Abstract
Severe acute brain injury (SABI) is a heterogeneous group of diseases that leave patients acutely neurologically devastated. This chapter discusses the unique challenges that clinicians face when caring for patients with SABI and their families with a focus on the acute setting, when shared decision making is challenged by significant prognostic uncertainty and the need for grieving family members to consider patient’s goals of care. Clinicians practicing in the emergency department, acute neurology or neuro-critical care setting need to master a set of distinct palliative care skills to help patients and families navigate the severe acute brain injury landscape. These skills are relevant to all clinicians, providing both primary and specialist palliative care. Using a case example, we provide recommendations regarding symptom management and caregiver support in the acute and chronic setting, and discuss communication skills for clinicians engaging with family members, especially around prognosis and treatment goals. Research and education needs in this area of palliative care are substantial.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Severe acute brain injury (SABI)
- Stroke
- Subarachnoid hemorrhage
- Post-anoxic encephalopathy
- Traumatic brain injury (TBI)
- Family conferences
- Goals of care
- Symptom management
- Prognostication
- Withdrawal of life support
Ms. B was a 55-year-old very active right-handed woman with untreated hypertension who woke up with left hemiplegia, severe dysarthria and increasing somnolence. Imaging revealed a large ischemic stroke in the territory of the right middle cerebral artery. She arrived in the Emergency Department alone, though her husband was immediately available by phone and agreed that everything should be done to keep her alive. She was intubated for airway protection, and admitted to the neurological intensive care unit.
Severe acute brain injury (SABI) is defined as an acute neurologic catastrophe, caused by one or more distinct disease processes. Examples include ischemic stroke, intracerebral and subarachnoid hemorrhages, traumatic or inflammatory brain injury, and postanoxic encephalopathy following cardiac arrest. These varied disease processes collectively account for over 14 million deaths annually and represent one of the leading causes of disability worldwide [1].
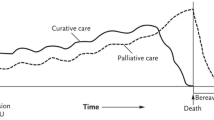
Regardless of the underlying cause, severe acute brain injury (SABI) results in a common clinical scenario with common unique challenges facing patients, their families and clinicians. These include a sudden, unexpected, and devastating neurologic insult, for which treatment decisions must be made quickly, typically with impaired consciousness and communication such that conversations about goals of care have to occur between clinicians and surrogate decision-makers, rather than with the patient themselves. Patients with SABI follow a distinct illness trajectory that we have proposed as the “fourth trajectory” (See Chap. 1 “Neuropalliative Care : Introduction”, Fig. 1.1), in which patients either die acutely, typically after withdrawal or withholding of life-sustaining interventions, or survive with a wide range of disability. Thus, specific approaches and considerations particular to the palliative care of patients and their families in this setting are required. These include early identification and management of pain and distressing symptoms, provision of psychosocial support for patients and their families, accurate prognostication, and sensitive conversations, typically with patient’s family, about prognosis, goals of care and treatment decisions.
Symptom Management I: The Acute Setting
Case continued
Ms. B appeared agitated during her first day of hospitalization. The nurses and physicians caring for her became concerned that she might be in pain due to facial grimacing and ventilator noncompliance, so treated her with small boluses of fentanyl.
The inability of patients to communicate their needs may lead to undertreatment of symptoms. Studies have shown that patients with stroke and aphasia receive fewer pain medications than those without, suggesting that pain and other distressing symptoms are underrecognized [2]. Clinicians must be aware of the prevalence of symptoms, especially pain and anxiety, and particularly attentive to their presence. Table 2.1 lists common symptoms after SABI and their suggested management. Treating any potential symptoms and sources of discomfort is important and is often more challenging in this setting given the need to rely on measures other than direct symptom reports from patients. Empiric trials based on clinical suspicion of symptoms can be a reasonable approach in this setting. If opioids or benzodiazepines are used in the acute setting, short-acting forms are preferred to avoid oversedation and clouding of the neurologic exam.
Objective assessment tools have been validated in critically ill, mechanically ventilated patients but are not specific to neurologically critically ill patients and may be helpful in evaluating the symptom burden in patients with communication barriers and altered sensorium. Examples of such tools include the Behavioral Pain Scale (BPS , Table 2.2) [11] and the Critical Care Pain Observation Tool (CPOT) [12]. However, the utility of these tools can be compromised in patients with SABI who may have limitations in these behaviors due to their underlying injury and tend to exhibit a broader range of behavioral responses to pain than other patient populations [13]. For pain, physiologic markers such as tachycardia and hypertension can be used as indicators in comatose and/or sedated patients, though these signs are nonspecific and can be affected by many other factors. Depression is common and similarly hard to recognize. Clinicians should screen patients regularly for depression and consider SSRIs, especially early in the course of stroke [3].
Myoclonus after hypoxic ischemic brain injury is characterized by abrupt, irregular contractions of muscles. It can occur early (acute) or late (chronic). Post-hypoxic myoclonic status epilepticus may portend a poor prognosis. The treatment of choice is benzodiazepines, though non-sedating anticonvulsants such as valproic acid or levetiracetam can also be used.
‘Storming’ or paroxysmal sympathetic hyperactivity is seen after various types of severe acute brain injury and characterized by episodes with various combinations of hyperthermia, hypertension, tachycardia, tachypnea, increased muscle tone, diaphoresis and other symptoms of sympathetic hyperactivity. Once causes such as seizures, infection, pain and/or metabolic derangements have been ruled out, first-line treatment consists of opioids , intravenous anesthetics such as propofol and beta-blockers (especially propranolol). Benzodiazepines and gabapentin may also be used [14] (See also Chap. 3 ‘Prolonged disorders of consciousness’).
Caregiver Support
Case continued
Ms. B.’s husband did not leave her bedside, and anxiously reported every movement he saw. His sons made sure he was eating, and the neuro-ICU staff provided him with pillows and a blanket. The physician team sat down with the family about 24 h after she presented to discuss the current situation and provide support.
Seeing a loved one experience any serious illness is incredibly challenging, and acting as a surrogate decision-maker for patients with critical illness has been associated with longer term psychiatric symptoms and syndromes such as post-traumatic stress symptoms [15], post-traumatic stress disorder [16], anxiety, and depression [17,18,19]. Early in the course of SABI, the clinical course can be rapidly changing and the ICU setting, in particular, can be unfamiliar and overwhelming to caregivers. Small gestures by ICU staff can go a long way in promoting comfort with family members – these include open visiting hours [20], comfortable waiting areas, refreshments, and facilities for showers and personal care [21].
Families describe a loss of personhood through brain injury, and identify the need for clinicians to maintain this personhood by talking to the patient, even when unresponsive, and by asking the family about the patient as a person, prior to this injury [21]. Clinicians can further support this awareness of patient personhood by defining surrogates’ responsibility for decision-making within a substituted judgment framework. In other words, clinicians can ask surrogates to communicate the voice of their loved one rather than making decisions in the best interest of their loved one. Some phrases that can be helpful in clarifying this for surrogates include:
-
“What we’re asking you to do is to bring Rita’s voice into the room. If she could be here right now talking with us about what’s happened, what do you think she would say?”
-
“We are not asking that you make decisions for Rita based on your own values – that’s an impossibly difficult position for you to be in. What’s most important is to get a sense of what Rita would want in this situation. Has she ever spoken about issues like this before?”
When discussing the patient’s condition, family members have expressed a need for hope when presented with uncertainty [21]. One helpful way to maintain hope with the family in a time of immense loss is by reframing the focus of hope. Clinicians can ask what families are hoping for and help them shift their hope, if not on survival, perhaps on re-uniting with a family member; if not on recovery to independence, perhaps on being able to participate in an important future event. (See Chaps. 18 “Spiritual Care” and 20 “Caregiver Assessment and Support”).
Given the “fourth trajectory” described above [22] and shown in (Fig. 2.1), the prolonged period of convalescence following hospitalization with significant debility and functional dependence also confers a large psychological, financial, and physical burden on caregivers. Many patients are discharged to skilled nursing facilities or adult family homes, and one in five patients with stroke require institutional care at 3 months after the acute event [23]. After patients are discharged from the acute care setting, outpatient follow-up is paramount for symptom identification and management, psychosocial support and ongoing conversations addressing goals of care as the patient’s condition evolves.
Severe Acute Brain Injury trajectory. The two red errors symbolize the two periods of treatment decisions described in the text (very early and early). (Adapted by permission from BMJ Publishing Group Limited from Creutzfeldt et al. [22])
Prior conversations about serious illness and health care directives can help guide surrogates and clinicians. However, the language in advance directives (ADs) is often vague and the applicability can be difficult to determine, failing to capture the uncertainty inherent in clinical medicine generally, and in the case of SABI, specifically. Physicians vary in the degree to which they capture uncertainty in their prognostic conversations with patients and their families. The uncertainty in outcomes present in the majority of patients with SABI makes interpretation of ADs challenging, though they may amplify a family’s understanding of their loved one’s wishes. Family members find ADs more useful than do physicians [24] – which may speak to physicians’ understanding of the nuance and complexity of a clinical situation and discomfort with the applicability of ADs. Moreover, treatment preferences are not always stable over time [25] which can also limit the applicability of ADs. Addressing goals of care more generally can open the door to a broader conversation about a patient’s values and priorities and help frame specific decisions about medical interventions in the context of a patient’s life.
Case continued
On hospital day 2, a meeting was conducted between Ms. B.’s family and the medical team. They continued to assert that she would want every possible intervention to improve her chances of meaningful recovery and consented to a decompressive hemicraniectomy, which she underwent that same day.
Estimating and Communicating Prognosis
Accurate prognostication in the setting of SABI is critically important to help surrogates with decision making. While the focus is often on the likelihood of survival, families want to know the likelihood and extent of functional recovery and quality of life after SABI. Centering the conversation on “How long?” and “How well?” can help focus discussions on both longevity and function/quality of life – which is particularly salient in the setting of SABI [26]. Threading the needle between optimism and pessimism, between hope and truth-telling, is one of the greatest challenges in communicating with families of patients with SABI. The presence of many different clinicians with discordant prognostic estimates can complicate communication and decision-making.
Although many individual signs and symptoms correlate with survival, the strength of these correlations is rarely strong enough to rely upon when prognosticating. To construct a more accurate short- and long-term prognosis, an enormous number of diagnostic tests, clinical severity grading scales and prognostic models have been described that are reviewed in detail elsewhere [27,28,29]. These scales use various clinical and radiological signs of illness severity to predict longer term mortality, and sometimes functional status. While such scales may allow for the use of multiple variables that add up to an approximate prognosis, these models are fairly limited in their ability to accurately prognosticate for individual patients, and are fraught with uncertainty and biases [30]. There are rare instances in SABI when prognostic markers have a high accuracy of a poor outcome prediction. These occur in patients who present with severe hypoxic ischemic encephalopathy (HIE) after cardiac arrest. In these patients, the absence of pupillary light or corneal reflexes on day 3 or absent cortical (N20) responses on somatosensory evoked potentials by day 1–3 uniformly predicts a poor prognosis defined as death or severe disability [31].
It usually takes at least 6 months after cardiac arrest or stroke [32] and 1–2 years after severe traumatic brain injury [33] until the stage of chronic recovery is reached. These time frames are typically marked by institutionalized care with extended use of life-sustaining treatment such as artificial hydration and nutrition or respiratory support, and complications and comorbidities leading to recurring hospitalizations. As clinicians discuss prognosis and treatment decisions with individual families in the acute stages, the range of possible outcomes needs to include the potential burden of continued acute and chronic treatment and considered alongside the patients’ previously stated or presumed values and goals. In the fast-paced, often chaotic environment of the Intensive Care Unit, it is important to address the potential for recovery and adaptation over a longer time horizon. (See Chap. 11, “Communicating Effectively”, Chap. 12 and “Prognostication”, and 13, “Improving Medical Decisions”).
Serious Illness Conversation Triggers
Discussions with surrogate decision-makers about prognosis, treatment decisions and goals of care are best thought of as a series of conversations, beginning early in hospitalization and occurring at regular intervals throughout the acute hospitalization. Certain clinical events and treatment decisions that occur in the course of SABI can function as watershed events or “serious illness conversation triggers” (Table 2.3), prompting the team and family to readdress prognosis and goals. During the initial hospitalization for SABI, there are typically two periods of treatment decisions: very early and early (Fig. 2.1).
-
Very early treatment decisions (hours to days).
Very early treatment decisions occur during the first hours to days of admission and include immediately life-saving procedures such as tPA and/or mechanical thrombectomy for ischemic CVA, temperature management for HIE, decompressive hemicraniectomy, ventricular drain placement or clot evacuation to manage of increased intracranial pressure. The acute need for intervention leaves little time for deliberation and these are often the first major decisions that families must make about whether to proceed with aggressive interventions. Deciding for such a life-saving intervention means deciding for survival with a wide range of disabilities. For example, after a large (‘malignant’) ischemic stroke (as described in our case), there is good evidence that decompressive hemicraniectomy (DHC) within 48 h decreases mortality from 70% to 20% in younger patients (<60 years), and to 35% in older patients; among the young ones who survive, one in two undergoing DHC will gain independence, among the older ones this proportion drops to one in ten [36, 37]. There is a well-described discrepancy between the interventions that healthy patients think they would want when presented with theoretical clinical scenarios, and the actual satisfaction of those who have received those interventions – particularly in the case of surgical decompression. Affective forecasting describes the process of predicting for oneself how one may feel in a future state. Patients often fail to predict how they will adapt to a new baseline, focusing more on what will change than what will stay the same, and underestimating their own ability to cope [38]. Most healthy people say that they would not want to undergo DHC in the setting of a malignant cerebral infarct if the outcome were moderate or severe impairment [39]. However, most patients who have undergone this procedure and their caregivers reported feeling satisfied with this decision, despite significant disability and say they would make the same decision again [40, 41]. When communicating with surrogate decision-makers, it can be helpful to educate them about this “disability paradox” or to help them “imagine the unimaginable” [42]: to help them imagine what life might be like, and to share the experiences of others as they try to imagine a life for their loved one that may feel unfamiliar and frightening.
