Abstract
Plant tissue culture and genetic transformation are powerful biotechniques for plant genetic improvement. For sesame, in vitro callus induction and differentiation with high efficiency are very difficult because of the specific characters of the species, and employing genetic transformation is too difficult. In the past forty years, many scientists tried a huge number of experiments in order to establish efficient callus induction and plantlet regeneration system in sesame. In this section, we present the history and the main progresses of sesame tissue culture and gene transformation research. Several successful examples for plant regeneration and gene transformation in sesame are listed. In addition, the main factors influencing the efficiency of tissue culture, plantlet regeneration, and gene transformation are discussed. The application prospect of tissue culture and gene transformation in sesame genetics and breeding research is also introduced.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Sesame belongs to the Pedaliaceae family and is originated from the tropical regions. Its rapid growth and development and short life-cycle (low to 70 d) character exhibit high potential for sesame to be a model crop for genetics research in key agronomic traits. However, sesame is regarded as one of the most recalcitrant crops for in vitro callus induction and differentiation (Were et al. 2006; Yadav et al. 2010; Raja and Jayabalan 2011; Zhang et al. 2019). In the past forty years, many researchers conducted technical optimization experiments in order to establish the effective techniques of tissue culture and plantlet regeneration in sesame. Early studies involved adventitious shoot induction techniques (George et al. 1987; Chen et al. 1994; Zhi et al. 1998; Saravanan and Nadarajan 2005; Seo et al. 2007; Chattopadhyaya et al. 2010; An 2009; An et al. 2011). Later, in order to increase the efficiency of multiple shoot formation and plantlet regeneration, many factors including genotype, explant type, explant pre-treatment method, culture medium and condition, and plant hormones and the concentration combinations have been investigated (Shi and Cai 1986, 1989a; Yi et al. 1997; Were et al. 2006; An 2009; Miao et al. 2012). At the same time, both Agrobacterium-mediated transformation and particle bombarding methods were also applied in sesame in order to set up the gene transformation technique (Chen et al. 1996; Yadav et al. 2010; Chowdhury et al. 2014). In recent a few years, the techniques of callus induction, differentiation, and plantlet regeneration have been established in sesame and are being applied for sesame transgene research (Mary and Jayabalan 1997; Were et al. 2006; Charaborti and Ghosh 2009). Fortunately, the Agrobacterium-mediated gene transformation system has been successfully established in sesame in recent a few years (Mitsuma et al. 2004; Chowdhury et al. 2017; Zhang et al. 2019), even though the transformation efficiency is still low. The findings provide the powerful technique system for exploring the molecular genetic basis of important agronomic traits and expanding the genetic basis of the cultivated sesame in near future.
6.2 Tissue Culture and Plant Regeneration in Sesame
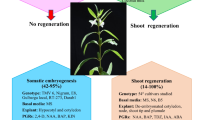
For sesame, studies of tissue culture and plant regeneration techniques initiated in the 1980s (Liu 1986; Liu et al. 1994; Qu et al. 1994; Li et al. 1996; Mary and Jayabalan 1997; Xu et al. 1997; Zhi et al. 1998; Baskaran and Jayabalan 2006; Wei et al 2007; Charaborti and Ghosh 2009; Cui et al 2010). At present, the tissue culture and plant regeneration techniques in sesame are mainly applied for two different purposes. One is to produce adventitious shoots and propagate valuable plants via explant induction, which is designated as organogenesis (direct way). The other is to form adventitious shoots or embryos via callus tissue induction, differentiate the callus tissues, and then develop regenerated plants, which is designated as somatic embryogenesis (indirect way) (Miao et al. 2012).
In the early stage of the tissue culture technology development in sesame, many scientists focused on the adventitious shoot induction techniques (George et al. 1987; Chen et al. 1994; Zhi et al. 1998; Saravanan and Nadarajan 2005; Seo et al. 2007; Chattopadhyaya et al. 2010; An 2009; An et al. 2011). Several reports showed that various explants such as seed, shoot tip, stem, and young leaf could be used to directly induce plexus bud and for propagation (George et al. 1987, 1989; Kwon et al. 1993; Rao and Vaidyanath 1997; Gangopadhyay et al. 1998; Were et al. 2006; Lokesha et al. 2007; Seo et al. 2007; Ahmed et al. 2008; Abdellatef et al. 2010; Chattopadhyaya et al. 2010; Raja and Jayabalan 2010; Honnale 2011; Raja and Jayabalan 2011; Shashidhara et al. 2011; Singh and Shagufta 2011; Lokesha et al. 2012; Wadeyar 2013; Malaghan et al. 2016; Pratik et al. 2016). However, inducing shoots from the above explants via callus induction and differentiation in sesame seems very difficult (Taskin and Turgut 1997). Till now, most of the regenerated plants in some sesame genotypes were induced from the adventitious buds which are induced through callus tissue induction and differentiation methods (Shi and Cai 1986, 1989a; Li et al. 1996; Miao et al. 2012; Bangaremma et al. 2013).
