Abstract
Stratification in lakes is a natural phenomenon caused by solar heating resulting in development of a thermocline. This becomes a barrier to heat, dissolved oxygen and dissolved nutrient transfer between the upper and lower water column. Nutrient runoff from land and phytoplankton growth in lakes can cause bottom waters to become oxygen depleted and potentially unsuitable as a habitat for aquatic biota. Conversely, reduced depth of mixing above the thermocline provides a high light field that enhances algal and cyanobacteria growth and, in nutrient rich conditions, the lake becomes degraded, the natural lake eutrophication process. Reducing the nutrient load on the lake may not be possible and alternative actions are required such as minimising or removing the magnifying effects of thermal stratification on the eutrophication process. This chapter describes issues associated with thermal stratification in lakes and examines the possible options for removing or preventing thermal stratification by mixing as a management strategy for the rehabilitation of degraded lakes. The most common mixing device is aeration, using bubble plumes to induce vertical movement of the water column. Sparge line aeration systems can be designed to suit most water bodies from small ponds to large reservoirs and natural lakes, with the maximum size being determined by economics: a generic design is described in detail. Also described are alternative water column mixing systems and the option of bottom water re-oxygenation without destratification. Discussions are based around strategies adopted for the ten water supply reservoirs for Auckland City (New Zealand), used as a case study.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Restoration of lakes involves multiple processes, many of them biological, but there are several engineering interventions that have been successfully used, such as diversions of eutrophic water sources away from less eutrophic lakes as was done with the Ohau Channel diversion wall between Lake Rotorua and Lake Rotoiti (BOPRC 2008). This chapter discusses two groups of engineering options that influence the in-lake processes of stratification and oxygen depletion. Alterations to these processes have been shown to be successful for lake management and restoration.
6.1.1 Thermal Stratification
Stratification in a lake water body is caused by density differences between water in the upper and lower water column (Fig. 6.1a). Thermal stratification is the process whereby such differences are due to temperature (rather than salinity) effects and results in the separation of the lake water column into three layers definable by the temperature-induced density differences: the epilimnion at the top is the warmest, the metalimnion in the middle is cooler than the epilimnion and the hypolimnion at the bottom is the coldest. The thermocline within the metalimnion is the zone is where the largest temperature change occurs with change in depth (Fig. 6.1b). As the temperature gradient across the thermocline increases, it becomes a barrier to the rapid mixing of heat and oxygen down to the hypolimnion and transporting nutrients released from the sediment up to the epilimnion. Eventually, the thermocline reduces these flux rates to the rate of diffusion.
Schematic diagram of a lake showing the concept of (a) fully mixed and (b) thermally stratified lake (redrawn from Gibbs and Hickey 2012)
In New Zealand’s warm-temperate climate, thermal stratification is a seasonal phenomenon with most lakes being fully mixed in winter and thermally stratified in summer. Such lakes are termed monomictic as they only stratify and then mix once per year. In countries of the Northern Hemisphere, lakes may also become ice covered in winter and so stratify and mix twice in a year (dimictic), a process that occurs in a very few high altitude South Island lakes. In this chapter, we only consider monomictic lakes.
6.1.2 Consequences of Thermal Stratification
6.1.2.1 Deoxygenation
Decomposition processes in the hypolimnion of the lake water column and sediments consume dissolved oxygen (DO). This process is referred to as hypolimnetic oxygen demand (HOD). HOD comprises oxygen loss from the water column plus sediment oxygen demand (SOD). If the loss of oxygen from the water column is greater than the rate of oxygen diffusion across the thermocline, the hypolimnion begins to become oxygen depleted. As the DO concentration reduces, electrochemical reduction and oxidation (REDOX) potentials in the water column reduce from positive to negative with the zero point coinciding with zero DO, a condition called anoxic. In this anoxic state, there is no free oxygen in the water column but there are oxygen-containing anions such as nitrate (NO3 –) and sulphate (SO4 2–), and oxygen in the organic molecules (e.g. sugars and fatty acids) in organic detritus settling out of the upper water column. As the REDOX potential becomes increasingly negative, bacteria in the sediment strip the oxygen from these molecules releasing ammonium (NH4+), hydrogen sulphide (H2S) and methane (CH4). When there are no more oxygen atoms available in the system, the condition is termed anaerobic. In the process of becoming oxygen depleted, there are several threshold DO concentrations where biological and geochemical changes occur. In general, the presence of most fish species and other non-microbial aquatic biota declines markedly when the DO concentration falls below 5 mg L–1.
6.1.2.2 Nutrient Release
At a DO concentration of about 5 mg L–1, manganese changes from insoluble manganic (Mn3+) form to soluble manganous (Mn2+) form and at an approximate DO concentration of <2 mg L–1 iron changes from insoluble ferric (Fe3+) form to soluble ferrous (Fe2+) form. Both iron and manganese can sequester (adsorb) soluble reactive phosphorus (SRP) (mostly PO4 3–) from the water in their insoluble forms, but release it when they dissolve under reduced oxygen concentrations (Fig. 6.2). This process is reversible, with SRP being sequestered or released depending on the DO concentration in the water column. The sequestration of SRP from the water column is not instantaneous, and there is a kinetic reaction time which allows a short window of time (hours to days, depending on algal abundance) when there is SRP free in the water column after the iron and manganese oxides have precipitated to the lake bed.
Biogeochemical processes in a lake that are directly controlled by oxygen. Upward arrows indicate increasing concentration of the soluble form while their horizontal position indicates the level of oxygen required for these transitions (redrawn from Gibbs and Hickey 2012)
Decomposition processes in the sediment render the sediment anaerobic from a few mm below the sediment surface. This means the Fe and Mn in the sediment are in their soluble forms. However, as these metals diffuse out of the sediment, they may encounter oxygen in the overlying water and precipitate as their insoluble forms at the sediment surface. Decomposition processes also release dissolved inorganic nitrogen (DIN) from the sediment in the form of ammonium-N (NH4-N). The NH4-N is continuously released from the sediment at all oxygen levels and it diffuses up out of the sediment. In the process, it must pass through the surface layer of sediment where, if the overlying water is aerobic, nitrifying bacteria can oxidise it to nitrate-N (NO3-N). If the water column is anoxic or anaerobic, nitrification does not occur and the water column accumulates elevated concentrations of NH4-N with no NO3-N. As oxygen levels in the overlying water increase from the anoxic state, the bacterially driven nitrification rate increases producing NO3-N, and both NH4-N and NO3-N can be present in the water column. At the point that nitrification rates exceed the rate of NH4-N release from the sediment, only NO3-N remains in the water column. If the NO3-N being produced is still within the surface sediment layer, denitrifying bacteria can reduce it to nitrogen gas (N2), which is lost from the water to the atmosphere. There is no similar loss mechanism for phosphorus (P).
6.1.2.3 Light Penetration
The release of SRP, NH4-N and NO3-N into the lake water column from both decomposition in the hypolimnion and in the sediments provides these nutrients for algal (phytoplankton) growth. The rate of phytoplankton growth at a specific depth in the water column depends, amongst other things, on the species, the water temperature and the depth of light penetration. The upper water column between the surface and the depth at which the light level is 1% of the surface illumination is called the euphotic zone. In this zone, there is sufficient light for photosynthesis and hence phytoplankton growth. With thermal stratification in summer, phytoplankton can circulate in the high light environment of the surface epilimnion and mixing may not carry them into low light conditions (i.e. Fig. 6.1). This positively influences growth rates. Thermal stratification, therefore, produces conditions that can enhance phytoplankton growth by constraining growth to the upper water column where they experience longer periods in the euphotic zone. The euphotic depth (1% light level) need not coincide with the thermocline. In high water quality lakes, the euphotic depth may be deeper than the thermocline while in degraded lakes it may be shallower. However, if there is no stratification, or the lake has been destratified, wind-induced mixing of the water column will generate vertical mixing currents that may carry phytoplankton cells through the depth of the water column (i.e. Fig. 6.1a condition). During that deep mixing cycle, they may experience considerable periods of time in low light conditions, and the growth rates will be reduced (Gibbs and Hickey 2012).
The length of time spent in the euphotic zone determines the rate at which phytoplankton biomass increases while the time spent in the dark determines the loss of phytoplankton biomass through respiration, which may ultimately lead to cell death. Zooplankton grazing also results in phytoplankton biomass reduction. The theoretical depth at which phytoplankton growth in the euphotic zone is matched by respiratory losses of phytoplankton biomass in the dark is known as the ‘critical depth’ (Sverdrup 1953). Phytoplankton biomass declines when the critical depth is exceeded. Artificially mixing the water column in stratified lakes by a process of ‘destratification’ can produce conditions where the critical depth is exceeded. This technique can, therefore, be used to limit algal growth and even control nuisance cyanobacteria blooms (Visser et al. 2015).
6.2 Lake Mixing
6.2.1 Natural Processes
Natural mixing of lakes occurs regularly in shallow lakes by strong winds. However, wind energy may generally not be strong enough to cause full depth mixing of deeper lakes and reservoirs during summer stratification (Lossow et al. 1998). This is because energy transfer from a surface wind wave down into a lake is through orbital rotation currents produced by the wave. The orbital velocities generally decrease with increasing depth and, in lakes of small to medium depth, and may only extend to a depth equivalent to ca. nine times the amplitude of the surface wave (Kumagai 1988). This effect is an important driver of thermal stratification in summer when wind speeds tend to be lower than at other times of year. In autumn, the cooling surface water will reduce the temperature gradient across the thermocline making it easier for wind-induced currents to mix the lake to the bottom (Gibbs et al. 2016). This event is called ‘turn-over’.
The potential for wind mixing may be assessed by using the Osgood index (US Department of Agriculture 1999) or the Wedderburn number (Wedderburn 1912). The Osgood Index is defined as the mean depth (z) of a waterbody in meters divided by the square root of the surface area (A) in km2, or z/A 0.5. It reflects the degree to which a lake or reservoir will mix because of forces of wind. Low numbers indicate a shallow, large lake that is readily mixed by wind, although during a period of calm days, it may become temporarily stratified. The Wedderburn number W gives an indication of how likely the lake is to mix and can be interpreted by:
-
W ≥ (L/4)h e—Strong thermal stratification, little mixing, small internal seiche amplitudes.
-
½ < W < (L/4)h e—Wind-induced mixing stronger that thermal stratification, more surface mixing than instability at the thermocline, large internal seiche amplitudes.
-
h e/L < W ≤ ½—High degree of mixing between the epilimnion and the hypolimnion, much upwelling at the thermocline (unstable) surface at the upwind end of the basin.
-
W ≤ h e/L—complete overturn (mixing).
Where h e is the epilimnion thickness at rest condition and L is the length of the lake basin.
Partial mixing of a thermally stratified lake will transfer nutrients from the hypolimnion into the epilimnion where they can support phytoplankton growth and in some cases even result in autumnal blooms. Full depth mixing in autumn disperses nutrients from the hypolimnion throughout the lake. These nutrients support phytoplankton growth and typically result in a spring growth that may reach bloom proportions. If the lake is deep and the hypolimnion has become anoxic or anaerobic, full depth mixing at turn-over may result in a drop in DO concentration throughout the lake water column, reflecting the volumetric mixing ratio of the hypolimnion/epilimnion and the conservation of mass of the DO concentration in each layer. This effect may last for a short period until the lake becomes fully re-oxygenated again, but may have adverse effects on aquatic biota that were living in the epilimnion.
6.2.2 Artificial Processes
The process of artificially mixing a lake is called destratification (Feature Box 6.1). The main reasons for artificially mixing a lake or reservoir are to raise the DO concentration in the bottom water and to improve water quality by preventing the release of Fe, Mn and SRP from the sediment. Therefore, the term aeration is used together with artificial mixing as the two processes act in concert. Achievement of aeration to the bottom of a lake requires full depth mixing of the water column to raise the DO concentration at the sediment–water interface above 5 mg L–1. Artificial mixing is achieved by establishing a circulation current that draws surface oxygenated water down to the bottom of the lake to replace bottom water raised to the surface by the mixing device. DO in the downward-circulation current will raise the DO concentration in the bottom water. This level of mixing may also allow nitrification of any released NH4-N to NO3-N at the sediment–water interface, thereby reducing NH4-N and DIN concentrations in the lake.
Artificial mixing to increase lake bottom water DO concentrations has the added advantage of increasing the aerobic habitat for fish and benthic biota. It may, in some cases, also have a potentially negative effect in raising the bottom water temperature thereby reducing cold water refuges for fish such as trout, forcing them to move into the colder inflow streams.
A further common reason for mixing a lake or reservoir is to disperse or prevent algal (and particularly cyanobacterial) blooms developing (Visser et al. 2015) by making use of the critical depth factor (see Sect. 6.1.2.3). Consequently, in a eutrophic lake or reservoir, full depth mixing may result in a change in the phytoplankton species composition from buoyant cyanobacteria, which can form surface blooms and scums in calm conditions, to diatoms and chlorophytes, which require turbulence to keep them suspended in the water column.
There is likely to be a financial constraint to the size of lake or reservoir that can be reasonably managed using this turbulent mixing technique. There are also issues associated with the lake water column structure to be considered when the mixing device is first turned on. These issues are described later in this chapter.
Box 6.1 Artificial Destratification
Bradford Sherman Reservoir Doctors Pty Ltd Canberra ACT AustraliaArtificial destratification is the mechanical manipulation of water circulation within a reservoir with the aim of weakening or eliminating the density stratification of the water column. Density stratification is a nearly ubiquitous feature of reservoirs globally and most commonly is the result of thermal stratification caused by seasonal warming of the upper regions of the water column in response to solar heating. It is quite common in temperate and tropical climates for thermal stratification to commence in early spring as both day length and air temperatures increase and to persist through late autumn.
Left undisturbed, density stratification can suppress vertical transport of heat, dissolved nutrients and dissolved oxygen to rates approaching as little as 1–10 times molecular diffusion levels. Vertical transport can be 10–100 times faster in actively mixing zones of the water column such as the surface layer, where wind mixing and cooling by evaporation and conduction supply mixing energy (turbulent kinetic energy, or TKE) and along the bottom boundaries where velocity shear (a change in velocity with distance from the boundary) introduces TKE.
The suppression of vertical transport arising from density stratification facilitates oxygen depletion of deeper waters as well as the release of nutrients and reduced forms of iron and manganese from anaerobic sediments. The severity of these water quality problems are reservoir-specific and reflect differences in local climate, inflows and outflows and pollutant loading. In addition, density stratification is frequently accompanied by relatively shallow (< 6 m deep) surface mixing layers (SMLs) (just 0.1 °C can be a sufficient temperature change to suppress transport downwards through the bottom of the SML) which provide support for the development of harmful algal blooms of buoyant algae by increasing the amount of light experienced by the algae within the SML. As a general rule, if the SML depth is less than three times the euphotic depth (the depth to which 1% of the light incident at the water surface penetrates), the light environment will be conducive to blue-green algal bloom formation.
Artificial destratification typically uses bubble plumes or mechanical mixers to induce vertical currents in a reservoir. A bubble plume introduced at the bottom of the water column will rise to the surface and lift the surrounding water with it. As the plume rises, it entrains water laterally from the reservoir so that by the time the plume reaches the water surface it contains a mixture of water drawn from nearly the full height of the water column. At the surface, the plume will have a temperature intermediate to that observed at the top and bottom of the water column. The plume spreads radially along the surface for only a few metres before it plunges back down through the water column until it reaches its level of neutral buoyancy, i.e. the level at which the temperature of the reservoir away from the bubble plume matches the temperature of the descending water. At the level of neutral buoyancy, an intrusion forms and the plume spreads horizontally until it reaches the boundaries of the reservoir. In its simplest form, this sets up two counter-rotating circulation gyres with flow moving away from the plumes at the level of the intrusion and moving towards the plume at the top and bottom of the water column.
It is important to remember that away from the bubble plumes themselves, and in the absence of strong winds or cooling, the water column of the reservoir will frequently not be turbulent apart from the boundaries of the intrusion flow generated by the plumes. In other words, the destratifier does not mix the reservoir directly. Instead, by inducing a net downwards movement of the water column away from the plume (to balance the amount of water entrained into the plume), the circulation that is established effectively shifts heat and oxygen vertically downwards at some characteristic velocity ‘w’. As the number of plumes increases, w increases along with the rate of reduction of density stratification. Eventually, the bottom temperature might increase enough so that normal nighttime cooling (it is generally penetrative convection and not wind mixing that causes deepening of the SML) or wind mixing is able to overcome the residual stratification and cause full mixing of the reservoir.
In theory, an artificial destratification system can be engineered to eliminate density stratification in almost any reservoir. However, effective destratification rapidly becomes more expensive as the depth and volume of a reservoir increase. When considering the use of artificial destratification to improve water quality, it is crucial to carefully consider what the problem is. Past reviews of artificial destratification in Australia (McAuliffe and Rosich 1989) have generally found that this technique is more effective at managing dissolved oxygen concentrations and reducing nutrient release from sediments and dissolved reduced forms of iron and manganese than it is at preventing the occurrence of blue-green algal blooms. In the first case, the design criteria is to ensure that the downwards transport of oxygen is sufficient to meet the in situ demand (which will increase if the temperature increases) whereas the latter case generally requires production of a deeper SML. Producing a deeper SML is a much more challenging problem, especially for storages > 10–20 GL in volume, because SML dynamics are much more sensitive to local climatic conditions. Fortunately, the physics of artificial destratification is well understood and there are several numerical models that can be employed to simulate the performance of the method for any particular reservoir.
6.2.3 Influence of Lake Size and Shape
The size and shape of the lake or reservoir may determine the type and number of mixing devices required to achieve a specific rehabilitation or water quality goal. Lakes with low shoreline to surface area ratios, i.e. lakes tending towards a circular shape, will generally be easier to mix than lakes with high shoreline to surface area ratios such as elongated drowned river valleys with side arms. Thus, small ‘round’ ponds (<1 ha) may be efficiently mixed with a single centrally located mixing device, such as motor driven pumps and fountains, mechanical paddles, air lift bubble plumes and solar-powered mixing devices. Water depth influences the efficacy of some mixing techniques, some are designed specifically for shallow water and others work best in deep water.
In contrast, larger lakes and lakes with complex basin morphometry, including elongated side arms, may require several mixing devices to achieve a particular level of mixing. There will be an upper limit to the size of the lake that it is possible to artificially mix cost effectively. Consequently, the economic returns of having a recreational facility provided by the lake should also be considered. An example of a large reservoir (72 m deep, surface area 622 ha or 6.22 km2 and capacity 139 million m3) that has successfully been mixed using aeration is El Capitan, a water supply reservoir for San Diego city in California (Fast 1968). This reservoir is elongated and is >3.5 times larger than the largest reservoir in Auckland city’s water supply network (Mangatawhiri Reservoir). Part of the economic benefits in El Capitan include recreational amenities such as sailing, water skiing, kayaking, fishing and swimming that offset the aeration costs.
6.2.4 Destratification and Aeration Mechanisms
There are several options for lake mixing (e.g. Singleton and Little 2006a, b) and destratification (Feature Box 6.2). Most of these either use air-lift techniques or propellers to induce vertical mixing currents to the surface or water jets to carry surface water down into the lake. In addition, there are several options for aerating or oxygenating the hypolimnion without mixing and destratification that have also been used. The first option we discuss is the Bubble-Plume Diffuser and its operation in non-stratified and stratified lake conditions.
Box 6.2 Artificial Destratification, Surface Mixers and Hypolimnetic Oxygenation
Justin D. Brookes Bas W. Ibelings grid.1010.0 0000 0004 1936 7304 The University of Adelaide Adelaide SA Australia grid.8591.5 0000 0001 2322 4988 Université de Genève Genève SwitzerlandThe thermal or density stratification that results from differential heating of surface waters can often lead to the deterioration of water quality. As water at the surface is heated, it becomes less dense (more buoyant) and floats on the cooler denser water below. A diurnally stratified lake or reservoir is typically characterised by a surface layer that stratifies during daytime and mixes nocturnally with convection as heat is lost to the atmosphere. A seasonally stratified lake or reservoir typically has a metalimnion that marks the zone of sharp temperature change between the epilimnion (surface water) and the hypolimnion (cooler bottom water) and persists over some months.
The bloom-forming cyanobacteria tend to dominate phytoplankton communities when lakes or reservoirs are stratified as they contain gas vesicles which provide buoyancy and overcome losses from sedimentation. Under these conditions of relative water column stability, algae that have a density greater than water, such as diatoms, will tend to settle out. Buoyant cyanobacteria, however, will float up and concentrate their biomass in the illuminated, near-surface mixed layer. The degree of mixing, therefore, plays a key role in determining phytoplankton competition and succession (Visser et al. 2015).
Density stratification within a lake or reservoir indicates there is no or restricted vertical mixing, limiting gas exchange with the atmosphere. Microbial activity in the sediments, termed the sediment oxygen demand, can utilise the available oxygen, significantly reducing the dissolved oxygen concentrations. This can lead to hypoxic or anoxic conditions in the diurnally stratified lower water layer or seasonal hypolimnion, with redox conditions that promote the flux of nutrients and metals from the bottom sediments. Reduced manganese will be released from sediments when dissolved oxygen concentrations in the overlying water are less than 4 mg L–1. Iron and phosphorus are released from the sediments at still lower oxygen concentrations. Iron and manganese are problematic as they can persist in reduced form during water treatment and cause ‘dirty water’ issues as they oxidise upon exiting the consumer’s tap. Nutrients released from the sediment are a concern as they can increase algal growth, with the cyanobacteria being of particular concern as they produce toxins and compounds that cause taste and odour issues for potable water.
Artificial destratification is a commonly used technique to disrupt or prevent the initiation or persistence of stratification, making the water column of a lake or reservoir more vulnerable to overturn from wind or convection, and increasing gas transfer to the hypolimnion. There are two distinct types of devices used for artificial destratification: bubble-plume aerators and surface mounted mechanical mixers. Bubble-plume aerators disrupt the stratification (Fig. 6.3) and transport gas and heat deeper in the water column. There are many examples of successful bubble-plume aerators. In Chaffey Dam (Australia), the years when the bubble-plume aerator was operated had considerably lower phosphorus concentrations in the hypolimnion than in years when the aerator was not operated (Sherman et al. 2000).
Artificial destratifiers are often deployed to control cyanobacteria. The theory is that phytoplankton will be mixed deeper in the water column and so become light limited. Furthermore, in a fully mixed water column, the advantage of cyanobacteria buoyancy is nullified. It is evident that stratification still occurs outside of the immediate influence of the bubble plume. Visser et al. (1996) demonstrated that Microcystis aeruginosa colonies remained positively buoyant close to a bubble plume in Lake Nieuwe Meer (The Netherlands) as the mean light that colonies experienced was low due to deep mixing and photosynthate (sugar) accumulation insufficient to overcome the buoyancy provided by gas vesicles. In contrast, Microcystis colonies in the more stratified regions further away from the aerator cells were not fully mixed, floated up and experienced higher mean light near the surface. Artificial mixing to control cyanobacteria is not always successful and should be carefully designed, taking into account key characteristics of the lake—like average and maximum depth—and characteristics of the dominant cyanobacteria—like size and flotation velocity (Visser et al. 2015).
The fact that intense temperature stratification can occur in the surface layers and support cyanobacterial populations, even when bubble-plume aerators are used, prompted trials using surface mounted mechanical mixers. These consist of large impellers that draw water through a draft tube in either an upward or downward flow configuration (see Fig. 6.4). Lewis et al. (2010) measured flow in a downward impeller configuration and showed that a small temperature difference between the surface water and the water at the depth of exit meant that the warmer buoyant water returned rapidly to the surface and mixing with the adjacent water was minimal. While the energy use of these systems is considerably less than bubble-plume aerators, the mixing is inefficient and the surface mounted mixers need considerably more maintenance. Small solar-powered mixers, for example, are ineffective at mixing lakes and have an extremely small zone of influence (Upadhay et al. 2013).
Artificial destratification not only mixes dissolved gas that diffuses into the sediment, but also transfers heat. The warmer temperatures may stimulate microbial activity, which will increase oxygen demand and may also increase the bottom-sediment contaminant fluxes if oxygen is in short supply. Hypolimnetic oxygenation is a possible alternative to artificial destratification as a method to limit metal and nutrient fluxes from sediment. A major benefit is that oxygen is delivered to the bottom waters and diffuses to the bottom sediment, but the lake is not destratified and so a cooler hypolimnion is maintained. Hypolimnetic oxygenation is becoming more widely used and may present a sound alterative to artificial destratification as the climate warms and vertical density stratification increases. It has less potential, however, to specifically remove the physical conditions that promote buoyant colonial cyanobacteria.
6.2.4.1 Bubble-Plume Diffuser
The simplest aerator is the bubble-plume system where compressed air is blown through pipes to one or more diffusers on the lake bed. The design of the diffuser produces a rising column of air bubbles which entrains bottom lake water into the plume and induces a vertical current in the water column (Schladow 1993). The depth of the air outlet is important—the greater the depth, the more efficient the mixing (Cooke et al. 1993).
A common misconception for rising air bubble plumes is that aeration is achieved by the dissolution of the oxygen from the bubbles into the water. In practice, this is only a small component of oxygen transfer. The major transfer of oxygen into water occurs at the lake surface when the oxygen-depleted water is brought into contact with air. Air bubble plumes are produced by forcing compressed air to the bottom of a lake or reservoir and allowing it to escape from a diffuser. A compressed air flow rate of 9.2 m3 min–1 km–2 lake area should be sufficient to achieve adequate surface re-aeration in most lakes (Lorenzen and Fast 1977).
For some reservoirs, it is common to use an aeration sparge line design where a long diffuser is attached to a weighted cable (ballast rope) deployed in a straight line along the deepest part of the water body and attached to an anchor block at each end. The length of the diffuser depends on the size of the water body and is designed to provide sufficient air lift in the bubble plume to mix the water body (Fig. 6.5).
Schematic diagram of a bottom mounted tubular aerator system. The length of the sparge pipe is typically 50–100 m and is positioned in the deepest part of the reservoir near the dam. The ballast rope is anchored at both ends and orientated along the deep axis of the reservoir so that it lies on top of the bed. The ballast rope can be 32 mm trawler rope or a similar sized plastic sheathed multi-core steel cable
The diffuser design (sparge pipe, Fig. 6.5) comprises a series of small 0.5–1 mm diameter holes (ports) through the top or sides of an Alkathene pipe attached to the ballast rope by tethers to keep it above the sediment (Fig. 6.6a). Because Alkathene is less dense than water, it will float and the tether length determines how high it will be above the sediment. The spacing between diffuser ports in the sparge pipe should be 0.1–0.2 times the depth of water above the pipe, e.g. in 30 m water depth the spacing would be around 3 m but in practice the maximum spacing is 300–600 mm apart, allowing the formation of a coherent bubble curtain.
In the example shown (Fig. 6.6a, cross section (left) and transverse section (right)), the sparge pipe is tethered about 1 m above the ballast rope. The end of the pipe is sealed to allow compressed air pressure inside the pipe to be forced air through the diffuser ports to form the bubble plume.
Also attached to the ballast rope directly is a buoyancy pipe which has an open end. In operation, this buoyancy pipe is full of water and is held down by the ballast rope. For servicing and maintenance, air is pumped into the buoyancy pipe and it will lift the ballast rope and sparge pipe to the surface. To allow the aeration system to settle back to the bottom, water is pumped through the buoyancy pipe to flush out the air. The valve manifold used at Opuha Reservoir near Timaru is shown in Fig. 6.7a.
There are several different designs for a sparge pipe diffuser. One alternative is to have the holes through the side of the pipe (Fig. 6.6b), so that there is always additional residual buoyancy due to air trapped in the upper part of the pipe when the system is not in use.
It is not necessary to empty the reservoir to install a sparge line aeration system. With the sparge line system assembled, it can be lain across the surface of the water body and the ends attached to the anchor blocks with sufficient slack to account for the water depth and bottom topography. Then the buoyancy tube is pumped full of water and the ballast rope will carry the assembly to the lake bed. Diver or drop-camera inspection is recommended to allow correct adjustment of the ballast rope length at the non-manifold end.
The size or length of the bubble plume required to mix a water body depends on the size of the water body and on the degree and strength of stratification. They all operate by the same principles but non-stratified and stratified lakes are discussed separately below.
6.2.4.2 Bubble-Plume Diffuser in a Non-stratified Water Body
Bubble-plume aerators operate by pumping air through a diffuser (sparge pipe) near the bottom of the reservoir. As the small bubbles rise to the surface, they entrain water and a rising plume of bubbles develops (Fig. 6.8a). As this plume rises to the surface the bubbles expand and entrain more ambient water into the plume and the volume of water in the plume increases. At the surface, the bubbles dissipate into the air and water from the plume spreads across the surface absorbing oxygen from the air before plunging back to a level of equivalent density. If the water column is almost isothermal (uniform temperature at all depths) and hence non-stratified, the water will continue moving horizontally away from the plume until it meets the edge of the lake, a barrier, or it gets caught in the downwards-moving water replacing the water entrained into the bubble plume. This flow pattern is similar to a conveyer belt (Fig. 6.9a) and will maintain vertical mixing in the water body (e.g. Fig. 6.1a). This flow pattern means the orientation of the aerator sparge pipe is important and can be used to focus the energy of the aerator along the axis of the lake or reservoir to best advantage.
Stylised representation of (a) the ‘conveyer-belt’ circulation pattern in an unstratified reservoir. Aeration occurs mostly in the indirect mixing zone. In (b) circulation pattern develops in a thermally stratified reservoir when the aeration system is first turned on. Nutrients from below the thermocline will be entrained by the rising plume and will stimulate algal growth in the upper water column. Because it may take 20–30 days of continuous aeration to mix the water body, there is sufficient time to develop an algal bloom, which may spread throughout the lake (redrawn from Gibbs and Hickey 2012)
The vertical flux (amount of water moved) in the plume is found by solving differential equations describing conservation of flux and momentum for vertical velocity and plume radius (Fig. 6.8b). Once established, the ‘conveyer-belt’ circulation can be maintained with less energy than was required to establish it, due to the hydraulic inertia of the water in the circulation current. Consequently, it is possible to turn the aerator system off for a period of several days and then back on for at least 2 days. For example, a 4-day-off/2-day-on cycle currently works well in the Auckland water supply reservoirs (observations from NIWA practical advice to Watercare Services Ltd for their reservoir management.) This mode of operation reduces running costs. The timing of this strategy is given in Sect. 6.2.5.
If the water column is non-stratified and relatively well mixed, the time to set up the conveyer-belt circulation pattern may be 5–10 days. It will take longer if the water column is thermally stratified.
6.2.4.3 Bubble-Plume Diffuser in a Stratified Water Body
If the water column is thermally stratified, a detrainment point (Schladow and Fisher 1995) will develop about the thermocline. This results in an intrusion current which will propagate horizontally away from the aerator plume at about the depth of neutral buoyancy generating a separate circulation current in the hypolimnion mirroring the circulation current in the upper water column (Fig. 6.10). As the intrusion moves through the reservoir, there is a return flow above and below the intrusion and these circulation cells may cause mixing between the surface layer and the hypolimnion and reduce the thickness of the thermocline. Given sufficient time and sufficient air, typically 20–30 days, the thermocline may disappear and the reservoir would then become fully mixed. Until full mixing is achieved, this indirect mixing circulation in a stratified lake is less efficient than direct mixing circulation in a non-stratified lake.
Bubble-plume representation in a thermally stratified water body. Detrained water leaves the bubble plume at the thermocline, reducing the overall efficiency of mixing (stylised image redrawn from Kirke 2000)
6.2.5 Morphology and Timing
The operation of a bubble-plume diffuser in a stratified lake requires consideration of: (a) the morphology of the lake, (b) the amount of air required, and (c) the timing of the aeration process.
-
(a)
The morphology (size and shape) of the lake is important in determining the efficacy of the mixing process in reservoirs and lakes in drowned river valleys. These valleys often have elongated side arms that are not fully or even partially mixed by the circulation currents from the main aeration system. The main consequence of having incomplete mixing or residual thermal stratification in the elongated side arms is that these locations may have smaller wind fetches than the main body of the lake or reservoir. Small fetches allow for calmer conditions that encourage the development of surface-forming algal or cyanobacterial blooms. To mitigate this problem, the side arms may require additional mixing devices installed.
-
(b)
The amount of air required is a function of the size of the waterbody to be mixed. Commercially available bubble-plume aerators delivering around 4.7 L s–1, which is 10 cubic feet per minute (cfm), are suitable for smaller lakes and medium-sized ponds. Using the rule of thumb for calculating compressed air flow rate requirements of 9.2 m3 min–1 km–2 lake area (Lorenzen and Fast 1977), the 4.7 L s–1 device would mix a water body with a lake area of around 3 ha. A flow rate of 9.2 m3 min–1 is equivalent to 153 L s–1, which is suitable for a lake of 100 ha. The actual air flow rate used in any particular lake will depend on the depth of the lake and the size of the compressor available which can exceed the hydrostatic pressure associated with that depth, i.e. at 40 m depth the hydrostatic pressure is 4 atmospheres or 3.86 bar. As a general rule, a small over capacity is better than not enough air. However, while there is merit in having over capacity, too much air may prevent stable bubble formation resulting in fizzing where the bubbles are too small to have effective lifting power and the mixing will be slower. Installation of a longer sparge line or additional sparge lines is more appropriate for larger lakes and reservoirs, e.g. El Capitan (Fast 1968).
-
(c)
The timing for turning on aeration is important (Chowdhury et al. 2014). Early aeration in a large reservoir, just before stratification establishes, may be a way to develop the conveyer-belt circulation pattern with minimal energy expenditure. However, if the aeration is too early, it may not be cost-effective and aeration that is too late, after the lake has thermally stratified and the hypolimnion has become oxygen depleted, will require considerably more energy than if started at the optimum time. Note that, if aeration is started after nutrients have accumulated in the hypolimnion, these may be dispersed into the upper water column with the unintended consequence of stimulating algal growth and potential for producing cyanobacteria blooms (Fig. 6.9b).
An example of how timing affects a water supply reservoir is shown in Fig. 6.11. A sparge pipe aerator system was installed in the Nihotupu reservoir at the end of 1999 and it was turned on whenever the reservoir showed thermal stratification and hypolimnetic deoxygenation (i.e. the conditions illustrated in Fig. 6.9b).
Time-series data from Lower Nihotupu reservoir in Waitakere Ranges, Auckland, showing surface and bottom temperatures, turn-on points for aeration (red arrows) and the cyanobacteria species abundance in cell ml–1. The horizontal line at 1000 cells ml–1 represents the drinking water standard for planktonic cyanobacterial cells. From 2005 to 2006, the aeration system was run continuously (data from Watercare Services Ltd.)
This mode of operation is referred to as ‘turn-on by calendar date’. In 2006, data from temperature/DO profiles and the timing guidelines (see Sect. 6.2.6) were used to inform aeration turn-on points. Although this reservoir is notorious for developing algal proliferations, the results were immediate and the cyanobacteria blooms disappeared (Fig. 6.12). The short-term exceptions in 2013 and 2014 occurred when there was a failure of the compressor during a hot calm period.
6.2.6 Aeration Protocols
When using temperature and DO data to control aeration, there is a recommended procedure to ensure aeration is turned on at the appropriate times ensuring cost-effective management (revised from Gibbs and Hickey 2012).
Oxygen concentrations and/or surface-to-bottom temperature differences are used as the triggers for the different stages for activating the aerator through spring and summer. An appropriate procedure is as follows:
-
1.
Temperature low and vertical profile uniform with DO near 100% saturation throughout: Aerator off; monitor monthly. (This represents the winter mixed condition.)
-
2.
Temperature higher by >1°C in surface than bottom water with DO in bottom water below 100% saturation but >8 g m–3: Aerator off; monitor fortnightly. (The lake is developing a weak thermal stratification.)
-
3.
Temperature higher by >1°C in surface than bottom water with DO in bottom water between 7 and 8 g m–3: Aerator off; monitor weekly. (The lake is thermally stratified and developing bottom water oxygen depletion but DO is above the action aeration threshold.) A caveat for this case is that if the temperature rises to >2°C warmer in surface than bottom water with DO in the bottom water <8 g m–3 but above 7 g m–3 (i.e. there is strong thermal stratification), then remedial action is required: Aerator on continuously; monitor weekly.
-
4.
Temperature higher by >1°C in surface than bottom water, with DO in bottom water <7 g m–3: Aerator on continuously; monitor weekly. (With DO > 6 g m–3, the aerator can rapidly mix the water column causing re-aeration to occur and this level is above the minimum of 5 g m–3 required for most fish species.)
-
5.
Temperature high and vertical profile uniform with DO concentrations uniform and >7 g m–3: Aerator cycled at 2 days on and 2 days off; monitor weekly. (The lake is mixed and aeration is in maintenance mode. The most appropriate cycle can be determined experimentally—if 2-days off does not cause a measurable decrease in the DO concentration and the development of thermal stratification in the water column, the off period can be increased by a day—if it does, then the off period should be reduced by a day. Minimum on period is 2 days and this can be increased if required to maintain a mixed water column and DO concentrations >7 g m–3.)
-
6.
Temperature lower than previous measurement and profile uniform with DO concentrations uniform and >7 g m–3: Aerator off; monitor weekly for a month then monthly. (This represents the autumn cooling phase of the annual cycle. The monitoring is continued at weekly intervals for a month after switch-off to ensure that the falling water temperature was not just a transient change in late summer.)
The bottom line is that if DO falls below the threshold of 7 g m–3 at any time of year, the aerator should be turned on until the threshold is exceeded again.
6.3 Mechanical Mechanisms
Where incomplete mixing or residual thermal stratification in elongated side arms of lakes and reservoirs occurs, separate and independent mixing devices may need to be installed at one or more locations within these side arms. There are a range of options for these smaller mixing devices, other than bubble-plume diffusers, that can be used to mix smaller lakes and ponds, as well as the side arms of large lakes.
These include mechanical mechanisms such as: low energy mixing impellors that draw bottom water up or forces surface water down through constraining draft tubes; high energy pumps that jet the water down at an angle, entraining air into the jet; and the classic vertical aeration pumps and fast splashing paddles used to aerate waste water treatment plant ponds. These latter are not discussed here.
6.3.1 Solar-Powered Water Mixers
Because lake side arms are sometimes remote and may be difficult to provide power to compressors, there is an opportunity to use solar-powered water mixer (SWM) devices (Upadhay et al. 2013). These devices are cost-effective where the cost of installing electricity to the remote site is greater than the cost of the SWM. The SWM is typically floated on the surface of the lake, with a low energy solar-powered electric motor turning the impellors. There are also mains-powered versions.
A commonly used SWM is the SolarBee® which floats on the lake surface and draws water up through a constraining tube, or ‘draft’ tube, and discharges it across the surface of the lake. The operating principle is similar to an air-lift bubble plume but without the bubbles. The main difference is that, because the propeller that moves the water is at the surface, a draft tube is required to determine the depth from which the water is drawn up. Typically, the draft tube is adjustable and can be positioned to draw water from almost any depth in the waterbody. Draft tubes range from 0.2 to 0.9 m diameter and have a maximum draw depth of 18–30 m (depending on the model). They are fitted with a base plate that only allows water entry horizontally thereby preventing scouring of the lake bed, if the draft tube intake has been positioned near the lake bed or allowing entry of water from a specific depth in the water column.
In operation, the SolarBee® draws deep water horizontally into the bottom of the draft tube, pulls it up the centre of the draft tube and discharges it across the surface of the reservoir, thereby generating a circulation flow pattern similar to the bubble-plume ‘conveyer belt’ current. This circulation pattern is claimed to be able to move from 284 to 2272 m3 h–1 and can slowly destratify a thermally stratified lake with an area of up to 14 ha. The mean water depth and thus the water volume are likely to be crucial design factors.
If the reservoir is thermally stratified, the bottom of the device can be set below the thermocline. The colder bottom water then mixes with warmer surface water as it is discharged and reoxygenated. This results in water of an intermediate temperature and density, which remains in the upper water column at a depth of equal density. By increasing the amount of water above the thermocline in this way, the thermocline is displaced downwards and will eventually reach the lake bed or the draw depth of the SWM device. Consequently, destratification is caused by downwards depression of the thermocline. If the bottom waters are anoxic and nutrient enriched, this option is not recommended.
Mixing processes with SWM devices are slow but efficient as the battery-powered motor runs continuously and the device keeps the circulation pattern operating. It works well in smaller ponds and lakes and should perform well in the confined shallow side arms of reservoirs. In larger ponds and lakes, several SWM devices may be required although a single unit may be sufficient to destratify a narrow side arm.
However, because the SWM is a low energy device, the destratification system using these devices must be designed to have sufficient water transfer to exceed the re-stratifying effects of solar heating. A significant problem with artificial mixing will arise if insufficient energy is used and the waterbody fails to mix within the time taken for natural re-stratification. This could be exacerbated by an inappropriately positioned mixing device that fails to deliver appropriate circulation. For example, if the lake or reservoir has become thermally stratified and the draft tube is set below the thermocline, the low energy SWM device will lift the nutrients accumulating in the hypolimnion and disperse these through the upper water column thereby stimulating algal growth. Alternatively, if the draft tube is set above the thermocline, the mixing currents developed will erode the top of the thermocline. If the velocity and turbulence of the mixing current are great enough compared with the strength and depth of the thermocline, the entire thermocline may eventually be eroded. In contrast, for weak currents only limited erosion of the thermocline would occur, possibly creating even sharper temperature gradients at the base of the mixing layer. Because sharp temperature gradients inhibit vertical turbulence, this would create greater resistance to further mixing (Kortmann et al. 1982). The latter outcome may be more likely for SWM devices.
The thicker the metalimnion, the shallower the gradients of temperature, oxygen and nutrients. This would facilitate the transfer heat and dissolved oxygen down through the water column and nutrients up from the hypolimnion. Conversely, the thinner the metalimnion and the stronger the temperature gradient, the harder it is for these transfer processes to occur, and the greater the resistance to mixing. Note that these gradients may also be affected by the concentration gradient.
6.3.2 Constrained Bubble Plumes
These devices use an air-lift bubble plume instead of the propeller inside the draft tube to induce upwards entrainment of bottom water. They have the same limitations as the SWM but can have an advantage where the compressed air supply provides greater mixing energy than the SWM propeller. However, in a situation where there is insufficient energy provided by the mixing device to mix the whole waterbody, the re-aerated bottom water may settle on or above the thermocline and move away from the mixing device as an intrusion layer.
An example of this occurred in Lake Rotoehu (an 8 km2 lake with multiple side arms) where an experimental air-lift bubble-plume design was being tested. The design comprised a large diameter (~3 m) tubular structure (mixing engine) (Fig. 6.13), which constrained the bubble plume causing the bubbles to draw water from below the thermocline in a horizontal current caused by the base plate and discharging them horizontally below the lake surface from the 90-degree bend at the top.
(a) Schematic of the draft tube and mixing engine (provided by Hans Burggraaf) showing the relative deployment position below the lake surface and the water flow directions, (b) a photo of the draft tube and mixing engine (on its side) being loaded onto a barge for deployment. For size perspective, the black floats are 900 mm diameter mussel buoys (photo by Andy Bruere, Bay of Plenty Regional Council)
In Lake Rotoehu, three mixing engines were deployed together upright, in a clover leaf pattern around a central axis, with the outflow openings pointing horizontally out into the lake at 120° from each neighbour. This produced a triangular flow pattern across the lake surface for a short distance from the mixing engines.
To determine the efficacy of the mixing engines, Rhodamine WT dye was injected into the bottom of one rising bubble plume and the path of the dye leaving the mixing engine was tracked using a fluorimeter. These results were collated with concentration and contoured to produce a visual representation of the water movement from the mixing engines (Fig. 6.14). The horizontal flow plume was lost at about 100 m from the mixing engine but was still detected by a bottom mounted acoustic doppler current profiler. The results imply that, to mix a large lake such as Lake Rotoehu with this design of mixing engine, there would be a requirement for multiple mixing engines set probably about 200–300 m apart all using the same amount of air. This would be consistent with the use of multiple aerator/oxygenation devices in larger lakes and reservoirs overseas (e.g. Beutel and Horne 1999).
Contour plot of the dye plume from the mixing engine in Lake Rotoehu using Surfer32 by Golden Software. Vertical lines are profiling points. Extrapolation between points used triangulation with a ratio of 12:1. The contour plot is a representation of the likely dye flow path based on the available data (redrawn from McBride et al. 2015)
The data from the Lake Rotoehu dye study (Fig. 6.14) indicated that the exit velocity from the mixing engine was insufficient to produce a strong lateral plume away from the mixing engine, and, therefore, there was insufficient mixing with warmer surface water to raise the temperature of the exit plume. Consequently, the exit water plunged within 10–15 m from the mixing engine and was entrained back through the thermocline to be drawn back into the mixing engine again. The vertical profiles through the dye plume showed a sharp cut off at the thermocline with no dye found below the thermocline away from the mixing engine (Fig. 6.14). This is consistent with a sharp thermocline at the time of the experiment. This phenomenon would be less likely to occur in a deeper lake where the added depth would allow the water velocity in the draft tube to increase and produce a stronger plume out of the exit tube.
By way of explanation, as the bubbles expand, they increase in cross-sectional area and volume. An increase in depth from 10 m (1 atmosphere pressure) to 20 m (2 atmospheres pressure) will require double the compressed air pressure to the diffuser to get the same initial bubble plume. This means the bubbles will increase in volume by a factor of four relative to the 10-m level and become even larger as they approach the surface. The original volume of water entering the draft tube remains unchanged and no water can be entrained through the sides of the tube. Because the volume (water plus air) is increasing inside a confining tube, the velocity must increase inside the draft tube towards the surface, i.e. a 20 m draft tube will have a faster exit velocity than a 10 m draft tube, and a 40 m draft tube should be faster still.
6.4 Selective Draw-Induced Stratification
A phenomenon that can be associated with constrained upwards circulation devices is the generation of a sharp artificial ‘metalimnion’ at the depth of the intake. With a base plate fitted, the draw is horizontal and confined to the gap between the base plate and the draft tube. If the water column is thermally stratified at the level of the draw, then upward movement of denser water below that depth will be suppressed and water entering the tube will tend to come from a horizontal layer at the level of the draw. As this water is removed, lighter, warmer water from higher levels in the water column will drop down to replace it, creating a temperature discontinuity at the level of the draw. The deeper water then becomes isolated and does not mix. That water will have the same characteristics as the hypolimnion of a thermally stratified waterbody.
This phenomenon, called draw-induced stratification (Fig. 6.15), is seen in reservoirs without mixing/destratification devices but where the offtake water is drawn constantly from the same depth (selective withdrawal). Selective withdrawal is commonly used for managing the quality of water discharged from a reservoir (Çalışkan and Elçi 2009).
Stylised diagram of a reservoir with three offtake depths. Continuous draw from a mid-depth in (a) causes stagnation or dead volume in the water below the draw depth because there is no mechanism for mixing oxygen below this depth. This would also happen if there was continuous draw from the lowest depth in (b), but then the dead volume would be minimal (redrawn from Gibbs and Hickey 2012)
6.4.1 Case Study: Upper Huia Reservoir
While selective draw-induced stratification might seem to be a problem, it can be used to advantage where there is no artificial mixing device available. A management strategy for selective withdrawal was developed on the upper Huia reservoir by Spigel and Ogilvie (1985) and demonstrates this. They examined the effect of prolonged draw from the mid-water column offtake valve over 4 years from 1980 to 1984. Spigel and Ogilvie (1985) found that continuous selective withdrawal from a fixed depth-induced density stratification at that depth. Essentially, water from below that depth was not drawn while the water above that depth was and became progressively warmer as the lake level fell bringing the warmer surface water closer to the draw depth. The resulting density stratification produced the same geochemical effects as normal thermal stratification, with water below the offtake valve depth becoming anoxic and nutrients and minerals released from the sediments accumulated in high concentrations (Fig. 6.16; years 1980–82).
Time series results of bottom water (a) temperature, (b) dissolved oxygen, (c) total manganese (Mn), (d) total iron (Fe) in the Upper Huia reservoir from 1980 to 1984. For explanation, see text (graph redrawn from Spigel and Ogilvie 1985)
From 1980 to 1983, the draw depth was the middle offtake valve at a depth of 17 m. The data (years 1980–1982) show slight warming in the bottom water through the summer and autumn as well as oxygen depletion beginning as early August/September (Fig. 6.16). As oxygen depletion progressed to a DO concentration of around 6 g m–3, manganese insoluble oxides began to reduce from manganic form (Mn3+) to manganous form (Mn2+), which is soluble, and Total Manganese concentrations began to rise (Fig. 6.16). As oxygen depletion progressed to a DO concentration of around 2 g m–3, iron in insoluble oxides began to reduce from ferric form (Fe3+) to ferrous form (Fe2+), which is soluble, and total iron concentrations began to rise (Fig. 6.16). These changes were consistent with the oxygen driven processes shown in Fig. 6.2.
Shifting the draw depth to the bottom offtake valve at a depth of 29 m in 1984 caused the water column above the draw depth to remain oxygenated and eliminated the mineral release from the sediments (Fig. 6.16; years 1983–4). This technique was used until summer 2000 when aerators were installed in the reservoirs. The disadvantage of destratifying and mixing a reservoir is that the whole water column warms to near-surface temperature (Fig. 6.16; years 1983–4 in temperature graph) thereby removing the cold-water refuge required for some aquatic biota and fish as well as the ability of the water supply company to provide a cold water supply to domestic and industrial clients. In addition, the warmer water increases the oxygen demand, and a balance between in-situ demand and supply from the atmosphere is needed. Where these effects pose a serious problem, techniques have been developed for oxygenating the hypolimnion without destratification, a process known as hypolimnetic aeration.
6.5 Oxygenation of the Hypolimnion Without Mixing
Where the cold bottom water layer in a lake or reservoir is important, i.e. needed as a refuge for some fish species or a town cold water supply, special aeration equipment that aerates just the hypolimnion is used (Beutel and Horne 1999). There are two main types of hypolimnetic aeration (Fig. 6.17):
-
1.
The equipment is mounted in the hypolimnion so that it aerates the hypolimnion only but has a small vent tube extending to the surface. The entrained water with dissolved oxygen is dispersed back into the hypolimnion below the thermocline without breaking the thermal stratification. Any bubbles rising from the aerator are directed to the surface through the vent tube (Fig. 6.17).
-
2.
The equipment is mounted at the surface and incorporates a return tube to carry the oxygenated water back down to the hypolimnion before it is dispersed. There are many designs for this ‘full-lift’ type hypolimnetic aeration system (e.g. Ashley 2000).
In both of these systems, aeration with pure oxygen is more effective than compressed air, which has only 20% oxygen. Using pure oxygen raises the running costs.
In both systems, if they are used in deep lakes, the aerator component needs to be a heavy walled tube to prevent ambient lake water pressure crushing the tube. Air bubbles inside the tube occupy some of the volume thereby reducing water density. Consequently, the pressure inside the tube is less than outside. Placement of the hypolimnetic aerator should be central at the deepest part of the lake. That will allow water to be drawn into the aerator from all directions and be dispersed back into the hypolimnion in all directions.
Much research has been undertaken on hypolimnetic aeration systems overseas and many devices have been designed (Prepas and Burke 1997; Lindeschmidt and Hamblin 1997; Ashley and Nordin 1999; Singleton and Little 2006a, b; Ashley et al. 2008, 2009, 2014; Barber et al. 2015) and reviewed (Beutel and Horne 1999; Gafsi et al. 2009; Ashley 2000). However, while hypolimnetic aeration works, it is more expensive to operate than most other systems for re-oxygenating the hypolimnion of a lake or reservoir. The major cost is equipment that is needed to confine the air or oxygen and prevent it from destratifying the lake. Few lakes in New Zealand would require this type of mixing device.
The main advantage of hypolimnetic aeration is that, because the hypolimnetic water is returned to the hypolimnion, there is no nutrient enrichment of the epilimnion and the hypolimnion is mixed and oxygenated but is not warmed. This provides a safe, cool refuge for fish species such a salmonids, which prefer temperatures below 22 °C. As most fish species can tolerate DO concentrations down to about 5 g m–3, it is not necessary to fully oxygenate the hypolimnion provided that this level of re-oxygenation is achieved. As stated earlier, a DO concentration of 5 g m–3 and above is also sufficient to control the release of minerals (Mn and Fe) and associated P from the sediments (Pakhomova et al. 2007; Hupfer and Lewandowski 2008).
Other hypolimnetic oxygenation system options include injection of micro oxygen bubbles or liquid oxygen directly into the water at the bottom (Beutel and Horne 1999). Applied at the correct rate, pure oxygen dissolves in the water without developing a bubble plume that would destratify the lake. This was the concept behind the original oxygen injection system (Speece et al. 1973).
More recently, a line diffuser system has been developed where pure oxygen is delivered to the hypolimnion through a porous hose (Gerling et al. 2014). The line diffuser system uses side stream supersaturation (SSS) and is designed to deliver large quantities of oxygen with maximum oxygen transfer efficiency through diffuser lines that may be 1–2 km long. The SSS system is claimed to have increased hypolimnetic dissolved oxygen concentrations at a rate of ~1 g m–3 week–1 without weakening stratification or warming the sediments. A nano-bubble technique is also being developed but has not been published yet.
6.6 Down-Flow Mixing Devices
Most of the devices discussed above for lake water quality improvement involve the vertical movement of water upwards. There are also a range of mixing devices that move water downwards. These include pump systems, which jet the water down through the thermocline (Fast and Lorenzen 1976; Lorenzen and Fast 1977; Gu and Stefan 1990; Cooke et al. 1993; Kirke 2000; Michele and Michele 2002). Such mechanical mixing devices can be small high speed pumps as used in oxidation ponds through to large diameter (>5 m in width), low speed and low energy impeller fans, with or without draft tubes, designed to push warmer surface water down through the thermocline.
These downward mixing devices work well in shallow waterbodies, but their operation works against the intrinsic buoyancy of warm water. Consequently, even though they entrain water in much the same way as a bubble plume, the plume may not extend to the lake bottom, whereas a bottom mounted bubble plume will always entrain hypolimnetic water up to the surface. If used when the lake is strongly stratified, the buoyant plume from a down-flow mixing device will return to the surface close to the intake and will be recycled, together with any nutrients entrained in the returning water. Consequently, these devices are best used in a pre-emptive mode where they maintain the waterbody in a mixed state before it begins to thermally stratify. In this way, they can be used to prevent thermal stratification developing if activated early in spring (e.g. Chowdhury et al. 2014; see also Sects. 6.2.5 and 6.2.6). In practice, these devices are used more to improve water quality by controlling algal blooms than to destratify or aerate a lake.
6.7 Computer Modelling
If the mixing device is too small to mix the whole lake water body then several devices may be needed. The efficacy of a mixing device can be simulated in a lake hydrodynamics model such as DYRESM (Dynamic Reservoir Simulation Model—Centre for inland Waters, University of Western Australia) (Moshfeghi et al. 2005), which will allow an aeration system to be designed for the specific water body. An air bubble destratification sub-model of DYRESM can be used to derive the design specifications of a bubbler system, and DYRESM can be run to predict the effect of bubbler on the reservoir destratification. The design of the aeration system requires information on a number of parameters, which can be provided from routine monitoring programmes or the use of high frequency data collection used in lake monitoring buoys (Read et al. 2011).
6.8 Aeration Case Studies
Aeration can be used in the restoration of degraded lakes by accelerating decomposition processes that reduce the organic content of the sediment. Over time (i.e. several years), continuous aeration during summer has been shown to reduce the sediment oxygen demand and eliminate periods of anoxia that would normally occur during summer stratification without aeration (Grochowska and Gawrońska 2004). Here, we discuss two contrasting New Zealand case studies where aeration has made significant improvements to lake quality: Opuha Dam and Virginia Lake.
6.8.1 Opuha Dam
The Opuha River flows into the Opihi River which is used for irrigation. Because water flows in the Opihi River system are unreliable in summer, local interests combined to construct the Opuha water supply scheme to provide water for urban consumption in Timaru and for farm irrigation. The scheme relies on the fact that the largest water flows into the Opuha River catchment occur during winter and early spring. This water could be stored in a reservoir and used to achieve more reliable monthly environmental flows and to satisfy the high water demand for irrigation in late spring and summer. Although the Opuha Dam and reservoir was originally conceived as an irrigation scheme for water-short areas of South Canterbury, the economics of the scheme as a stand-alone urban water supply/irrigation proposal were marginal so to ensure a more viable scheme, a power station was added, to utilise the energy available in the head of water held by the dam.
6.8.1.1 Background
The Opuha dam is an earth dam (see Fig. 6.7) constructed at the confluence of the North and South Opuha Rivers in South Canterbury. The lake behind the dam is 3.3 km long, has an area of 710 ha, a maximum depth of about 40 m and a storage capacity of 91 million m3. The off take is at a depth of 30 m, 10 m above the lake bed, discharging water through the power station at a constant 16 m3 s–1, when operating. The power station is used for peak power generation in the morning and evening and is off during the middle of the day and at night, a total of about 10 h day–1, producing unstable flows in the Opuha River downstream.
About a kilometre below the dam is a weir, which is designed to act as a regulation pond that is used to balance the intermittent discharge flow from the power station. This produces a less variable discharge of water released into the Opuha River throughout the day for irrigation and environmental flow downstream of the dam. All water for irrigation must pass through the power station and is drawn from the Opuha River below the lower weir.
The scheme is subject to constraints, in respect of minimum water flows down the Opuha and Opihi Rivers and the use of water stored behind the dam, by conditions applying to the granting of consents under the Resource Management Act (RMA) 1991. The RMA consents require that the Opuha scheme must be managed to ensure (in the following order of priority):
-
A minimum water flow downstream of the dam weir of 1.5 m3 s–1 (to ensure satisfactory conditions for fish life in the Opuha River).
-
A minimum specified environmental flow in the Opihi River at Pleasant Point (varying from 3.5 to 8.5 m3 s–1 specified for particular months) which may require the release of water from the dam and which requires the continuous calculation of a river system flow balance for the Te Ngawai, Opihi and Opuha Rivers matched with offtakes for irrigation both upstream and downstream of Pleasant Point and urban water supply for Timaru.
-
The rate of filling of the dam is maximised by restricting winter electricity generation to achieving only the minimum flows above, to ensure that it is full at 1 October each year, the start of the drought season.
Only after meeting the consent requirements can Opuha Water Limited manage water flows from the dam.
6.8.1.2 Water Quality Problems
The water quality in the lake initially deteriorated, with high dissolved colour, high nutrient concentrations and no oxygen in the bottom waters in summer (Hawes and Spigel 1999; Meredith 1999). Consequently, water released from the lake in summer was adversely impacting on the downstream river environment. Subsequent formal review of dam consent conditions required installation of continuous monitoring of stratification, deoxygenation and operation of an in lake aeration system. These were operational by 2001, and the degraded water quality conditions have been avoided and effectively managed since.
Excessive nuisance growths of algal mats in the Opuha River below the downstream weir have been a reoccurring problem since the dam was commissioned. These have included prolific growths of toxic cyanobacteria mats and later the introduced algae Didymosphenia geminata. These growths have also proliferated in reaches of the Opihi River below the confluence with the Opuha River (Lessard et al. 2013) and result from excessive flow regulation, a lack of ‘flushing flows’ and an armoured or embedded river bed material. There is on-going examination of mechanisms to address these issues, including elements of redesign of the downstream weir.
6.8.1.3 Design Issues
There are four major design issues with the Opuha Dam:
-
1.
Having a single offtake valve near the bottom of the lake. This restricts the quality of the water being discharged to the worst possible quality in the hypolimnion, when the lake is stratified, without the option of discharging cleaner oxygenated water from higher up the water column (Lessard et al. 2013).
-
2.
It is primarily an irrigation dam, not a hydroelectric reservoir. This means that, although the outlet valve always discharges at 16 m3 s–1 because it flows through the turbine, it only operates for brief periods of time. Consequently, the deep offtake, which the designers anticipated would act as a deep withdrawal system to entrain the oxygenated surface waters with that flow (see Sect. 6.4), has little effect on the lake water quality. This management strategy is only effective where there is continuous flow. With an area of 710 ha, the short duration flow of 16 m3 s–1 is unlikely to have a substantial effect on the oxygen levels in the deep water basin near the dam wall.
-
3.
Stratification. Large deep reservoirs such as Opuha Dam are likely to thermally stratify due to solar heating in summer and deoxygenate such that active lake water quality management systems need to be built into the design. Primary consideration should be given to installing an aeration system to keep the water column mixed during summer and thus prevent bottom water anoxia developing. Stratification in a lake is not always driven by solar heating. If the inflow streams are colder than the main body of the lake, they can form density currents that flow down the lake bed and form a cold water layer at the bottom of the lake. This will initially be well-oxygenated water but will rapidly lose that oxygen if the density current has a high biochemical oxygen demand (BOD).
In Lake Opuha, the two sub-alpine river inflows are almost always colder than the lake and always form density currents. They are also highly turbid after rainfall events and, therefore, have high BOD loads. Because of the location of the offtake, the turbid water overwhelms the offtake and turbid water is discharged from the power station into the downstream environment.
-
4.
Flushing flows. There is no requirement for periodic discharge of flushing flows from the dam to maintain downstream river habitats. Allowing only regulated low flows from the downstream weir eliminates flushing flows and has generated the problems of regulated flow downstream (nuisance algae, armoured bed etc.). Flushing flows ‘rumble’ the river bed stones, sloughing off periphyton and redistributing fine sediment rather than letting it consolidate and smother benthic invertebrate habitats between and beneath stones.
6.8.1.4 Mitigation Measures
After initial problems of deoxygenation in the hypolimnion were identified, an aeration system was installed in Opuha Dam (illustrated in Fig. 6.7). Ideally, the offtake system should have multiple valves at different levels to allow management of the water quality being discharged to the downstream environment. This requires a water quality monitoring programme to provide the information needed to allow adaptive management of the discharge water quality. A tower used to raise and lower the control plug on the offtake valve has been fitted with monitoring instruments to provide information for adaptive management of the lake.
A bubble-plume aeration system was installed in Opuha Dam in October 2003 using air supplied with a 11.3 m3 min–1 (400 cfm) compressor. This has the theoretical capacity to mix a lake with a surface area of 1.22 km2 based on the 9.2 m3 min–1 km–2 lake area guideline of Lorenzen and Fast (1977). The design of this aeration system has been described above (Figs. 6.6 and 6.7). Although the engineers have been enthusiastic about the results, they acknowledge that they may have overstated the case and the rate of aeration is slow. Actual data indicates that a period of 14 days aeration increased the DO saturation concentration by 14%.
Overall, operation of the aeration system in Opuha reservoir has been successful, and the incidence of thermal stratification with concomitant anoxic events is rare rather than common. A larger capacity air compressor system is being installed to provide un-interrupted aeration during the summer months, and the aeration mixing protocols are proactive using the monitoring data for management decision making.
6.8.2 Virginia Lake
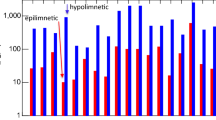
6.8.2.1 Background
Virginia Lake in Whanganui was known to Māori as Rotokawau and was an important eeling reserve. Kawau, the name of the black shag, refers to the large number of shags that used to frequent the lake. Europeans settled around the lake in the 1850s, and the Borough Council bought the lake in 1874 as a water supply reservoir. A dam and offtake structure controlled the water level in the lake, which was a spring-fed with high quality water. It was fashionable to have a house overlooking the lake, and the native evergreen vegetation was replaced with deciduous European trees. The gardens developed around the lake form a popular recreational park where visitors can picnic and feed the multitude of ducks, geese and swans.
Virginia Lake has an area of 4.5 ha, a volume of 540,000 m3, a maximum depth of around 20 m and a residence time of around 1.6 years. The outflow is via a surface skimmer drop shaft at the offtake structure and this serves to control the lake level. The lake thermally stratifies at a depth of about 6 m and only mixes briefly due to the shelter provided by the tall trees around the lake shore.
6.8.2.2 Water Quality Problems
Virginia Lake thermally stratifies in winter (Fig. 6.18) and the hypolimnion becomes oxygen depleted to the point of anoxia in spring (Gibbs 2004). Phosphate and ammonium released from the sediments drive the algal blooms in spring. The intensity of the algal bloom reduces the nutrient concentrations in the surface waters as photosynthesis causes oxygen super-saturation and high pH in the epilimnion as CO2 and bicarbonate are removed from the water. With pH values above 9.2, on occasion, concentrations of ammonia develop to toxic levels (MfE 2014). When the algal bloom is comprised of cyanobacteria, it will drift inshore and the ammonia may result in fish kills. As the cyanobacteria senesce, they may also release toxins.
Temperature structure in the water column at the critical winter mixing time. Each line represents the temperature at a specific depth. The water column is mixed when all the temperature lines merge into one. In this 2007 data set, the lake was mixed for 8 days (data from Colin Hovey, Whanganui District Council)
Winter mixing in 2007 occurred just before the 30 July profile indicating some re-oxygenation of the hypolimnion before it became anoxic again by 20 September. A cyanobacteria bloom in September caused DO super-saturation of the surface waters. Photosynthesis by cyanobacteria removed the carbonate and bicarbonate from the upper water column causing the pH to rise above 9.2, a level where ammoniacal nitrogen in the water column is in the form of ammonia (NH3) which is toxic to aquatic biota including fish.
There are several factors that have combined to cause the water quality problems in Virginia Lake. These include:
-
Changing the vegetation from evergreen natives to deciduous exotic species has deposited a large amount of leaf carbon in the lake as a pulse in autumn, which may be one of the drivers of sediment oxygen demand as it decomposes. The very short mixing period in winter may not provide sufficient re-oxygenation of the bottom water to balance this seasonal input.
-
Development of housing serviced by septic tanks in close proximity to the lake has contaminated the surface groundwater aquifer with nutrient-rich leachate (e.g. Gibbs 1977a, b) which can enter the lake.
-
Introduction of geese and swans to the lake has destroyed the marginal vegetation that would otherwise act as a buffer zone to remove the nutrients from the groundwater. Without the buffer zones shore line erosion is occurring, reducing the clarity of the lake through increased suspended solids.
-
The introduction of coarse fish (pest species) will be impacting the regrowth of these buffer zone plants.
-
Feeding of large numbers of ducks by the public introduces large amounts of bread, which settles to the sediments either directly or via faecal material from the water fowl and adds to the sediment oxygen demand. It also adds to the nutrient load on the lake. For example, Burns and Singleton (1994) determined that the TP input from ducks represented 34% of the annual external load on 54 ha Lake Rotoroa, Hamilton.
-
Draining storm water away from the lake and having a surface outflow has reduced the flushing rate.
6.8.2.3 Design Issues and Mitigation Measures
The lake has a water fountain which is likely to degas the surface water and reduce the super-saturation slightly. A Solar Bee mixer has also been used as a mitigation measure. While this appeared to reduce the extent of the algal bloom, the water column profiles suggest that it may have sharpened the thermocline reducing the diffusive nutrient flux from the hypolimnion.
Restoration action for Virginia Lake could include
-
Aeration from near the bottom to keep the lake fully mixed throughout the year. Turn-on would need to coincide with the brief period of winter mixing to reduce the energy otherwise required to break the thermal stratification. At that time, it would be unlikely to alter the magnitude of the spring algal bloom that occurs after mixing. By pumping air through the aeration mixer, the water column would have more CO2 which would tend to reduce the pH below 9, thereby eliminating the production of toxic ammonia. Because the water column would be fully mixed, cyanobacteria would be less likely to become dominant and the magnitude of algal growth could be reduced due to critical depth (see Sect. 6.1.2.3) effects.
-
Increase bottom water exchange. In the absence of an aeration system, the outflow could be engineered to include a hypolimnetic siphon (Fig. 6.19) which would discharge the anoxic, nutrient-rich bottom water from the lake instead of the nutrient-depleted aerated surface water. The outflow water would need to be treated to introduce oxygen and reduce the nutrient concentrations that would otherwise impact the downstream environment.
Fig. 6.19 Schematic of a hypolimnetic siphon for Virginia Lake. This uses the existing offtake drop shaft outflow pipes but has an intake at the bottom of the lake. The open head tube sets the water level so that bottom water is drawn continuously. The height of the head tube could be set at the maximum lake level under heavy rain events to prevent flooding
-
The diversion/piping of additional clean water into the lake as a surface inflow would enhance the flushing via a hypolimnetic syphon.
-
Reducing road runoff would reduce the nutrient load on the lake.
-
Reducing the numbers of geese and swans on the lake would allow the riparian buffer zones to re-establish and would reduce the nutrient load from groundwater on the lake. Even though the area now has reticulated waste water, the historical legacy of septic tank effluent will still be travelling in the groundwater to the lake, along with more recent nutrients from lawn and garden fertilisers.
-
Educate the public that feeding the ducks so much bread is adversely affecting the lake water quality.
-
The sediments could be treated to reduce nutrient release. Sediment capping to retain the P in the sediment has the potential to reduce the abundance of algae and dominance of cyanobacteria due to P-limitation.
Virginia Lake is a classic example of how a lake (natural or artificial) can become degraded through small apparently innocuous changes in the lake catchment. It is also a prime example of where an aeration/destratification system would make an immediate improvement to the lake water quality. The Whanganui District Council observes in their Lake Management Plan (WDC 2009) that ‘the water quality of Virginia Lake has been improved since the installation of a Solar Bee lake aeration device in December 2007’. However, ‘whilst there has been a considerable reduction in the amount of toxic blue-green algae (cyanobacteria) blooms, rich algae populations are still present in the Lake’ (WDC 2009).
6.9 Future Developments
Techniques for destratification and mixing of lakes to manage water quality have improved rapidly over the last few decades as the processes driving deoxygenation and lake degradation become better understood. The rehabilitation potential of traditional and new engineering devices needs to be tempered with the need to meet the requirements of the end user of the water, be it the biota living in the lake or the external users of the lake water for drinking, irrigation or recreation. The bottom line is that, whatever device is used, it must be fit-for-purpose.
Key points to consider will include:
-
Size and depth: the mechanical devices need to match the size of the lake and problem. Insufficient mixing power may exacerbate the problem while too much power is a waste of energy and may cause enhanced turbidity. There will be an upper limit to the size of lake that can be mixed economically.
-
The end use of the water should be decided as part of the engineering design phase. It may not be necessary to fully re-oxygenate a lake to achieve a water quality that meets the requirement for fish, irrigation or many recreational pursuits. Conversely, full mixing and oxygenation will be required for municipal drinking water supplies.
-
Understanding the seasonal cycle and succession of phytoplankton species will enable an aeration system to be activated pro-actively to prevent cyanobacteria growth and proliferation. Getting the timing correct by using feedback from monitoring programmes is a relatively new concept that has proved very effective in medium to large water supply lakes.
-
Using multiple mechanical devices to mix larger lakes is a possibility that needs further investigation.
-
Combinations of different mechanical devices may be needed to mix lakes with complex morphology, and solar-powered devices with storage batteries will improve with the rapidly advancing technologies in these areas.
-
Pre-emptive mixing is likely to be more cost effective than destratification.
-
Destratifying a lake to begin the rehabilitation process can have unexpected short-term effects. These should be discussed with a competent lake water quality person before any action is taken.
-
Modelling should be used as a tool to check whether the equipment suggested for a specific restoration project is fit-for-purpose, i.e. is it feasible that it will perform as anticipated or could it have unexpected results.
It is likely that future developments in this area for lake restoration will be a closely coordinated combination of engineering and ecological approaches.
References
Ashley K (2000) Recent advances in hypolimnetic aeration design. Verh Internat Verein Limnol 27:2256–2260
Ashley K, Nordin R (1999) Lake aeration in British Columbia: applications and experiences. In: Murphy T, Munawar M (eds) Aquatic restoration in Canada, Ecovision world monograph series. Backhuys, Leiden, pp 87–108
Ashley KI, Mavinic DS, Hall KJ (2008) Oxygenation performance of a laboratory-scale Speece Cone hypolimnetic aerator: preliminary assessment. Can J Civil Eng 35:663–675
Ashley KI, Mavinic DS, Hall KJ (2009) Effect of orifice diameter, depth of air injection, and air flow rate on oxygen transfer in a pilot-scale, full lift, hypolimnetic aerator. Can J Civil Eng 36:137–147
Ashley K, Fattah K, Mavinic D, Kosari S (2014) Analysis of design factors influencing the oxygen transfer of a pilot-scale Speece Cone hypolimnetic aerator. J Environ Eng 140:04013011
Barber T, Ashley K, Mavinic D, Christison K (2015) Superoxygenation: analysis of oxygen transfer design parameters using high-purity oxygen and a pressurized column. Can J Civ Eng 42:737–746
Beutel MW, Horne AJ (1999) A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv Manag 15:285–297
BOPRC (2008) Ohau Channel diversion wall. Information sheet prepared by Bay of Plenty Regional Council. https://www.boprc.govt.nz/environment/water/rotorua-lakes/ohau-channel-diversion-wall/. Accessed 8 Sep 2018
Burns NM, Singleton A (1994) An estimated phosphorus budget for Lake Rotoroa. NIWA consultancy report number HCC004 to Hamilton City Council. National Institute of Water and Atmospheric Research, Hamilton, New Zealand
Çalışkan A, Elçi Ş (2009) Effects of selective withdrawal on hydrodynamics of a stratified reservoir. Water Resour Manag 23:1257–1273
Chowdhury AS, Hasan K, Alam K (2014) The use of an aeration system to prevent thermal stratification of water bodies: pond, lake and water supply reservoir. Appl Ecol Environ Sci 2:1–7
Cooke DG, Welch EB, Peterson S, Newroth PR (1993) Restoration and management of lakes and reservoirs, 3rd edn. Taylor & Francis, CRC Press, London
Fast AW (1968) Artificial destratification of El Capitan Reservoir by aeration. Part I: effects on chemical and physical parameters. State of California, the Resources Agency, Department of Fish and Game. Fish Bull 141:98p
Fast AW, Lorenzen MW (1976) Synoptic survey of hypolimnetic aeration. J Environ Eng Div 102:1161–1173
Gafsi M, Kettab A, Saadia Benmamar S, Benziada S (2009) Comparative studies of the different mechanical oxygenation systems used in the restoration of lakes and reservoirs. J Food Agric Environ 7:815–822
Gerling AB, Browne RG, Gantzer PA, Mobley MH, Little JC, Carey CC (2014) First report of the successful operation of a side stream supersaturation hypolimnetic oxygenation system in a eutrophic, shallow reservoir. Water Res 67:129–143
Gibbs MM (1977a) Soil renovation of effluent from a septic tank on a lake shore. N Z J Sci 20:255–263
Gibbs MM (1977b) Study of septic tank system on a lake shore: temperature and effluent flow patterns. N Z J Sci 20:55–61
Gibbs M (2004) Rotokawau-Virginia lake study: a preliminary assessment. NIWA Client report HAM2004-112 to Wanganui Water Services. National Institute of Water and Atmospheric Research, Hamilton, New Zealand
Gibbs M, Hickey C (2012) Guidelines for artificial lakes: before construction, maintenance of new lakes, rehabilitation of degraded lakes. NIWA report HAM2011-045 prepared for Ministry of Science and Innovation, National Institute of Water and Atmospheric Research, 157 pp. http://www.envirolink.govt.nz/assets/Envirolink/Guidelines-for-artificial-lakes.pdf. Accessed 8 Sep 2018
Gibbs M, Abell J, Hamilton D (2016) Wind forced circulation and sediment disturbance in a temperate lake. N Z J Mar Freshwater Res 50:209–227
Grochowska J, Gawrońska H (2004) Restoration effectiveness of a degraded lake using multi-year artificial aeration. Pol J Environ Stud 13:671-681
Gu R, Stefan HG (1990) Jet mixing in lake or reservoir stratification simulations. Lake Reserv Manag 6:165–174
Hawes I, Spigel R (1999) Report on water quality in the Opuha Dam. NIWA consultants report CHC99/12 to the Opuha Dam Company. National Institute of Water and Atmospheric Research, Christchurch, New Zealand
Hupfer M, Lewandowski J (2008) Oxygen controls the phosphorus release from lake sediments—a long-lasting paradigm in limnology. Int Rev Hydrobiol 93:415–432
Kirke BK (2000) Circulation, destratification, mixing and aeration: why and how? Water, July/August, 24–30
Kortmann RW, Henry DD, Kuether A, Kaufman S (1982) Epilimnetic nutrient loading by metalimnetic erosion and resultant algal responses in Lake Waramaug, Connecticut. Hydrobiologia 91:501–510
Kumagai M (1988) Predictive model for resuspension and deposition of bottom sediment in a lake. Jap J Limnol 49:185–200
Lessard J, Hicks DM, Snelder TH, Arscott DB, Larned ST, Booker D, Suren AM (2013) Dam design can impede adaptive management of environmental flows: a case study from the Opuha Dam, New Zealand. Environ Manag 51:459–473
Lewis DM, Lambert MF, Burch MD, Brookes JD (2010) Field measurements of mean velocity characteristics of a large-diameter swirling jet. J Hydraul Eng ASCE 136:642–650
Lindeschmidt K-E, Hamblin PF (1997) Hypolimnetic aeration in Lake Tegel, Berlin. Water Res 31:1619–1628
Lorenzen MW, Fast R (1977) A guide to aeration/circulation techniques for lake management, Res Ser EPA-600/3-77-004. U.S. Environment Protection Agency, Washington, DC
Lossow K, Gawrońska H, Jaszczułt R (1998) Attempts to use wind energy for artificial destratification of Lake Starodworskie. Pol J Environ Stud 7:221–227
Martinez LE (1995) Métodos para oxigenación de embalses de centrales hidroeléctricas. BSc thesis, Faculty of Engineering, National University Autonomous of México, Mexico City, Mexico
McAuliffe TF, Rosich RS (1989) Review of artificial destratification of water storages in Australia. Urban Water Research Association of Australia, Melbourne, Australia, 233pp
McBride CG, Tempero GW, Hamilton DP et al (2015) Ecological effects of artificial mixing in Lake Rotoehu. University of Waikato, Environmental Research Institute report 59 prepared for Bay of Plenty Regional Council. University of Waikato, Hamilton, New Zealand
Meredith AS (1999) Water quality of Lake Opuha: limnological issues in a newly formed lake. Environment Canterbury Unpublished Report, Christchuch, New Zealand
MfE (2014) National Policy Statement for Freshwater Management 2014 (see National Objective Framework (NOF) attribute for ammonia nitrogen). Ministry for the Environment, Wellington, New Zealand, 34p. http://www.mfe.govt.nz/publications/rma/nps-freshwater-management-2014/nps-freshwater-management-jul-14.pdf
Michele J, Michele V (2002) The free jet as a means to improve water quality: destratification and oxygen enrichment. Limnologica 32:329–337
Moshfeghi H, Etemad-Shahidi A, Imberger J (2005) Modelling of bubble plume destratification using DYRESM. J Water Supply Res Technol—AQUA 54:37–46
Pakhomova SV, Hall POJ, Kononets MY et al (2007) Fluxes of iron and manganese across the sediment-water interface under various redox conditions. Mar Chem 107:319–331
Prepas EE, Burke JM (1997) Effects of hypolimnetic oxygenation on water quality in Amisk Lake, Alberta, a deep, eutrophic lake with high internal phosphorus loading rates. Can J Fish Aquat Sci 54:2111–2120
Read JS, Hamilton DP, Jones ID, Muraoka K, Winslow LA, Kroiss R, Wu CH, Gaiser E (2011) Derivation of lake mixing and stratification indices from high-resolution lake buoy data. Environ Model Softw 26:1325–1336
Schladow SG (1993) Lake destratification by bubble plume systems: a design methodology. ASCE J Hydraul Eng 119:350–368
Schladow SG, Fisher IH (1995) The physical response of temperate lakes to artificial destratification. Limnol Oceanogr 40:359–373
Sherman BS, Whittington J, Oliver RL (2000) The impact of destratification on water quality in Chaffey Dam. Arch Hydrobiol 55:15–29
Singleton VL, Little JC (2006a) Designing hypolimnetic aeration and oxygenation systems—a review. Environ Sci Technol 40:7512–7520
Singleton VL, Little JC (2006b) Designing hypolimnetic aeration and oxygenation systems—a review. Supporting information: early design studies, nomenclature, tables, figures, and literature cited. Environ Sci Technol 40:S1–S18
Speece RE, Rayyan F, Murfee G (1973) Alternative considerations in the oxygenation of reservoir discharges and rivers. In: Speece RE, Malina JF Jr (eds) Applications of commercial oxygen to water and wastewater systems. Center for Research in Water Resources, Austin, TX, pp 342–361
Spigel RH, Ogilvie DJ (1985) Importance of selective withdrawal in reservoirs with short residence times: a case study. In: Proceedings of the 21st congress of the International Association for Hydraulic Research, Melbourne, 19–23 August 1985, National Conference Publication no. 85/13, vol 2. The Institution of Engineers, Australia, pp 275–279
Sverdrup HU (1953) On conditions for the vernal blooming of phytoplankton. Journal du Conseil International pour l’Exploration de la Mer 18:287–295
Upadhay S, