Abstract
Imaging Crohn’s disease poses significant challenges, particularly in precisely defining disease activity and monitoring the adequacy of therapeutic response. Observations based on the structure of the bowel and extra-enteric tissues are the mainstay of radiological interpretation. However, functional techniques which extract information beyond simple anatomy also show considerable promise and may contribute to individualised management of Crohn’s disease patients. In this chapter, we summarise the importance and challenges posed by cross-sectional imaging of the bowel, focusing on magnetic resonance enterography (MRE). We then consider MR techniques that provide functional evaluation of the bowel over and above structure, including diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE) imaging and assessment of bowel motility. Finally, we summarise the emerging data on the potential utility of positron emission tomography-magnet resonance imaging (PET-MRI) and positron emission tomography-computed tomography (PET-CT).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Crohn’s disease
- Inflammatory bowel disease
- Imaging
- Radiology
- Magnetic resonance enterography
- MRE
- Diffusion weighted imaging
- DWI
- Dynamic contrast-enhanced magnetic resonance imaging
- DCE-MRI
- CINE
- Motility
- Positron emission tomography
- PET-MRI
6.1 Introduction
Crohn’s disease (CD), an idiopathic inflammatory bowel disorder, can affect any part of the gastrointestinal (GI) tract from the mouth to anus [1,2,3,4]. Classically, there is a discontinuous pattern of inflammation with diseased segments separated by areas of unaffected normal bowel, termed ‘skip lesions’. Inflammation confined to the small bowel accounts for around 30% of cases, another 20% involve only the large bowel, with the remainder involving both the small bowel and colon [5]. The phenotype of CD is highly diverse ranging from superficial mucosal ulceration to penetrating transmural inflammation, inflammatory and fibrotic stricturing disease, fistula and abscess formation as well as a plethora of extra-intestinal manifestations [6,7,8]. These macroscopic bowel changes have been utilised in cross-sectional imaging to identify and quantify structural transmural damage and disease activity [9, 10].
At the microscopic level, there are many well-recognised features seen in CD including an increase in lamina propria cellularity, oedema, crypt irregularities, inflammatory cellular infiltrate, increased microvessel density, granuloma formation, lymphoid aggregates, changes in mural perfusion and fibrosis [11]. These histopathological changes underlie the utility of functional MRI techniques considered in this chapter.
In the following discussion, we will first briefly review the importance and challenges posed by imaging the structure of the bowel by cross-sectional imaging, with a focus on magnetic resonance enterography (MRE). This is covered in depth in other chapters. We will then consider MR techniques that provide functional evaluation of the bowel over and above structure, including diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE) imaging and assessment of bowel motility. We then review emerging data on the potential utility of positron emission tomography-magnetic resonance imaging (PET-MRI) and positron emission tomography-computed tomography (PET-CT).
6.1.1 The Importance and Challenges of Enteric Assessment in Crohn’s Disease
Accurate characterisation of disease status is fundamental to optimised patient management [1, 4]. This principle holds true across the full spectrum of CD care, from preoperative planning [12] to serial reassessment in patients receiving immune modulators [13]. The unpredictable, progressive and relapsing nature of the disease process requires periodic lifelong evaluation by radiological and endoscopic means [1, 8, 9, 14].
As discussed in other chapters, computed tomography (CT) enterography is a valuable technique, offering high-resolution imaging from a single breath-hold, and its high diagnostic yield is well described [15,16,17,18]. However, as detailed below, many functional cross-sectional imaging techniques such as assessment of motility or dynamic contrast enhancement require multiple image acquisitions at high temporal resolution, and given its associated radiation burden, the role of CT in functional bowel imaging is currently limited. This is particularly relevant in CD given that most patients present when young, with a peak incidence between 15 and 25 years [19]. Audits suggest approximately 10% of CD patients are exposed to potentially harmful doses of radiation [20], with over 15% acquiring a cumulative radiation dose that potentially increases their risk of cancer by over 7% [21]. Most of the data regarding functional imaging in CD has been acquired using radiation-free techniques, such as small bowel ultrasound and MRI, with an emphasis on the latter [22, 23].
6.1.2 Routine Applications of MRE
As discussed in previous chapters, MRE of the small bowel affords a high-tissue-contrast examination with multiplanar assessment of the abdomen and pelvis, without radiation exposure, and is increasingly used in clinical practice for assessment of disease activity, extent and complications and for monitoring response to therapy [24,25,26].
6.1.2.1 Technique
A full description of MRE technique and sequence selection is given in Chap. 5. In brief, enteric luminal distension is achieved by oral ingestion of a hyperosmolar, biphasic liquid [27]. This permits luminal distention for the duration of the examination with low signal intensity on T1-weighted sequences, permitting visualisation of small bowel enhancement and high signal intensity on T2-weighted sequences to identify bowel wall thickening. Sufficient luminal distension is vital as collapsed bowel can obscure or even mimic disease [23]. Modern scanners facilitate rapid acquisition of images, and a typical protocol may include fast spin echo (FSE) T2-weighted sequences (with and without fat saturation), steady-state free-precession gradient echo (SSFP GE) sequences without fat saturation and unenhanced and gadolinium-enhanced T1-weighted sequences. Typical sequences and an example of a good quality examination are illustrated in Fig. 6.1.
MRI sequences in a standard MRE protocol; (a, b) Coronal steady-state free precession gradient echo (SSFP/Trufi) with good terminal ileum (white circle) distention. (c) Axial T2 Trufi. (d, e) Coronal and (f, g) axial T2 half-Fourier single-shot fast echo (HASTE) sequences with/without fat saturation, respectively
6.1.2.2 Interpretation of MRE
Routine clinical evaluation of MRE is based predominantly around the structure of the bowel and extra-enteric tissues. Morphological observations such as bowel wall thickness and the signal on T2- and post-contrast T1-weighted images form the bedrock of interpretation. As discussed fully in Chap. 10, various MRE activity scores have been developed and validated to aid phenotyping of disease activity in order to optimise delivery of target-directed therapy [28,29,30]. Examples include the magnetic resonance of activity (MaRIA) score (1.5 × bowel wall thickness (mm) × relative contrast enhancement (RCE) + 5 × oedema +10 × ulceration), the Crohn’s Disease MRE Index (CDMI) (1.79 + 1.34 mural thickness score + 0.94 mural T2 signal score) [31] and its extended derivative, the magnetic resonance enterography global score (MEGS) [32]. MaRIA and CDMI are both reproducible between radiologists and highly sensitive for detecting and grading active CD [31, 33, 34]. At present, however, such scores are used mainly for research rather than in routine clinical practice.
Despite this emphasis on the structural information afforded by MRE, a range of functional techniques have been developed which further probe the underlying pathophysiology of CD, such as cellularity, vascular changes and peristaltic activity. Such techniques have potential as imaging biomarkers of inflammatory activity, both for disease phenotyping and therapeutic monitoring.
6.1.3 Diffusion-Weighted Magnetic Resonance Imaging (DWI)
6.1.3.1 Basic Principles Underpinning DWI
Diffusion of water molecules in biological tissues reflects the underlying architecture and histological properties [35]. Evaluating the diffusion of water molecules can therefore be exploited to model internal tissue structure and physiology. Changes in water motility occur as a result of interactions with cell membranes and macromolecules and changes in the tissue environment, which alter the Brownian motion and distribution of fluids. Pathological processes such as inflammation can disturb and alter the relative amount of diffusion occurring in the affected area. The differences in the diffusional properties between tissues can therefore be interrogated to reveal pathophysiological abnormalities and are the principles underpinning diffusion-weighted magnetic resonance imaging (DWI). Since its inception in 1985, DWI has evolved and is employed in many clinical applications, including neuroradiology, oncological and, more recently, in abdominal imaging [36].
Acquisition of DWI sequences utilises a T2-weighted fat-suppressed MR sequence in combination with a diffusion gradient, measured by a diffusion coefficient, the b-value [37]. As the b-value increases, there is a corresponding reduction in the signal in areas of free diffusion. Conversely, increasing the diffusion coefficient results in a slower decline of the signal in areas of restricted diffusion such as areas of increased cellularity. The acquired images undergo post-processing to extract parametric maps, and signal decay is modelled. Most commercial MR scanners make use of the monoexponential fitting method, which offers a single parametric map referred to as the apparent diffusion coefficient (ADC) map [38]. By providing numerical values, ADC mapping permits quantification of diffusion restriction.
