Abstract
In the present study, the biosorption of Astrazon Blue FGRL (AB) which is one of the cationic dyes most commonly used in nylon and acrylic textiles from aqueous solution was studied onto tea waste (tea dust discharged after using), a waste lignocellulosic material. The effects of different parameters including biosorbent dosage, initial pH, contact time, initial dye concentration, and temperature were studied. Tea waste was characterized by Brunauer-Emmett-Teller (BET) surface area, FTIR, and SEM. The experimental equilibrium data were fitted to the Langmuir and Freundlich isotherms. The Freundlich isotherm model fitted to the experimental data better than the Langmuir isotherm. The maximum biosorption capacity, q max, was found to be 263.16 mg/g. The experimental data were discussed in detail comparing with some other low-cost adsorbents reported for AB removal in the previous literature, considering q max, adsorbent surface area, experimental conditions, isotherm models, and thermodynamics of the AB adsorption. The thermodynamic data indicated that AB biosorption was feasible but nonspontaneous, endothermic, and a chemisorption reaction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Discharge of colored wastewaters from textile industries into natural streams causes several environmental problems which is accompanied by the necessity of removal of the dyes from wastewater before discharging into water bodies. In wastewater treatment, various methods such as biological treatment, coagulation, membrane separation, advanced oxidation, and adsorption are used to remove dye.
Adsorption is a simple, very efficient separation process. It is easily operated. Therefore, among the mentioned techniques above, it attracts extensive attention. However, the use of inexpensive adsorbents having high adsorption capacity is a very important issue in terms of adsorption technology [1]. Powdered and granular activated carbons are the most commonly used adsorbents in the treatment of industrial wastewater. However, they are not low cost. Whereas, adsorbents to be used in wastewater treatment should be low cost, easily available, and environmentally friendly and have high adsorption capacity. Agro-based waste materials/by-products, industrial by-products, and biomasses such as bacteria, yeast, and fungi do meet these requirements. Lignin and cellulose are the two major constituents found in agro-based waste materials and by-products. Besides, these materials may also contain alcohols, aldehydes, ketones, and carboxylic, phenolic, and ether groups, which are the other polar functional groups of lignin [2]. Nowadays, the agro-based waste materials/by-products , which are lignocellulosic materials, attract particular interests due to their environmentally friendly properties, inexpensiveness, and easy availability as an adsorbent.
In this study, the removal of Astrazon Blue FGRL (AB) dye by tea waste (tea dust discharged after use), which is a lignocellulosic material, was investigated. AB is a cationic dye which is widely used for dyeing acrylic and nylon. These groups of dyes have complex chemical structures. Therefore, they are not destroyed by chemical, physical, and biological treatment [3]. Even at low concentrations, the majority of the cationic dyes are harmful to human beings and toxic to microorganisms [4].
Up to now, researchers have worked on the utilization of macroalga C. lentillifera [5], dried biomass of baker’s yeast [6], fly ash, apricot stone activated carbon [7], dried sea grape (Caulerpa lentillifera) [8], and sepiolite [7, 9] for Astrazon Blue FGRL (AB) removal from aqueous solution . However, according to our literature knowledge, the utilization of tea waste for adsorbing AB has not been reported.
Tea plant (Camellia sinensis (L) Kuntze) is grown in various countries in the world. After tea leaves were dried and cured by tea factories, it is widely consumed as a beverage. Turkey is one of the largest producers of tea in the world. Tea plant is usually grown in Rize, Turkey. In Turkey and many other parts of the world, it is one of the most popular and inexpensive beverages. The used tea after preparation of this beverage is completely disposed of as a waste. It is therefore available in huge amounts as solid wastes from houses, cafes, and restaurants in nearly all parts of the world.
In the scope of this study, the effect of various operational parameters (adsorbent dosage, contact time, initial dye concentration, pH, temperature) on AB removal was studied. The biosorption equilibrium isotherms and thermodynamic parameters of AB dye on tea waste were evaluated. The AB removal using tea waste was compared and discussed in detail with various low-cost adsorbents reported in the literature, in the light of the adsorbent surface area, experimental conditions, isotherm models, and thermodynamic and maximum adsorption capacity.
