Abstract
Secondary materials, that contain relatively high concentrations of platinum group metals, (PGMs) are treated in copper smelting process. The PGMs lost in the slag is increasing with increasing quantities of scrap treated amount. To determine the portion of the PGMs chemically dissolved and that associated with the mechanically trapped matte in the slag will be a key factor to improve the recovery of those metals. An experimental study was carried out to determine the distribution of palladium between the FeOx–SiO2 slag and the liquid Cu2S–FeS matte at 1573 K and a fixed partial pressure of SO2 of 0.1 atm. It was found that the distribution ratios are around 10−3 for platinum and palladium. The distribution ratios show a tendency to increase when the grade of matte is increased above 60 mass% Cu. In addition, the solubility of platinum in FeOx–SiO2 slag equilibrated with a pure palladium and the Pd-Cu alloy was determined at 1573 K and the range of oxygen partial pressure from 10−9 to 10−7 atm. The solubility of palladium in the slag tends to increase with increasing oxygen partial pressure and activity of CuO0.5 in the slag.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
There is an increasing trend in copper smelters to process secondary materials, which contain relatively high concentrations of precious metals such as gold, silver, platinum, palladium and rhodium. As a consequence of the increasing of amount of secondary materials treated, the precious metals lost in the slag phase is also increasing. The ratio of the precious metals chemically dissolved and that associated with the mechanically trapped matte in the slag will be a key factor in improving the recovery of those precious metals. The distribution ratio of silver metals between iron silicate slag and Cu2S–FeS matte at 1573 K has been reported by Roghani et al. [1]. The distributions of Ag, Au, Pd, Pt, and Rh between copper matte and silica-saturated iron silicate slag were determined at 1250–1350 °C by Avarmaa et al. [2].
The present study was carried out at the oxygen smelting conditions with pSO2 = 0.1 atm and controlled partial pressures of O2 and S2. The distribution ratios of platinum group metals (PGMs) of platinum and palladium between iron silicate slag and Cu2S–FeS matte were measured at 1573 K. The results are intended to indicate the lowest content of PGMs that would be expected in the industry and also a give clear indication of the range of matte grade where recovery of precious metals can be optimized.
The distribution of PGMs has been found to increase with increasing concentration of copper in the slag and increasing oxygen partial pressure. The solubility of palladium in the FeOx–SiO2 slag equilibrated with a pure palladium and the Pd–Cu alloy has been is investigated at 1573 K and the range of oxygen partial pressure from 10−9 to 10−7 atm controlled with CO–CO2 gas mixture. Nakamura and Sano reported the solubility of platinum in molten BaO–CuOx, BaO–MnOx, CaOsatd–SiO2–FeOx, KO0.5–SiO2, NaO0.5–SiO2, and NaO0.5–PO2.5 slag [3, 4]. Shuto et al. measured the ruthenium solubility in the CaO–SiO2, Na2O–SiO2, and Na2O–SiO2–Al2O3 slag [5]. There are no reports of the palladium solubility in the FeOx–SiO2 slags.
Experimental
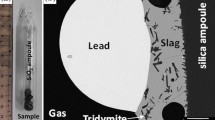
The experimental apparatus used in this study is shown in Fig. 1. The furnace consists of a silicon carbide heating element and an alumina reaction tube. CO–CO2–SO2 gas mixtures with a flow rate at 120 mL/s were used to control the partial pressures of S2, O2 and SO2. CO2 gas passed through a P2O5 column to trap moisture. The gas mixture was introduced into an alumina reaction tube with an inner diameter of 6 × 10−4 m and a height of 0.6 m. The partial pressures of S2, O2 and SO2 were calculated by using FactSage software [6]. The experiments were made under the partial pressure of SO2 at 0.1 atm under conditions typical of oxidation of the matte with air.