6.1.3.2 Application of DWI in Crohn’s Disease
DWI has potential to provide information related to tissue perfusion, vascular leakage and water diffusion in CD. The inflammatory process in CD results in infiltration of the lamina propria and submucosal layer by lymphoid aggregates and inflammatory cells [11]. The resultant increase in cellularity, viscosity, granuloma formation and dilated lymphatics, all contribute to altered diffusion. Characteristic ulceration in CD is also associated with lymphoid aggregation, increased cell density and reduction of the extracellular space, all factors that can also restrict diffusion. This restricted diffusion manifests as high signal on high b-value images with corresponding low ADC values on ADC maps and is typically seen in active CD. However the exact microscopic basis for diffusion restriction in CD remains incompletely understood [28]. It is known, for example, that fibrosis also reduces the ADC, and bowel wall perfusion also likely influences the signal such that DWI does not simply reflect inflammatory activity [39]. Nevertheless, multiple studies using a range of reference standards have shown restricted diffusion in actively inflamed bowel segments [40,41,42,43,44,45,46].
DWI may have both clinical and research applications in CD. It has potential to improve MRE sensitivity for disease detection, and thereafter in assessing disease activity and response to therapeutic interventions. Finally, it may mitigate against the need to administer intravenous contrast.
6.1.3.3 Technical Aspects
Unlike routine MRI protocols which are now relatively well standardised, the technical parameters used for DWI remain heterogenous. Both 1.5-T and 3-T scanners can be used and both platforms appear adequate. Most studies use a single b-value or multiple b-values, with the highest ranging between 500 and 1000 s/mm2, although there is a dearth of data pertaining to the optimal values [23, 28]. Feng et al., for example, reported that a high b-value of 1500 s/mm2 was most suitable on their 3T platform [47].
Fasting is recommended between 2 and 6 h before the examination, to minimise peristalsis. Although it is feasible to perform DWI without antiperistaltic agents [48, 49], recent prospective data revealed that omission resulted in significantly reduced sensitivity for detecting bowel inflammation and underestimation of the extent of disease [50]. Adequate bowel distension, generally considered essential for a successful enterographic examination, is achieved by administering an oral contrast agent 45–60 min prior to image acquisition (see Chap. 5) [23]. Enteroclysis techniques, which require intestinal intubation under fluoroscopic guidance with associated radiation burden, have not yielded superior accuracy [51] and are therefore not recommended in routine protocols [23]. Only a handful of studies have reported on the fidelity of DWI for CD in the absence of an oral contrast agent, with varying success [42, 46, 52]. However, routine use of oral contrast is generally adopted, aiding standardisation and increasing consistency of results [28].
It is recommended that the patient is placed prone when performing DWI to improve distension of the bowel and to minimise both peristalsis and motion artefact, although superior accuracy compared to the supine position is yet to be formally demonstrated [53]. Some patients tolerate the prone position poorly, and there is an increased risk of vomiting, but otherwise this is the preferred posture for acquisition of images [23].
DW images are most often taken in the transverse plane as they are less prone to motion artefact than those acquired coronally. Navigator-trigger techniques or breath-holding can be used, with the advantage of reducing breathing motion artefact. However, these approaches increase acquisition time because images can only be obtained in certain respiratory phases, and potential reductions in signal-to-noise ratio can therefore impact on image quality. A signal averaging technique is therefore frequently employed, permitting the patient to breathe freely whilst images are acquired, increasing the signal-to-noise ratio. The use of free breathing protocols is therefore widespread [23].
Fat saturation or suppression techniques are important adjuncts to DWI to nullify the intrinsic high signal of mesenteric fat due to its short T1 relaxation time. The methods used are variable, but commonly a spoiler gradient is employed which saturates the fat signal, thereby suppressing it.
The DWI sequences performed at our institution are provided in Table 6.1.
6.1.3.4 Technical Limitations of DWI
Artefacts may be encountered when performing enteric DWI for several reasons. The required long acquisition times can result in motion artefacts. Gating techniques, faster imaging acquisition methods such as echo planar or parallel imaging, the use of antiperistaltic agents prior to the examination and novel motion compensation software algorithms are all strategies that can be employed to mitigate this [28]. The T2 shine-through effect is due to the intrinsic high signal of fluid within the bowel lumen and/or bowel wall and can give the appearance of restricted diffusion if only high B value images are reviewed. It is important to correlate with ADC maps where areas of restricted diffusion (low ADC) can be differentiated from areas of T2 shine-through (which exhibit a high ADC value). T2 shine-through effects can be minimised by using short echo times and high b-values.
6.1.3.5 Detecting Active Bowel Inflammation in Crohn’s Disease
Many studies have explored the efficacy of simple qualitative evaluation of DWI to identify active CD, both in the small [29, 40, 42, 44, 45, 54,55,56,57,58,59,60] and large bowel [29, 40, 42, 44, 46, 55, 57,58,59,60]. They differ in their standard of reference for disease activity and variably employ conventional imaging, surgical findings, histopathology, endoscopy and MRE activity scores. They also differ in how they define abnormality on DWI imaging. Most compare the bowel wall signal to an internal reference such as adjacent normal bowel [40, 61] or the spleen [55, 62], for example. Some use a binary yes/no to define active disease [55] (Figs. 6.2 and 6.3), whilst others use grading systems from 1 to 4, for example [61]. The calculated ADC is also used by some studies, with heterogenicity in suggested cut-offs for active disease. Comparison across studies is therefore rather challenging.
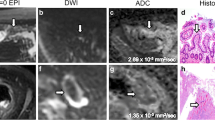
Typical appearance of a long segment (>10 cm) of very active terminal ileal (white arrow and circled area) Crohn’s on conventional sequences and DWI. Mural thickening, oedema and ulceration are shown on axial Trufi (a) and HASTE sequences without (b) and with (c) fat saturation. Axial DWI images show increased mucosal signal on (d, B = 0; and e, B = 600 s/mm2). The ADC map (f) confirms a corresponding low signal area of restricted diffusion (white circles)
Utility of diffusion-weighted imaging (DWI) in diagnosing terminal ileal Crohn’s disease—The terminal ileum is under distended on the (a) axial T2 HASTE sequence, (b) axial HASTE fat saturated sequence and (c) coronal HASTE fat saturated sequence and so indeterminate for Crohn’s disease involvement. (d) Axial DWI-weighted image (B value = 600 s/mm2) shows abnormal increased linear high signal in the bowel wall suggestive of ileitis
Overall, the reported diagnostic performance of DWI is variable, with studies citing sensitivities and specificities between 68–100% and 51–100%, respectively. A systematic review and meta-analysis incorporating studies up to March 2015 concluded that although DWI sequences had good sensitivity in detecting inflammatory CD lesions, specificity was compromised [63]. The overall reported sensitivity and specificity were 92.9% (95% CI, 85.8–96.6%) and 91% (95% CI, 79.7–96.3%), respectively, but the authors noted significant heterogeneity across studies. Those studies not blinding readers to conventional sequences and/or using contrast-enhanced MRE as the standard of reference reported higher accuracy than studies not employing this methodology. Indeed, restricting the analysis to only studies using an endoscopic or a surgical reference standard resulted in an overall sensitivity for DWI of 84% with specificity of 73%. The relatively low specificity for DWI was underlined by a study by Kim et al. using a colonoscopic standard of reference [55]. Although adding DWI to conventional sequences increased sensitivity from 62 to 83% (mainly due to detection of subtle mucosal disease in the colon), specificity fell from 94% to just 60%. Most false positives occurred in the colorectum. The jejunum is another common site of DWI false positives, likely related in part to inadequate bowel distension (Figs. 6.4 and 6.5), and lymphoid hyperplasia of the terminal ileum can also be indistinguishable from active CD on DWI [64].
False positive DWI, a common finding in under distended bowel, particularly in the jejunum (arrows), as seen on (a) coronal HASTE and with fat saturation (b). Apparent restricted diffusion as demonstrated on the DWI and corresponding ADC map (B = 600 s/mm2) (c, d), but Cine Trufi T2 (e) sequence slabs show normal motility and distension of the corresponding bowel
False positive DWI, of the terminal ileum (arrows), which is partially collapsed as seen on coronal HASTE without (a) and with (b) fat saturation, although little in the way of mural thickening. Apparent restricted diffusion as demonstrated on the DWI (c) and corresponding ADC map (B = 600 s/mm2) (d). The terminal ileum and ileocaecal valve (arrow head) were normal at CT colonography (e–g)
6.1.3.6 Quantitative Assessment of Inflammation
The ability of DWI to quantify the severity of bowel inflammation in CD has also been explored. Various groups have either used stand-alone ADC values on their own or combined DWI with morphological parameters from conventional MRE sequences. For example, the Clermont score comprises ADC values and several conventional MRE observations and is defined as 1.646 × bowel thickness –1.321 × ADC + 5.613 × oedema +8.306 × ulceration +5.039 [40]. The Clermont score is highly correlated with both the MaRIA [40, 45] and the Simplified Endoscopic Score for Crohn’s Disease (SES-CD) [54, 65].
