor insertion has little influence on synaptic plasticity; the authors rather suggest that LTP is expressed on the presynaptic site in a bidirectional, graded fashion (Enoki et al. 2009).
A novel form of plasticity at synapses with GluA3-containing AMPA receptors has been demonstrated in Schaffer collateral synapses. GluA2/3 subunit-containing receptors are in a low-conductance state but upon a rise in intracellular cAMP levels shift to a high-conductance state and lead to the potentiation of the synapse (Renner et al. 2017).
The majority of Schaffer collateral synapses contains one active zone with several docked vesicles (Harris and Sultan 1995; Schikorski and Stevens 1997). The probability of release at various release sites arising from the same axons is highly variable (Rosenmund et al. 1993). With increased release probability, more glutamate is released to the synaptic cleft; this is possible when more than one vesicle fuses with the presynaptic membrane in response to a single action potential. The probability of multivesicular release increases with release probability (Oertner et al. 2002). Multivesicular release is likely to occur at synapses with higher number of docked vesicles, which is generally observed in larger synapses (Harris and Sultan 1995).
In recent years, several studies showed the diverse effect auxiliary proteins can have on AMPA receptor functions. AMPA receptors can assemble with a wide range of auxiliary subunits belonging to four distinct families: TARP (Jackson and Nicoll 2011), cornichons (Schwenk et al. 2009), shisas (Farrow et al. 2015), and the germ cell-specific gene 1-like protein (Schwenk et al. 2012). The first three can effectively increase the mean channel conductance (Coombs et al. 2012; Jackson et al. 2011; Tomita et al. 2005; Shi et al. 2010), TARPs being the most effective among them. TARPs also influence deactivation kinetics and extra- and intracellular polyamine block of GluA2-lacking AMPA receptors (Jackson et al. 2011; Soto et al. 2009; Cho et al. 2007; Milstein et al. 2007).
NMDA Receptors
receptors are abundantly presentNMDA at excitatory synapses in the CA1 region of the hippocampus, and the number of NMDA receptors in individual synapses shows very little variability (Petralia et al. 1994b; Racca et al. 2000). The number of NMDA receptors activated by synaptic stimulation is small, only 1–5 NMDA channels open during synaptic transmission at low frequencies, and this value is small enough to render quantal dendritic NMDA responses undetectable at the soma through physiological measurements (Nimchinsky et al. 2004). In contrast to AMPA receptors, the number of NMDA receptors expressed at synapses does not show activity-dependent changes.
Furthermore, dendritic NMDA current amplitudes do not change with distance from the soma, and their kinetics and quantal parameters are very similar in proximal and distal dendrites (Andrasfalvy and Magee 2001) (Table 4).
Kainate Receptors
Kainate receptor activation in the CA1 region leads to the depression of glutamatergic transmission (Chittajallu et al. 1996; Frerking et al. 2001; Kamiya et al. 1996; Vignes et al. 1998). Several mechanisms were suggested to explain this effect: direct action via presynaptic receptors, indirect action via somatodendritic kainate receptors , and also both ionotropic and metabotropic modes of action were postulated. Kainate agonist depresses glutamatergic transmission via a decrease in quantal content; in agreement with this finding, little or no evidence of postsynaptic modulation was observed. Rather, the experimental data indicate that kainate receptors are directly activated on the presynaptic terminal and are coupled to G-proteins (Frerking et al. 2001).
Beside the direct action on pyramidal cells, kainate receptors are also involved in the modulation of network activity via the regulation of inhibitory neurons and their synaptic inputs. Dendritic kainate receptor activation results in the increased activity of inhibitory neurons which provide increased inhibitory input onto pyramidal cells. This postsynaptic effect is mediated by GluK1 subunit-containing receptors (Cossart et al. 1998). Excitatory input onto somatostatin -containing interneurons shows unusually robust short-term facilitation, and the activation of presynaptic kainate receptors contributes to this effect, enabling a target cell-specific mechanism leading to the preferential activation of a subset of interneurons following repeated stimuli (Sun and Dobrunz 2006) (Fig. 6). This effect is mediated by GluK1 and GluK2 subunit-containing, calcium-permeable kainate receptors (Sun et al. 2009). Finally, GABAergic neurotransmission is also regulated by presynaptic kainate receptors. This action mostly affects inhibitory connections between interneurons and may serve to enhance interneuron-interneuron interactions (Cossart et al. 2001).