6.2.1 Somatic Embryo Formation
Somatic embryogenesis belongs to indirect way to realize the plant regeneration. As to the various explants, switching organ growth to the de-differentiation and callus formation is the first culture strategy. Even though somatic embryos could form from callus tissues induced from hypocotyl explants, the differentiation of somatic embryos into new sesame plantlets is difficult (Mary and Jayabalan 1997). The failure or low rate of callus differentiation, low repeatability of successful methods, or significant effect of genotypes could result into the low ratio of embryo formation in sesame (Mary and Jayabalan 1997; Were et al. 2006; Charaborti and Ghosh 2009; Tiwari et al. 2011). Miao et al. (2012) investigated the effects of explant type, hormone type, and hormone concentration on callus induction and plantlet regeneration from various genotypes of the cultivated sesame (S. indicum), S. radiatum, S. schinzianum, and hybrids of the interspecific cross S. schinzianum × S. indicum, and established a highly efficient system of callus induction, differentiation, and plantlet regeneration for the Sesamum genus. Meanwhile, George et al. (1987) detected the embryo-like structure of callus tissues which were derived from hypocotyl segments. However, the embryo-like cells did not form shoots. Xu et al. (1997) obtained somatic embryos on the surface of the induced callus tissues. The somatic embryos in late maturation stage formed cotyledon, shoot apex, and radical tissue after several rounds of subculture. Liu et al. (1994) successfully induced somatic embryos from embryonic callus tissues through suspension shock culture. They also investigated the variation of hormone level during the somatic embryo development. The results showed that the endogenous hormone content in cells increased, as the embryonic callus formed, while the exogenous hormones are necessary to induce explant de-differentiation. In addition, main research results suggested that the genotype was the key factor to limit the application of callus induction and plantlet regeneration methods in sesame (Mary and Jayabalan 1997; Were et al. 2006; Charaborti and Ghosh 2009).
6.2.2 Genotype
Genotype is a key factor affecting the efficiency of tissue culture techniques in sesame. In the previous reports, many sesame germplasm accessions and wild Sesamum species including S. schinzianum, S. radiatum, and S. occidentalis have been studied for plant regeneration (Table 6.1). Different genotypes exhibited different capacity of plant regeneration. Kwon et al. (1993) compared the callus induction rate of two cultivars and S. orientate which was collected from Ghana. The results showed that S. orientate exhibited significantly higher rate than cultivated sesame. Rao and Vaidyanath (1997) compared the nine sesame varieties with the wild species S. occidentalis cultured in the studied medium. The results indicated that the average shoot number of S. occidentalis was much higher. Miao et al. (2012) examined the genotype effect of three cultivars, two wild species S. radiatum and S. schinzianum, and five distant hybrid lines of S. schinzianum × S. indicum on callus induction and shoot regeneration. The results confirmed that genotype determined the callus induction rate, callus quality, and the final plant regeneration rate. The highest frequency of callus inducement and differentiation in the wild species was 97.50% and 94.02%, respectively, higher than those in cultivars (highest to 40.60% and 8.16%, respectively). Interestingly, the distant hybrids showed medium frequency of callus inducement (46.67%) and differentiation (89.29%) because of their hybridized genetic background. Therefore, optimizing the regeneration technique for different genotypes is necessary in sesame tissue culture research. George et al. (1989) performed the shoot induction using seven cultivars. Of the seven cultivars, the maximum number of the produced multiple buds varied from 15 to 18 per explants, and 80% explants could develop buds. The minimum of the produced multiple buds was 4–5, and only 44% explants developed buds. The genotype of donors indeed could influence in vitro propagation of explants, although shoot tip induction is a reliable method in sesame.
6.2.3 Explant Type
In sesame, many types of explants such as immature embryo, mature embryo, anther, seed, hypocotyl, cotyledon, shoot tip, stem segment with axillary, de-embryonated cotyledon, root, and transverse thin cell layer of the internodes have been tested to induce callus or adventitious shoot (Table 6.1). The induction frequency of callus tissues and plant regeneration varied with the various explant types and sources. In general, the induction rate of callus tissues of hypocotyls is significantly higher than cotyledon and other explants. Meanwhile, the efficiency of callus induction and regeneration was affected by other factors, including explant age and collection time, development stage of donor plants, nutritional condition, and cultivation condition. Liu et al. (1990) found that anthers of plants cultured in autumn were more suitable for callus induction than summer planted materials. To rescue the immature embryos, especially from the interspecific hybrid seeds, about 10-day-old embryos after pollination are ideal for plant regeneration (Unpublished data, Haiyang Zhang).
6.2.4 Plant Growth Regulators
Besides the above factors, plant growth regulators and nutrition substances are also the main factors determining the tissue culture efficiency in sesame. George et al. (1987) cultured the hypocotyls and cotyledon explants on MS medium without hormones and found all the explants necrosed without any morphogenetic response. In sesame, common cytokinins, such as 6-BA (6-benzylaminopurine), KT (kinetin), TDZ (thidiazuron, B1), and ZT (zeatin) and auxins, such as 2,4-D (2,4-dichlorophenoxy acetic acid), NAA (naphthalene acetic acid), IAA (indole acetic acid), and IBA (indole-3-butyric acid) are widely applied for tissue culture (George et al. 1987; Were et al. 2006; Ahmed et al. 2008; Charaborti and Ghosh 2009; Raja and Jayabalan 2011). In addition, some growth regulating substances, such as GA3 (gibberellic acid), AgNO3, and activated charcoal are also added in culture media to regulate the growth and development of various tissues. Studies showed that the specific hormone combinations with various concentrations could induce different morphogenetic responses of explants in sesame (Baskaran and Jayabalan 2006). Singh et al. (2006) found that both 2,4-D and 6-BA could reduce the formation of callus tissue in sesame. Miao et al. (2012) also detected that 2,4-D had the function in promoting the cell division and expansion which took the important function in increasing the callus induction efficiency. However, the induced calli were apt to forming roots, rather than shoot differentiation. Meanwhile, 6-BA could accelerate cell division and were favorable for the formation of high-quality calli in sesame.
