Abstract
α-Emitting radionuclides have radiobiological properties that make them particularly attractive for the therapy of a number of difficult-to-treat diseases—such as cancers that are blood-borne or disseminated throughout the body—as well as residual cancer remaining after surgery. While there are many α-emitting radionuclides in nature, only ten have been identified as potentially useful for medical applications. And unfortunately, most of the members of this useful subset are difficult to obtain or are very expensive to produce. Despite these limitations, however, the enticing prospect of using these radionuclides to address medically unmet needs has prompted efforts to attach them to disease-targeting vectors and test their therapeutic efficacy in animal models of disease. Both metallic and non-metallic α-emitting radionuclides have been explored for targeted therapy, and as such, a variety of approaches to incorporating these radionuclides into targeting vectors have been developed, including direct covalent modification and the use of bifunctional chelators. While a fair number of bifunctional chelators have been developed, only a handful—e.g. CHX-A″-DTPA, DOTA, and HOPO—can be used for both α-emitters and radionuclides with imaging properties. In contrast, one of the α-emitting radionuclides that is not readily chelated, 211At, can be attached to disease-targeting agents through efficient electrophilic aromatic substitution reactions using trialkylstannyl intermediates or direct labeling on aromatic boron cage moieties. Radiopharmaceuticals containing α-emitting radionuclides are undergoing preclinical investigations for treatment of a number of cancers as well as the treatment of drug-resistant bacterial and viral infections. Furthermore, an α-emitting radiopharmaceutical—223RaCl2 (Xofigo™; Bayer HealthCare Pharmaceuticals, Inc., Whippany NJ, USA)—is an FDA-approved product, and several other radiopharmaceuticals containing α-emitting radionuclides have progressed to clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- α-Emitting radionuclides
- Targeted α therapy
- Theranostic radiopharmaceuticals
- Astatine-211
- Actinium-225
- Bismuth-212/213
- Radium-223/224
- Terbium-149
- Thorium-226/227
Fundamentals
Radiopharmaceuticals bearing α-emitting radionuclides have generated significant interest for cancer therapy and the treatment of viral- or bacterial-related diseases [1,2,3,4,5,6]. This enthusiasm stems from the fact that α particles travel short distances in tissues but have excellent cell-killing properties when carrier molecules bearing α-emitting radionuclides are bound to—or internalized within—target cells. This combination of traits allows targeted α-emitting radiopharmaceuticals to kill single cells while having minimal toxicity to non-targeted tissues. However, three obstacles have hampered the development of radiopharmaceuticals containing α-emitting radionuclides: (1) the low availability of the radionuclides, (2) a critical need for the development of appropriate carriers or targeting vectors, and (3) the requirement to develop chemistry that keeps the α-emitting radionuclide attached to the disease-targeting carrier and its metabolites in vivo. In this chapter, the radiobiological rationale for the interest in α-emitting radiopharmaceuticals will be explained, as well as the process of identifying and producing medically useful α-emitting radionuclides. In addition, the chemistry underpinning the incorporation of α-emitting radionuclides into targeting vectors will also be addressed in conjunction with a discussion of the issues surrounding the in vivo stability of α-emitting radiopharmaceuticals.
Details
While there has been a recent surge in the development and evaluation of α-emitting radiopharmaceuticals, it should be noted that the very first radionuclide used for targeted radiotherapy of any kind was in fact an α-emitting radionuclide. In 1903—only 5 years after the discovery of radioactive elements—an article appeared in the “Medical Record” journal describing the potential use of radium (226Ra) rays in the treatment of cancer [7]. The use of radium for the treatment of cancer, which included both irradiation with external sources as well as the ingestion or injection of salts, increased greatly in the two decades after the turn of the twentieth century [8, 9]. This movement prompted the Curies to donate virtually all of the radium they isolated to physicians for the treatment of cancer. Ultimately, this generosity resulted in Marie Curie having to come to the US in 1921 to obtain a single gram of the radionuclide—paid for by donations obtained by “women of America”—so she could continue her research. Unfortunately, without regulations on radioactive materials, radium was used in many non-medical applications that resulted in toxicity to people [10, 11], and interest in this form of therapy diminished. Thankfully, however, much more knowledge about radiochemistry, radiobiology, and radiopharmaceutical development has been gained in the past 100 years, so the potential of α-emitting radiopharmaceuticals for therapy is now beginning to be realized. It is an interesting coincidence that the first US FDA-approved α-particle-emitting radiopharmaceutical is also based on a radioisotope of radium: 223RaCl2 (Xofigo™).
Radiobiological Effects of α-Emission
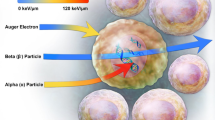
Since the first medical uses of radioactivity in the early 1900s, much has been learned about the effects of radiation on biological materials [12]. It is this knowledge that allows investigators to develop new therapeutic radiopharmaceuticals based on the expected biological responses in target and non-target tissues. To better understand why α-emitters have garnered so much interest as radionuclides for therapy, one only needs to contrast their physical and radiobiological properties with those of the more commonly used β particle emissions. Some important differences in the physical properties of these two particle types are shown in Table 1. It should be noted that the mass of an α particle is 7468 times that of a β particle(!) This large difference in mass—along with differences in the energy, velocity, and charges on the particles—produces very different interactions with biological materials. While it is hard to relate to a ~7,500-fold difference in mass, one can get some appreciation for this by visualizing the damage done to a factory (representing a cell) by a very fast-moving military battle tank weighing 55,000 kg compared to that done by a fast-moving bowling ball weighing 7.3 kg. Indeed, the energy deposited by an α particle can be 1000 times that of a β particle per unit distance traveled. This high deposition of energy over a short distance is referred to as having a high linear energy transfer (or high LET), a term often used in discussions of expected radiobiological response [13].
It should be noted that the distance that an α-particle travels in tissue—16–75 μm—is only a few cell diameters (10–30 μm for eukaryotic cell), whereas a β particle can travel several hundred cell diameters. The 3–8 MeV of energy that an α-particle deposits over the short range of its travel results in a high LET (keV/μm), which in turn results in a high relative biological effectiveness (RBE) when compared to β particles [14]. Radiobiological cell survival studies have shown that the most effective LET for killing mammalian cells is around 100 keV/μm [13]. It has been proposed that at this optimal LET, the ionization events coincide with the diameter of DNA double stands (~2 nm), resulting in lethal double strand breaks in cells. Higher LET radiation (e.g. 200 keV/μm) has similar cell-killing properties, but the extra energy deposition might be considered “wasted.” However, as α particles interact with biological material, the α energy deposition decreases rapidly on its path, so an average energy of greater than 100 keV might be of value in cell killing. This highly efficient cell killing when an α particle transverses a cell negates two important factors that usually affect cell survival when irradiating with lower LET radiation: the dose rate effect and the oxygen effect. When cells are damaged by low LET dose rates, cellular repairs can occur during the period of irradiation, making it harder to kill the cells. Similarly, if oxygen concentrations are low or absent—as in hypoxic and necrotic tissues—it can be more difficult to kill cells with radiation.