Kainate responses in the CA1 area of the hippocampus. Kainate activation depresses AMPA (Aa) and NMDA (Ab) (Frerking et al. 2001) receptor-mediated events at the Schaffer collateral synapses. Kainate receptor-specific antagonist reduces facilitation at inputs onto somatostatin -positive interneurons (Sun and Dobrunz 2006)
Metabotropic Glutamate Receptors
Metabotropic receptors have diverse effects in the CA1 area of the hippocampus: they directly excite pyramidal cells (Gereau and Conn 1995) and interneurons (McBain et al. 1994), they decrease excitatory and inhibitory synaptic transmission, and they influence long-term plasticity (Bortolotto et al. 1999). Direct excitation of pyramidal cells and interneurons is mainly mediated by Group I metabotropic receptors localized postsynaptically on the somato-dendritic membrane (Gereau and Conn 1995; McBain et al. 1994). In interneurons located in the stratum oriens/alveus, activation of mGluR1α leads to Ca2+ signals resulting from Ca2+ influx through transient potential channels and Ca2+ release from intracellular stores. Dendritic Ca2+ transients resulting from mGluR5 activation are solely mediated by intracellular Ca2+ release. Furthermore, long-term plasticity at oriens/alveus interneurons is influenced by mGluR1- and mGluR5-specific signaling (Le Vasseur et al. 2008; Topolnik et al. 2006).
Glutamate release is suppressed by Group I and Group III metabotropic receptors at Schaffer collateral synapses (Gereau and Conn 1995), while the selective Group II receptors antagonist DCG-IV does not have influence on excitatory neurotransmission (Kamiya et al. 1996). Both Group I and Group III metabotropic receptors are localized presynaptically as evidenced by the effect observed on mEPSC frequency and paired-pulse facilitation (Gereau and Conn 1995), but the two receptors are regulating glutamatergic neurotransmission through different mechanisms. Group II metabotropic receptors are only expressed in granule cells in the adult hippocampus (Tanabe et al. 1992; Tanabe et al. 1993); however activation of group II mGluRs was shown to influence glutamatergic transmission, and this effect is mediated by mGluR3 receptors expressed on glia cells (Winder et al. 1996).
The amplitude of GABA-mediated synaptic events is also reduced by group I mGluR agonist; however this effect can effectively be blocked by the pre-application of CB1 receptor antagonist indicating that mGluR1/5 receptors are not situated directly at the inhibitory presynaptic terminals (Neu et al. 2007). Group III mGluRs are known to present at GABAergic presynaptic terminals (Shigemoto et al. 1997), and activation of these receptors leads to decreased inhibitory transmission among GABAergic cells. These receptors are likely to sense glutamate spillover from neighboring excitatory afferent terminals (Semyanov and Kullmann 2000).
Experimental Techniques
The key techniques used for the investigation of glutamatergic signals are intracellular and patch-clamp recordings. These experimental approaches are described in detail in Chapter X (Marco Martina). Synaptic inputs are evoked with either electric or osmotic stimuli. Alternatively, glutamate can be applied to the tissue by either bath application or focal pressure injection. A more precise approach is the recently developed technique using glutamate uncaging. With the latter approach, synapses on a specific subcellular element can selectively be activated (i.e., dendrite vs. soma). Activation of selected subpopulation of synapses or limited number of synapses can be challenging in in vitro slices, given the complex network connections in the tissue. Under these circumstances, paired recordings can be used to identify synaptic contacts between individual cells where both the pre- and postsynaptic element can be anatomically identified. Recordings from synaptically connected neuron pairs are harder when the pre- and postsynaptic cells are relatively far away from each other, as the probability of functional connections decreases with distance. In these cases, direct stimulation of presynaptic terminals could provide information about individual synaptic contacts. This is only possible when the presynaptic terminal is large, like the mossy fiber boutons. Optogenetic approaches in combination with two-photon imaging allow the simultaneous activation and imaging of small groups of synapses; however the activation of single synapses with optogenetic tools still remains challenging.