-
Early treatment decisions (weeks)
Once patients have moved into the more subacute phase of their illness, the need for decision-making around tracheostomy and percutaneous enteral gastrostomy (PEG) tube placement can serve as another watershed moment for family conferences (Fig. 2.1, blue arrow). At least one in 20 patients with stroke are discharged from the acute care hospital with a feeding tube [23] - this number varies widely across hospitals [43]. Among patients with severe traumatic brain injury who underwent PEG tube placement, one in three were independent at 1 year; in that same small study, the persistence of a PEG tube at 3 months was associated with much greater disability, as only 5% of patients achieved independence [44]. In stroke patients who undergo PEG placement, 2-year mortality may be as high as 66% [45]. Among survivors, about one in ten regain independence, while all others will have a varying range of long-term disability, the risk for which increases with age [45].
The indication for tracheostomy placement in critically ill patients is to facilitate weaning from mechanical ventilation, for long-term airway protection, or a combination of the two. Approximately one in ten of all (medical and surgical) patients who receive mechanical ventilation will go on to receive a tracheostomy; the majority of patients with tracheostomies are discharged to long-term care facilities [46], and 1 year survival may be as low as 10%, although these numbers are not specific to patients with SABI [47].
The decision for placement of a PEG or tracheostomy after SABI, ideally, would be guided by evidence- and preference-based prognostication, i.e. by predicting the degree of future recovery and dependence as well as the patient’s ability and willingness to adapt to such a life. However, uncertainties and biases in prognostication are common after SABI [48], especially early in the course of the illness, when surrogates and clinicians are faced with the decision to either shift to comfort measures only or continue a potentially burdensome treatment for a time that some may perceive as too long [49, 50]. To accommodate this tension, a third strategy is recommended as an alternative to all-or-nothing approaches, often referred to as a time-limited trial: clinicians and surrogate decision makers agree to use certain medical therapies – such as trial of nasogastric feeding before PEG placement – “over a defined period of time to observe if the patient improves or deteriorates according to agreed-upon clinical outcomes.” [49] Engaging in such a trial requires clinicians to educate the families about what to look out for and to provide a clear follow up plan to re-evaluate the clinical situation (See Chap. 13, “Improving Medical Decisions”). Transitioning to comfort measures only can be more challenging in the later subacute and chronic setting if there is no acute event to prompt that transition. Outpatient follow-up with neurologists, primary care providers, and palliative medicine specialists can help frame and guide decision-making for patients and families in the longer term.
Establishing Goals of Care
Developing trust takes time. Additionally, surrogate decision-makers need time to fully grasp the nature of what is occurring and the implications of medical decision-making. Thus – addressing goals of care and patient values in the setting of SABI is best viewed not as a single event but as a series of conversations over time that frames medical decisions within the greater context of a patient’s values and priorities. If consistent with a patient’s goals of care, aggressive measures with thorough and careful attention to medical details and family communication early on in the disease course can demonstrate to surrogates that the care team is deeply invested in the best clinical outcome for their loved one. If and when a poor prognosis becomes clear, or the patient decompensates further, families are more likely to trust negative prognostic data provided by that same team. Furthermore, setting the stage for the future can help families “hope for the best and prepare for the worst” [51]. (See Chap. 11, “Communicating Effectively”) Sample language early in the hospital course might include:
-
“I hear you telling me that Gary would want ‘everything done’. Right now, we are doing everything we can to keep him alive – we’re making sure he’s getting enough oxygen by putting in a breathing tube and connecting him to a breathing machine. We’re keeping a careful eye on his blood pressure and may need to think about surgery to reduce the pressure around his brain. I’m hopeful that he will improve, and I also want to let you know that I’m worried things could get worse. I’ll be talking to you a lot over the next few days, and I’m going to be honest with you about what’s going on.”
-
“We’re going to do everything we can to try to make Gary better. If we get to the point where I think that’s not possible, I’m going to let you know that too. I also want you to let me know if we get to a point when you feel that he would no longer want the aggressive treatment we’re providing.”
Best Case, Worst Case, and Most Likely Case
Because prognosis is often uncertain in the setting of SABI, presenting best, worst, and most likely outcomes can be one strategy to help families manage the uncertainty associated with recovery [42, 52]. Some possible phrases might include:
-
“Because we don’t have a crystal ball, I can’t tell you for certain what the future holds for Tom. I think that the best case scenario is that he recovers enough to be able to talk and interact with the people he cares about – he would likely still need help with his usual daily activities like eating, dressing, and bathing, but, with enough help, might be able to return home eventually. I think the worst case scenario is that he does not wake up and will need life support to keep his body alive for the long-term. I think the most likely scenario is somewhere in between – awake, able to track your movement around the room, but not able to talk and interact with you and the family. What do you think about all of that?”
-
“I’d like to talk to you about what we call an ‘acceptable level of better’. How much better do you think Tom would have to be to have a life that is meaningful for him?”
-
“If Tom could be a part of this conversation now, talking to us about his wishes, what do you think he would say? If he could tell us about what’s most important – longevity or living as long as possible, comfort, and independence – which one do you think he’d value the most?”