The radiobiological properties of α particle-emitting radiopharmaceuticals make them of particular interest for the treatment of disseminated (micro)metastatic disease and blood-related cancers. Indeed, it has been hypothesized that as few as one transversal of a cell (nucleus) by a single α particle can kill that cell, whereas it might take 400 β particle transversals to kill the same cell. It is generally believed that radiopharmaceuticals containing β-emitters might be more effective at treating large solid tumors than radiopharmaceuticals containing an α-emitter. This belief stems from two phenomena: the expression of target antigen within tumors can be highly heterogeneous, and it can be difficult to access cells in necrotic portions of the tumor. Both of these factors favor radiation delivered over longer distances (a radiation field). However, some investigators believe the fact that α therapy is not affected by the dose rate or the oxygen present in the target tissue may provide advantages over β particle-emitting radionuclides even in treating large tumors. They note that treatment with α-particles may require multiple administrations (fractionated doses) of the radiopharmaceutical to be effective in larger tumors.
Medically Useful α-Emitters
Although there are a large number of α-emitting radionuclides, only a few of them have been identified as appropriate for use in radiopharmaceuticals [15]. There are several important factors to consider when determining whether an α-emitting radionuclide is suitable for the development of therapeutic radiopharmaceuticals: (a) the abundance of α emissions; (b) its physical half-life; (c) the daughters it produces, along with their half-lives and emissions; (d) the availability of facile production routes; and (e) the cost of its production. For example, a radionuclide that has a low abundance of α-emissions may be unattractive, as more radioactivity is required to deliver the α dose than in cases where a radionuclide has a high abundance of α emissions. The requirement for higher quantities of radioactivity being used may result in higher non-target toxicity. The half-life of the radionuclide is also important, as too short a half-life (e.g. <30 min) may preclude the radiopharmaceutical from reaching its target in vivo before a large portion has decayed. In addition, the short half-life makes it very difficult to prepare the radiopharmaceutical and conduct quality control on it before significant amounts decay. In contrast, too long a half-life (e.g. over a month) prolongs the treatment period, which is not desired since many patients are medically compromised. Another important consideration is the nature of the daughter nuclides produced when the α-emitter decays. The ideal situation is that the radionuclide decays to a stable isotope so that no additional radioactive materials are generated. However, this does not generally occur. Rather, the radionuclide often decays to another radionuclide, which then decays to yet another radionuclide, and so on. If an α-emitting radionuclide is produced from the decay of the parent radionuclide, the resultant daughter or daughters could redistribute in the body and cause unwanted toxicity.
Table 2 lists ten α-emitting radionuclides that have been identified as candidates for medical application [15]. The selection of an α-emitting radionuclide to develop a therapeutic radiopharmaceutical might ideally be accomplished by assessing the intended application and matching the properties of the radionuclide to the disease being treated and the targeting vector to be used. In reality, however, the primary factors in the selection of a radionuclide from the table have all too often been the availability and cost of the radionuclide. Another important consideration in the selection of an α-emitting radionuclide is the chemistry for incorporating it into a targeting molecule. These factors must be considered before entering clinical trials and developing a marketable product. Fortunately, the logistics and economics of availability can be overlooked in the selection of a radionuclide used in early exploratory evaluations, as the availability can change dramatically when technology is advanced.
The decay characteristics of α-emitting radionuclides are critical in the selection of a suitable α-emitter. Table 2 provides information on the energy of α and photon emissions for each radionuclide listed. Major α-emission energies are listed for each radionuclide in the table, and the photon emissions are provided to allow for evaluation of whether each isotope can be used for imaging and quantification of the activity in the patient’s organs and tissues. With the move toward theranostic radiopharmaceuticals in personalized or precision medicine, radionuclides that provide “imageable” photons might be favored over others that do not. Many of the photon emissions of the listed radionuclides occur in low abundance, calling into question whether imaging could actually be practically accomplished. It is important to note that one radionuclide—bismuth-213 (213Bi)—has a 440 keV γ emission in high enough abundance to be used for single-photon emission computed tomography (SPECT), and indeed, imaging has been conducted when this isotope was used. In addition, given that 213Bi is a daughter of 225Ac, its 440 keV γ emission can also be used for that radionuclide as well. Another radionuclide, terbium-149 (149Tb), has many photon emissions that might be used for SPECT, but it also has a 511 keV γ emission from positron annihilation that can be used for positron emission tomography (PET) [16]. It is apparent from entries in Table 2 that alternative theranostic pair radionuclides will be required for imaging of most radiopharmaceuticals containing an α emitter.
The daughter radionuclides produced during the decay of an α-emitting radionuclide are also of high importance in selecting a suitable α-emitter. The four panels (a–d) in Fig. 1 show the daughters produced during the decay of 225Ac, 213Bi, 227Th, 223Ra, 224Ra, 212Bi, and 226Th (highlighted in red). All of the radionuclides except for 226Th are produced via naturally occurring radioactive decay processes occurring in the earth’s crust. With the exception of the 212Bi and 213Bi, the α-emitters in Fig. 1 identified as acceptable for use in humans (in red) are associated with four or five α emissions arising from their initial decay and their daughters’ decay before ultimately decaying to a stable nuclide. Having four or five α decays associated with a radionuclide has been cited as an advantage for therapy, as there is an increased dose to the target tissue. However, the α decay chain can also introduce an unwanted source of toxicity depending on the nature of the daughter radionuclides produced and their half-lives. Although very stable attachments of α-emitting radionuclides to targeting vectors can be obtained, the recoil energy from alpha decay is so high that the resultant daughter nuclide will no longer be associated with the targeting vector. If the released daughter nuclide—or one of the subsequent daughter nuclides—has a long enough half-life, it can redistribute within the body and irradiate non-target tissues elsewhere [17].
Radionuclide decay schemes showing α-emitting radionuclides of interest for use in therapeutic radiopharmaceuticals (in red). Panel A shows the natural decay scheme (part of Neptunium Series) for 225Ac and 213Bi. Panel B shows the natural decay scheme (part of Actinium Series) for 227Th and 223Ra. Panel C shows the natural decay scheme (part of Thorium Series) for 224Ra and 212Bi. Panel D shows the decay series (that feeds into the Uranium Series) for 226Th. Radionuclide half-lives were obtained from the National Nuclear Data Center Chart of Nuclides, Brookhaven National Laboratory. https://www.nndc.bnl.gov/chart/. (Accessed 6 Apr 2018)
For example, during the decay of 225Ac (see Fig. 1, Panel A), 213Bi is formed, which has a long enough half-life (i.e. 45.6 min) to redistribute to non-target tissues. In the decay of 224Ra (see Fig. 1, Panel C), another bismuth nuclide—212Bi—is formed. 212Bi may end up in non-target tissues, as the 212Pb (t1/2 ~ 10.6 h) formed in the decay of 224Ra can redistribute prior to its decay to 212Bi. In fact, the redistribution of 212Pb seems very likely, and thus the redistribution of 212Bi is also highly likely. The chief concern with the redistribution of 213Bi or 212Bi is the natural sequestration of bismuth (and thus these radionuclides) in the kidney. Methods to remove bismuth isotopes from the kidney have been somewhat successful, but the potential for kidney damage should be of concern when using 225Ac or 224Ra. Fortunately, thus far no significant kidney toxicity has been noted in clinical trials involving the use of 225Ac.