The Future
Recent developments in optogenetics and high-resolution imaging have opened the window to functional questions that are very specific to a group of neurons or receptors and to the combination of these two. With these developments in imaging and genetic techniques, synaptic inputs onto specific subset of cells can be studied in isolation, and target cell specificity of various inputs established. We will see large body of data generated on cell-type and receptor subunit-specific signaling. In addition, using live imaging with high temporal and spatial resolution allows the simultaneous investigation of large population of cells. All this information will require modeling strategies to help better understand the role of a certain type of synapse in network activity and in the control of input/output properties of large population of neurons.
References
Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G (1998) GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci 18(9):3386–3403
Adesnik H, Nicoll RA, England PM (2005) Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron 48(6):977–985
Aksoy-Aksel A, Manahan-Vaughan D (2015) Synaptic strength at the temporoammonic input to the hippocampal CA1 region in vivo is regulated by NMDA receptors, metabotropic glutamate receptors and voltage-gated calcium channels. Neuroscience 309:191–199
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195(1):51–86
Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31(3):571–591
Amaral DG, Ishizuka N, Claiborne B (1990) Neurons, numbers and the hippocampal network. Prog Brain Res 83:1–11
Andrasfalvy BK, Magee JC (2001) Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci 21(23):9151–9159
Arrigoni E, Greene RW (2004) Schaffer collateral and perforant path inputs activate different subtypes of NMDA receptors on the same CA1 pyramidal cell. Br J Pharmacol 142(2):317–322
Barberis A, Sachidhanandam S, Mulle C (2008) GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci 28(25):6402–6406
Berzhanskaya J, Urban NN, Barrionuevo G (1998) Electrophysiological and pharmacological characterization of the direct perforant path input to hippocampal area CA3. J Neurophysiol 79(4):2111–2118
Bischofberger J, Geiger JR, Jonas P (2002) Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci 22(24):10593–10602
Blaschke M, Keller BU, Rivosecchi R, Hollmann M, Heinemann S, Konnerth A (1993) A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc Natl Acad Sci U S A 90(14):6528–6532
Bortolotto ZA, Fitzjohn SM, Collingridge GL (1999) Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol 9(3):299–304
Bowie D, Mayer ML (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15(2):453–462
Breustedt J, Schmitz D (2004) Assessing the role of GLUK5 and GLUK6 at hippocampal mossy fiber synapses. J Neurosci 24(45):10093–10098
Calixto E, Galván EJ, Card JP, Barrionuevo G (2008) Coincidence detection of convergent perforant path and mossy fibre inputs by CA3 interneurons. J Physiol 586(11):2695–2712
Capogna M (2004) Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway-CA1 EPSCs. Eur J Neurosci 19(10):2847–2858
Castillo PE, Malenka RC, Nicoll RA (1997) Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388(6638):182–186
Chamberlain SE, Sadowski JH, Teles-Grilo Ruivo LM, Atherton LA, Mellor JR (2013) Long-term depression of synaptic kainate receptors reduces excitability by relieving inhibition of the slow afterhyperpolarization. J Neurosci 33(22):9536–9545
Chamberland S, Evstratova A, Tóth K (2014) Interplay between synchronization of multivesicular release and recruitment of additional release sites support short-term facilitation at hippocampal mossy fiber to CA3 pyramidal cells synapses. J Neurosci 34(33):11032–11047
Chamberland S, Evstratova A, Tóth K (2017) Short-term facilitation at a detonator synapse requires the distinct contribution of multiple types of voltage-gated calcium channels. J Neurosci 37(19):4913–4927
Chicurel ME, Harris KM (1992) Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol 325(2):169–182
Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM (1996) Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379(6560):78–81
Chiu CQ, Castillo PE (2008) Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology 54(1):68–78
Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR (2007) Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron 55(6):890–904
Colbert CM, Levy WB (1992) Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors. J Neurophysiol 68(1):1–8