Anticipatory guidance is a way to help patients and family prepare for anticipated developments, expect complications and plan for potential decisions that may ensue.
-
“I’m worried that, down the road, Nancy’s condition might worsen – people with her type of brain injury often get infections, for example, and that could make things worse. I’m worried that aggressive care in the ICU at that point might really make her more uncomfortable. With that in mind, I think it would be reasonable to continue with what we’re doing right now, and also plan that, in the future, if she gets worse, that we won’t escalate her care, or bring her back to the ICU, because we’d see that as a sign that she probably wasn’t going to get well enough to return home. What do you think?”
Shared Decision-Making: Balance Between Paternalism and Autonomy
Decision control can be viewed as existing on a spectrum with patient autonomy on one end in which patients and/or surrogates make decisions independently, and paternalism (parentalism) on the other, in which clinicians make decisions on behalf of patients. (See Chap. 13, “Improving Medical Decisions”) In between is shared decision-making, in which patients and clinicians share responsibility and make decisions together in a collaborative fashion. Multiple critical care societies have come to consensus [53, 54] that shared decision-making is a best practice, though in reality, patients and surrogates are variable in the amount of control they prefer to have over complex medical decision making vs. letting the physician decide [55, 56].
SABI presents a clinical scenario in which clinicians may have a great deal of experience, and surrogates usually have very little. Clinicians therefore have an opportunity to share their experience and make recommendations that are in line with a patient’s stated values [48]. Directiveness by physicians is more appropriate when prognosis is certain. In the setting of SABI, communicating prognostic uncertainty is one of the greatest communication challenges. Making serious decisions in the face of clinical uncertainty is one of the key struggles that family members face. It can be a temptation to present prognosis in more certain terms in a well-intentioned effort to ease the burden of decision-making, but physicians have a moral obligation to communicate honestly with patients and their surrogates. How physicians discuss prognosis [57] and goals of care has a significant impact on the decisions that patients and families make. In this situation of substantial uncertainty, clinicians have to be humble and sensitive to the power of our words to impact the lives of our patients and of their loved ones.
Case continued
Mrs. B. spent about 2 weeks in the neuro-intensive care unit and was eventually discharged to a rehabilitation facility with persistent left hemiparesis and neglect, some cognitive deficits and a PEG tube. Over the next several years, she was able to live independently with her husband, but continued to have severe L sided pain which limited her mobility and her ability to participate in hiking and many of the other outdoor activities that had given her joy and connected her to a social community.
Symptom Management II: The Sub-acute and Chronic Setting
Survivors of SABI can have a high chronic symptom burden, with a high prevalence of fatigue, major depressive disorder, generalized anxiety, and chronic pain (Table 2.1). Even patients with good recovery after stroke can suffer from depression, cognitive impairment and trouble reintegrating into normal living [58]. Fatigue is reported in up to 50% of stroke survivors, and around a third experience depression and/or anxiety [59]. Similar numbers have been reported after traumatic brain injury [60] and cardiac arrest [61]. After evaluating and treating for secondary causes of fatigue, including depression and sleep apnea (estimated in over half of patients with ischemic stroke) [62], management of fatigue should start with behavioral approaches such as sleep hygiene and exercise; medications such as modafinil or methylphenidate may be considered in refractory situations. Post-stroke depression can be treated effectively with SSRIs, ideally in combination with psychotherapy [59], and some suggest that SSRIs may help prevent depression following TBI [63]. One quarter of stroke survivors experience pain [64], so careful attention to the its diagnosis and management with both pharmacologic and non-pharmacologic approaches is important in the long-term setting (see Table 2.1).
Case continued
Ms. B, now 65 years old and 10 years after her first stroke, presented to the emergency room with sudden onset confusion, right hemiplegia and left gaze preference. She had no advance directive documented. Her head CT demonstrated a left thalamic intraparenchymal hemorrhage with intraventricular extension. Her family consented to emergent placement of an external ventricular drain (EVD), but on hospital day 3, she suffered a worsening in her neurologic status with increasing somnolence, no spontaneous eye opening or movement; and eyes with downward gaze. With stimulation, she had spontaneous movement of left upper and lower extremities and weak but purposeful withdrawal to noxious stimuli. Repeat imaging demonstrated evidence of a delayed EVD tract-associated hemorrhage.
The neurology team met with the family, who indicated they did not think that Ms. B would want to live with a significant decline in her functional status. They felt that she could “barely tolerate” the pain and functional limitations associated with her prior ischemic stroke. The neurology team met with the patient’s sons and husband. After discussing the “best case” and “most likely” scenarios, the decision was made to transition to comfort measures only. She died peacefully one day later, surrounded by family.