Experimental
Biosorbent
Tea dust discharged after use (tea waste) was collected from households and used without any additional pretreatment except for washing and sieving to obtain the desired adsorbent sizes. The Turkish tea waste was washed several times with boiled water and then washed with an adequate amount of distilled water until the washing water contained no color. The washed tea was dried in an oven at 70–80 °C for a few hours and then sieved to the particle size of 0.8–1 mm. The dried material was stored in dark glass bottles for further use.
Chemicals
Astrazon Blue FGRL (AB) was obtained by DyStar, from Turkey. This dye consisted of two main components which were C.I. Basic Blue 159 and C.I. Basic Blue 3 (Fig. 1). The ratio of the two components was 5:1 (w/w).
Chemical structures of (a) C.I. Basic Blue 159 and (b) C.I. Basic Blue [7]
A 1000 mg/L stock solution of the AB was prepared in distilled water. The solution was diluted to the required concentration for the experiments.
Characterization of Biosorbent
In this study, the characterization of tea waste was performed by utilizing Brunauer-Emmett-Teller (BET) surface area, Fourier transform infrared (FTIR) spectroscopy (Shimadzu model FTIR-8201 PC (1000–4000 cm−1), and scanning electron microscopy (SEM) (Hitachi 2300 Scanning Electron Microscope). The analysis of Brunauer-Emmett-Teller (BET) surface area was carried out in the METU Central Laboratory-Training Centre in Ankara, Turkey.
Batch Biosorption Experiments
In the batch biosorption experiments , the tea waste was weighed and then placed in 250 mL stoppered Erlenmeyer flasks containing 50 mL of dye solution. The flasks were then placed in a water-bath shaker and agitated at a stirring rate of 200 rpm. The suspensions were filtered, and the concentrations of AB in the filtrate were analyzed using a UV/VIS spectrophotometer (Perkin Elmer UV/VIS spectrometry) by monitoring the absorbance (at 599 nm). The experiments were conducted with varying biosorbent dosages (50, 100, 200, 300, 400, 600 g/L), contact times (5, 15, 30, 45, 60, 75 min), initial AB concentrations (25, 50, 100, 200 mg/L), initial pH (2.0, 4.0, 6.0, 8.0, 10.0), and temperature (20, 30, 40, 50 °C). The desired pH of the solution was adjusted using dilute HCl and/or dilute NaOH.
The amounts of dye uptake per unit weight of the biosorbent q e (mg/g) and dye removals (%) were calculated by the following equations, respectively:
where C 0 and C e are the initial and equilibrium concentrations of dye in the solution (mg/L), respectively, V is the total volume of the dye solution (L), and M is the mass of the biosorbent used (g).
To ensure the repeatability of the data, the biosorption experiments were performed at least twice, and the mean values were presented.
Theory
Equilibrium Isotherms
Langmuir model (Eq. 3) and Freundlich model (Eq. 4) are expressed in linear form as follows [10, 11]:
where C e is the equilibrium dye concentration (mg/L), q e is the amount of dye uptake per unit weight of the biosorbent (mg/g), q max is the maximum biosorption capacity of the biosorbent (mg/g), b is the Langmuir isotherm constant (L/mg), and K F (mg/g) and 1/n are the constants representing the biosorption capacity and the biosorption intensity, respectively.
Thermodynamic
The thermodynamic parameters (the change in free energy (ΔG), enthalpy (ΔH), and entropy (ΔS)) were calculated using the following equations:
where K d is the distribution coefficient for the adsorption; ΔH, ΔS, and ΔG are the changes in enthalpy, entropy, and Gibb’s free energy, respectively; R is the gas constant; T is the absolute temperature; q is the equilibrium concentration of dye cations sorbed onto biosorbent (mg/L); and C e is the equilibrium dye concentration (mg/L).
Results and Discussion
Characterization of Tea Waste
Specific Surface Area
The Brunauer-Emmett-Teller (BET) surface area of the tea waste as a natural material with plant origin was determined as 0.871 m2/g. It can be said that the BET surface area of the tea waste is significantly smaller than that of the activated carbon. It is known that the surface area of several activated carbons used for wastewater treatment is about 1000 m2/g [12].
Lignocellulosic adsorbents (natural raw materials mainly with plant origin) such as agro-based waste materials usually exhibit low surface area. Physicochemical modifications of these materials can enlarge surface area, type of adsorbing sites, porosity, etc. Thus, sorptive capacity can be improved. However, it is noted that the modification process may compensate for the cost of additional processing [13]. Therefore, tea waste was not subjected to any pretreatment.