As to hormone combination, George et al. (1987) studied the function of cytokinin and auxin combinations in sesame. When NAA (0.2 mg·L−1) in combination with BA, KIN, ZEA, or 2iP was used, the callus tissues with moderate growth rate were observed at the fourth week after culture. George et al. (1989) found that shoot tip with cotyledon pretreated with cytokinins showed high induction frequency of multiple buds, of which 6-BA (8 mg·L−1) was the most favorable cytokinin to form multiple buds, while 2ip (8 mg·L−1) exhibited the lowest effect.
Meanwhile, Kwon et al. (1993) screened a combination of 1–2 mg·L−1 NAA and 0.2–0.6 mg·L−1 BAP which was effective for callus induction from hypocotyl and cotyledon explants. The embryo-like structures were formed in hypocotyl-derived calli on the medium supplemented with only 1 mg·L−1 2,4-D. Addition of casein hydrolysate (1–2 g·L−1) to the regeneration media containing 0.1 mg·L−1 NAA plus 1–4 mg·L−1 BAP could effectively increase the formation rate of adventitious shoots from the hypocotyl- (22–42%) and cotyledon-derived calli (16–34%), respectively, in S. orientale. In general, high concentration of BAP (3–4 mg·L−1) in combination with casein hydrolysate could favor the formation of multiple shoots, while low concentration (1–2 mg·L−1) induced the formation of single shoot. Under the condition of low BAP concentration, addition of casein hydrolysate with high concentration also tended to induce the formation of multiple shoots. Meanwhile, the differentiation of roots was easily induced from the regenerated shoots on 1/2 MS medium supplemented with 0.5 mg·L−1 NAA. Till now, there are rarely any reports on the mechanism of hormone function in sesame callus differentiation. To set up the stable and high-efficient callus induction and differentiation system in sesame, more studies of explant de-differentiation regulation and the response mechanism of explants on hormone combinations should be conducted in future.
6.2.5 Culture Medium
In the process of in vitro tissue culture, various culture media with different nutritional components and concentrations should be supplied for specific tissues at specific growth stages. MS medium is the most common medium applied for sesame tissue culture, while 1/2MS sometimes was used for seed germination and root formation of the regenerated shoots (Yi et al. 1997; An et al. 2011; Miao et al. 2012). Other media such as B5, Nitsch, LS, and White have been applied for sesame tissue culture and plant regeneration. Li and chen (1990) found that MS, B5, and LS medium gave insignificant effects on the induction of sesame callus tissue. Yi et al. (1997) detected that the MS medium was the most optimum medium for callus induction of black sesame. Rao and Vaidyanath (1997) investigated the induction of multiple shoots in MS, LS, Camborg’s, and L6 medium, respectively. The induction percentage of multiple shoots varied from 38 to 100% for different genotypes on different media. Percentage of multiple shoot response of genotype T-85, TC-25, Co-I, and Rajeshwari reached 100% on all the four types of media. The lowest percentage of 38% was for the genotype Krishna on B5 medium.
6.2.6 Other Additives and Treatments
In addition to the above main factors such as genotype, plant growth regulator, and explants which affect callus induction and shoot regeneration, some other additives and treatments such as glutamine, casein hydrolysate, L-tyrosine, activated charcoal, and ethylene inhibitors including AgNO3 and CoCl2 also have certain effects on sesame tissue culture. Shi and Cai (1986) added casein hydrolysate in the regeneration induction medium for sesame stem section culture and found its benefit for shoot regeneration. Kwon et al. (1993) also found that addition of casein hydrolysate effectively increased the formation rate of adventitious shoots from hypocotyl- and cotyledon-derived calli in S. orientale. In sesame anther culture, addition of 300 mg·L−1 L-tyrosine did not promote the formation of pollen callus but presented toxic function for explant culture.
In addition, Abdellatef (2010) studied the effect of ethylene inhibitors AgNO3 and CoCl2 on sesame tissue culture and found that AgNO3 increased the number of shoots from 2.7 to 3.7 per explant, and the shoot length increased from 1.3 to 2.9 cm accordingly. Meanwhile, addition of 5 mg·L−1 CoCl2 induced the root length from 3.5 cm to 17 cm. The stimulative effect might result from a reduction of ethylene concentration or inhibition of ethylene action.
Xu et al. (1997) found that addition of 0.5% activated charcoal and 3% mannitol medium had good effects on embryos development. However, Shi and Cai (1989b) detected that addition of 0.2% activated carbon took no effects on callus induction and regeneration for sesame anther culture.