EOL Care Including Hospice
End of life care for patients with SABI includes both the care patients receive in the hospital and the care they may receive in other settings, including skilled nursing facilities, inpatient hospices, and home. In the hospital setting, palliative care services are a resource to assist with complex medical decision-making, direct efforts at symptom management, and navigate challenging family dynamics.
Hospice can add an additional layer of support for patients in the terminal stage of their disease, either at home or in an institutional setting. Patients with stroke or coma are considered to be eligible for hospice if they meet the following Medicare Guidelines (See Chap. 16). These guidelines are meant to standardize criteria for this disease category but clinicians should assess specific needs and prognostic estimates individually for each patient when setting a treatment plan.
-
An inability to maintain hydration and caloric intake with one of the following:
-
Poor functional status with Palliative Performance Scale [65] score <40%.
-
Weight loss >10% during the past 6 months or >7.5% in past 3 months;
-
Serum albumin <2.5 g/dL;
-
Current history of pulmonary aspiration without response to interventions;
-
Sequential calorie counts documenting inadequate caloric/fluid intake;
-
Dysphagia severe enough to prevent the patient from receiving food and fluids necessary to sustain life, and patient does not receive artificial nutrition and hydration.
-
Brain Death
Some patients with a catastrophic, irreversible brain injury may progress to brain death [66]. While the concept of brain death is usually clear to most clinicians, it is often very challenging for families to grasp: the brain-dead patient in the intensive care unit does not appear deceased but still feels warm, has a beating heart and vital signs. Key to communication is to be pro-active if at all possible: to have early, honest conversations with the family of a patient with a progressively worsening severe acute brain injury and to prepare them for anticipated developments. If the outcome is clear and hopeless, the family also needs to be given the opportunity to discuss possible organ donation with a representative of an organ donation agency. Families should also be informed that adventitious and often complex movements can occur due to retained lower-level reflexes. In rare occasions, families are unable to accept brain death as death. In addition to providing continued emotional support and repeated conversations with the family, options include continuation of organ support for a few more days while involving the hospital ethics committee, spiritual care specialists if relevant and, eventually, the court [67].
Research Agenda
Educational needs exist for both neurology and palliative care clinicians, and for both trainees and those with an established career. Communication training should be prioritized that teaches clinicians to deliver serious news in an effective and empathic manner, that assists families with difficult treatment decisions and supports them through these. This communication would ideally be somewhat standardized – a common language around severe acute brain injury and prognostic uncertainty as detailed in this chapter may help medical teams and families work together towards a patient-centered approach. Research agenda items include the need for better prognostic models for patients with SABI – enhanced prognostication would make communication easier for clinicians, and would certainly ease some of the decisional burden that surrogates face. We need to identify best ways to integrate primary and specialist palliative care into the care of patients with SABI, especially around shared decision-making and family engagement. Hospice eligibility criteria after SABI have yet to be developed and validated.
Take Home Messages
-
SABI is a heterogeneous category of diseases that are characterized by a sudden, catastrophic neurologic event.
-
Patients with SABI typically lack decisional capacity in the acute setting, so goals of care discussions typically occur with surrogate decision-makers.
-
The initial phase of illness usually occurs in the emergency department and critical care setting: unfamiliar and fast-paced environments which can be uncomfortable for family members suddenly thrust into the role of surrogate decision-maker
-
Addressing goals of care in the setting of SABI is best done in a series of conversations, often marked in time by critical decisions that need to be made – around the decision to pursue decompressive hemicraniectomy, and, later, tracheostomy and PEG tube placement.
-
Balancing uncertainty and clarity can be a major challenge in communicating with families of patients with SABI.
References
Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448–57.
Kehayia E, Korner-Bitensky N, Singer F, Becker R, Lamarche M, Georges P, et al. Differences in pain medication use in stroke patients with aphasia and without aphasia. Stroke. 1997;28(10):1867–70.
Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):123–30.
Slooter AJ, Van De Leur RR, Zaal IJ. Delirium in critically ill patients. Handb Clin Neurol. 2017;141:449–66. Netherlands: 2017 Elsevier B.V.
Diamond AL, Callison RC, Shokri J, Cruz-Flores S, Kinsella LJ. Paroxysmal sympathetic storm. Neurocrit Care. 2005;2:288–91. United States2005.
Ko SB, Kim CK, Lee SH, Bae HJ, Yoon BW. Morphine-sensitive paroxysmal sympathetic storm in pontine intracerebral hemorrhage. Neurologist. 2010;16:384–5. United States2010.
Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol. 2013;12:597–608. England: 2013 Elsevier Ltd.
Oh H, Seo W. A comprehensive review of central post-stroke pain. Pain Manag Nurs. 2015;16:804–18. United States: 2015 American Society for Pain Management Nursing. Published by Elsevier Inc.
Hinkle JL, Becker KJ, Kim JS, Choi-Kwon S, Saban KL, McNair N, et al. Poststroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American Heart Association. Stroke. 2017;48:e159–70. United States: 2017 American Heart Association, Inc.