Fourier Transform Infrared (FTIR) Analyses
In this study, the chemical characterizations were studied by Fourier transform infrared (FTIR) spectroscopy in order to identify the functional groups that might have participated in the biosorption and also indicate the surface site(s) on which biosorption has taken place. The FTIR spectra for tea waste before and after dye biosorption are shown in Fig. 2. The FTIR spectral characteristics of the tea waste are shown in Table 1. The spectral data obtained from FTIR analyses in the present study were found to be similar to the ones (bonded OH groups, aliphatic C–H group, secondary amine group, etc.) obtained from some other studies on heavy metal or dye biosorption by tea (tea dust discharged after use, waste tea leaves from tea factory, etc.).
From the FTIR spectra, various functional groups were detected on the surface of the biosorbent sample before and after biosorption. As can be seen from Fig. 2, the spectra display the number of adsorption peaks. Figure 2 also shows that the comparison of the spectra of the tea waste unloaded and loaded with dye showed changes in the absorption intensities of various peaks.
According to the spectra, it can be said that the biosorbent exhibits a complex nature. Vien-Lin et al. [14] revealed that the troughs due to the bonded OH groups can be observed in the range of 3340–3380 cm−1. Fazal and Rafique [15] reported that the trough was observed at 3404.47 cm−1 (–OH, –NH). In our study, the bonded OH groups were seen at 3310.6 cm−1 before adsorption. The band changed after biosorption (3298.1 cm−1). The considerable shift to the lower wave number suggested that chemical interactions between the dye cations and the hydroxyl (OH) groups occurred on the tea waste surface [15].
The bands observed at about 2850.2 and 2850.6 cm−1 before and after biosorption could be assigned to the aliphatic C–H group [16, 17]. A shoulder at wave number 1732.4 cm−1 was observed. It might be due to the carbonyl stretch of unionized carboxylate [18]. As stated by Malkoc and Nuhoglu [17], the peaks observed at 1511 and 1546 cm−1 correspond to the secondary amine group. We observed this peak at 1516 cm−1. Symmetric bending of CH3 was observed to shift to 1464 cm−1.
Auta and Hameed [19] revealed that 1034.20 cm−1 band width had some molecules containing sulfur/oxygen bonds (S〓O). We observed this peak at 1037.5 cm−1. It was reported that the peaks observed at 1148 and 619 cm−1 could be assigned to C–O stretching of ether groups and –CN stretching, respectively [20]. In our study, the C–O stretching of ether groups was seen at 1164.9 and 1155.7 cm−1. –CN stretching was seen at 661.09 and 661.38 cm−1.
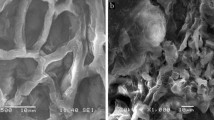
SEM Micrographs
The SEM micrographs of the tea waste before and after dye biosorption are given in Fig. 3, respectively. It can be seen that the tea waste mainly consists of fibers with open stomata. Similar finding for green tea residue has been reported by Yang and Cui [21]. As can be seen from Fig. 3, a significant difference does not exist in the surface morphology of the tea waste before and after dye biosorption.
The Effect of Biosorbent Dosage
The effect of biosorbent dosage on AB removal is shown in Fig. 4. The percentage of dye removal increased when the dosage of the tea waste per liter of solution was increased. This trend is a result of the increased adsorbent surface area and the availability of more adsorption sites arising from the increased dosage of the adsorbent [18]. On the contrary, the dye uptake (q) decreased with an increase in the biosorbent dosage. As stated by Jiang et al. [22], this may be due to the presence of more surface area for a fixed amount of dye. Similar findings for AB adsorption onto macroalga Caulerpa lentillifera [5], cationic dye methylene blue adsorptions onto tea waste [18], and sugar beet pulp [23] have been reported.
The Effect of pH
The effect of initial pH on the biosorption of AB onto tea waste was investigated by varying the initial pH from 2 to 10, under a constant initial dye concentration of 50 mg/L, adsorption time of 30 min, biosorbent size of 0.8–1.0 mm, and a tea waste dosage of 5.0 g/L. The results of the pH studies are shown in Fig. 5. As known, the solution pH affects the surface binding sites of the adsorbent and the degree of ionization of the dye [24].