Contractor A, Swanson G, Heinemann SF (2001) Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron 29(1):209–216
Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT et al (2003) Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J Neurosci 23(2):422–429
Coombs ID, Soto D, Zonouzi M, Renzi M, Shelley C, Farrant M et al (2012) Cornichons modify channel properties of recombinant and glial AMPA receptors. J Neurosci 32(29):9796–9804
Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y (1998) GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1(6):470–478
Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y et al (2001) Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29(2):497–508
Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y et al (2002) Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35(1):147–159
Dahl D, Sarvey JM (1989) Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci U S A 86(12):4776–4780
Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF (2003) Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci 23(22):8013–8019
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51(1):7–61
Donevan SD, Rogawski MA (1995) Intracellular polyamines mediate inward rectification of ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A 92(20):9298–9302
Durand GM, Kovalchuk Y, Konnerth A (1996) Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381(6577):71–75
Empson RM, Heinemann U (1995a) Perforant path connections to area CA1 are predominantly inhibitory in the rat hippocampal-entorhinal cortex combined slice preparation. Hippocampus 5(2):104–107
Empson RM, Heinemann U (1995b) The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice. J Physiol 484(Pt 3):707–720
Enoki R, Hu YL, Hamilton D, Fine A (2009) Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron 62(2):242–253
Farrow P, Khodosevich K, Sapir Y, Schulmann A, Aslam M, Stern-Bach Y et al (2015) Auxiliary subunits of the CKAMP family differentially modulate AMPA receptor properties. elife 4:e09693
Frerking M, Ohliger-Frerking P (2002) AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci 22(17):7434–7443
Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA (2001) Kainate receptors depress excitatory synaptic transmission at CA3-->CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci 21(9):2958–2966
Geiger JR, Jonas P (2000) Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron 28(3):927–939
Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P et al (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15(1):193–204
Geiger JR, Lübke J, Roth A, Frotscher M, Jonas P (1997) Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18(6):1009–1023
Gereau RW, Conn PJ (1995) Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci 15(10):6879–6889
Hallermann S, Pawlu C, Jonas P, Heckmann M (2003) A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc Natl Acad Sci U S A 100(15):8975–8980
Han ZS, Buhl EH, Lörinczi Z, Somogyi P (1993) A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci 5:395–410
Harris KM, Sultan P (1995) Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology 34(11):1387–1395
Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287(5461):2262–2267
Henze DA, Card JP, Barrionuevo G, Ben-Ari Y (1997) Large amplitude miniature excitatory postsynaptic currents in hippocampal CA3 pyramidal neurons are of mossy fiber origin. J Neurophysiol 77(3):1075–1086
Henze DA, McMahon DB, Harris KM, Barrionuevo G (2002a) Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal. J Neurophysiol 87(1):15–29
Henze DA, Wittner L, Buzsáki G (2002b) Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci 5(8):790–795
Heuss C, Scanziani M, Gähwiler BH, Gerber U (1999) G-protein-independent signaling mediated by metabotropic glutamate receptors. Nat Neurosci 2(12):1070–1077
Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J et al (2007) Developmental expression of Ca2+−permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J Neurosci 27(43):11651–11662
Isaac JT, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54(6):859–871
Ito K, Contractor A, Swanson GT (2004) Attenuated plasticity of postsynaptic kainate receptors in hippocampal CA3 pyramidal neurons. J Neurosci 24(27):6228–6236
Jackson AC, Nicoll RA (2011) The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70(2):178–199
Jackson AC, Milstein AD, Soto D, Farrant M, Cull-Candy SG, Nicoll RA (2011) Probing TARP modulation of AMPA receptor conductance with polyamine toxins. J Neurosci 31(20):7511–7520
Jarsky T, Roxin A, Kath WL, Spruston N (2005) Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci 8(12):1667–1676