Kim JS. Post-stroke mood and emotional disturbances: pharmacological therapy based on mechanisms. J Stroke. 2016;18(3):244–55.
Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29(12):2258–63.
Rijkenberg S, Stilma W, Endeman H, Bosman RJ, Oudemans-van Straaten HM. Pain measurement in mechanically ventilated critically ill patients: behavioral pain scale versus critical-care pain observation tool. J Crit Care. 2015;30(1):167–72.
Arbour C, Gélinas C. Behavioral and physiologic indicators of pain in nonverbal patients with a traumatic brain injury: an integrative review. Pain Manag Nurs. 2014;15(2):506–18.
Meyfroidt G, Baguley IJ, Menon DK. Paroxysmal sympathetic hyperactivity: the storm after acute brain injury. Lancet Neurol. 2017;16(9):721–9.
Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987–94.
Zimmerli M, Tisljar K, Balestra GM, Langewitz W, Marsch S, Hunziker S. Prevalence and risk factors for post-traumatic stress disorder in relatives of out-of-hospital cardiac arrest patients. Resuscitation. 2014;85:801–8. Ireland: 2014 Elsevier Ireland Ltd.
Pochard F, Azoulay E, Chevret S, Lemaire F, Hubert P, Canoui P, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: ethical hypothesis regarding decision-making capacity. Crit Care Med. 2001;29(10):1893–7.
Pochard F, Darmon M, Fassier T, Bollaert PE, Cheval C, Coloigner M, et al. Symptoms of anxiety and depression in family members of intensive care unit patients before discharge or death. A prospective multicenter study. J Crit Care. 2005;20(1):90–6.
Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–78.
Whitton S, Pittiglio LI. Critical care open visiting hours. Crit Care Nurs Q. 2011;34:361–6. United States2011.
Schutz RE, Coats HL, Engelberg RA, Curtis JR, Creutzfeldt CJ. Is there hope? Is she there? How families and clinicians experience severe acute brain injury. J Palliat Med. 2017;20(2):170–6.
Creutzfeldt CJ, Longstreth WT, Holloway RG. Predicting decline and survival in severe acute brain injury: the fourth trajectory. BMJ. 2015;351:h3904.
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. United States2012.
Leder N, Schwarzkopf D, Reinhart K, Witte OW, Pfeifer R, Hartog CS. The validity of advance directives in acute situations. Dtsch Arztebl Int. 2015;112(43):723–9.
Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc. 2003;51(10):1398–403.
Holloway RG, Gramling R, Kelly AG. Estimating and communicating prognosis in advanced neurologic disease. Neurology. 2013;80(8):764–72.
Hwang BY, Appelboom G, Kellner CP, Carpenter AM, Kellner MA, Gigante PR, et al. Clinical grading scales in intracerebral hemorrhage. Neurocrit Care. 2010;13(1):141–51.
Sharma K, Stevens RD. Determinants of prognosis in neurocatastrophes. Handb Clin Neurol. 2017;140:379–95.
Holloway RG, Arnold RM, Creutzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(6):1887–916.
Creutzfeldt CJ, Holloway RG, Curtis JR. Palliative care: a core competency for stroke neurologists. Stroke. 2015;46(9):2714–9.
Young GB, Doig G, Ragazzoni A. Anoxic-ischemic encephalopathy: clinical and electrophysiological associations with outcome. Neurocrit Care. 2005;2:159–64. United States2005.
Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81(18):1588–95.
Whyte J, Nakase-Richardson R, Hammond FM, McNamee S, Giacino JT, Kalmar K, et al. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch Phys Med Rehabil. 2013;94(10):1855–60.
Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365(9461):764–72.
Bistrian BR, Askew W, Erdman JW Jr, Oria MP. Nutrition and traumatic brain injury: a perspective from the Institute of Medicine report. JPEN J Parenter Enteral Nutr. 2011;35(5):556–9.
Juttler E, Unterberg A, Woitzik J, Bosel J, Amiri H, Sakowitz OW, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091–100.
Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–22. England2007.
Halpern J, Arnold RM. Affective forecasting: an unrecognized challenge in making serious health decisions. J Gen Intern Med. 2008;23(10):1708–12.
Klein A, Kuehner C, Schwarz S. Attitudes in the general population towards hemi-craniectomy for middle cerebral artery (MCA) infarction. A population-based survey. Neurocrit Care. 2012;16(3):456–61.
Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg. 2012;117(4):749–54.
Green TL, Newcommon N, Demchuk A. Quality of life and caregiver outcomes following decompressive hemicraniectomy for severe stroke: a narrative literature review. Can J Neurosci Nurs. 2010;32(2):24–33.
Ubel PA, Loewenstein G, Schwarz N, Smith D. Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57–62. United States2005.