As can be seen from Fig. 5, the dye uptake increased as the initial pH increased from pH 2 to 4 and then slightly decreased at pH beyond 5. AB is a cationic dye, which exists in aqueous solutions in the form of positively charged ions. As stated by various researches [24, 25], dye removal is inhibited at low pH (less than 4.0). This can be explained by the high concentration of protons in the solution which leads to positive charge density on dye binding sites. As described by Uddin et al. [18], when the pH increases, the surface of the tea waste may become negatively charged as a result of the adsorption of OH−, and the carboxyl groups of the tea waste may become deprotonated. Thus, negatively charged biosorption sites are produced. Accordingly, the biosorption process is favorably preferred by means of the electrostatic forces of attraction.
However, as can be seen from Fig. 5, the dye biosorption at alkali pH is slightly decreased. This behavior may be due to the competition of OH− [26]. A similar trend has been observed elsewhere [26, 27].
In this study, the initial pH of 5.0 was selected as the more adequate value for the other biosorption experiments. As stated by Liu and Huang [28], the pH values of the cationic dye solutions are generally below 6.0, and instead of the alkaline mediums, the cationic dyes become stable in the acidic mediums. Moreover, the pH values of the cationic dye effluents arising from the dyeing processes range from 4.0 to 7.0.
The Effect of Initial Concentration and Contact Time
The effect of contact time and initial dye concentration on dye removal is shown in Fig. 6. As can be seen from Fig. 6, an increase in the initial dye concentration leads to an increase in the dye uptake. In other words, the more concentrated the dye solution, the higher the biosorption capacity. Presumably, this trend results from the high driving force for mass transfer in high dye concentration [29]. The resistance to dye uptake decreases as the mass transfer driving force increases [7, 30].
The results were in agreement with Karagözoglu et al. [7], Marungrueng and Pavasant [5], and Ongen et al. [9] who studied the adsorption of AB from aqueous solutions . The increased dye uptakes with increasing initial concentrations have also been observed in the adsorption of a cationic dye, methylene blue, onto tea waste [18] and rejected tea [30].
As can be seen from Fig. 6, rapid dye uptake values were obtained in the initial stages of contact time. However, as the contact time increased, biosorption became progressively slower. The fast removal of dye in the initial stage and thereafter saturation gradually could also occur as a consequence of a large number of surface sites which were initially available for the biosorption of the dye molecules . However, steric repulsion could occur between the solute molecules, which consequently leads to a slower biosorption process [31]. Similar phenomena have been observed in the adsorption of AB onto sepiolite, fly ash, and apricot stone activated carbon [7].
Equilibrium Studies
In this study, the Langmuir and Freundlich isotherm models were used for the mathematical statement of the dye biosorption onto tea waste. Fitting of the Freundlich model to the experimental data was better than the Langmuir model . The constants and correlation coefficients (r) of the Langmuir and Freundlich isotherm models for AB biosorption on tea waste are presented in Table 2. The equilibrium isotherm of AB in aqueous solutions onto tea waste is shown in Fig. 7.
In the Freundlich equation, the surface is assumed to be heterogeneous. Therefore, the reason for the better fit of the Freundlich isotherm to the experimental data (r: 0.9565) may be a consequence of the heterogeneous distribution of the active sites on the tea waste surface [32]. The Langmuir isotherm is known with its dominant ion-exchange mechanism. On the other hand, the Freundlich isotherm exhibits adsorption-complexation reactions that occur in the biosorption process [33].
The Comparison in Detail of the AB Adsorption Capacities of Various Low-Cost Adsorbents Reported in the Literature
Up to now, some kinds of biomasses (macroalga Caulerpa lentillifera, baker’s yeast), natural materials (sepiolite), industrial waste products (fly ash), and activated carbon prepared from agricultural by-product (apricot stone activated carbon) have been used for AB removal by various researchers [5,6,7,8,9]. The comparison of the maximum adsorption capacity (q max) and the adsorbent surface area of the tea waste tested in this study with these adsorbents reported in the literature is given in Table 3. Besides, the experimental conditions employed in these studies, isotherm models, thermodynamics, and kinetics related to AB removal by these adsorbents, are presented in this table.