Again, given the lack of a single reference standard for Crohn’s disease activity, authors have assessed DWI against a variety of reference standards, including MRE parameters [44, 66] and activity scores [40, 45, 54], endoscopic scores [54, 62, 67] and histopathological systems derived from surgical specimens [39]. In general, most studies report high correlation between DWI-/ADC- and MRE-derived activity scores and weak to moderate correlation against independent standard of reference such as endoscopy or histopathology. In study of 55 terminal ileum segments, Caruso et al. compared both the Clermont score and ADC to the reference standards of MaRIA and SES-CD. The correlation coefficients between Clermont and ADC with MaRIA were 0.91 and −0.80, respectively. When using the SES-CD score as the reference standard, the correlation coefficients with Clermont and ADC were 0.76 and −0.63, respectively [54]. Tielbeek et al. compared ADC to an acute inflammation scored derived from histopathological specimens in 27 bowel segments and reported a correlation coefficient of −0.33 [39]. Most recently, in a series of 39 terminal ileum segments, Seo et al. noted a correlation coefficient of 0.87, when comparing a semi-quantitative DWI score with endoscopy [62]. Pendsé et al. found that faecal calprotectin levels were significantly higher when DWI was abnormal in the small bowel and/or colon [14]. Of promise, abnormal DWI signal had a sensitivity of 83% for active CD based on faecal calprotectin as an independent reference standard, but specificity was disappointing at only 52%.
Although the literature suggests a relationship between DWI signal/ADC and the severity of bowel inflammation, the relationship is complex, and it is unlikely that a simple measure such as ADC can ever capture the complexity of the pathological changes of CD. For example, Tielbeek et al. found that ADC correlated well with histopathologically quantified bowel wall fibrosis, but not with inflammation [39], a finding recently reproduced by Li et al. [58].
6.1.3.7 Treatment Response Evaluation
The utility of DWI to monitor the effects of therapy has been investigated by a few groups (Figs. 6.6 and 6.7). Bhatnagar et al. studied 23 patients monitored by DWI following treatment with tumour necrosis alpha antagonist therapy. They reported that ADC values increased significantly in clinical responders, with no significant change seen in patients who did not respond to immunosuppressive therapy [68]. Similarly, a Korean study of 18 patients found that endoscopic improvement of disease following medical therapy is associated with reduced mural diffusion restriction and higher ADC values [69]. Conversely, in a paediatric cohort, Dillman et al. reported that changes in ADC were just 58% sensitive and 52% specific for identifying response to tumour necrosis alpha antagonist therapy [70].
Changes in DWI following treatment. There is a mildly thickened and active terminal ileal activity in a Crohn’s patient, on coronal HASTE with fat saturation (a) and DWI (b), with mildly restricted diffusion on the ADC map (c). Subsequent follow-up on treatment 1 year later (d–f) still demonstrates some mild mural thickening, but the degree of restricted diffusion has improved to the level of adjacent/background bowel activity (arrows)
Changes in DWI following treatment. There is mildly thickened and active neo-terminal ileal activity in a Crohn’s patient with an ileo-colonic anastomosis shown on pre- (a) and post-contrast (b) fat saturation T1 sequences and DWI B = 600 s/mm2) (c) with moderately restricted diffusion on the ADC map with (d). Subsequent follow-up 2 years later (e–h) demonstrates improved features with no mural oedema on fat sat HASTE sequence (f), reduced signal on DWI (g) and less restricted diffusion (h)
The utility of DWI to monitor treatment response therefore currently remains uncertain, and further research is required.
6.1.3.8 Replacement of IV Contrast Administration
Whilst administration of IV gadolinium remains part of standard MRE protocols (Fig. 6.8) [14], there are concerns about retention in the brain [71], and its use is occasionally contraindicated, such as in pregnancy, in individuals with documented allergy to contrast and in those with severely impaired renal function. There is therefore interest in replacing IV enhanced sequences with DWI.
Typical hyper-enhancement of active terminal ileal (arrow) Crohn’s disease after IV gadolinium administration. (a) Pre-contrast coronal T1-weighted sequences with fat saturation. Post-contrast (b) coronal and (c) axial T1-weighted sequences with fat saturation show avid predominantly mucosal enhancement of the terminal ileum
In a non-inferiority study, Seo et al. reported that the diagnostic performance of T2-weighted images combined with DWI was equivalent to conventional MRE with T1 post IV contrast images [62]. It should be noted, however, that in this study the median CDEIS of the terminal ileum was 15.5, suggesting high prevalence of very active disease. Furthermore, of eight bowel segments with penetrating disease, T2-weighted images combined with DWI missed a 2 cm sinus tract and incorrectly labelled 2.2 cm abscess as a phlegmon and a phlegmon as an entero-cutaneous fistula. All penetrating complications were correctly diagnosed using conventional MRE with T1 post IV contrast images.
In a paediatric cohort, Dubron et al. also reported that DWI was more accurate in detecting disease than contrast-enhanced imaging [72]. Notwithstanding these findings some mild lesions that are identifiable with contrast-enhanced MRE may be poorly detected on DWI [55, 62], In the author’s institution, DWI has replaced T1-weighted post-contrast imaging in patients undergoing follow-up MRE for known CD, although this approach remains under investigation [28].
6.1.3.9 What Is the Clinical Role of DWI?
As discussed above, the evidence suggests that DWI used in combination with either T2-weighted sequences or T1-weighted sequences enhanced with gadolinium may improve the sensitivity of MRE for inflammatory lesions in CD, particularly if mild in severity [46, 55, 59]. However, there is an associated reduction in specificity. Furthermore, there may be no diagnostic advantage over conventional sequences for detecting more established inflammation, for example, when refining recruitment to clinical trials [29]. Recent consensus guidelines suggest that the use of DWI in routine MRE protocols remains optional [14] and, if used, DWI must always be used in conjunction with conventional MRE sequences.
At the author’s institution, DWI is performed routinely and has replaced IV gadolinium in outpatient MRE follow-up of CD patients. We use it as an adjunct to conventional sequences and to help identify potential subtle disease, particularly in the colon, and provide a rapid overview of disease status. We do not use formal ADC measurements but subjectively evaluate DWI-weighted images as part of disease response evaluation, although priority is always placed on the findings on conventional sequences, notably T2-weighted images. We administer IV contrast in patients with known penetrating disease and in all hospital inpatients on the assumption that they are at higher risk of extra-enteric complications.
6.1.3.10 Summary
There is good evidence that active CD results in restricted diffusion within the bowel wall, although fibrosis has a similar effect. Sensitivity for subtle or early disease may be increased by adding DWI to conventional sequences, but specificity is reduced using this approach. DWI may have a role as an alternative to post IV gadolinium sequences in selected patients. The role of DWI in quantifying the severity of inflammation and assessing response to medical therapy requires further investigation before widespread clinical adoption.
6.1.4 Dynamic Contrast-Enhanced (DCE) Magnetic Resonance Perfusion Imaging
6.1.4.1 Basic Principles Underpinning DCE
The continual injury and regenerative process that occur in the bowel of CD patients lead to angiogenesis, driven by inflammation, hypoxia and the immune response [73]. This in turn results in an influx of inflammatory cells and greater expression of endothelial cells, inducing pro-inflammatory mechanisms and cytokine secretion. Indeed, the role of angiogenesis in CD has been extensively studied for over 50 years [74] and has been a therapeutic target [75]. Advanced functional imaging techniques able to estimate angiogenesis and quantify tissue haemodynamics have been invaluable in the study of tumour angiogenesis [76], with increasing interest in translating these methods to CD [77].
6.1.4.2 Applications of DCE-MRI in Crohn’s Disease
It has long been established that there are marked macro and microscopic vascular changes in CD. Indeed, conventional angiography has been used in the past for diagnosis given the abnormal strictured and tortuous vessel anatomy adjacent to affected bowel [78], and in the era of cross-sectional imaging, engorgement of the vasa recta (comb sign) is often associated with active disease [9, 79]. Increased bowel wall enhancement following intravenous contrast administration on CT, MRE and ultrasound has been strongly linked to disease activity [80,81,82] and, as fully explored in Chap. 10, forms part of many imaging activity scores.