When employing 227Th there is also a concern about redistribution of its daughter 223Ra (see Fig. 1, Panel B), since the half-life of 223Ra is certainly long enough (11.4 days) for significant redistribution to occur. Fortunately, a lot is known about the distribution and toxicity of 223Ra in humans, as it is an approved therapeutic radiopharmaceutical (223RaCl2). Importantly, 223Ra is not sequestered in normal tissues but rather on bone surfaces (or eliminated quickly through the hepatobiliary system). This knowledge of the distribution and toxicity profile of 223Ra makes the use of 227Th very attractive for targeted alpha therapy . Another thorium isotope, 226Th (see Fig. 1, Panel D), is also very interesting for use in therapeutic applications. While 226Th’s short half-life (t½ = 30.6 min) will limit its applications, the three α-emitting daughters leading up to 210Pb (t½ = 22.2 years) have very short half-lives, making it unlikely that they will redistribute. In contrast, it is very likely that the long-lived 210Pb produced will redistribute, but its lack of damaging particle emissions seems unlikely to cause toxicity. 149Tb (Fig. 2, Panel A) has only one α emission, but it is produced in low abundance (e.g. 16.7%). It should be noted that there are several radioactive daughters produced from its decay. Even though these radionuclides do not have α-emissions and will not give a large radiation dose to tissues, radioactivity will likely remain in the patient for an extended time. Another α-emitting radionuclide, 211At, has a branched decay path (Fig. 2, Panel B) that provides 100% α emission. 211At is a very attractive α-emitting radionuclide as it has no α-emitting daughters to cause toxicity through redistribution, but it must be pointed out that 211At has a long-lived 207Bi daughter that could remain in the body for an extended period of time.
Schemes showing the production routes and decay schemes for 149Tb (Panel A) and 211At (Panel B). Radionuclides of interest are in red and stable nuclides are in green. Radionuclide half-lives were obtained from the National Nuclear Data Center Chart of Nuclides, Brookhaven National Laboratory. https://www.nndc.bnl.gov/chart/. (Accessed 6 Apr 2018)
Production of Radionuclides
The paucity of production methods and the high costs associated with producing the α-emitting radionuclides of interest have limited their use in preclinical and clinical investigations. Many of the radionuclides studied have been obtained from natural radioactive sources or produced in highly specialized irradiation and isolation facilities. Another issue in obtaining them is that the radionuclides are particularly difficult to handle and purify. These costly facility and difficult technical barriers may ultimately preclude the use of some of the α-emitting radionuclides of interest. Additionally, the radionuclides with half-lives less than 1 day can have limited availability because much (or all) of the radionuclide might be lost in transit. It can be very difficult to prepare a radiopharmaceutical from the short half-lived radionuclides, as they decay rapidly during the processes of radiolabeling, conducting quality control assessments, and transferring to a patient injection area for administration. Fortunately, all the short-lived α-emitting radionuclides of interest, except 149Tb, have longer-lived parent radionuclides that can be used as generators. The use of a generator system allows for the production of the radionuclide at specialized facilities and shipment to multiple sites for the isolation of the α-emitting daughter radionuclide and on-site production of the radiopharmaceutical. As can be seen in the decay schemes in Fig. 1, 225Ac can be used as a generator for 213Bi [18]; 224Ra can be used as a generator for 212Bi [19]; and 230U can be used as a generator for production of 226Th [20]. Also, as shown in Fig. 2, radon-211 (211Rn) can be used as a generator for 211At, potentially allowing broader distribution for radiopharmaceutical development and application.
Table 3 lists some possible production routes for the radionuclides of interest and their parent (generator) radionuclides. Some of the very long-lived radionuclides—for example, 238U, 232Th, and 226Ra—can be irradiated to produce many of the longer-lived parent nuclides, such as 227Ac, 228Th, 229Th, 230Pa, and 211Rn. Thus, the radionuclides listed can be used to produce most of the α-emitting radionuclides of interest. An example in which difficulties in the preparation and isolation of the radionuclide may ultimately preclude investigation is fermium-255 (255Fm). While 255Fm has favorable radiochemical properties, such as a reasonable half-life, a decay pathway that ends in a long-lived daughter (251Cf; t1/2 = 898 years), and the availability of an einsteinium-255 (255Es; t1/2 = 39.8 days) generator system, it is unlikely that sufficient quantities of 255Es or 255Fm will ever be produced for the development of radiopharmaceuticals. In the 1950s, trace quantities of 255Fm were obtained in debris from the first US hydrogen bomb and in Sweden from the irradiation of uranium-238 (238U) with oxygen-16 (16O) atoms. The production of quantities of 255Fm on the order of 37 MBq (1 mCi) was carried out by the neutron activation of curium in the High Flux Isotope Reactor (HIFR) at Oak Ridge National Laboratory, but much more would have to be made to develop and test radiopharmaceuticals containing this radionuclide. Similarly, another man-made radionuclide (not listed in Table 1)—253Es (t1/2 = 20.5 days)—has been suggested as a possible therapeutic α-emitter for human use, but both its production [21] and the production of useful quantities of a possible generator (253Cf; t1/2 = 17.8 days) would also be extremely difficult. Thus, at this time, neither of these α-emitting radionuclides is really practical for development of therapeutic radiopharmaceuticals.
Another α-emitting radionuclide that is very difficult to produce is terbium-149 (149Tb). It can be produced using heavy-ion irradiations of lanthanide isotopes [22, 23] (as listed in Table 3) as well as by high-energy proton spallation reactions. High-energy proton spallation (1–1.2 GeV) reactions on tantalum foil targets, coupled with mass separation, have provided enough quantities, e.g. 1 GBq (37 mCi), of 149Tb to conduct animal studies [24] and conduct PET imaging [25]. Although 149Tb is difficult to produce, the fact that it is a theranostic radionuclide that could be harnessed for therapy as well as PET and SPECT imaging makes it of high interest (Table 2). One can hope that the development of new accelerator technology might make this radionuclide more available in the future for the development of radiopharmaceuticals.
Bonding and Chelation
Another challenge in creating effective α-emitting radiopharmaceuticals has been the development of chemical methods for stably attaching the radionuclide to disease-targeting carrier molecules. Because of the highly cytotoxic nature of α emissions, it is of paramount importance that the radionuclide remain stably attached to the carrier molecule and its metabolites while in the body. If the radionuclide becomes detached from the disease-targeting molecule, the therapy will less efficacious for the quantity of activity injected. Further, it is likely to be more toxic, possibly decreasing the therapeutic window to a point at which treatment with the radiopharmaceutical is not viable. Thus, the bioconjugation method used to attach the radionuclide to the targeting vector is absolutely critical. The low availability and high cost of α-emitting radionuclides has proven troublesome in this regard, too, as these issues have made it difficult to fully optimize bonding or chelation methods. In addition, it is important to note that of the isotopes we have discussed, only terbium and bismuth have stable isotopes. As a result, the characterization of the products in chelation or bonding studies involving the other elements is quite difficult, since macroscopic analytical techniques (e.g. NMR, crystallography) are not feasible. An example of this is the difficulty in characterizing 211At-labeled compounds. There are no stable isotopes of astatine, and a radioactivity quantity as high as 2 GBq (54 mCi) of 211At is only ~26 ng, making the physical characterization of 211At-containing radiopharmaceuticals very difficult if not impossible. Importantly, iodinated (and radioiodinated) derivatives can be used as chromatographic standards for 211At-labeled compounds. While this approach generally provides retention times that indicate approximately where the corresponding 211At-labeled compound might elute, it does not necessarily provide unequivocal proof that a radiochromatographic peak in that area comes from an 211At-labeled compound with the expected structure.