Indeed, every single adsorbent has distinct physical and chemical characteristics including porosity, surface morphology, surface area, stability, and physical strength. In addition, depending on the experimental conditions, the adsorption capacities of the adsorbents vary. Thus, it is difficult to make a comparison of the adsorption performances [34]. However, as can be seen from Table 3, q max of the tea waste is similar to or greater than those reported for other kinds of adsorbent. Despite the tea waste’s low surface area, it exhibited a high biosorption capacity for AB. The high biosorption capacity of the tea waste may be attributed to its cellulosic structure which has a high affinity for dyes [35].
When Table 3 was examined in detail, it was seen that:
-
1.
An interesting feature of the adsorbents is that, on the contrary to what was expected, q max values of most of the adsorbents with high surface area were lower than those of the adsorbents with low surface area such as the tea waste. For example, the surface areas of the apricot stone activated carbon and the sepiolite samples are 566 m2/g [7], 234.3 [7], and 377.916 m2/g [9], respectively. However, the sepiolite samples have higher q max values than those of the apricot stone activated carbon. In spite of the fact that the surface area of fly ash [7] is lower than that of the dried sea grape (macroalga Caulerpa lentillifera) [8], its q max value is higher than that of the dried sea grape (macroalga Caulerpa lentillifera). The reason may probably be due to the effect of pore diameter as well as the varying experimental conditions. As also pointed out by Punjongharn et al. [8], the effect of the pore diameter is more important than that of the surface area. Cationic dye molecules could hardly enter the small pores of the adsorbent, and access to the binding sites inside the pores becomes difficult.
-
2.
AB adsorption by macroalga Caulerpa lentillifera [5], dried sea grape (macroalga Caulerpa lentillifera) [8], sepiolite [9], and the tea waste (this study) is best described by the Freundlich adsorption isotherm model which may show adsorption-complexation reactions that occur in the adsorption process. The Langmuir isotherm model established the best prediction for the adsorption of AB by dried biomass of baker’s yeast [6], apricot stone activated carbon [7], sepiolite [7], and fly ash [7]. As stated above, the Langmuir isotherm is known with its dominant ion-exchange mechanism.
-
3.
Although macroalga Caulerpa lentillifera and sepiolite are tested for AB removal from aqueous solution by various researchers [5, 7, 9], q max values found for these adsorbents by various researchers are different from each other. It can be said that, as expected, this trend is due to the different experimental conditions.
-
4.
q max and b values of all the adsorbents (apricot stone activated carbon, sepiolite, fly ash, macroalga Caulerpa lentillifera, and dried biomass of baker’s yeast) reported in the literature except for the sepiolite tested by Ongen et al. [9] clearly increased depending on the temperature. The adsorption of AB on various adsorbents as well as the tea waste is endothermic in nature.
The Effect of Temperature
The effect of temperature on AB biosorption was studied at the dye concentration of 50 mg/L and contact time of 30 min under the optimum experimental conditions of biosorbent size of 0.8–1.0 mm, biosorbent dosage of 5.0 g/L, and at pH of 5.0, and the results are shown in Fig. 8. The dye uptake, q, on the tea waste increased with the increasing temperature (Table 4), indicating that a high temperature favors AB removal by biosorption on tea waste (Fig. 8) and the biosorption of AB on tea waste was endothermic in nature.
Although adsorption reactions are normally exothermic, the adsorption experiments for different temperatures in this study show endothermic results. It also implies that the adsorption process is dominated by the diffusion process. As stated by Li et al. [36], the diffusion is an endothermic process. Therefore, it can be said that the interparticle diffusion rate of the adsorbate molecules into the pores increased with an increase in the temperature. When the temperature increases, the molecular mobility increases, and the solution viscosity decreases. The increase in the molecular mobility and the decrease of solution viscosity enhance the diffusion rate of dye through the boundary layer and within the internal pores of the tea waste particles [30]. Similar effects on the removal of cationic dyes were reported by other researchers for temperature [24, 28, 30].
The calculated thermodynamic parameters (∆G, ∆H, and ∆S) are given in Table 4. The positive and weak value of ∆G indicates that the biosorption of AB dye is feasible but nonspontaneous [37]. Also, as can be seen from Table 4, the positive values of ∆G increase with increasing temperature. It can be said that this indicates the presence of an energy barrier at high temperature in biosorption [38].