A total 6 g of pre-melted slag of approximate composition of 65 mass%FeO-35 mass%SiO2 was equilibrated with a same amount of Cu2S–FeS in a magnesia crucible with an inner diameter of 0.011 m and a height of 0.05 m. The master slag was synthesized in an iron crucible. The Cu2S and FeS were prepared by vacuum sealing the required metals and sulfur in a quartz ampoule. The temperature of the sample was measured by another Pt/Pt-Rh thermocouple attached to the magnesia crucible. Preliminary experiments have clarified that the equilibrium could be made in a restricted time of less than 24 h by adjusting the Cu2S–FeS content of the starting alloy phase so that it is near in composition to that of the estimated equilibrium value reported by Roghani et al. [1]. The sample was cooled rapidly by flushing a large amount of argon gas onto a surface of the slag layer to prevent the segregation of the slag components during the solidification. The chemical analysis was made for the solidified matte and slag specimens after physical separation of the phases using an ICP analysis.
The experimental apparatus and procedure of the palladium solubility in the iron silicate slag are same as in the previous study of the distribution of the precious metals between the slag and matte. A mixture of CO–CO2 was used to control pO2. The starting slag had 65 mass%FeOx-35 mass%SiO2 were synthesized in an iron crucible. 10 g of the slag and 0.2 g of pure solid palladium or 1 g of Pd–Cu alloy were put in a magnesia crucible with an inner diameter of 0.018 m and a height of 0.04 m. The sample was heated at 1573 K for 24 h, which was long enough to reach the equilibrium, as confirmed in previous study [1]. After that, it was cooled rapidly by flushing a large amount of argon gas onto a surface of the slag layer during the solidification. The palladium and copper in the slag sample were analyzed by an ICP analysis.
Results and Discussion
Distribution of Platinum and Palladium Between Slag and Matte
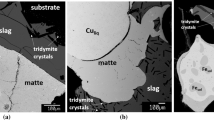
The 65 mass%FeOx-35 mass%SiO2 slag and Cu2S–FeS equilibrium was carried out at the oxygen-smelting conditions with pSO2 = 0.1 atm and controlled partial pressures of S2 and O2 at 1573 K. The slag separated completely from the Cu2S–FeS matte with copper concentration from 50 to 75 mass%. The MgO concentration in the present slag at 1573 K was less than 8 mass%. The affect of MgO in the distribution of minor elements can be assumed small. Experimental data obtained on the copper content in the slag as a function of copper content in the matte are shown in Fig. 2. The copper concentration in the slag increases with increasing matte grade and increase remarkably in the range of higher matte grade. The copper concentration in the slag at 1573 K and pSO2 of 0.1 atm determined by Roghani et al. shows an agreement with the present results [1].
The distribution ratio of platinum and palladium between the 8 mass%MgO-35 mass%SiO2-FeOx slag and Cu2S–FeS matte phases is defined by following equation:
and distribution ratio was determined from the chemical analysis of the slag and matte phases.
The distribution ratios of palladium and platinum are shown in Fig. 3. It was found that the distribution ratios are approximately 0.001 for platinum and palladium. It is noted that in the range of matte grade between 40 and 65 mass% of copper there is no appreciable variation in the distribution ratios. However, above 60 to 65 mass% of copper in the matte the distribution ratios increase with increasing copper content in the matte. This behavior resembles a tendency of dissolution of copper in the slag. The distribution ratio of the precious metals may depend on the solubility of copper in the slag.
Solubility of Palladium in the FeOx–SiO2 Slag
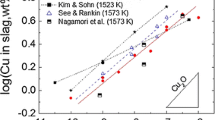
The platinum solubility in the iron silicate slag was measured under controlled pO2 using a mixture of CO–CO2 gases. The palladium concentration in the slag as a function of partial pressure of oxygen at 1573 K are shown in Fig. 4 as compare with the platinum solubility determined by us [7]. As shown in figure, the palladium solubility in the slag is in this range from 7 to 20 mass ppm for the range of conditions investigated and increase with increasing oxygen partial pressure.
The platinum solubility in the iron silicate slag equilibrated with the Pd–Cu alloy was determined at 1573 K. The activity of Cu and Pd in the liquid Cu–Pd alloy at 1573 K were calculated with the thermodynamic data base of FactSage software 6.3 [6]. The copper concentration in the slag, as shown in Fig. 5, increases with increasing activity of copper in the Pd–Cu alloy and decreases with decreasing partial pressure of oxygen. When activity of copper is unity, extrapolated copper solubility as the fixed oxygen partial pressure agree with the reported values by Takeda [8].