The choice of a bonding or chelation method used with α-emitting radionuclides is also somewhat dependent on the emissions from decay of the radionuclide of interest (or its prompt daughter). The ideal scenario is to use a chelation or bonding method that can bind the α-emitting radionuclide and a radionuclide that is useful for imaging. In general, it is important to conduct imaging (PET or SPECT) both to determine if a therapeutic radiopharmaceutical will be efficacious in a particular patient and to follow the course of therapy. If the therapeutic radionuclide has a gamma emission useful for imaging, it is considered a theranostic radionuclide. If, on the other hand, the therapeutic radionuclide does not have an “imageable” gamma, but another isotope of that element does have an “imageable” gamma, this is considered a theranostic radionuclide pair. Theranostic pairs are of high value: the same labeling chemistry can be used for both radionuclides, and identical in vivo behavior can be expected for each agent. A third situation is where the therapeutic radionuclide is not theranostic and does not have a theranostic pair. In this case, one must use an “imageable” radionuclide of another element to create a diagnostic scout probe for the therapy. Since the same element is not used, a chelation/bonding reagent is chosen such that binding/bonding of both elements results in high in vivo stability. The two agents in this situation are typically referred to as a “theranostic matched pair.”
Most of the α-emitting radionuclides of interest are radiometals, with the exception of the halogen 211At. As with the positron- and β-emitting radiometals described in other chapters, the attachment of α-emitting radiometals to carrier molecules can be accomplished by complexation with chelators having the appropriate functional groups. The design of the ligand for stable bonding is based on the chemical nature, preferred oxidation states, and preferred coordination number of the radiometal. A tremendous amount of effort has been dedicated to the creation of effective chelators [26, 27]. An in-depth discussion of the many different types of chelators is beyond the scope of this chapter. However, extensive reviews of the ligands used for the chelation of α-emitting radionuclides have been published [15, 28]. Despite the large number of chelators that have been prepared and tested, there are only a few that are routinely used for labeling targeting molecules with α-emitting radiometals. It is important to note that in addition to the functional part of the chelator that binds the radiometal in question, the molecule must also include a functional group that allows for bioconjugation to disease-targeting molecules. As a result, these modified chelators are typically known as “bifunctional chelators.” The most commonly used reactive functional groups on bifunctional chelators are amine-reactive “active esters,” such as N-hydroxysuccinimidyl or tetrafluorophenyl esters that form amide bonds and phenyl isothiocyanates that form thiourea bonds. Another common reactive functional group is a maleimide, which reacts with sulfhydryl groups to form thioether bonds.
Generally speaking, there two approaches have been used for the radiolabeling targeting molecules with α-emitters: (1) radiolabeling the bifunctional chelator prior to its attachment to the targeting molecule and (2) radiolabeling the bifunctional chelator after its attachment to the targeting molecule (reaction paths A and B, respectively in Fig. 3). The second approach is typically preferred because it often results in much higher radiolabeling yields, and it is easier to characterize and evaluate the target binding properties of the chelator-bearing targeting vector prior to radiolabeling. However, due to the radiolabeling conditions of some chelators as well as the possibility of side reactions during some radiosyntheses, the first approach has been used in some cases.
General scheme depicting alternate reaction paths for radiolabeling of a monoclonal antibody (mAb) . Reaction Path A depicts radiolabeling of bifunctional chelator or bonding moiety in the first step, followed by conjugation of the radiolabeled reagent with the mAb in a second step. Reaction Path B depicts conjugation of the bifunctional chelator or bonding moiety in the first step, followed by radiolabeling of the mAb conjugate in a second step. The circled X signifies a functional group on the chelator or bonding moiety that is reactive with a functional group on the mAb. The radioactivity emblem is representative of an α-emitting radionuclide
The structures of the most commonly used bifunctional chelators are shown in Fig. 4, and information on which chelators have been used with each radionuclide is included in Table 4. In general, acyclic ligands such as DTPA and its analog CHX-A″-DTPA have fast radiolabeling kinetics under mild reaction conditions, traits that are important when working with radionuclides that have short half-lives or sensitive biomolecules that require mild conditions (e.g. proteins). However, while acyclic chelators do have fast complexation kinetics, their complexes are often not stable to in vivo demetallation. In contrast, more rigid macrocyclic ligands such as DOTA can require harsh reaction conditions to form complexes, but the resultant complex is often more stable in vivo. Because peptides and small molecule targeting moieties are generally less sensitive to high reaction temperatures and lower pH used to facilitate chelation, DOTA can be used to radiolabel them.
The chemistry of bonding astatine to targeting molecules merits a separate discussion as it is fundamentally different from that of the radiometals discussed above. Astatine is a halogen, and it undergoes reactions similar to the other halogens. Interestingly, when astatine was first isolated and its chemistry was evaluated, it was noted that its chemical properties were more similar to its metallic neighbor polonium than its nearest halogen neighbor iodine [29]. More recently, calculations of condensed astatine have shown that it has quite different properties from other halogens (e.g. monoatomic) and is metallic in nature [30]. While investigations have been conducted to determine if it can be chelated, no chelators that are stable in vivo have yet been found. Some radiolabeling studies involving the binding of 211At to chelated rhodium and iridium have been published [31], but the in vivo stability and general utility of this approach remain undefined.
Similar to other halogens, astatine undergoes electrophilic or nucleophilic substitution reactions. Significant effort has been put into development of methods to label molecules with astatine. In those studies, it has been noted that astatine’s reactions are generally similar to that of radioiodine, but the properties of the radiolabeled molecules can be quite different, resulting in radioiodine being a poor surrogate for astatine. Nucleophilic substitution reactions involving astatine have not been used much, as they generally require more stringent reaction conditions than electrophilic substitution reactions. However, some nucleophilic substitution reactions using iodonium salt intermediates appear to have promise. It should be noted that electrophilic reactions on activated aromatic compounds such as phenols (e.g. tyrosine moieties on proteins) can provide a labeling approach, but the resultant astatinated molecules are readily deastatinated. In contrast, the astatination of non-activated or deactivated aromatic compounds provides compounds that are stable (in vitro). Of particular importance has been the electrophilic substitution of non-activated aromatic compounds that is facilitated by organometallic intermediates, including organomercury, organosilanes, and organostannanes [32]. Both trimethyl and tri-n-butyl organostannanes have proven to be the intermediates of choice for these reactions. Very high astatine labeling yields (>95%) can be achieved using these intermediates.
The critical issue with astatine labeling methods is that most result in an astatine-labeled molecule that is unstable in vivo [33]. Not surprisingly, this has made it particularly difficult to develop radiopharmaceuticals containing astatine. This instability appears to be related to the in vivo metabolism of the astatine-labeled biomolecule, as the same conjugates are often quite stable in vitro. However, the short half-life of astatine is an advantage when the carrier molecule is slowly metabolized—as in the case of monoclonal antibodies (mAb)—because the astatine undergoes decay more rapidly than the protein is metabolized, so the presence of free 211At is kept to a minimum.