The thermodynamic parameters ∆H and ∆S for the biosorption of AB by tea waste were obtained from the slope and the intercept of a plot of ln K d versus 1/T (Fig. 8). The positive change of 19.609 kJ/mol in ΔH indicates that, as stated above, the biosorption of AB by the tea waste is endothermic. This finding was similar to the other studies on the biosorption of AB. For instance, positive enthalpy of adsorption was also observed in the adsorption of AB on sepiolite, fly ash and apricot stone activated carbon [7], C. lentillifera [5], and dried biomass of baker’s yeast [6]. The positive value of ΔH has also been observed in the adsorption of methylene blue onto tea waste [26] and NaOH-modified rejected tea [39]. Besides, the positive value of ΔH (19.609 kJ/mol) indicates chemical adsorption [5, 6].
The negative value of ΔS (−82.477 J/mol K) normally indicates that a significant change does not occur in the internal structure of the tea waste during biosorption of AB [40]. Besides, the negative sign of the entropy indicates that at last things become more organized than at the beginning [4].
The values of T AΔS were determined from the experimental data, where T A represents the average values of the range of temperature used for the adsorption studies. It was found to be ΔH < −T AΔS. This indicates that, even though the contribution of ΔH is not negligible, the influence of entropy is more important than that of the enthalpy in activation [40].
Cost Estimation
The cost-effective and economic removal of dyes from industrial wastewaters is possible if low-cost and easily available adsorbents are used. In Turkey as well as in many parts of the world, the tea waste is an easily available material from the houses, cafeterias, and restaurants as a waste. As stated by Amarasinghe and Williams [41], an appropriate mechanism should be considered for the collection and storing of the tea waste.
Hydroxyl and carboxylic groups in agricultural wastes make them amenable to easy desorption and regeneration with basic or acid solution [13]. However, it can be said that the regeneration of the tea waste is not required due to its abundant availability and low cost. It can also be disposed of after use without the need for expensive regeneration.
In the world market, activated carbon has a price that changes over a wide range (US$ 3–12 for kg) depending on the origin, quality, and quantity [42]. The cheapest commercially available activated carbon available in Turkey is US$ 1.5 for kg. As stated above, tea waste, which is a household waste, is available in huge amounts. Therefore, the total cost of tea waste will be extremely low when compared with those of the activated carbon and some other adsorbents for AB dye. For cost analysis, the expenses for collection and storing can be considered.
As stated by Baek et al. [25], the dye-loaded adsorbent can be disposed of by incineration to prevent further impact on the environment. The dye-loaded tea waste can be utilized as a fuel in the boilers/incinerators after dried. Thus, both energy recovery from the used biosorbent and the safe disposal of the biosorbed dye can be obtained by providing a greener solution.
Conclusion
In this study, the utilization of the tea waste (tea dust discharged after use), which is a green environmentally friendly adsorbent, in the removal of toxic AB dye from aqueous solution was reported.
The main conclusions can be summarized as follows:
-
1.
When the optimum experimental conditions were determined as a function of the process parameters such as the biosorbent dosage, initial AB concentration and contact time, initial pH, and temperature, it was seen that the values of the biosorbent dosage, contact time, initial pH, and temperature were found to be 5.0 g/L, 30 min, 5.0, and endothermic, respectively.
-
2.
The uptake of the dye, q, decreased at low initial pH (less than 4.0) and increased when the temperature of the aqueous medium increased. The effect of initial pH and temperature on AB removal using the other low-cost adsorbents (sepiolite, fly ash, apricot stone activated carbon, C. lentillifera, and dried biomass of baker’s yeast) reported in the literature exhibited similar trends to the tea waste.
-
3.
The Freundlich equation fitted well to the experimental data. The maximum biosorption capacity, q max, was found to be 263.16 mg/g by the Langmuir isotherm.
-
4.
It is worth mentioning that when q max values obtained for biosorption of AB onto tea waste (this study) and various other low-cost adsorbents reported in the literature were compared with each other, it was seen that q max values of the adsorbents with low surface area such as the tea waste were higher than those of most of the adsorbents with high surface area. It was realized that the adsorbents with high surface area do not always exhibit high q max values. However, it should not be forgotten that the adsorption capacities of the adsorbents differ depending on the experimental conditions.
-
5.