Solubility of palladium in the slag equilibrated the Pd–Cu alloy as function of the activity of palladium shown in Fig. 6. The activities of palladium were estimated with FactSage software 6.3 [6]. The solubility of the palladium depends on the activity of the palladium in the alloy, and it will be large value with the increase of the palladium concentration in the alloy. However, palladium solubility decreases with increasing palladium activity.
The palladium solubility is influenced by oxygen partial pressure and alloy composition. The activity coefficient of PdO for the slag, γPdO, can be regarded as a normalization of the palladium solubility. The following reaction and equation are available to calculate the activity coefficient:
where K and aPd are the equilibrium constant of reaction (2) and activity of palladium in the Pd–Cu alloy calculated with the FactSage software 6.3 [6], respectively. MPd is atomic weight of palladium, nT is total mole constituents in 100 g of the slag which are FeOx, SiO2 and PdO. Based on the measured palladium solubility and the oxygen partial pressure of experimental conditions, γPdO was derived as a function of copper content in the slag. Figure 7 shows the relationship between the activity coefficients of PdO in the slag and the copper solubility in the slag. The activity coefficients of palladium decrease with increasing of the copper content in the slag. It is found that the palladium solubility and the activity coefficient of palladium in the slag depend on the copper content in the slag. Therefore, lower copper content in the slag tend to lower slag loss of the palladium.
Conclusions
As part of the fundamental study related smelting of copper sulfide and the recycling of electronic materials, the phase relation between the FeOx–SiO2 slag and the Cu2S–FeS melt and the distribution of platinum and palladium between these phases in a magnesia crucible was investigated at 1573 K under controlled fixed pSO2 at 0.1 atm and pO2 and pS2 in ranges between 6.3 × 10−9 ~ 2.5 × 10−8 and 5 × 10−4 ~ 0.01 atm, respectively.
It is suggested from the distribution ratios that platinum and palladium are preferentially dissolved in the matte phase. The distribution ratio is around 0.001 for Pt and Pd. The distributions increased at matte concentrations greater than 60 mass% Cu, and tended to be clearing dependent on matte grade.
The palladium solubility in the iron silicate slag was measured under controlled pO2 using a mixture of CO–CO2 gases. The palladium solubility in the slag increases with increasing oxygen partial pressure. The activity coefficient of palladium in the slag depends on the copper concentration in the slag. Therefore, lower copper content in the slag tend to lower slag loss of the precious metals.
References
Rogani G, Takeda Y, Itagaki K (2000) Phase equilibrium and minor elements distribution between FeOx-SiO2-MgO-based slag and Cu2S-FeS Matte at 1573 K under high partial pressure of SO2 metal. Mater Trans B 31B:705–712
Avarmaa K, O’Brien H, Johto H, Taskinen P (2015) Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. J Sustain Metall 1:216–228. https://doi.org/10.1007/s40831-015-0020-x
Nakamura S, Sano N (1997) The influence of basicity on the solubility of platinum in oxide melts. Metall Mater Trans B 28B:103–108
Nakamura S, Iwasawa K, Morita K, Sano N (1998) Dissolution mechanism of platinum in basic fluxes metall. Mater Trans B 29B:411–414
Shuto H, Okabe TH, Morita K (2011) Ruthenium solubility and dissolution behavior in molten slag. Mater Trans 52:1899–1904
Bale CW, Chartrand P, Degterov SA, Eriksson G, Hack K, Mahfond RB, Melancon J, Pelton AD, Petersen S (2002) FactSage thermochemical software and databases. Calphad 26:189–228
Baba K, Yamaguchi K (2013) The solubility of platinum in the FeOx-SiO2 slag at 1573 K. J MMIJ 129:208–212
Takeda Y (1993) Miscibility gap in the CaO-SiO2-Cu2O-Fe3O4 system under copper saturation and distribution of impurities. Mater Trans JIM 34:937–945
Acknowledgements
This work has been supported financially by High-Efficiency Rare Element Extraction Technology of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), which is to be acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Yamaguchi, K. (2018). Thermodynamic Study of the Equilibrium Distribution of Platinum Group Metals Between Slag and Molten Metals and Slag and Copper Matte. In: Davis, B., et al. Extraction 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95022-8_63
Download citation
DOI: https://doi.org/10.1007/978-3-319-95022-8_63
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95021-1
Online ISBN: 978-3-319-95022-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)