An alternate approach for astatine labeling is the use of anionic aromatic boron cage moieties, in which the 211At is bound to an aromatic boron atom rather than a carbon atom. Boron-halogen bonds are in general stronger than carbon-halogen bonds, particularly in aromatic compounds. In studies directed at boron neutron capture therapy, aromatic boron cage moieties—such as the closo-decaborate2− moiety (empirical formula of B10H10 2−)—have been shown to have low toxicity. Furthermore, the dianionic aromatic nature of the closo-decaborate2− moiety makes it extremely reactive with electrophilic astatine, resulting in high radiochemical labeling yields. These factors, along with the fact that the aromatic boron cage moieties are similar in size to a phenyl ring, make them very attractive for use in radiohalogenations. This labeling approach has been shown to provide 211At-labeled compounds that are stable to in vivo deastatination [34]. While the use of anionic boron cage moieties for labeling proteins with astatine has been very successful, their use in labeling small molecules has not been demonstrated and may be questionable. This is because the anionic charge on the borate labeling moiety can potentially change the in vivo pharmacokinetics and tissue/cell penetration of the small molecule targeting agent. It is apparent that additional astatine labeling methods are needed to develop a broader array of astatinated radiopharmaceuticals.
A description of the most common bifunctional reagents used to modify disease-targeting and receptor-binding molecules for radiolabeling with α-emitting radionuclides is provided in the following sections. Examples of two mAb labeling approaches are also provided for actinium-225 and astatine-211 (see Fig. 3).
Actinium-225
Initial chelation studies with 225Ac were conducted with the commonly used acyclic chelators EDTA and DTPA as well as the latter’s more sterically restricted methyl (e.g. 1B4M-DTPA) and cyclohexyl (e.g. CHX-A″-DTPA) congeners. These ligands were modified with isothiocyanato-benzyl (Bn-NCS) functional groups for conjugation to proteins (see Fig. 4). Unfortunately, when the protein conjugates were radiolabeled with 225Ac, none of them provided adequate in vivo stability for use as radiopharmaceuticals. The macrocyclic DOTA ligand was subsequently studied as an alternative. A DOTA-NHS derivative (in which an N-hydroxysuccinimide ester is attached to an acetate side group) was conjugated with a monoclonal antibody (mAb), and radiolabeling with 225Ac was evaluated. The initial radiolabeling studies provided very low radiochemical yields (<1%) when directly labeling the mAb, so an alternate 2-step labeling approach was evaluated (see Reaction Path A, Fig. 3). In this 2-step approach, the bifunctional chelator isothiocyanato-benzyl-DOTA (DOTA-Bn-NCS) was radiolabeled in the first step to provide an 225Ac-labeled amine-reactive intermediate 2 that was subsequently conjugated to a mAb to give the radiolabeled mAb 3. This approach provided higher radiochemical yields (~10%) than the first approach, but these yields remained quite low [35]. More recently, a direct labeling method (see Reaction Path B, Fig. 3) in which the mAb is conjugated with DOTA-Bn-NCS prior to radiolabeling with 225Ac was reported [36]. This approach provided ~95% labeling yield of the radioimmunoconjugate, and the 225Ac-labeled antibody was found to be stable in vivo. The difference in direct labeling yields from the DOTA-NHS and DOTA-Bn-NCS conjugates—which have 3 or 4 carboxylate groups available, respectively—is striking. Other larger macrocyclic ligands similar to DOTA have been tested for labeling mAbs. Interestingly, a pentaaza-chelate (15 atom ring; PEPA) mAb conjugate was found to be unstable in vivo, whereas the mAb conjugate of a hexaaza-derivative (18 atom ring; HEHA-Bn-NCS) was found to be stable [37]. Unlike more sensitive mAbs, DOTA-bearing small molecules and peptides can be labeled under elevated temperatures to obtain high radiochemical yields. Other ligands that might improve the labeling conditions for 225Ac are currently under investigation, but it is likely that DOTA-Bn-NCS will continue to be used for 225Ac labeling in the future.
Bismuth-212/213
The half-lives of 212Bi and 213Bi are very short (60.6 min and 45.6 min, respectively) for developing radiopharmaceuticals, so the ligand and reaction conditions used in labeling the targeting vector must provide very rapid radiolabeling. Early chelation studies involved the use of a bifunctional DTPA derivative; however, the resulting radioimmunoconjugates were found to be unstable to in vivo demetallation. Because of the requirement for rapid labeling and the reaction conditions needed to label DOTA-bearing mAbs with bismuth, the use of DOTA derivatives was not favorable. Thus, considerable efforts were undertaken to find more stable chelator for 212Bi and 213Bi. Since the DTPA ligand provided rapid labeling, DTPA derivatives with rigidifying backbone modifications were developed [27]. These included DTPA variants bearing methyl-substituted backbones (e.g. 1B4M-Bn-NCS) as well as backbones that incorporated a cyclohexyl group (CHX-A″-DTPA-Bn-NCS) (see Fig. 4). Unfortunately, substitutions on the DTPA backbone introduced epimers and diastereomeric pairs that affected the in vivo stability of the chelate complexes. More information on the in vivo stability differences observed for the backbone-modified DTPA derivatives can be obtained from a review on the labeling chemistry of α-emitters [15]. At present, CHX-A″-DTPA-Bn-NCS might be considered the bifunctional chelator of choice for labeling heat- and pH-sensitive biomolecules with 212/213Bi. It must be emphasized that the CHX-A″-DTPA ligand does not provide high in vivo stability for bismuth radionuclides, but it has adequate stability for use with slowly metabolized molecules such as mAbs. In contrast, the stability of bismuth-labeled CHX-A″-DTPA chelate complex may not be adequate for labeling small molecules and rapidly metabolized proteins or peptides. Importantly, small molecules and peptides can generally withstand the reaction conditions required to label DOTA derivatives with bismuth isotopes. Examples in which DOTA has been incorporated into a disease-targeting small molecule include a 213Bi-labeled DOTATOC [38] and a 213Bi-labeled biotin-DOTA derivative [39]. Reaction temperatures of 80–100 °C were used to obtain these two products in just 5 min reaction time. Importantly, 213Bi can be used to prepare theranostic radiopharmaceuticals, as it has a 440 keV photon γ emission that can be used for imaging.
Fermium-255
It appears that there are no examples of 255Fm chelation for in vivo use, but studies evaluating binding of 254Fm with DTPA suggest that the cyclohexyl-bearing derivative CHX-A″-DTPA-Bn-NCS might facilitate the labeling of proteins. However, considering the ~20 h half-life of 255Fm, it would be best if a macrocyclic bifunctional chelator such as DOTA-Bn-NCS be evaluated as a labeling moiety. The important point is that it is unlikely that 255Fm will be made available for developing radiopharmaceuticals due to the difficulty of its production.
Radium-223/224
Because of its availability, there is a high interest in the coupling of radium radionuclides—particularly 223Ra—to disease-targeting vectors. Radium’s chemistry is similar to that of barium, and in aqueous solution, it is found almost exclusively in the +2 oxidation state [40]. While there have been many attempts to find a ligand that will enable the stable in vivo chelation of radium nuclides, none has been developed so far. Attempts with DTPA, DOTA, and calix[4]arene tetraacetic acid have shown that the calix[4]arene provided the most stable complexes, but that stability was not sufficient for in vivo use [41]. As an alternative to chelation, incorporation of radium isotopes into nanoparticles may provide an approach that is successful for in vivo applications [42].