Meanwhile, some scientists found a few culture treatments could give aid to optimize the tissue culture techniques. To study the effect of cytokinins on multiple bud induction in the various sesame cultivars, George et al. (1989) soaked the seeds in the 6-BA, zeatin, or 2iP solution, respectively for 72 h and then put all the treated seeds on MS medium for germination and further multiple shoot induction. As a result, the induction frequency of the multiple shoot buds from the explants pretreated with cytokine increased. Rao and Vaidyanath (1997) considered that the total number of shoots induced from the explants pretreated by GA3 was higher than those of control and the seedlings pretreated with BAP in different varieties and wild species. Moreover, dark treatment was also detected as a crucial step for shoot regeneration from cotyledon in sesame.
6.3 Application of Tissue Culture Techniques in Interspecific Hybridization in Sesame
Plant tissue culture technique is developed based on the theory of plant cell totipotency and the specific character of tissue regeneration. At present, the tissue culture techniques in sesame are mainly applied for rescuing the easily abortive embryos and precious germplasm materials, overcoming the incompatibility of distant hybridization in interspecific hybridization, and facilitating genetic modification. To improve resistance level of sesame to biotic and abiotic stresses, scientists performed interspecific hybridization research since 1950s (Joshi 1961). However, the low frequency of interspecific hybrids and the high incompatibility between the cultivated sesame and most of the wild Sesamum species limit the application of the elite genotypes of the wild species in sesame breeding (Joshi 1961; Subramanian 2003; Nimmakayala et al. 2011; Zhang et al. 2013, 2019; Yang et al. 2017). Only the progeny derived from the cross between S. malabaricum (2n = 26) and the cultivated sesame exhibited high fertility rate under natural growth conditions (Nimmakayala et al. 2011). Moreover, to obtain hybrids from the crosses between the cultivated sesame and other remote wild Sesamum species and interspecific hybrids with different chromosome karyotypes, such as S. schinzianum (2n = 64), S. radiatum (2n = 64), S. alatum (2n = 26), and S. indicatum (hybrid species derived from S. indicum and S. prostratum) (Mukherji 1947), the embryo rescue and tissue culture techniques should be applied (Nimmakayala et al. 2011; Miao et al. 2012; Zhang et al. 2013; Yang et al. 2017; Zhao et al. 2018). To assess the interspecific hybrids, some specific simple sequence repeat (SSR) markers have been screened and utilized (Chinese patent no. ZL. 201210318496.8). The findings thus give impetus to the interspecific hybridization breeding in sesame.
6.4 Genetic Modification in Sesame
Plant genetic transformation system includes the recombinant DNA technology and the tissue culture skills, which introduce the exogenous genes or foreign DNA fragments into the receptor genomes and transmits the new genetic information into the offsprings. Sesame is one of the most recalcitrant crops for tissue culture and plantlet regeneration. Thus, compared with cereals and other oilseed crops, the development of genetic transformation techniques in sesame is relatively lagging. In order to develop an efficient genetic modification technique in sesame, many researchers focused on exploring the genetic modification techniques, especially the Agrobacterium-mediated transformation and particle bombarding methods via adventitious shoot formation (Chen et al. 1996; Yadav et al. 2010). In the past three decades, the main transgenic methods such as biolistic or particle gun bombardment, Agrobacterium-mediated transformation, pollen-tube channel transformation, and explant dipping methods including floral dipping, pollen infiltration, and suspension drop have been used in sesame (Table 6.2). Of these the former two transformation methods depend on the tissue culture and shoot regeneration system.
6.4.1 Genetic Modification Via Micro-Particle Bombardment in Sesame
As a direct gene transfer method for forming transgenic plants, micro-particle bombardment technique was developed in the 1980s. Till now, the technique is widely applied for transgene delivery in various crops (Christou 1995; Breitler et al. 2002). Particle bombardment can directly transfer the foreign DNA molecules into the cells or tissues, independent of cell or tissue type. Chen et al. (1996) performed particle bombardment using cotyledon as explant with the aid of the direct multiple shoot induction techniques. Bhattacharyya et al. (2015) also analyzed the effect of particle bombardment transformation technique on 5-day-old apical tissues of sesame seedlings (cv. Rama). The results of PCR, RT-PCR, western blot, and enzymatic assay showed that four transformants carrying the exogenous bar (bialaphos resistance) gene with single integration site in the genome. The mean transformation frequency touched 15.84%.
6.4.2 Agrobacterium-Mediated Genetic Modification in Sesame
Differing from the particle bombardment method, Agrobacterium-mediated genetic transformation is easy to obtain more transformants with low copy of exogenous genes. Some advantages such as high conversion efficiency and low equipment cost make the Agrobacterium-mediated genetic transformation widely used in gene function research, biological metabolite production, and transgenic breeding. For example, the Agrobacterium rhizogenes-mediated transformation is mainly used to culture the transformed hairy roots as a plant organ bioreactor and to produce valuable substances such as the recombinant proteins and pharmaceutically important secondary metabolites (Jin et al. 2005).