Studies have also shown that there is increased microvascular (MVL) density observed in intestinal tissue, which shows positive correlation with CD activity [83, 84]. However, the relationship between neoangiogensis and disease activity is complex. For example, it has been shown that bowel wall enhancement is correlated with the number of years post-diagnosis in patients with chronic disease and is also related to tissue hypoxia [85].
In conventional MRE and CTE protocols, images are acquired at one or two time points after administration of a single bolus of intravenous contrast (Chap. 5). These permit either qualitative or quantitative assessment of the relative enhancement of the bowel wall to look for the hyper enhancement associated with CD (Chap. 10). Dynamic contrast-enhanced imaging conversely acquires multiple images through the bowel with high spatial and temporal resolution to probe the local haemodynamics of the tissue.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in particular is an established technique that affords the opportunity to probe vascular structure and function. Applying DCE-MRI to CD to quantify physiological parameters related to enteric perfusion has the potential to provide greater insights into activity status than single-time point post-contrast images. Specifically, detailed functional data is derived from changes in blood flow and vascular permeability, as well as extravascular volumes. In this way, there is the potential to not only better differentiate active from inactive disease but also better grade the severity of disease. Furthermore, DCE imaging technique may play a role in furthering our understanding of the pathogenesis of CD and could help tailor therapy on a more individual basis.
6.1.4.3 Technical Aspects
A variety of pulse sequences are available for post-contrast for T1-weighted imaging including inversion recovery, gradient echo, fast spin echo and echo planar imaging. Gradient echo sequences (2D and 3D acquisitions) are frequently used as part of MRE protocols (Chap. 5) balancing signal to noise, bowel coverage, temporal resolution and production of artefacts. Prior to DCE, patient preparation is crucial; ingestion of oral contrast and administration of spasmolytic is routine.
DCE protocols acquire multiple images through the bowel wall often for several minutes (typically around 5–7 min) such that signal intensity can be plotted against time in the form of a time-intensity curve (TIC). The required temporal resolution is dependent on the planned analysis method. Simple evaluation of curve shape requires moderate temporal resolution achievable on most MRI scanners. The more quantitative analysis techniques described below require greater temporal resolution, and formal pharmacokinetic modelling requires resolutions at least below 5 seconds per volume [86] and ideally less; protocols achieving less than 1 second per volume are now possible [87]. It may be necessary to compromise on the volume of tissue interrogated as it is very difficult to image the whole small bowel volume with such high temporal resolution. Many workers therefore pre-identify the bowel section for DCE interrogation and limit the anatomical coverage. Thereafter, the problem of motion correction (peristalsis and breathing) must be overcome. Breath-hold protocols or those relying on respiratory triggering are often used, but this results in “gaps” in the data between acquisitions which may impact on quantitation particularly if a “gap” occurs around the crucial time of peak enhancement. Free-breathing protocols solve the problem of missing data, but motion correction is challenging. Various correction algorithms have been used including respiratory tracking (often of the diaphragm), volume preserving non-rigid registration, principal component registration [88], robust data decomposition registration [89] and more recently template-based registration [87]. Simpler approaches such as manually positioned ROIs whilst rejecting motion-corrupted images are also widely used in the literature.
Even when good quality motion-corrected DCE data is available, analysis is subject to many potential sources of error. Inhomogeneity in the B1 field is usually present across the imaged volume which impacts on quantitation. Thereafter, formal pharmacokinetic modelling requires calculation of the T1 relaxation time, knowledge of contrast volume/rate of administration, measurement or estimate of signal changes in nearby vascular structures (arterial input function), conversion TIC curves to concentration curves and finally, mathematical modelling.
It is of note that the linear relationship between iodine concentration and Hounsfield units means that DCE using CT is potentially more robust than MRI and is widely used in oncological imaging. However, as discussed above, the radiation doses associated with CT DCE protocols are currently generally viewed as prohibitive in young patients with non-neoplastic diseases, such as Crohn’s disease.
A range of semi-quantitative measures can be derived from TIC, such as enhancement ratio, slope of enhancement and time to peak, which do not rely on complex mathematical models but nonetheless do require good-quality DCE data. These parameters can be rapidly derived but may not be reproducible for inter-patient comparison, particularly across different MRI platforms. Quantitative measures can be calculated based on the pharmacokinetics of the contrast agent as it traverses from the intravascular to extravascular-extracellular space, using mathematical models [90]. Most workers in CD have used the two-compartment Tofts model [90] from which commonly reported parameters are K trans and V e, measures of the volume transfer constant between intravascular space and extravascular-extracellular space and of extracellular extravascular volume per unit of volume tissue, respectively.
6.1.4.4 Detecting Active Bowel Inflammation in Crohn’s Disease Semi-Quantitative DCE
As noted above, in general, increased bowel wall enhancement is a well-recognised finding suggesting active inflammation in CD [33, 91, 92] and is covered elsewhere in this volume. As well as facilitating quantitation of physiological parameters of perfusion, DCE-MRI allows the assessment of bowel wall enhancement with respect to time.
The literature concerning the use of DCE in CD is subject to the same variations in applied standards of reference than those described above for DWI. A range of reference standards have been used including endoscopy, surgical specimens, CRP and patient activity scores such as Crohn’s Disease Activity Index (CDAI) and Harvey-Bradshaw Index (HBI). This complicates comparison between studies, but a defined association between semi-quantitative measures of DCE and disease activity has emerged.
Pupillo et al. reported a significant correlation between the peak of contrast uptake and severity of disease, assessed using the Crohn’s Disease Activity Index (CDAI) [93]. They also noted a positive association between time to reach plateau and CDAI score, although statistical significance was not reached. In a 16-patient prospective cohort study, Del Vescovo et al. compared the enhancement kinetics of the bowel wall parietal layers with the degree of CD activity (measured using CDAI, CRP and histology) [94]. In patients with active disease, there was a rapid rise of mucosal-submucosal enhancement, with the peak reached 3 min after contrast administration. These changes were not seen in those with clinically inactive disease. These findings have been replicated in other studies comparing both diseased and normal bowel [95, 96] and clinically active versus dormant CD [97, 98]. Giusti et al., for example, reported that the maximum enhancement and slope of enhancement were significantly higher in patients with active disease defined using the CDAI, imaging patients over 6 min after the contrast injection [98]. Similarly, Rottgen et al. studied 26 patients with histologically proven CD and found that the slope of the contrast enhancement correlated significantly with local inflammation evaluated by ileocolonscopy [99]. Florie et al. used a free-breathing three axial slice protocol through abnormal bowel with a temporal resolution of 4–6 s over 2 min. They found a significant correlation between the bowel wall enhancement ratio, clinical grade of CD and the CDAI score [100]. More recently, in a prospective series of 33 CD patients, Ziech and co-workers described a significantly greater maximum enhancement in both severe and mild CD when compared to normal bowel mucosa [101], and using surgical resection specimens, Tielbeek et al. reported a positive correlation between the T1 ratio, maximum enhancement and initial slope of increase and a histological transmural acute inflammation score (AIS) [39].
6.1.4.5 Quantitative Assessment of Bowel Inflammation
Promising data from semi-quantitative analysis of DCE as described above has generated great interest in the possible opportunities afforded by DCE-MRI. Utilising quantitative, rather than semi-quantitative, measures could provide functional data directly relating to physiological parameters of vascular perfusion in the bowel wall of CD patients. An additional benefit is that comparisons could potentially be made between patients across different scanners, if the correct QA systems are in place. However, despite this, relatively few studies have used formal pharmacokinetic modelling in CD.
A pilot study of 51 bowel segments (19 with inflammation) in 11 patients that used endoscopic and surgical standards of reference tested a DCE protocol with a temporal resolution of 5–12 s in which image acquisition was over 5–7 min [96]. Inflamed bowel had greater K trans and V e values than normal bowel. Similarly, in a study of 21 patients principally designed to compare methods for estimating the arterial input function, van Schie et al. reported a good correlation between K trans and CDEIS of 0.73 (p < 0.001) [102].
Zu et al. studied 32 patients with CD and 18 healthy volunteers and compared DCE parameters against the CRP as a marker of disease activity [103]. K trans, K ep and V e were all higher in the CD patients than controls, and both K trans and V e were correlated to CRP (r = 0.72, p < 0.001, and r = 0.53, p = 0.002), respectively.
However, a study by Taylor et al. did not corroborate the link between disease activity and quantitative DCE parameters. They found no correlation between quantitative parameters and clinical nor histological markers of inflammatory CD using surgical resection specimens [85].
These conflicting data highlight the need for further studies to further explore and clarify the utility of these quantitative measures.