Jonas P, Major G, Sakmann B (1993) Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol 472:615–663
Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H (1994) Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12(6):1281–1289
Kahle JS, Cotman CW (1989) Carbachol depresses synaptic responses in the medial but not the lateral perforant path. Brain Res 482(1):159–163
Kamboj SK, Swanson GT, Cull-Candy SG (1995) Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol 486(Pt 2):297–303
Kamiya H, Shinozaki H, Yamamoto C (1996) Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol 493(Pt 2):447–455
Kilbride J, Rush AM, Rowan MJ, Anwyl R (2001) Presynaptic group II mGluR inhibition of short-term depression in the medial perforant path of the dentate gyrus in vitro. J Neurophysiol 85(6):2509–2515
Koh DS, Burnashev N, Jonas P (1995) Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol 486(Pt 2):305–312
Kullmann DM (1994) Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron 12(5):1111–1120
Kwon HB, Castillo PE (2008a) Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron 60(6):1082–1094
Kwon HB, Castillo PE (2008b) Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 57(1):108–120
Lambert JD, Jones RS (1989) Activation of N-methyl-D-aspartate receptors contributes to the EPSP at perforant path synapses in the rat dentate gyrus in vitro. Neurosci Lett 97(3):323–328
Lambert JD, Jones RS (1990) A reevaluation of excitatory amino acid-mediated synaptic transmission in rat dentate gyrus. J Neurophysiol 64(1):119–132
Larkman AU, Jack JJ, Stratford KJ (1997) Quantal analysis of excitatory synapses in rat hippocampal CA1 in vitro during low-frequency depression. J Physiol 505(Pt 2):457–471
Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT et al (2001) A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron 32(4):697–709
Lawrence JJ, Grinspan ZM, McBain CJ (2004) Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol 554(Pt 1):175–193
Le Vasseur M, Ran I, Lacaille JC (2008) Selective induction of metabotropic glutamate receptor 1- and metabotropic glutamate receptor 5-dependent chemical long-term potentiation at oriens/alveus interneuron synapses of mouse hippocampus. Neuroscience 151(1):28–42
Lei S, McBain CJ (2002) Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron 33(6):921–933
Li XG, Somogyi P, Ylinen A, Buzsáki G (1994) The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol 339(2):181–208
Liao D, Hessler NA, Malinow R (1995) Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375(6530):400–404
Macek TA, Winder DG, Gereau RW, Ladd CO, Conn PJ (1996) Differential involvement of group II and group III mGluRs as autoreceptors at lateral and medial perforant path synapses. J Neurophysiol 76(6):3798–3806
Magee JC, Cook EP (2000) Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci 3(9):895–903
Masurkar AV, Srinivas KV, Brann DH, Warren R, Lowes DC, Siegelbaum SA (2017) Medial and lateral entorhinal cortex differentially excite deep versus superficial CA1 pyramidal neurons. Cell Rep 18(1):148–160
McBain CJ, Dingledine R (1993) Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol 462:373–392
McBain CJ, DiChiara TJ, Kauer JA (1994) Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci 14(7):4433–4445
McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK et al (2007) Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317(5834):94–99
McNaughton BL (1980) Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res 199(1):1–19
Megías M, Emri Z, Freund TF, Gulyás AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102(3):527–540
Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA (2007) TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron 55(6):905–918
Min MY, Asztely F, Kokaia M, Kullmann DM (1998) Long-term potentiation and dual-component quantal signaling in the dentate gyrus. Proc Natl Acad Sci U S A 95(8):4702–4707
Mulle C, Sailer A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I et al (1998) Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392(6676):601–605
Neu A, Földy C, Soltesz I (2007) Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol 578(Pt 1):233–247
Nimchinsky EA, Yasuda R, Oertner TG, Svoboda K (2004) The number of glutamate receptors opened by synaptic stimulation in single hippocampal spines. J Neurosci 24(8):2054–2064
O’Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A et al (1999) Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science 286(5442):1180–1184
Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K (2002) Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci 5(7):657–664
Otmakhova NA, Otmakhov N, Lisman JE (2002) Pathway-specific properties of AMPA and NMDA-mediated transmission in CA1 hippocampal pyramidal cells. J Neurosci 22(4):1199–1207
Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ (2005) mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46(1):89–102
Pelkey KA, Yuan X, Lavezzari G, Roche KW, McBain CJ (2007) mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology 52(1):108–117
Pelletier MR, Kirkby RD, Jones SJ, Corcoran ME (1994) Pathway specificity of noradrenergic plasticity in the dentate gyrus. Hippocampus 4(2):181–188
Petralia RS, Yokotani N, Wenthold RJ (1994) Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci 14(2):667–696
Petralia RS, Wang YX, Wenthold RJ (1994a) Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol 349(1):85–110
Petralia RS, Wang YX, Wenthold RJ (1994b) The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci 14(10):6102–6120
Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR et al (2007) GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A 104(29):12181–12186
Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crépel V et al (2013) Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb Cortex 23(2):323–331
Price CJ, Karayannis T, Pál BZ, Capogna M (2005) Group II and III mGluRs-mediated presynaptic inhibition of EPSCs recorded from hippocampal interneurons of CA1 stratum lacunosum moleculare. Neuropharmacology 49(Suppl 1):45–56
Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P (2000) NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci 20(7):2512–2522
Reagh ZM, Yassa MA (2014) Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci U S A 111(40):E4264–E4273
Rebola N, Sachidhanandam S, Perrais D, Cunha RA, Mulle C (2007) Short-term plasticity of kainate receptor-mediated EPSCs induced by NMDA receptors at hippocampal mossy fiber synapses. J Neurosci 27(15):3987–3993
Remondes M, Schuman EM (2002) Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature 416(6882):736–740
Renner MC, Albers EH, Gutierrez-Castellanos N, Reinders NR, van Huijstee AN, Xiong H et al (2017) Synaptic plasticity through activation of GluA3-containing AMPA-receptors. elife 6
Rollenhagen A, Sätzler K, Rodríguez EP, Jonas P, Frotscher M, Lübke JH (2007) Structural determinants of transmission at large hippocampal mossy fiber synapses. J Neurosci 27(39):10434–10444
Rosenmund C, Clements JD, Westbrook GL (1993) Nonuniform probability of glutamate release at a hippocampal synapse. Science 262(5134):754–757
Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C (2005) Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci 25(50):11710–11718
Rush AM, Kilbride J, Rowan MJ, Anwyl R (2002) Presynaptic group III mGluR modulation of short-term plasticity in the lateral perforant path of the dentate gyrus in vitro. Brain Res 952(1):38–43
Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH, Mulle C (2009) Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J Neurosci 29(15):5000–5008
Salin PA, Scanziani M, Malenka RC, Nicoll RA (1996) Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A 93(23):13304–13309
Sambandan S, Sauer JF, Vida I, Bartos M (2010) Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus. J Neurosci 30(35):11826–11837
Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA (1997) Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature 385(6617):630–634
Schikorski T, Stevens CF (1997) Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci 17(15):5858–5867
Schmitz D, Frerking M, Nicoll RA (2000) Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron 27(2):327–338
Schmitz D, Mellor J, Nicoll RA (2001) Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science 291(5510):1972–1976
Schmitz D, Mellor J, Breustedt J, Nicoll RA (2003) Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci 6(10):1058–1063
Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B et al (2009) Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323(5919):1313–1319
Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Müller CS et al (2012) High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74(4):621–633
Semyanov A, Kullmann DM (2000) Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron 25(3):663–672
Shi Y, Suh YH, Milstein AD, Isozaki K, Schmid SM, Roche KW et al (2010) Functional comparison of the effects of TARPs and cornichons on AMPA receptor trafficking and gating. Proc Natl Acad Sci U S A 107(37):16315–16319
Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M et al (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17(19):7503–7522
Smith MA, Ellis-Davies GC, Magee JC (2003) Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol 548(Pt 1):245–258
Soto D, Coombs ID, Renzi M, Zonouzi M, Farrant M, Cull-Candy SG (2009) Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat Neurosci 12(3):277–285
Spruston N, Jonas P, Sakmann B (1995) Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol 482(Pt 2):325–352
Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE et al (2011) Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci 14(7):866–873
Sun HY, Dobrunz LE (2006) Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci 26(42):10796–10807
Sun HY, Bartley AF, Dobrunz LE (2009) Calcium-permeable presynaptic kainate receptors involved in excitatory short-term facilitation onto somatostatin interneurons during natural stimulus patterns. J Neurophysiol 101(2):1043–1055
Szabadics J, Soltesz I (2009) Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J Neurosci 29(13):4239–4251
Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen OP (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci 2(7):618–624
Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S (1992) A family of metabotropic glutamate receptors. Neuron 8(1):169–179
Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S (1993) Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci 13(4):1372–1378
Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW et al (2011) Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci 31(27):10009–10018
Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR et al (2005) Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435(7045):1052–1058
Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC (2006) mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol 575(Pt 1):115–131
Tóth K, McBain CJ (1998) Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1(7):572–578
Tóth K, McBain CJ (2000) Target-specific expression of pre- and postsynaptic mechanisms. J Physiol 525(Pt 1):41–51
Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ (2000) Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20(22):8279–8289
Valenzuela-Harrington M, Gruart A, Delgado-García JM (2007) Contribution of NMDA receptor NR2B subunit to synaptic plasticity during associative learning in behaving rats. Eur J Neurosci 25(3):830–836
Vignes M, Clarke VR, Parry MJ, Bleakman D, Lodge D, Ornstein PL et al (1998) The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology 37(10–11):1269–1277
von Kitzing E, Jonas P, Sakmann B (1994) Quantal analysis of excitatory postsynaptic currents at the hippocampal mossy fiber-CA3 pyramidal cell synapse. Adv Second Messenger Phosphoprotein Res 29:235–260
Walker HC, Lawrence JJ, McBain CJ (2002) Activation of kinetically distinct synaptic conductances on inhibitory interneurons by electrotonically overlapping afferents. Neuron 35(1):161–171
Wang W, Trieu BH, Palmer LC, Jia Y, Pham DT, Jung KM et al (2016) A primary cortical input to Hippocampus expresses a pathway-specific and endocannabinoid-dependent form of long-term potentiation. eNeuro 3(4)
Washburn MS, Dingledine R (1996) Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther 278(2):669–678
Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y (1998) Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci 10(2):478–487
Wilson DI, Watanabe S, Milner H, Ainge JA (2013) Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus 23(12):1280–1290
Winder DG, Ritch PS, Gereau RW, Conn PJ (1996) Novel glial-neuronal signalling by coactivation of metabotropic glutamate and beta-adrenergic receptors in rat hippocampus. J Physiol 494(Pt 3):743–755
Witter MP (1993) Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus, 3 Spec No, 33–44
Wyeth MS, Pelkey KA, Petralia RS, Salter MW, McInnes RR, McBain CJ (2014) Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J Neurosci 34(2):622–628
Yeckel MF, Kapur A, Johnston D (1999) Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci 2(7):625–633
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tóth, K. (2018). Glutamatergic Neurotransmission in the Hippocampus. In: Cutsuridis, V., Graham, B., Cobb, S., Vida, I. (eds) Hippocampal Microcircuits. Springer Series in Computational Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-99103-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-99103-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99102-3
Online ISBN: 978-3-319-99103-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)