Terbium-149
The fact that there are several radioisotopes of terbium that have emissions for imaging and therapy makes the α-particle-emitting 149Tb attractive to develop--> theranostic radiopharmaceuticals [43]. It has been demonstrated that terbium radioisotopes, including 149Tb, can be readily chelated by CHX-A″-DTPA and DOTA. While this is the case, the difficulty in production of 149Tb calls into question the potential of this radionuclide for the development of theranostic radiopharmaceuticals.
Thorium-226/227
The α-emitting isotopes of thorium—226Th and 227Th—have very disparate half-lives: 30.6 min and 18.7 days, respectively. As a result, it seems that different ligand types (e.g. acyclic vs. macrocyclic) could be used as bifunctional chelators for labeling radiopharmaceuticals with each of these isotopes. As with the short-lived bismuth isotopes, DTPA derivatives such as CHX-A″-DTPA may be useful for radiopharmaceuticals employing 226Th [44]. Given its much longer half-life, 227Th may be best suited for use with antibody-based vectors, thus making macrocyclic chelators attractive. The labeling of mAbs with 227Th has been accomplished using DOTA-Bn-NCS as the bifunctional chelator [45], but the labeling conditions are not optimal and the labeling yields were low. More recently, an octadentate bifunctional chelator containing hydroxypyridinone (HOPO) moieties—(Me-3,2-HOPO) 4-Bn-NCS (see Fig. 4)—has been developed and facilitates the labeling of mAbs with 227Th under mild conditions [46]. The chelation of 227Th occurred within 30 min and provided >96% labeling yield, making this chelator attractive for labeling with 226Th as well. In vivo studies have shown that (Me-3,2-HOPO)4-chelated 227Th has good in vivo stability. While 226Th and 227Th do not emit imageable photons in high enough abundance for use in theranostics, HOPO chelators have been shown to bind the positron-emitting radionuclide zirconium-89 (89Zr) [47], perhaps allowing for the development of matched Th/Zr theranostic pairs. Other bifunctional chelators with functional groups similar to the HOPO ligands—such as carboxy-pyridyl derivatives having denticities of 8 (octapa-Bn-NCS) and 10 (decapa-Bn-NCS)—might also be used for theranostic applications in which the chelation of two different radionuclides are required [26].
Lead-212/Bi-212
The short half-life of 212Bi severely limits its application in therapeutic radiopharmaceuticals. However, investigators have noted that the parent radionuclide 212Pb, which has a 10.6 h half-life, might be used as an in vivo generator to produce 212Bi for therapeutic uses. Thus, studies to find an appropriate chelator for 212Pb were conducted. While some of the studies were directed at acyclic ligands, the majority of chelation studies have been conducted with macrocyclic ligands. Of several different macrocyclic chelators, DOTA appeared most stable for both lead and bismuth. Studies with DOTA-Bn-NCS conjugated to antibodies provided data that suggested the 212Pb was being released in vivo, so another DOTA derivative that had amide bonds rather than the free carboxylates, denoted TCMC-Bn-NCS (see Fig. 4), was prepared and tested as it had been previously shown to be particularly stable to the release of chelated lead [27]. The labeling of small molecule targeting agents with 212Pb has been primarily accomplished through the incorporation of DOTA into the small molecule. The issue that arises with this 212Pb/212Bi “in vivo generator” approach is that some (>30%) of the 212Bi is released from the chelator upon the decay of 212Pb, and a significant portion of this released 212Bi redistributes to the kidneys. While agents can be administered to release the 212Bi from the kidneys, the potential for latent kidney toxicity remains, so the development of new approaches to the chelation of 212Pb are needed.
Astatine-211
Radiolabeling with 211At is very different from radiolabeling with the other α-emitting radionuclides, as (so far) no methods for the stable chelation of this element from the halogen family have been demonstrated. Being a halogen, 211At can be bound to other molecules through nucleophilic and electrophilic reactions. A large number of studies have been carried out using labeling methods based on these approaches, but many reactions result in products that are not stable in vivo [33]. However, it was found that non-activated aromatic ring-containing bifunctional reagents such as meta- or para-astatobenzoate esters (Fig. 5) could be radiolabeled and then conjugated with intact mAbs to provide radioimmunoconjugates that are reasonably stable to in vivo deastatination. The result reinforces the notion that in vivo stability is a function of the rate of metabolism of the astatinated radiopharmaceutical, as small molecules and antibody fragments containing the same astatobenzoate functionalities are quite unstable in vivo. Unfortunately, due to the insolubility of the tri-n-butylstannylbenzoate moiety and the high toxicity of the trimethylstannylbenzoate moiety, the stannylbenzoates are generally radiolabeled prior to conjugation with proteins. This two-step labeling approach (see Reaction Path A, Fig. 3) results in a moderate overall radiolabeling yield (e.g. 40–50%). An alternative to the benzoate esters is to use phenethylsuccinimide NHS ester conjugates, i.e. p-PESA-NHS (see Fig. 5) [48].
Chemical structures of benzoate esters , phenethylsuccinimide ester, and closo-decaborate(2-) derivatives used to prepare radiohalogen-labeled monoclonal antibody (mAb) conjugates. In the aryl compounds either a SnBu3 or SnMe3 labeling group was used, and the radioiodinated derivatives that have been prepared are shown for the demonstration of their theranostic potential. In the depicted closo-decaborate2− cage structure, the open circles represent boron atoms, and the protons attached to the boron atoms are left off for simplicity (as in other aromatic rings)
The in vivo instability and low labeling yields of the phenyl ring-based conjugates led to the development of new reagents for labeling targeting vectors with 211At that rely upon the formation of aromatic boron-astatine bonds. The underlying concept here is that boron-halogen bonds are generally more stable than carbon-halogen bonds, and aromatic-halogen bonds are more stable than aliphatic-halogen bonds. A number of different aromatic boron cage molecules have been prepared, conjugated with antibody fragments, and tested in vivo to evaluate their stability. The nonahydro-closo-decaborate2− aromatic moiety was found to provide the best properties for labeling proteins with 211At. Two bifunctional variants of the closo-decaborate2− moiety—isothiocyanato-phenethylureido (B10-NCS) and maleimido-trioxadiamine (B10-Mal) derivatives—have been used extensively to radiolabel intact antibodies and antibody fragments (see Fig. 5). The use of these bifunctional reagents has provided immunoconjugates that can be rapidly labeled (under 2 min) to give high radiochemical labeling yields (80–95%) and have been found to be stable to in vivo deastatination [34]. While the closo-decaborate2− moiety has provided excellent results for labeling proteins, this moiety may be problematic when incorporated into some small molecule carriers, as the dianionic charge may negatively affect their in vivo targeting, cell penetration, and pharmacokinetics. This has yet to be determined, however. It should be noted that astatinated benzoate derivatives are being used to prepare mAb-based radiopharmaceuticals, as they undergo minimal deastatination due to the slow metabolism of intact mAbs. Furthermore, an approach that allows for the direct labeling of mAbs conjugated with the trimethylstannylbenzoate moiety has been developed. The direct labeling approach uses a large excess of N-iodosuccinimide to cleave the trimethylstannyl group after astatination [49], alleviating the issue of toxicity of the stannylbenzoate conjugate. An improvement in radiolabeling yield was obtained, but the yields were not as high as those obtained using the closo-decaborate2−-based conjugates. It should also be noted that the arylstannanes and closo-decaborate2− moieties readily react with radioiodine and radiobromine, making it possible to develop theranostic matched pair radiopharmaceuticals.