In the past two decades, scientists applied the tissue induction and plantlet regeneration techniques in the Agrobacterium-mediated gene transformation in sesame (Taskin et al. 1999; Yadav et al. 2010; Chowdhury et al. 2014). Taskin et al. (1999) confirmed that sesame was an amenable crop for Agrobacterium-mediated transformation; however, no transformants generated due to low efficient regeneration system in sesame. Yadav et al. (2010) established the Agrobacterium-mediated transformation technique in sesame for the first time, based on the adventitious shoot induction technique. PCR, RT-PCR, and southern blot analysis showed that the transformation frequency reached up to 1.01%. Meanwhile, Chowdhury et al. (2014) performed the Agrobacterium-mediated transformation using de-embryonated cotyledons at shoot development stage. The transgenic adventitious shoots were developed based on somatic embryo induction and differentiation techniques. To screen the positive transgenic lines, the optimized medium containing 50 mg·L−1 kanamycin and 500 mg·L−1 cefotaxime was applied in the study. As a result, the highest plantlet regeneration rate and transformation efficiency in sesame reached 52.00% and 42.66%, respectively. GUS histochemical activity and RT-PCR assay results exhibited the high efficiency of the Agrobacterium-mediated transformation technique with low copy of the exogenous gene nptII.
To data, there are two successful examples for the application of the Agrobacterium-mediated gene transformation in sesame have been reported (Mitsuma et al. 2004; Chowdhury et al. 2017). One is the transformation of a carrot calmodulin gene (cam-4) into the wild species S. schinzianum (2n = 64) through the A. tumefaciens infection on the stems of seedlings (Mitsuma et al. 2004). Both southern hybridization and RT-PCR proved the integration and expression of cam-4 gene in the transformed Sesamum plants. Moreover, overexpression of cam-4 gene enhanced the biosynthetic activity of phenylpropane derivatives in the transformed plants (Mitsuma et al. 2004). The other is the transformation of osmotin-like protein (OLP) gene (SindOLP) into the cultivated sesame cv. VR1-1 (Chowdhury et al. 2014, 2017). A total of 13 T0 lines carried single copy of SindOLP gene were obtained under the kanamycin screening.
6.4.3 Pollen-Tube Channel Transformation in Sesame
Compared with the above two methods for plant gene transformation, the pollen-tube channel method is relatively simple with low cost, regardless of the tissue culture stage. For sesame, the pollen-tube channel transformation method is rarely used. Zhen et al. (2004) performed the transformation of the exogenous DNA from the wild sesame and sorghum into the cultivated sesame via pollen-tube channel method. To establish a novel plant bioreactor based on sesame oil body expression system, Li et al. (2016) transformed the binary vector PBI121 containing the insulin gene into the sesame cultivar Jiheizhi no. 1 via pollen-tube pathway. The transformed plantlets with kanamycin resistance were testified by PCR and PCR-southern hybridization. The results showed that the insulin gene was integrated into the genome of transformants and passed to the T5 generation. The findings proved the feasibility of the genetic transformation of the exogenous insulin gene into sesame oil body via the pollen-tube-mediated method. In addition, Were (2006) transformed the green fluorescent protein (GFP) reporter gene and neomycin phosphotransferase (NPTII) gene into sesame genome via floral dip with Agrobacterium suspension before pollination. The highest mean transformation rate reached 49.8%. The above rare results showed the high potential of uncommon dipping transformation method in sesame.
6.5 T-DNA Insertion Mutant Library in Sesame
In recent few years, in order to improve the genetic modification techniques in sesame, a highly efficient callus induction and differentiation technique and the adventitious shoot induction technique were systematically developed in sesame and several wild species (An et al. 2011; Miao et al. 2012). Subsequently, an efficient Agrobacterium-mediated transformation technique via callus induction and differentiation was established in sesame, S. latifolium, and S. radiatum for the first time (partial results shown in Fig. 6.1) (unpublished data, Haiyang Zhang). The results showed that both callus type and genotype significantly affected the transformation efficiency (unpublished data, Haiyang Zhang).
Histochemical assay of positive transformants with transient and stable GUS expression. a Nontransformed hypocotyl explant (control). b Transient gus expression in hypocotyl. c Nontransformed callus tissue (control). d Transient gus expression in callus. e–h Leaf, flower, capsule, and seed, respectively in the nontransformed plantlet (control). i–l Stable gus expression of leaf, flower, capsule, and seed, respectively in the positive T0 transformant. (Provided by Ming Ju)
In the study, the pBI121 vector containing a neomycin phosphotransferase gene (Npt-II) and a β-glucuronidase (Uid A) gene was transferred into sesame and several wild Sesamum species using the transformation system. The embryonic calli (type III, annotated by Miao et al. (2012)) with high quality were induced from the A. tumefaciens-infected explants on the selection medium (i.e., basal MS + 0.1 mg·L−1 NAA + 2 mg·L−1 6-BA + 30 g·L−1 sucrose + 400 mg·L−1 cefotaxime + 25 mg·L−1 kanamycin). The histochemical activity of GUS expression proved the positive transformants (Fig. 6.1). As a result, 353 transgenic lines for Sesamum were generated. The high transformation efficiency reached to 7.5%. In addition, RT-PCR results indicated that 59.7% of the assayed transgenic lines presented the low copy number of the disarmed T-DNA insertions (≤2) (Unpublished data, Haiyang Zhang). The results proved the high efficiency of the Agrobacterium-mediated transformation technique in sesame and its key function in genetic transformation and functional genomics research in sesame.