6.1.4.6 Treatment Response Evaluation
There are emerging data assessing the efficacy of DCE-MRI to evaluate response to therapy in CD. In a study of 27 patients who received tumour necrosis factor alpha antagonist therapy and followed up by DCE-MRI, Bhatnagar et al. found a significant difference in a number of quantitative measures in treatment responders, not seen in those who did not exhibit a clinical response to treatment [68]. Specifically K trans and slope of enhancement changed significantly in clinical responders but not in nonresponders.
Similarly, Zhu and colleagues studied 22 CD patients treated by faecal microbiota transplantation (FMT) and found a significant difference in the K trans and blood volume, measured by DCE-MRI in those demonstrating a clinical response [103].
6.1.4.7 What Is the Clinical Role of DCE-MRI in Crohn’s Disease?
It is known that enteric contrast enhancement is related to CD activity. Simple qualitative grading or signal intensity measurements of a single post-contrast dataset are effective and widely used in clinical practice. DCE holds promise as a more quantitative method to assess abnormal perfusion associated with CD, but protocols are relatively complex, requiring high temporal resolution over long periods, usually with post-processing motion correction. Analysis is prone to error and requires relatively complex modelling. Whilst there is good date suggesting semi-quantitative parameters are related to disease activity, it is not clear if these add anything to the simpler evaluation of enhancement on a single-time point post-contrast dataset performed as part of routine MRE protocols. Thus, DCE is rarely used clinically and is limited to mainly a research setting. Similarly, formal quantitative modelling of DCE shows promise as a biomarker of disease activity and marker of therapeutic response but is to date mainly a research tool. Overall DCE is not currently considered a mandatory component of MRE protocols but can provide useful quantitative measures of contrast enhancement and can be used as an experimental adjunct [14].
6.1.4.8 Summary
There are profound vascular changes related to CD. Whilst these are driven by several factors, data suggests there is a link with inflammatory activity. There is a reasonable evidence base linking semi-quantitative measures of contrast enhancement with inflammatory burden in CD. Notwithstanding, quantitative measures that directly measure physiological parameters facilitate more robust comparisons between patients. Future studies are needed to clarify the utility of quantitative measures and further explore the role of DCE-MRI as a means of assessing therapeutic response.
6.1.5 Cine-MRE
Traditional fluoroscopic studies have long demonstrated altered motility in bowel segments affected by inflammation in CD. However, the clinical utility of this observation has until recently received little attention as this modality imparts ionising radiation and lacks methods of quantification, and use has generally fallen out of favour [104]. The ability of MRI to safely capture bowel motility has however rekindled interest.
6.1.5.1 Application of Cine MRE in Crohn’s Disease
The bowel wall contains smooth muscle cells which drive peristalsis through coordinated contraction of the circular muscle layer. This is controlled by the plexus myentericus of Auerbach which has also been described as the “brain of the gut”. There are broadly two types of contractility with postprandial actions and fasting activity. Postprandial actions such as peristalsis and segmentation occur after ingesting food to mix it and aid its absorption. Fasted contractility such as the migrating motor complex occurs between meals and is thought to be for maintaining bowel health and function.
The underlying mechanisms for reduced motility in Crohn’s affected bowel are multifactorial, but inflammatory and fibrotic infiltration, neuritis within the bowel wall and systemic effects of the inflammatory burden mediated via hormonal and neuronal pathways all likely play a role.
6.1.5.2 Technical Aspects
Hardware and software advances mean that bowel motility can be captured as part of routine MRE protocols, using standard MRI platforms [105]. The bowel is prepared using a standard oral contrast agent (Chap. 5), and this contrast volume stimulates bowel motility. Most commonly, motility is usually captured using 2D fast “cine” sequence such as T2-weighted steady-state free precession (SSFP) or echo-planar cine sequences. Data suggests temporal resolution of one image per second for at least 15 s during a breath-hold is adequate to meaningfully capture segmental bowel motility [106]. 3D sequences remain under investigation, as do techniques based on the insertion of “taglines” into the image acquisition to measure motility over time [107].
Images are typically acquired as a coronal slab (usually 1 cm thick) during a breath-hold, which is repeated after moving the position of the slab to cover the whole small bowel volume.
The recent advances in MR small bowel motility imaging have mainly been due to the development of post-processing software solutions that can reliably quantify motility; visual inspection lacks reproductivity, and manual measurements are prohibitively time-consuming for clinical use [108].
There are various software solutions available such as semiautomated bowel lumen calibre measurement [109] and displacement mapping [110] which uses registration techniques to produce a surrogate of motility based on modelling of intensity changes over time caused by bowel motility and movement of luminal content. One commonly applied technique uses the Jacobian determinant calculated from the deformation fields in a manually drawn region of interest over the bowel. In a 2D image, this represents the area change that each individual pixel in an image undergoes when being transformed to the equivalent pixel in a reference image (Fig. 6.9) [110].
Quantitative evaluation of dynamic motility data. A dynamic series has been processed with a specialised non-rigid registration algorithm (GIQuant, Motilent, Ford, UK) to produce a motility map (a, b white arrows) with areas of high motility attracting a high motility score [c regions of interest (ROI) around terminal ileum (black arrow) motility score is 0.055 vs. small bowel (white arrow) score of 0.343]. The motility score is based on the size of the deformation field produced by each of the image time points in the series to the registration target image or reference (d). A visual representation of the deformation fields can be seen in (e). Areas of high motility will undergo greater deformation over time
The motility sequence parameters performed at our institution are provided in Table 6.1.
6.1.5.3 Detecting Active Bowel Inflammation in Crohn’s Disease
Most work researching the use of motility imaging in Crohn’s disease has investigated the link between reduced segmental motility and the underlying inflammatory burden, although Froehlich et al. tested the additional diagnostic impact of motility sequences over conventional MRE protocols [111]. In a study of 40 patients with histologically proven CD, cine MRE was able to identify 35 more CD-specific findings than conventional MRE alone (p = 0.0007).
Menys et al. studied 28 patients with CD, all undergoing cine MRE with true fast imaging, steady-state precession (true FISP) sequences [112]. A software-quantified motility index was calculated from the terminal ileum and inflammatory activity measured using the endoscopic acute inflammation score (eAIS) based on histological analysis of endoscopic biopsies. Quantified terminal ileum motility correlated negatively with both eAIS (r = −0.52, p = 0.005) and an MR activity index of CD incorporating recognised parameters including T2 signal intensity and wall thickness (r = −0.7, p < 0.001). Cullman et al. reported similar findings in a cohort of 43 patients, although also reported that reduction in motility was associated with chronic as well as acute histological changes in the small bowel [113].
Hahnemann et al. reported that bowel segments with stigmata of CD on MRE had lower motility than non-affected bowel [114], a finding reproduced by Akerman and colleagues in 127 nonselected patients undergoing MRE [115]. Motility of the terminal ileum was significantly lower when compared to healthy controls (p = 0.018), and subgroup analysis revealed that this difference persisted in patients with CD limited to the small bowel (p = 0.002).
The effect of treatment on motility has also been investigated [116]. In a cohort of 46 patients undergoing anti-TNFα therapy, responders (defined by change in physician global assessment, HBI, CRP and MRI activity score) had significantly greater improvement in segmental motility than their nonresponding counterparts (p < 0.001). Improved MRI-measured motility was 93.1% sensitive (95% CI: 78.0–98.1%) and 76.5% specific (95% CI: 52.7–90.4%) for anti-TNFα response. Importantly, improvement in motility occurred as early as 12 weeks, suggesting motility may improve before some structural markers of activity on MRE.
Crohn’s disease patients often report persistent symptoms such as abdominal pain, bloating and diarrhoea, even when the disease is in apparent remission with little or no inflammatory activity. MRI has been used to measure motility in apparently healthy bowel not directly affected by Crohn’s disease in order to investigate whether aberrant motility underlies such persistent symptoms.
In a prospective study of 53 CD patients, Menys et al. demonstrated a significant negative correlation between motility variance (i.e. how much variation there was in the motility of apparently normal small bowel) and patient self-reported well-being (r = −0.4, p = 0.003), pain (r = −0.27, p = 0.05) and diarrhoea (r = −0.4, p = 0.0025) [117]. There was also a negative correlation with calprotectin, a marker of activity (r = −0.33, p = 0.015), a finding also reported by Bickelhaupt et al. who found contraction frequency was inversely correlated to calprotectin levels [118]. These data suggest subclinical inflammation may have a systemic effect on general gut motility likely via hormonal and/or neuronal pathway and is linked to persistent abdominal symptoms.