The foregoing description of labeling methods are cursory in nature, as they do not include descriptions of reagents or reaction conditions and do not include a fairly large number of the alternative reagents studied. Of course, one must read reviews and original literature publications to obtain that information. Instead, the descriptions provided cover what might be identified as the most important findings in this area, but such classification is subjective in nature.
Controversial Issues
There are several controversial issues that come up when discussing the potential of α-emitters radionuclides in treating human disease. Some of those issues include (1) the question of how stable the radionuclide bond to the targeting molecule needs to be to develop a useful targeted α-emitting radiopharmaceutical, (2) the belief that only one α-emitting radionuclide can be used for any or all applications, (3) the tendency to dismiss the issue of the redistribution and toxicity of α daughter radionuclides, and (4) the belief that targeted α-emitting radionuclides cannot effectively treat solid tumors. Coming to a consensus on how to address these issues is not easy, as the answers are based on the scientific environment and personal beliefs of individual researchers. Some comments on these issues follow.
With regard to the toxicity introduced by the redistribution of a radionuclide and/or its daughter radionuclides (issues #1 and #3 above), the answer is admittedly not yet clear for α-emitting radiopharmaceuticals. It is likely to be highly dependent on the quantity of radionuclide administered, the natural distribution of the radionuclide and its daughter radionuclides, the rate of release from the organ or tissue, and many other factors. With regard to one α-emitting radionuclide being useful for all/many medical applications, it is important to note that the low availability of α-emitting radionuclides has often resulted in investigators using the same radionuclide with different types of disease-targeting carrier molecules (e.g. antibodies, peptides, small molecules) rather than using a radionuclide which has a half-life or decay characteristics that best suites the carrier molecule and disease to be treated. Thus, a particular radionuclide can become that investigator’s favorite nuclide. While this belief may have merit for some applications, it seems that short half-lived radionuclides might be optimal in some applications (e.g. marrow conditioning) and less optimal in others (e.g. solid tumors). The issue of addressing the belief that--> targeted α-emitters cannot be used to treat solid tumors is perhaps the easiest to address, as it can be—and is currently being—tested, so a definitive answer will be obtained for some cancer types.
The Future
Based on the encouraging results obtained in several ongoing preclinical studies, it seems very likely that α-emitting radiopharmaceuticals will be used in the therapy of human diseases in the future. Perhaps one measure of whether a targeted α-emitting radionuclide might be useful in the therapy of cancer and other human diseases such as viral and bacterial infections is its advancement into clinical trials. While not the first to enter clinical trials, 223Ra (223RaCl2; Xofigo™) was the first α-emitting radiopharmaceutical to obtain approval for use in the therapy of metastatic bone cancer (albeit only for non-resectable metastatic prostate cancer at this time). Presently, five other α-emitting radionuclides listed in Table 2—225Ac, 213Bi, 211At, 212Pb/212Bi, and 227Th—are currently in clinical studies. One of the most important issues that will need to be addressed to bring radiopharmaceuticals to clinical practice is overcoming the low supply of α-emitting radionuclides. Fortunately, the US Department of Energy (US DOE) has focused on providing the quantities of 225Ac and other α-emitting radionuclides required for the commercialization of radiopharmaceuticals containing them. The US DOE is also setting up a network of universities that could ultimately provide regional production of 211At for preclinical and early clinical investigations.
The Bottom Line
The promise of α-emitting radionuclides for treating human diseases is being tested in a number of preclinical and clinical evaluations. Some important points about the development of α-emitting radiopharmaceuticals are listed below:
-
Blood-borne and metastatic diseases should be the focus when developing α-emitting radiopharmaceuticals, as radiopharmaceuticals can be of the highest utility and value in these areas.
-
At present, the supply of most α-emitting radionuclides is not adequate for routine clinical use, but work is being done to develop larger supplies, including the development of new, less costly methods for their production.
-
Highly selective disease-targeting agents with renal or hepatobiliary excretion of metabolites need to be developed to minimize off-target toxicity.
-
Methods for stably attaching α-emitting radionuclides to disease-targeting agents have been developed for some radionuclides, and new reagents are being developed for the ones that do not presently have suitable methods for stable attachment.
-
α-Emitting radionuclides which have short half-lives can be used with less stable chelators or bonding agents if the carrier molecule is slowly metabolized.
-
Methods for removing any free radionuclides from the blood and/or organs need to be developed to minimize the effects of the release of radionuclides from their carrier molecules or the release of their daughters after decay.
References
Vaidyanathan G, Zalutsky MR. Targeted therapy using alpha emitters. Phys Med Biol. 1996;41(10):1915–31.
McDevitt MR, Sgouros G, Finn RD, Humm JL, Jurcic JG, Larson SM, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25(9):1341–51.
Brechbiel MW. Targeted alpha-therapy: past, present, future? Dalton Trans. 2007;43:4918–28.
Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted alpha-particle therapy. J Nucl Med. 2005;46(Suppl 1):199S–204S.
Couturier O, Supiot S, Degraef-Mougin M, Faivre-Chauvet A, Carlier T, Chatal JF, et al. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med Mol Imaging. 2005;32(5):601–14.
Wadas TJ, Pandya DN, Sai KKS, Mintz A. Molecular targeted alpha-particle therapy for oncologic applications. Am J Roentgenol. 2014;203(2):253–60.
Cleaves MA. Radium: with a preliminary note on radium rays in the treatment of cancer. Med Rec. 1903;64(16):601–6.
Newcomet WS. Internal administration of radium. In: Radium and radiotherapy: radium, thorium, and other radio-active elements in medicine and surgery. New York: Lea & Febiger; 1914. p. 97–105.
Simpson FE. Radium therapy. St. Louis: C.V. Mosby Company; 1922.
Clark C. Radium girls: women and industrial health reform 1910–1935. Chapel Hill: University of North Carolina Press; 1997.
Rowland RE. Radium in humans: a review of U.S. Studies. In: Do U, editor. Energy. Chicago: University of Chicago; 1994. p. 233.
Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2006.
Hall EJ, Giaccia AJ. Linear energy transfer and relative biologic effectiveness. Radiobiology for the radiologist. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev. 2008;60(12):1402–6.
Wilbur DS. Chemical and radiochemical considerations in radiolabeling with α-emitting radionuclides. Curr Radiopharm. 2011;4(3):214–47.
Muller C, Vermeulen C, Koster U, Johnston K, Turler A, Schibli R, et al. Alpha-PET with terbium-149: evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm Chem. 2016:1–5.
de Kruijff RM, Wolterbeek HT, Denkova AG. A critical review of alpha radionuclide therapy-how to deal with recoiling daughters? Pharmaceuticals (Basel). 2015;8(2):321–36.
McDevitt MR, Finn RD, Sgouros G, Ma D, Scheinberg DA. An 225Ac/213Bi generator system for therapeutic clinical applications: construction and operation. Appl Radiat Isot. 1999;50(5):895–904.
Atcher RW, Friedman AM, Hines JJ. An improved generator for the production of 212Pb and 212Bi for 224Ra. Appl Radiat Isot. 1988;39:283–6.