In order to explore the unknown genes related to agronomic traits in sesame, Henan Sesame Research Center, Henan Academy of Agricultural Sciences (HSRC, HAAS), China, recently established a sesame T-DNA insertion mutant library for the first time based on the above techniques (Unpublished data, Haiyang Zhang). Till now, the mutation library consists of 520 mutant lines. PCR assay and phenotypic mutation investigation results showed that the mutation frequency of T-DNA insertion in the library reaches to 2.4%. About 50% of mutated traits were stably inherited into progeny. Meanwhile, the mutated traits were involved in leaf type, stem strength, capsule size, and environmental tolerance and resistance.
Furthermore, to explore the candidate genes affected by T-DNA insertion, the genetic characterization of a subset of 28 positive transgenic T0 lines were analyzed using the new-generation genome re-sequencing technique (Unpublished data, Haiyang Zhang). The results indicated that the average insert number was 1.7. About 54% of the mutated lines presented one inserted site in the whole genome sequences. A total of 21 insertion sites of T-DNA fragment were found located into the 5’ or 3’ UTR regions of genes, nine in gene exons, seven in gene introns, four in 3’ UTR region, and eight in intergenic regions. The high-efficient genetic transformation techniques and the constructed T-DNA insertion library supply the solid basis for the molecular genetics research in sesame.
References
Abdellatef E, Ahmed MMM, Daffalla HM, Khalafalla MM (2010) Enhancement of adventitious shoot regeneration in sesame (Sesamum indicum L.) cultivar promo KY using ethylene inhibitors. J Phytol 2:61–67
Ahmed MMM, Abdellatef E, Khalafalla MM (2008) In vitro multiple shoot induction and plant regeneration in elite Sudanese sesame cultivars (Sesamum indicum L.). Amer-Euras J Sustain Agri 2:308–314
An JW, Miao HM, Wei SL, Guo WZ, Zhang HY (2011) Study on Induced factors affecting adventitious bud formation from cotyledon in sesame. J Henan Agric Sci 10:44–47
An (2009) adventitious shoot induction and plant regeneration from cotyledon of sesame (Sesamum indicum L.). Thesis, Nanjing agriculture university, Nanjing, China
Bangaremma SW, Lokesha R, Gayatree GS, Sharanamma, (2013) Sesame shoot regeneration using different combinations of growth regulators. Mol Plant Breed 4:267–269
Baskaran P, Jayabalan N (2006) In vitro mass propagation and diverse callus orientation on Sesamum indicum L.- an important oil plant. J Agri Technol 2:259–269
Bhattacharyya J, Chakraborty A, Mitra J, Chakraborty S, Pradhan S et al (2015) Genetic transformation of cultivated sesame (Sesamum indicum L. cv Rama) through particle bombardment using 5-day-old apical, meristematic tissues of germinating seedlings. Plant Cell Tiss Org Cult 123:455–466
Breitler JC, Labeyrie A, Meynard D, Legavre T, Guiderdoni E (2002) Efficient microprojectile bombardment-mediated transformation of rice using gene cassettes. Theor Appl Genet 104:709–719
Charaborti P, Ghosh A (2009) Variation in callus induction and root-shoot bud formation depend on seed coat of sesame genotypes. Res J Bot 5:14–19
Chattopadhyaya B, Banerjee J, Basu A, Sen SK, Maiti MK (2010) Shoot induction and regeneration using internodal transverse thin cell layer culture in Sesamum indicum L. Plant Biotechnol Rep 4:173–178
Chen ZK, Wang JL, Zhi YB, Yi ML (1994) Study of multiple bud induction and shoot regeneration in sesame. Henan Agric Sci 11:10–12
Chen Z, Zhi Y, Yi M, Wang J, Liang X et al (1996) Transformation of engineered male sterile gene and establishment of transgenic plants in sesame (Sesamum indicum L.). Acta Agri Bor-Sin 11(4):33–38
Chowdhury S, Basu A, Kundu S (2014) A new high-frequency Agrobacterium-mediated transformation technique for Sesamum indicum L. using de-embryonated cotyledon as explant. Protoplasma 251:1–16
Chowdhury S, Basu A, Kundu S (2017) Overexpression of a new osmotin-Like protein gene (SindOLP) confers tolerance against biotic and abiotic stresses in sesame. Front Plant Sci 8:410
Christou P (1995) Strategies for variety-independent genetic transformation of important cereals, legumes and woody species utilizing particle bombardment. Euphytica 85:13–27
Cui YH, Sun Y, Liu WP (2010) Study on in vitro culture of hypocotyl of black sesame. Chin J Guizhou Sci 28:67–72
Gangopadhyay G, Poddar R, Gupta S (1998) Micropropagation of sesame (Sesamum indicum) by in vitro multiple shoot production from nodal explants. Phytomorphology 48:83–90