6.1.5.4 What Is the Clinical Role of Cine MRI?
There is increasing evidence that reduced segmental small bowel motility is correlated to inflammatory activity in Crohn’s disease and that motility improves with successful treatment. However, like DWI and DCE-MRI, consensus recommendations state that motility sequences are optional as part of MRE protocols [14]. In the author’s institution, motility sequences are acquired routinely and analysed subjectively by radiologists to help grade activity in stricturing disease as an adjunct to standard morphological criteria and to help confirm normality of poorly distended bowel loops (Figs. 6.10 and 6.11).
6.1.5.5 Summary
The link between MRI quantified small bowel motility, inflammatory burden and treatment response is being established. However, multicentre studies are currently underway to further test this association and to confirm whether it is robust across a range of patients, MRI scanners and radiological observers. Further mechanistic research is also underway investigating the use of MRI in quantifying aberrant bowel motility in symptomatic Crohn’s disease patients. Small bowel motility is an exciting technique which will likely play an increasing role in the imaging of Crohn’s disease.
6.1.5.6 PET/MRI and PET/CT
There has been interest in applying hybrid imaging techniques, combining positron emission tomography (PET) with MRI or CT to CD, inspired by the successes in other clinical settings such as oncology [119]. A commonly used radiotracer in PET imaging, 18F-fluorodeoxyglucose (18F-FDG), permeates cell membranes via specific channel proteins and tends to accumulate in regions where there is high glucose uptake and utilisation, for example, in inflammatory cells [120]. Indeed, 18F-FDG uptake has been shown to correlate with active inflammation in CD [121]. Hybrid imaging combining PET with CT is well described, and several studies have shown its ability to detect active CD in the small and large bowel [122, 123], but the radiation burden that it confers is a major limitation. In a prospective study of 28 patients using PET-CT, there was significantly greater radiotracer uptake in areas of active CD when compared to normal bowel wall segments [124]. Saboury et al. correlated FDG PET parameters with an endoscopic score of disease activity in 22 patients and found significant correlation between CDEIS and standardised uptake value (SUV) max [125]. Using MRI as a standard of reference, Russo et al. investigated PET-CT for treatment follow-up [126]. Thirteen patients underwent PET-CT and MRI before and 12 weeks after starting antitumour necrosis factor-alpha therapy. There were significantly greater falls in SUVmax in those who responded than in those who did not.
The possibility of amalgamating the anatomical detail afforded by MR with the detailed physiological molecular data that PET provides, thereby minimising radiation exposure, is highly appealing. To date, few studies have evaluated the clinical utility of PET/MRI, and whether it leads to an incremental benefit over performing these studies alone remains unclear [127]. Pellino et al. compared the accuracy and clinical impact of PET/CT with PET/MRI and MR alone, in a prospective study of 28 CD patients [128]. Despite all three modalities being equally accurate in detecting active disease, PET/MRI was more accurate than PET/CT in detecting fibrotic and extra-luminal disease. The latter translated to significantly reduced operating times, with a direct impact on clinical outcome. In a retrospective study of 19 CD patients, the same research group evaluated PET/MRI to discern fibrotic and inflammatory stricturing disease using the gold standard of histology from surgical specimens [129]. The authors found that the multiplication of the PET/MRI biomarkers of signal intensity (SI), PET maximum standardised uptake value (SUVMax) and ADC was significantly different between the fibrosis only, active inflammation and fibrosis with active inflammation groups. Their observation that PET/MRI could differentiate between fibrotic disease with minimal inflammation and fibrosis with a high associated inflammatory burden could be of considerable practical importance, as these clinical entities are managed in different ways [130]. The findings were, however, at odds to those of a previous study which found no difference in the ability of PET, MRI or US to classify the nature of Crohn’s strictures [131].
6.1.5.7 Summary
Undoubtedly, more studies are needed to assess the role of PET/MRI in CD. Differentiating inflammatory from fibrotic strictures is a promising potential application accepting that PET does expose patients to radiation. Furthermore, combining the morphological detail of MRI, along with advanced sequences like DWI and DCE-MRI and the metabolic data from PET, could offer exciting means of acquiring detailed functional data in CD.
6.2 Chapter Summary
Routine clinical evaluation of MRE is based predominantly around the structure of the bowel and extra-enteric tissues. However, techniques capturing the functional abnormalities in the bowel show considerable promise, particularly DWI and motility imaging. PET MRI remains under active investigation. Perhaps the most promising potential role of functional techniques is in the assessment of disease activity and therapeutic monitoring. More research is needed, for example, to confirm the reproducibility of functional techniques across institutions and to confirm the diagnostic benefit over standard MRI evaluation, but it seems likely that they will play an increasing role in the evaluation of CD.
References
Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818–25.
Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–32.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29.
Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27.
Farmer RG, Hawk WA, Turnbull RB Jr. Clinical patterns in Crohn’s disease: a statistical study of 615 cases. Gastroenterology. 1975;68:627–35.
Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205.
Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–82.
Lichtenstein GR, Hanauer SB, Sandborn WJ, Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83. quiz 4, 84
Benitez JM, Meuwis MA, Reenaers C, Van Kemseke C, Meunier P, Louis E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut. 2013;62:1806–16.
Pariente B, Peyrin-Biroulet L, Cohen L, Zagdanski AM, Colombel JF. Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol. 2011;197:42–9.
Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–51.
Otterson MF, Lundeen SJ, Spinelli KS, et al. Radiographic underestimation of small bowel stricturing Crohn’s disease: a comparison with surgical findings. Surgery. 2004;136:854–60.
Travis SP, Stange EF, Lemann M, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55(Suppl 1):i16–35.
Taylor SA, Avni F, Cronin CG, et al. The first joint ESGAR/ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol. 2017;27:2570–82.
Hara AK, Leighton JA, Heigh RI, et al. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128–34.
Lee SS, Ha HK, Yang SK, et al. CT of prominent pericolic or perienteric vasculature in patients with Crohn’s disease: correlation with clinical disease activity and findings on barium studies. AJR Am J Roentgenol. 2002;179:1029–36.
Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113–21.
Higgins PD, Caoili E, Zimmermann M, et al. Computed tomographic enterography adds information to clinical management in small bowel Crohn’s disease. Inflamm Bowel Dis. 2007;13:262–8.
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94.
Chatu S, Subramanian V, Pollok RC. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:529–39.
Desmond AN, O’Regan K, Curran C, et al. Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524–9.
Allen PB, De Cruz P, Lee WK, Taylor S, Desmond PV, Kamm MA. Noninvasive imaging of the small bowel in Crohn’s disease: the final frontier. Inflamm Bowel Dis. 2011;17:1987–99.
Dohan A, Taylor S, Hoeffel C, et al. Diffusion-weighted MRI in Crohn’s disease: current status and recommendations. J Magn Reson Imaging. 2016;44:1381–96.
Bruining DH, Bhatnagar G, Rimola J, Taylor S, Zimmermann EM, Fletcher JG. CT and MR enterography in Crohn’s disease: current and future applications. Abdom Imaging. 2015;40:965–74.
Rimola J, Panes J, Ordas I. Magnetic resonance enterography in Crohn’s disease: optimal use in clinical practice and clinical trials. Scand J Gastroenterol. 2015;50:66–73.
Kumar S, Hakim A, Alexakis C, et al. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015;30:86–91.
Grand DJ, Guglielmo FF, Al-Hawary MM. MR enterography in Crohn’s disease: current consensus on optimal imaging technique and future advances from the SAR Crohn’s disease-focused panel. Abdom Imaging. 2015;40:953–64.
Park SH. DWI at MR Enterography for evaluating bowel inflammation in Crohn disease. AJR Am J Roentgenol. 2016;207:40–8.
Rimola J, Alvarez-Cofino A, Perez-Jeldres T, et al. Increasing efficiency of MRE for diagnosis of Crohn’s disease activity through proper sequence selection: a practical approach for clinical trials. Abdom Radiol (NY). 2017;42:2783–91.
Bhatnagar G, Von Stempel C, Halligan S, Taylor SA. Utility of MR enterography and ultrasound for the investigation of small bowel Crohn’s disease. J Magn Reson Imaging. 2017;45:1573–88.
Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080–8.
Makanyanga JC, Pendse D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014;24:277–87.
Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–20.
Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–68.
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–7.
Chilla GS, Tan CH, Xu C, Poh CL. Diffusion weighted magnetic resonance imaging and its recent trend-a survey. Quant Imaging Med Surg. 2015;5:407–22.
Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797–810.