George BP, Kelly AG, Schneider EB, Holloway RG. Current practices in feeding tube placement for US acute ischemic stroke inpatients. Neurology. 2014;83(10):874–82.
Godbolt AK, Stenberg M, Jakobsson J, Sorjonen K, Krakau K, Stalnacke BM, et al. Subacute complications during recovery from severe traumatic brain injury: frequency and associations with outcome. BMJ Open. 2015;5(4):e007208.
Meisel K, Arnold RM, Stijacic Cenzer I, Boscardin J, Smith AK. Survival, functional status, and eating ability after percutaneous endoscopic gastrostomy tube placement for acute stroke. J Am Geriatr Soc. 2017;65:1848–52.
Mehta AB, Syeda SN, Bajpayee L, Cooke CR, Walkey AJ, Wiener RS. Trends in tracheostomy for mechanically ventilated patients in the United States, 1993–2012. Am J Respir Crit Care Med. 2015;192(4):446–54.
Cox CE, Martinu T, Sathy SJ, Clay AS, Chia J, Gray AL, et al. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009;37(11):2888–94; quiz 904.
Creutzfeldt CJ, Holloway RG. Treatment decisions after severe stroke: uncertainty and biases. Stroke. 2012;43:3405–8. United States2012.
Quill TE, Holloway R. Time-limited trials near the end of life. JAMA. 2011;306:1483–4. United States2011.
Schenker Y, Crowley-Matoka M, Dohan D, Tiver GA, Arnold RM, White DB. I don’t want to be the one saying ‘we should just let him die’: intrapersonal tensions experienced by surrogate decision makers in the ICU. J Gen Intern Med. 2012;27(12):1657–65.
Disraeli B. The Wondrous Tale of Alroy. Philadelphia: Carey, Lea, and Blanchard; 1833. 227 p.
Simpkin AL, Schwartzstein RM. Tolerating uncertainty – the next medical revolution? N Engl J Med. 2016;375(18):1713–5.
Davidson JE, Powers K, Hedayat KM, Tieszen M, Kon AA, Shepard E, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35(2):605–22.
Carlet J, Thijs LG, Antonelli M, Cassell J, Cox P, Hill N, et al. Challenges in end-of-life care in the ICU. Statement of the 5th International Consensus Conference in Critical Care: Brussels, Belgium, April 2003. Intensive Care Med. 2004;30(5):770–84.
Heyland DK, Cook DJ, Rocker GM, Dodek PM, Kutsogiannis DJ, Peters S, et al. Decision-making in the ICU: perspectives of the substitute decision-maker. Intensive Care Med. 2003;29(1):75–82.
Heyland DK, Tranmer J, O’Callaghan CJ, Gafni A. The seriously ill hospitalized patient: preferred role in end-of-life decision making? J Crit Care. 2003;18:3–10. United States: 2003 Elsevier, Inc.
Murphy DJ, Burrows D, Santilli S, Kemp AW, Tenner S, Kreling B, et al. The influence of the probability of survival on patients’ preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330(8):545–9.
Kapoor A, Lanctot KL, Bayley M, Kiss A, Herrmann N, Murray BJ, et al. “Good Outcome” isn’t good enough: cognitive impairment, depressive symptoms, and social restrictions in physically recovered stroke patients. Stroke. 2017;48:1688–90. United States: 2017 American Heart Association, Inc.
Creutzfeldt CJ, Holloway RG, Walker M. Symptomatic and palliative care for stroke survivors. J Gen Intern Med. 2012;27(7):853–60.
Seel RT, Macciocchi S, Kreutzer JS. Clinical considerations for the diagnosis of major depression after moderate to severe TBI. J Head Trauma Rehabil. 2010;25(2):99–112.
Moulaert VRM, van Heugten CM, Gorgels TPM, Wade DT, Verbunt JA. Long-term outcome after survival of a cardiac arrest: a prospective longitudinal cohort study. Neurorehabil Neural Repair. 2017;31(6):530–9.
Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73(16):1313–22.
Jorge RE, Acion L, Burin DI, Robinson RG. Sertraline for preventing mood disorders following traumatic brain injury: a randomized clinical trial. JAMA Psychiat. 2016;73(10):1041–7.
Jonsson AC, Lindgren I, Hallstrom B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry. 2006;77(5):590–5.
Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12(1):5–11.
Wijdicks EF, Varelas PN, Gronseth GS, Greer DM. American Academy of N. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74(23):1911–8.
Burkle CM, Pope TM. Brain death: legal obligations and the courts. Semin Neurol. 2015;35(2):174–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Isaac, M., Creutzfeldt, C.J. (2019). Severe Acute Brain Injury. In: Creutzfeldt, C., Kluger, B., Holloway, R. (eds) Neuropalliative Care. Springer, Cham. https://doi.org/10.1007/978-3-319-93215-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-93215-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93214-9
Online ISBN: 978-3-319-93215-6
eBook Packages: MedicineMedicine (R0)