From the thermodynamic parameters (E a, ΔG, ΔH, and ΔS), it was concluded that the biosorption process takes place by chemical adsorption (probably indicating adsorbent/dye complexation) and is endothermic in nature.
-
6.
It can be said that the biosorption of AB onto the tea waste is a complex process. It is thought that ion-exchange, complexation, and electrostatic interactions play an important role in the whole biosorption process of the tea waste for AB removal, as revealed for metal sorption using tea industry waste by Cay et al. [33].
-
7.
In spite of the fact that the tea waste has a significantly low surface area compared to the commercial activated carbons, the use of tea waste for biosorption of AB from wastewater streams seems to be feasible. From the economical point of view, the tea waste can be used as an alternative adsorbent to activated carbon. It has a low cost and good affinity for AB.
Further studies on AB biosorption using tea waste are currently underway. The kinetics and column studies will be the subject of the next study.
References
Jia Z, Li Z, Ni T, Li S (2017) Adsorption of low-cost absorption materials based on biomass (Cortaderia selloana flower spikes) for dye removal: kinetics, isotherms and thermodynamic studies. J Mol Liq 229:285–292
Pagnanelli F, Mainelli S, Veglio F, Toro L (2003) Heavy metal removal by olive pomace: biosorbent characterization and equilibrium modeling. Chem Eng Sci 58:4709–4717
Eren E, Afsin B (2007) Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated sepiolite surfaces. Dyes Pigments 73:162–167
Hong J-M, Lin B, Jiang J-S, Chen B-Y, Chang C-T (2014) Synthesis of pore-expanded mesoporous materials using waste quartz sand and the adsorption effects of methylene blue. J Ind Eng Chem 20:3667–3671
Marungrueng K, Pavasant P (2006) Removal of basic dye (Astrazon Blue FGRL) using macroalga Caulerpa lentillifera. J Environ Manag 78:268–274
Farah JY, El-Gendy N, Farahat LA (2007) Biosorption of Astrazone Blue basic dye from an aqueous solution using dried biomass of Baker’s yeast. J Hazard Mater 148:402–408
Karagözoglu B, Tasdemir M, Demirbas E, Kobya M (2007) The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: kinetic and equilibrium studies. J Hazard Mater 147:297–306
Punjongharn P, Meevasana K, Pavasant P (2008) Influence of particle size and salinity on adsorption of basic dyes by agricultural waste: dried Seagrape (Caulerpa lentillifera). J Environ Sci 20:760–768
Ongen A, Ozcan HK, Elmaslar Ozbas E, Balkaya N (2012) Adsorption of Astrazon Blue FGRL onto sepiolite from aqueous solutions. Desalin Water Treat 40:129–136
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Culp RL, Wesner GM, Culp GL (1978) Handbook of advanced wastewater treatment. Van Nostrand Reinholds Company, New York
Johnson TA, Jain N, Joshi HC, Prasad S (2008) Agricultural and agro-processing wastes as low cost adsorbents for metal removal from wastewater: a review. J Sci Ind Res 67:647–658
Vien-Lin D, Colthup NB, Fateley WG, Grasselli JC (1991) The handbook of infrared and raman characteristic frequencies of organic molecules. Academic Press, San Diego
Fazal A, Rafique U (2012) Acid/base treated spent black tea characterization of functional groups and lead sorptive mechanism. Int J Chem Environ Eng 3:217–224
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Bioresour Technol 61:221–227
Malkoc E, Nuhoglu Y (2006) Removal of Ni(II) ions from aqueous solutions using waste of tea factory: adsorption on a fixed-bed column. J Hazard Mater B135:328–336
Uddin MT, Islam MA, Mahmud S, Rukanuzzaman M (2009) Adsorptive removal of methylene blue by tea waste. J Hazard Mater 164:53–60
Auta M, Hameed BH (2011) Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem Eng J 171:502–509
Malkoc E, Nuhoglu Y (2006b) Fixed bed studies for the sorption of chromium(VI) onto tea factory waste. Chem Eng Sci 61:4363–4372
Yang X, Cui X (2013) Adsorption characteristics of Pb(II) on alkali treated tea residue. Water Res Ind 3:1–10