Barral M, Taouli B, Guiu B, et al. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology. 2015;274:45–63.
Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24:619–29.
Hordonneau C, Buisson A, Scanzi J, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol. 2014;109:89–98.
Oto A, Kayhan A, Williams JT, et al. Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33:615–24.
Kiryu S, Dodanuki K, Takao H, et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging. 2009;29:880–6.
Freiman M, Perez-Rossello JM, Callahan MJ, et al. Characterization of fast and slow diffusion from diffusion-weighted MRI of pediatric Crohn’s disease. J Magn Reson Imaging. 2013;37:156–63.
Neubauer H, Pabst T, Dick A, et al. Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol. 2013;43:103–14.
Buisson A, Joubert A, Montoriol PF, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther. 2013;37:537–45.
Oussalah A, Laurent V, Bruot O, et al. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056–65.
Feng Q, Yan YQ, Zhu J, Tong JL, Xu JR. Optimal b value of diffusion-weighted imaging on a 3.0T magnetic resonance scanner in Crohn’s disease. World J Gastroenterol. 2014;20:12621–7.
Grand DJ, Beland MD, Machan JT, Mayo-Smith WW. Detection of Crohn’s disease: comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol. 2012;81:1735–41.
Grand DJ, Kampalath V, Harris A, et al. MR enterography correlates highly with colonoscopy and histology for both distal ileal and colonic Crohn’s disease in 310 patients. Eur J Radiol. 2012;81:e763–9.
Park SH, Huh J, Park SH, Lee SS, Kim AY, Yang SK. Diffusion-weighted MR enterography for evaluating Crohn’s disease: effect of anti-peristaltic agent on the diagnosis of bowel inflammation. Eur Radiol. 2017;27:2554–62.
Stanescu-Siegmund N, Nimsch Y, Wunderlich AP, et al. Quantification of inflammatory activity in patients with Crohn’s disease using diffusion weighted imaging (DWI) in MR enteroclysis and MR enterography. Acta Radiol. 2017;58:264–71.
Jesuratnam-Nielsen K, Logager VB, Rezanavaz-Gheshlagh B, Munkholm P, Thomsen HS. Plain magnetic resonance imaging as an alternative in evaluating inflammation and bowel damage in inflammatory bowel disease—a prospective comparison with conventional magnetic resonance follow-through. Scand J Gastroenterol. 2015;50:519–27.
Cronin CG, Lohan DG, Mhuircheartaigh JN, et al. MRI small-bowel follow-through: prone versus supine patient positioning for best small-bowel distention and lesion detection. AJR Am J Roentgenol. 2008;191:502–6.
Caruso A, D’Inca R, Scarpa M, et al. Diffusion-weighted magnetic resonance for assessing ileal Crohn’s disease activity. Inflamm Bowel Dis. 2014;20:1575–83.
Kim KJ, Lee Y, Park SH, et al. Diffusion-weighted MR enterography for evaluating Crohn’s disease: how does it add diagnostically to conventional MR enterography? Inflamm Bowel Dis. 2015;21:101–9.
Qi F, Jun S, Qi QY, et al. Utility of the diffusion-weighted imaging for activity evaluation in Crohn’s disease patients underwent magnetic resonance enterography. BMC Gastroenterol. 2015;15:12.
Foti PV, Farina R, Coronella M, et al. Crohn’s disease of the small bowel: evaluation of ileal inflammation by diffusion-weighted MR imaging and correlation with the Harvey-Bradshaw index. Radiol Med. 2015;120:585–94.
Li XH, Sun CH, Mao R, et al. Assessment of activity of Crohn disease by diffusion-weighted magnetic resonance imaging. Medicine (Baltimore). 2015;94:e1819.
Sato H, Tamura C, Narimatsu K, et al. Magnetic resonance enterocolonography in detecting erosion and redness in intestinal mucosa of patients with Crohn’s disease. J Gastroenterol Hepatol. 2015;30:667–73.
Church PC, Greer MC, Cytter-Kuint R, et al. Magnetic resonance enterography has good inter-rater agreement and diagnostic accuracy for detecting inflammation in pediatric Crohn disease. Pediatr Radiol. 2017;47:565–75.
Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol. 2009;16:597–603.
Seo N, Park SH, Kim KJ, et al. MR Enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology. 2016;278:762–72.
Choi SH, Kim KW, Lee JY, Kim KJ, Park SH. Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2016;22:669–79.
Plumb AA, Pendse DA, McCartney S, Punwani S, Halligan S, Taylor SA. Lymphoid nodular hyperplasia of the terminal ileum can mimic active crohn disease on MR enterography. AJR Am J Roentgenol. 2014;203:W400–7.
Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–12.
Ream JM, Dillman JR, Adler J, et al. MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol. 2013;43:1077–85.
Buisson A, Hordonneau C, Goutte M, Boyer L, Pereira B, Bommelaer G. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment Pharmacol Ther. 2015;42:452–60.
Bhatnagar G, Dikaios N, Prezzi D, Vega R, Halligan S, Taylor SA. Changes in dynamic contrast-enhanced pharmacokinetic and diffusion-weighted imaging parameters reflect response to anti-TNF therapy in Crohn’s disease. Br J Radiol. 2015;88:20150547.
Huh J, Kim KJ, Park SH, et al. Diffusion-weighted MR Enterography to monitor bowel inflammation after medical therapy in Crohn’s disease: a prospective longitudinal study. Korean J Radiol. 2017;18:162–72.
Dillman JR, Smith EA, Sanchez R, et al. DWI in pediatric small-bowel Crohn disease: are apparent diffusion coefficients surrogates for disease activity in patients receiving infliximab therapy? AJR Am J Roentgenol. 2016;207:1002–8.
Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–32.
Dubron C, Avni F, Boutry N, Turck D, Duhamel A, Amzallag-Bellenger E. Prospective evaluation of free-breathing diffusion-weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br J Radiol. 2016;89:20150840.
Alkim C, Alkim H, Koksal AR, Boga S, Sen I. Angiogenesis in inflammatory bowel disease. Int J Inflam. 2015;2015:970890.
Bacaner MB. Quantitative measurement of regional colon blood flow in the normal and pathological human bowel. Gastroenterology. 1966;51:764–77.
Rutella S, Fiorino G, Vetrano S, et al. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn’s disease. Am J Gastroenterol. 2011;106:762–70.
Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002;16:407–22.
Oommen J, Oto A. Contrast-enhanced MRI of the small bowel in Crohn’s disease. Abdom Imaging. 2011;36:134–41.
Boijsen E, Ekman CA, Lundh G. Selective splanchnic angiography. Adv Surg. 1968;3:13–73.
Pendse DA, Makanyanga JC, Plumb AA, et al. Diffusion-weighted imaging for evaluating inflammatory activity in Crohn’s disease: comparison with histopathology, conventional MRI activity scores, and faecal calprotectin. Abdom Radiol (NY). 2017;42:115–23.
Choi D, Jin Lee S, Ah Cho Y, et al. Bowel wall thickening in patients with Crohn’s disease: CT patterns and correlation with inflammatory activity. Clin Radiol. 2003;58:68–74.
Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010;30:367–84.
Roccarina D, Garcovich M, Ainora ME, et al. Diagnosis of bowel diseases: the role of imaging and ultrasonography. World J Gastroenterol. 2013;19:2144–53.
Alkim C, Savas B, Ensari A, et al. Expression of p53, VEGF, microvessel density, and cyclin-D1 in noncancerous tissue of inflammatory bowel disease. Dig Dis Sci. 2009;54:1979–84.
Danese S, Fiorino G, Angelucci E, et al. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: a pilot study. World J Gastroenterol. 2010;16:2396–400.
Taylor SA, Punwani S, Rodriguez-Justo M, et al. Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination—pilot study. Radiology. 2009;251:369–79.
Makanyanga J, Punwani S, Taylor SA. Assessment of wall inflammation and fibrosis in Crohn’s disease: value of T1-weighted gadolinium-enhanced MR imaging. Abdom Imaging. 2012;37:933–43.
Li Z, Tielbeek JAW, Caan MWA, et al. Expiration-phase template-based motion correction of free-breathing abdominal dynamic contrast enhanced MRI. IEEE Trans Biomed Eng. 2015;62:1215–25.
Melbourne A, Atkinson D, White MJ, Collins D, Leach M, Hawkes D. Registration of dynamic contrast-enhanced MRI using a progressive principal component registration (PPCR). Phys Med Biol. 2007;52:5147–56.
Hamy V, Dikaios N, Punwani S, et al. Respiratory motion correction in dynamic MRI using robust data decomposition registration—application to DCE-MRI. Med Image Anal. 2014;18:301–13.
Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32.
Maccioni F, Viscido A, Broglia L, et al. Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging. 2000;25:219–28.
Sempere GA, Martinez Sanjuan V, Medina Chulia E, et al. MRI evaluation of inflammatory activity in Crohn’s disease. AJR Am J Roentgenol. 2005;184:1829–35.
Pupillo VA, Di Cesare E, Frieri G, Limbucci N, Tanga M, Masciocchi C. Assessment of inflammatory activity in Crohn’s disease by means of dynamic contrast-enhanced MRI. Radiol Med. 2007;112:798–809.
Del Vescovo R, Sansoni I, Caviglia R, et al. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn’s disease. Abdom Imaging. 2008;33:417–24.
Knuesel PR, Kubik RA, Crook DW, Eigenmann F, Froehlich JM. Assessment of dynamic contrast enhancement of the small bowel in active Crohn’s disease using 3D MR enterography. Eur J Radiol. 2010;73:607–13.
Oto A, Fan X, Mustafi D, et al. Quantitative analysis of dynamic contrast enhanced MRI for assessment of bowel inflammation in Crohn’s disease pilot study. Acad Radiol. 2009;16:1223–30.
Horsthuis K, Nederveen AJ, de Feiter MW, Lavini C, Stokkers PC, Stoker J. Mapping of T1-values and gadolinium-concentrations in MRI as indicator of disease activity in luminal Crohn’s disease: a feasibility study. J Magn Reson Imaging. 2009;29:488–93.
Giusti S, Faggioni L, Neri E, et al. Dynamic MRI of the small bowel: usefulness of quantitative contrast-enhancement parameters and time-signal intensity curves for differentiating between active and inactive Crohn’s disease. Abdom Imaging. 2010;35:646–53.
Rottgen R, Grandke T, Grieser C, Lehmkuhl L, Hamm B, Ludemann L. Measurement of MRI enhancement kinetics for evaluation of inflammatory activity in Crohn’s disease. Clin Imaging. 2010;34:29–35.
Florie J, Wasser MN, Arts-Cieslik K, Akkerman EM, Siersema PD, Stoker J. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol. 2006;186:1384–92.
Ziech ML, Lavini C, Caan MW, et al. Dynamic contrast-enhanced MRI in patients with luminal Crohn’s disease. Eur J Radiol. 2012;81:3019–27.
van Schie JJN, Lavini C, van Vliet LJ, et al. Estimating the arterial input function from dynamic contrast-enhanced MRI data with compensation for flow enhancement (II): applications in spine diagnostics and assessment of Crohn’s disease. J Magn Reson Imaging. 2017;47(5):1197–204.
Zhu J, Zhang F, Zhou J, Li H. Assessment of therapeutic response in Crohn’s disease using quantitative dynamic contrast enhanced MRI (DCE-MRI) parameters: a preliminary study. Medicine (Baltimore). 2017;96:e7759.
Patel P, Ormanoski M, Hoadley KM. Magnetic resonance enterography findings in Crohn’s disease in the pediatric population and correlation with fluoroscopic and multidetector computed tomographic techniques. J Clin Imaging Sci. 2011;1:41.
Maccioni F, Patak MA, Signore A, Laghi A. New frontiers of MRI in Crohn’s disease: motility imaging, diffusion-weighted imaging, perfusion MRI, MR spectroscopy, molecular imaging, and hybrid imaging (PET/MRI). Abdom Imaging. 2012;37:974–82.
de Jonge CS, Gollifer RM, Nederveen AJ, et al. Dynamic MRI for bowel motility imaging-how fast and how long? Br J Radiol. 2018:20170845.
de Jonge CS, Smout A, Nederveen AJ, Stoker J. Evaluation of gastrointestinal motility with MRI: advances, challenges and opportunities. Neurogastroenterol Motil 2018;30(1). doi: https://doi.org/10.1111/nmo.13257.
Ghobrial PM, Neuberger I, Guglielmo FF, et al. Cine MR enterography grading of small bowel peristalsis: evaluation of the antiperistaltic effectiveness of sublingual hyoscyamine sulfate. Acad Radiol. 2014;21:86–91.
Bickelhaupt S, Froehlich JM, Cattin R, et al. Software-assisted quantitative analysis of small bowel motility compared to manual measurements. Clin Radiol. 2014;69:363–71.
Odille F, Menys A, Ahmed A, Punwani S, Taylor SA, Atkinson D. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn Reson Med. 2012;68:783–93.
Froehlich JM, Waldherr C, Stoupis C, Erturk SM, Patak MA. MR motility imaging in Crohn’s disease improves lesion detection compared with standard MR imaging. Eur Radiol. 2010;20:1945–51.
Menys A, Atkinson D, Odille F, et al. Quantified terminal ileal motility during MR enterography as a potential biomarker of Crohn’s disease activity: a preliminary study. Eur Radiol. 2012;22:2494–501.
Cullmann JL, Bickelhaupt S, Froehlich JM, et al. MR imaging in Crohn’s disease: correlation of MR motility measurement with histopathology in the terminal ileum. Neurogastroenterol Motil. 2013;25:749-e577.
Hahnemann ML, Nensa F, Kinner S, et al. Quantitative assessment of small bowel motility in patients with Crohn’s disease using dynamic MRI. Neurogastroenterol Motil. 2015;27:841–8.
Akerman A, Mansson S, Fork FT, et al. Computational postprocessing quantification of small bowel motility using magnetic resonance images in clinical practice: an initial experience. J Magn Reson Imaging. 2016;44:277–87.
Plumb AA, Menys A, Russo E, et al. Magnetic resonance imaging-quantified small bowel motility is a sensitive marker of response to medical therapy in Crohn’s disease. Aliment Pharmacol Ther. 2015;42:343–55.
Menys A, Makanyanga J, Plumb A, et al. Aberrant motility in unaffected small bowel is linked to inflammatory burden and patient symptoms in Crohn’s disease. Inflamm Bowel Dis. 2016;22:424–32.
Bickelhaupt S, Pazahr S, Chuck N, et al. Crohn’s disease: small bowel motility impairment correlates with inflammatory-related markers C-reactive protein and calprotectin. Neurogastroenterol Motil. 2013;25:467–73.
Sotoudeh H, Sharma A, Fowler KJ, McConathy J, Dehdashti F. Clinical application of PET/MRI in oncology. J Magn Reson Imaging. 2016;44:265–76.
Malide D, Davies-Hill TM, Levine M, Simpson IA. Distinct localization of GLUT-1, -3, and -5 in human monocyte-derived macrophages: effects of cell activation. Am J Phys. 1998;274:E516–26.
Neurath MF, Vehling D, Schunk K, et al. Noninvasive assessment of Crohn’s disease activity: a comparison of 18F-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am J Gastroenterol. 2002;97:1978–85.
Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med. 2007;48:1053–9.
Bettenworth D, Reuter S, Hermann S, et al. Translational 18F-FDG PET/CT imaging to monitor lesion activity in intestinal inflammation. J Nucl Med. 2013;54:748–55.
Groshar D, Bernstine H, Stern D, et al. PET/CT enterography in Crohn disease: correlation of disease activity on CT enterography with 18F-FDG uptake. J Nucl Med. 2010;51:1009–14.
Saboury B, Salavati A, Brothers A, et al. FDG PET/CT in Crohn’s disease: correlation of quantitative FDG PET/CT parameters with clinical and endoscopic surrogate markers of disease activity. Eur J Nucl Med Mol Imaging. 2014;41:605–14.
Russo EA, Khan S, Janisch R, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in the monitoring of inflammatory activity in Crohn’s disease. Inflamm Bowel Dis. 2016;22:2619–29.
Jadvar H, Colletti PM. Competitive advantage of PET/MRI. Eur J Radiol. 2014;83:84–94.
Pellino G, Nicolai E, Catalano OA, et al. PET/MR versus PET/CT imaging: impact on the clinical management of small-bowel Crohn’s disease. J Crohns Colitis. 2016;10:277–85.
Catalano OA, Gee MS, Nicolai E, et al. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology. 2016;278:792–800.
Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–84.
Lenze F, Wessling J, Bremer J, et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis. 2012;18:2252–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, S., Rao, N., Taylor, S.A. (2019). Functional Cross-Sectional Imaging Techniques in Crohn’s Disease. In: Rimola, J. (eds) Cross-Sectional Imaging in Crohn’s Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-96586-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-96586-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96585-7
Online ISBN: 978-3-319-96586-4
eBook Packages: MedicineMedicine (R0)