George L, Bapat VA, Rao PS (1987) In vitro multiplication of sesame (Sesamum indicum) through tissue culture. Ann Bot-London 60:17–21
George L, Bapat VA, Rao PS (1989) Plant regeneration in vitro, in different cultivars of sesame ( Sesamum indicum, L.). Proc Plant Sci 99:135–137
Honnale HN (2011) Callus induction and organogenesis in Sesamum indicum L. CV E8. Curr Trends Biotechnol Pharmacy 6:1330–1332
Jin UH, Chun JA, Han MO, Li JW, Yi YB et al (2005) Sesame hairy root cultures for extra-cellular production of a recombinant fungal phytase. Process Biochem 40:3754–3762
Joshi AB (1961) Sesamum. Indian Central Oilseed Committee Hyderabad, India, p 109
Kwon TH, Abe T, Sasahara T (1993) Efficient callus induction and plant regeneration in Sesamum species. Plant Tissue Cult Lett 10:260–266
Li B, Chen R (1990) Tissue culture and plantlet regeneration of sesame. J Shanxi Univ 13:307–310
Li M, Zhang J, Wang Q, Yang YW, Wang ZS (1996) Callus induction and organogenesis of Sesamum indicum L. hypocotyl. J Henan Normal Uni 1:61–64
Li X, An SJ, Shao TM, Chai XQ, Liu P et al (2016) Insulin gene transformation into sesame via pollen-tube pathway. Chin J Jiangsu J Agr Sci 32(5):1013–1017
Liu M (1986) Preliminary report on tissue culture experiment of sesame. Chin J Henan Agric Sci 2:15–16
Liu M (1990) Anther culture of sesame (Sesamum indicum L.). Henan Sci 2:55–61
Liu SL, Wang YD, Luo S, Zhang J, Han BW (1994) Somatic embryogenesis from hypocotyl culture of sesame and changes of endogenous hormones and soluble proteins during embryogenesis. J Agric Biotechnol 2:44–49
Lokesha R, Shashidhara N, Janagoudar BS (2007) Callus induction and plant regeneration in sesame (Sesamum indicum L.) through direct seeding. Plant Cell Biotechnol Mol Biol 8:85–88
Lokesha R, Rahaminsab J, Ranganatha ARG, Dharmataj P (2012) Whole plant regeneration via adventitious shoot formation from de-embryonated cotyledon explants of sesame (Sesamum indicum L.). World J Nuclear Sci Technol 2:47–51
Malaghan S, Lokesha R, Savitha R, Ranganatha A (2013) Adventitious shoot regeneration in Sesame (Sesamum indicum L.) (Pedaliaceae) via deembryonated cotyledonary explants. Res J Biol 1:31–35
Malaghan S, Lokesha R, Revadi S (2016) Whole plant regeneration through direct organogenesis in sesame (Sesamum indicum L.). Green Farming 7:1034–1039
Mary RJ, Jayabalan N (1997) Influence of growth regulators on somatic embryogenesis in sesame. Plant Cell Tiss Org Cult 49:67–70
Miao HM, Ju M, Wei LB, Ma Q, Zhang HY (2012) Establishment of sesame callus induction and shoot regeneration system. Chin Bull Bot 47:162-170
Mitsuma S, Ishigaki E, Sugiyama R, Tetsuya A, Kuoji Y et al (2004) Activation of phenylpropanoid metabolism in sesame by over-expression of carrot calmodulin gene. Biol Pharm Bull 27:1621–1625
Mukherji S (1947) Relation of total soluble solids in the cell sap of Sesamum species to the degree of susceptibility and resistance to Antigastra (Lepidoptera–Pyralidæ) attack. Nature 160:95–96
Nimmakayala P, Perumal R, Mulpuri S, Reddy UK (2011) Sesamum. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Springer, Berlin, Heidelberg, pp 261–273
Pratik P, Shilpa B, Kokiladevi E (2016) Efficacy of plant growth hormones for shoot induction and regeneration in Sesame (Sesamum indicum L.). Res J Biotechnol 11(10):27–30
Qu Z, Wu XY, Xia FJ (1994) Embryo rescue and plant regeneration of inerspecific hybridization between S. indicum and a wild specie S. schizinixianum. Chin J Oil Crops 1:33–35
Raja A, Jayabalan N (2010) Callus induction and plantlet regeneration from leaf explants of Sesame (Sesamumindicum L. cv. SVPR - 1). J Swamy Bot Club 27:93–98
Raja A, Jayabalan N (2011) In vitro shoot regeneration and flowering of sesame (Sesamum indicum L.) cv. SVPR-1. J Agri Technol 7:1089–1096
Rao KR, Vaidyanath K (1997) Induction of multiple shoots from seedling shoot tips of different varieties of Sesamum. Indian J Plant Physiol 2:257–261
Saravanan S, Nadarajan N (2005) Effect of media supplements on in vitro response of sesame (Sesamum indicum L.) genotypes. Res J Agric Biol Sci 1:98–100
Seo HY, Kim YJ, Park TI, Kim HS, Yun SJ et al (2007) High-frequency plant regeneration via adventitious shoot formation from deembryonated cotyledon explants of Sesamum indicum L. Vitro Cell Dev Biol 43:209–214
Shashidhara N, Santosh D, Ravikumar H, Ashoka N, Dhanalakshmi T et al (2011) Exogeneous and endogeneous contaminations in sesame tissue culture—Boon or Bane. Intl J Agric Environ Biotechnol 4:103–106
Shi SW, Cai M (1986) Presentation of plant regeneration of the stem segment culture in wild sesame Congo. Chi J Oil Crops 4:67–68
Shi SW, Cai M (1989) Callus induction and plantlet regeneration from hypocotyls of Sesamum indicum. J Plant Physiol 04:40
Shi SW, Cai M (1989) Effect on inducing factors on pollen callus formation of in vitro cultured anthers in sesame (Sesamum indicum L.). Chin J Oil Crops 2:45–49
Singh KD, Shagufta K (2011) In vitro regeneration of sesame (Sesamum indicum L.) – —an important medicinal oil crop. Crop Res 42(1 to 3):125–130
Singh RP, Singh SP, Pransad BK, Singh BD (2006) Multiple plantlets regeneration in tissue culture of sesame (Sesamum indicum L.). Res Crops 7:760–764
Subramanian M (2003) Wide crosses and chromosome behavior in Sesamum. Madras Agric J 90:1–15
Sudhaker D, SreeRangasamy SR, Liang GQ (1990) Sesame seed embryo culture. Chin J Oil Crops 3:102
Taskin KM, Turgut K (1997) In vitro regeneration of Sesame (Sesamum indicum L.) Turk J Bot 21:15–18
Taskin KM, Ercan AG, Turgut K (1999) Agrobacterium tumefaciens-mediated transformation of sesame (Sesamum indicum L.). Turk J Bot 23:291–296
Tiwari S, Kumar S, Gontia I (2011) Mini review: biotechnological approaches for sesame (Sesamum indicum L.) and niger (Guizotia abyssinica L. f. Cass). Asia Pac J Mol Biol Biotechnol 19:2–9
Wadeyar BS (2013) Sesame shoot regeneration-using different combinations of growth regulators. Mol Plant Breed 4:267–269
Wadeyar BS, Lokesha R (2011) Studies on high frequency shoot regeneration in sesame (Sesamum indicum L.). Plant Tiss Cult Biotechnol 21:45–52
Wei SL, Zhang HY, Zheng YZ, Zhang TD, Mei HX et al (2007) Study on callus induction in cotyledon and hypocotyl of sesame. J Henan Agric Sci 2:41–45
Were BA (2006) Genetic improvement of oil quality in sesame (Sesamum indicum L.). Thesis, Swedish University of Agricultural Science, Uppsala, Swedish
Were BA, Gudu S, Onkware AO, Carlsson AS, Welander M (2006) In vitro regeneration of sesame (Sesamum indicum L.) from seedling cotyledon and hypocotyl explants. Plant Cell Tiss Org Cult 85:235–239
Xu ZQ, Jia JF, Hu ZD (1997) Somatic Embryogenesis in Sesamum indicum L. cv. Nigrum. J Plant Physiol 150:755–758
Yadav M, Chaudhary D, Sainger M, Jaiwal PK (2010) Agrobacterium tumefaciens-mediated genetic transformation of sesame (Sesamum indicum L.). Plant Cell Tiss Organ Cult 103:377–386
Yang M, Liu H, Zhou T, Qu H, Yang Y et al (2017) Production and identification of F1 interspecific hybrid between Sesamum indicum and wild relative S. indicatum. Sci Agri Sin 50(10):1763–1771
Yi Y, Zhang H, Zuo T, Wang ZZ (1997) Study on the culture of different explants of black seedcoat seame in vitro. Acta Agri Bor-Occiden Sin 6(4):26–29
Yifter M, Sbhatu DB, Mekbib F, Abraha E (2013) In vitro regeneration of four Ethiopian varieties of sesame (Sesamum indicum L.) using anther culture. Asian J Plant Sci 12:214–218
Yu ZG (2005) Study on callus inducement and differentiation of black sesame seed. J Yangtze Univ Agric Sci 2:73–75
Zhang HY, Miao HM, Zhang TD, Wei LB, Li C et al (2013) Biological characters of interspecific hybrid progenies between Sesamum indicum L. and wild relatives (Sesamum schinzianum Asch, Sesamum radiatum Schum & Thonn). Sci Agri Sin 46(19):3965–3977
Zhang HY, Miao HM, Ju M (2019) Potential for adaptation to climate change through genomic breeding in sesame. In: Kole C (ed) Genomic designing of climate-smart oilseed crops. Springer, Cham, Switzerland, pp 374–376
Zhao RH, Miao HM, Ma Q, Chen CB, Song WQ et al (2018) Karyotype comparison analysis of the wild species Sesamum alatum and the cultivated sesame. Acta Scientiarum Naturalium Universitatis Nankaiensis 5(05):27–36
Zhen ZG, Duan Y, Wang XL, Cui XH, Sun MY et al (2004) Breeding of sesame lines with high resistance introduced with foreign DNA by pollen tubes path. Chin J Oil Crop Sci 26:31–34
Zhi YB, Jiang WS, Yi ML, Chen ZK (1998) Study on influence of regeneration frequency of in vitro culture of cotyledon of sesame. Chin J Xinyang Agric College 8:12–15
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Miao, H., Ju, M., Wang, H., Zhang, H. (2021). Tissue Culture and Genetic Transformation in Sesame. In: Miao, H., Zhang, H., Kole, C. (eds) The Sesame Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-319-98098-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-98098-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98097-3
Online ISBN: 978-3-319-98098-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)