Jiang Z, Xie J, Jiang D, Yan Z, Jing J, Liu D (2014) Enhanced adsorption of hydroxyl contained/anionic dyes on nonfunctionalized Ni@SiO2 core-shell nanoparticles: kinetic and thermodynamic profile. Appl Surf Sci 292:301–310
Malekbala MR, Hosseini S, Yazdi SK, Soltani SM, Malekbala MR (2012) The study of the potential capability of sugar beet pulp on the removal efficiency of two cationic dyes. Chem Eng Res Design 90(5):704–712
Sánchez-Martín J, González-Velasco M, Beltrán-Heredia J, Gragera-Carvajal J, Salguero-Fernández J (2010) Novel tannin-based adsorbent in removing cationic dye (methylene blue) from aqueous solution: kinetics and equilibrium studies. J Hazard Mater 174:9–16
Baek MH, Ijagbemi CO, Se-Jin O, Kim DS (2010) Removal of malachite green from aqueous solution using degreased coffee bean. J Hazard Mater 176:820–828
Giahi M, Rakhshaee R, Bagherinia MA (2011) Removal of methylene blue by tea wastages from the synthesis waste waters. Chinese Chem Letters 22:225–228
Kumar KV, Sivanesan S, Ramamurthi V (2005) Adsorption of malachite green onto Pithophora sp., a fresh water algae: equilibrium and kinetic modeling. Process Biochem 40:2865–2872
Liu MH, Huang JH (2006) Removal and recovery of cationic dyes from aqueous solutions using spherical sulfonic lignin adsorbent. J Appl Polym Sci 101:2284–2291
Chen H, Zhao J, Dai G, Wu J, Yan H (2010) Adsorption characteristics of Pb(II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves. Desalination 262:174–182
Nasuha N, Hameed BH, Mohd Din AT (2010) Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater 175:126–132
Dhodapkar R, Rao NN, Pande SP, Kaul SN (2006) Removal of basic dyes from aqueous medium using a novel polymer: Jalshakti. Bioresour Technol 97:877–885
Rubin E, Rodriguez P, Herrero R, Cremades J, Barbara I, Sastre de Vicente ME (2005) Removal of methylene blue from aqueous solutions using as biosorbent Sargassum muticum: an invasive macroalga in Europe. J Chem Technol Biotechnol 80:291–298
Cay S, Uyanık A, Ozaşık A (2004) Single and binary component adsorption on copper (II) and cadmium (II) from aqueous solution using tea industry waste. Sep Purif Technol 38:273–280
Sharma P, Kaur R, Baskar C, Chung W-J (2010) Removal of methylene blue from aqueous waste using rice husk and rice husk ash. Desalination 259:249–257
El Ashtoukhy ESZ (2009) Loofa egyptiaca as a novel adsorbent for removal of direct blue dye from aqueous solution. J Environ Manag 90:2755–2761
Li K, Zheng Z, Huang X, Zhao G, Feng J, Zhang J (2009) Equilibrium, kinetic and thermodynamic studies on the adsorption of 2-nitroaniline onto activated carbon prepared from cotton stalk fibre. J Hazard Mater 166:213–220
Gueu S, Yao B, Adouby K, Ado G (2007) Kinetics and thermodynamics study of lead adsorption on to activated carbons from coconut and seed hull of the palm tree. Int J Environ Sci Technol 4:11–17
Yazdani M, Mahmoodi NM, Arami M, Bahrami H (2012) Surfactant-modified feldspar: isotherm, kinetic, and thermodynamic of binary system dye removal. J Appl Polym Sci 126:340–349
Nasuha N, Hameed BH (2011) Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem Eng J 166:783–786
Eren E (2009) Investigation of a basic dye removal from aqueous solution onto chemically modified Unye bentonite. J Hazard Mater 166:88–93
Amarasinghe BMWPK, Williams AR (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132:299–309
Kavitha D, Namasivayam C (2008) Capacity of activated carbon in the removal of acid brilliant blue: determination of equilibrium and kinetic model parameters. Chem Eng J 139:453–461
Acknowledgments
This research was supported by the Research Project Unit at Istanbul University (Project No: BYP–5090).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Balkaya, N. (2019). Biosorption of Dye from Aqueous Solutions by a Waste Lignocellulosic Material. In: Balkaya, N., Guneysu, S. (eds) Recycling and Reuse Approaches for Better Sustainability. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-95888-0_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-95888-0_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95887-3
Online ISBN: 978-3-319-95888-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)