Morgenstern A, Lebeda O, Stursa J, Bruchertseifer F, Capote R, McGinley J, et al. Production of 230U/226Th for targeted alpha therapy via proton irradiation of 231Pa. Anal Chem. 2008;80(22):8763–70.
Kulyukhin SA, Auerman LN, Novichenko VL, Mikheev NB, Rumer IA, Kamenskaya AN, et al. Production of microgram quantities of einsteinium-253 by reactor irradiation of californium. Inorg Chim Acta. 1985;110:25–6.
Zaitseva NG, Dmitriev SN, Maslov OD, Molokanova LG, Starodub GY, Shishkina TV, et al. Terbium-149 for nuclear medicine. The production of 149Tb via heavy ions induced nuclear reactions. Czechoslov J Phys. 2003;53:A455–A8.
Beyer G-J, Comar JJ, Dakovic M, Soloviev D, Tamburella C, Hagebo E, et al. Production routes of the alpha emitting 149Tb for medical application. Radiochim Acta. 2002;90:247–52.
Beyer GJ, Miederer M, Vranjes-Duric S, Comor JJ, Kunzi G, Hartley O, et al. Targeted alpha therapy in vivo: direct evidence for single cancer cell kill using 149Tb-rituximab. Eur J Nucl Med Mol Imaging. 2004;31(4):547–54.
Muller C, Vermeulen C, Koster U, Johnston K, Turler A, Schibli R, et al. Alpha-PET with terbium-149: evidence and perspectives for radiotheranostics. EJNMMI Radiopharm Chem. 2016;1:1–5.
Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43(1):260–90.
Brechbiel MW. Bifunctional chelates for metal nuclides. Q J Nucl Med Mol Imaging. 2008;52(2):166–73.
Hassfjell S, Brechbiel MW. The development of the a-particle emitting radionuclides 212Bi and 213Bi, and their decay chain related radionuclides, for therapeutic applications. Chem Rev. 2001;101(7):2019–36.
Corson DR, MacKenzie KR, Segre E. Artificially radioactive element 85. Phys Rev. 1940;58:672–8.
Hermann A, Hoffmann R, Ashcroft NW. Condensed astatine: monatomic and metallic. Phys Rev Lett. 2013;111(11):116404.
Pruszynski M, Bilewicz A, Zalutsky MR. Preparation of Rh[16aneS4-diol]211At and Ir[16aneS4-diol]211At complexes as potential precursors for astatine radiopharmaceuticals. Part I: synthesis. Bioconjug Chem. 2008;19(4):958–65.
Wilbur DS. Radiohalogenation of proteins: an overview of radionuclides, labeling methods, and reagents for conjugate labeling. Bioconjug Chem. 1992;3(6):433–70.
Wilbur DS. [211At]Astatine-labeled compound stability: issues with released [211at]astatide and development of labeling reagents to increase stability. Curr Radiopharm. 2008;1:144–76.
Wilbur DS, Chyan MK, Nakamae H, Chen Y, Hamlin DK, Santos EB, et al. Reagents for astatination of biomolecules. 6. An intact antibody conjugated with a maleimido-closo-decaborate(2-) reagent via sulfhydryl groups had considerably higher kidney concentrations than the same antibody conjugated with an isothiocyanato-closo-decaborate(2-) reagent via lysine amines. Bioconjug Chem. 2012;23(3):409–20.
McDevitt MR, Ma D, Simon J, Frank RK, Scheinberg DA. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl Radiat Isot. 2002;57(6):841–7.
Maguire WF, McDevitt MR, Smith-Jones PM, Scheinberg DA. Efficient 1-step radiolabeling of monoclonal antibodies to high specific activity with 225Ac for alpha-particle radioimmunotherapy of cancer. J Nucl Med. 2014;55(9):1492–8.
Deal KA, Davis IA, Mirzadeh S, Kennel SJ, Brechbiel MW. Improved in vivo stability of actinium-225 macrocyclic complexes. J Med Chem. 1999;42(15):2988–92.
Norenberg JP, Krenning BJ, Konings IRHM, Kusewitt DF, Nayak TK, Anderson TL, et al. 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res. 2006;12(3, Pt. 1):897–903.
Park SI, Shenoi J, Pagel JM, Hamlin DK, Wilbur DS, Orgun N, et al. Conventional and pretargeted radioimmunotherapy using bismuth-213 to target and treat non-Hodgkin lymphomas expressing CD20: a preclinical model toward optimal consolidation therapy to eradicate minimal residual disease. Blood. 2010;116(20):4231–9.
Kirby HW, Salutsky ML. The radiochemistry of radium, U.S. Atomic Energy Commission NAS-NS 3057. Springfield: National Academy of Sciences; 1964. p. 172.
Gott M, Steinbach J, Mamat C. The radiochemical and radiopharmaceutical applications of radium. Open Chem. 2016;14(1):118–29.
Rojas JV, Woodward JD, Chen N, Rondinone AJ, Castano CH, Mirzadeh S. Synthesis and characterization of lanthanum phosphate nanoparticles as carriers for Ra-223 and Ra-225 for targeted alpha therapy. Nucl Med Biol. 2015;42(7):614–20.
Muller C, Zhernosekov K, Koster U, Johnston K, Dorrer H, Hohn A, et al. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for alpha- and beta- radionuclide therapy: an in vivo proof-of-concept study with a new receptor-targeted folate derivative. J Nucl Med. 2012;53(12):1951–9.
Le Du A, Sabatie-Gogova A, Morgenstern A, Montavon G. Is DTPA a good competing chelating agent for Th(IV) in human serum and suitable in targeted alpha therapy? J Inorg Biochem. 2012;109:82–9.
Larsen RH, Borrebaek J, Dahle J, Melhus KB, Krogh C, Valan MH, et al. Preparation of TH227-labeled radioimmunoconjugates, assessment of serum stability and antigen binding ability. Cancer Biother Radiopharm. 2007;22(3):431–7.
Ramdahl T, Bonge-Hansen HT, Ryan OB, Larsen S, Herstad G, Sandberg M, et al. An efficient chelator for complexation of thorium-227. Bioorg Med Chem Lett. 2016;26(17):4318–21.
Deri MA, Ponnala S, Kozlowski P, Burton-Pye BP, Cicek HT, Hu C, et al. P-SCN-Bn-HOPO: a superior bifunctional chelator for 89Zr ImmunoPET. Bioconjug Chem. 2015;26(12):2579–91.
Yordanov AT, Garmestani K, Zhang M, Zhang Z, Yao Z, Phillips KE, et al. Preparation and in vivo evaluation of linkers for 211At labeling of humanized anti-Tac. Nucl Med Biol. 2001;28(7):845–56.
Lindegren S, Andersson H, Back T, Jacobsson L, Karlsson B, Skarnemark G. High-efficiency astatination of antibodies using N-iodosuccinimide as the oxidising agent in labelling of N-succinimidyl 3-(trimethylstannyl)benzoate. Nucl Med Biol. 2001;28(1):33–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wilbur, D.S. (2019). The Radiopharmaceutical Chemistry of Alpha-Emitting Radionuclides. In: Lewis, J., Windhorst, A., Zeglis, B. (eds) Radiopharmaceutical Chemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-98947-1_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-98947-1_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98946-4
Online ISBN: 978-3-319-98947-1
eBook Packages: MedicineMedicine (R0)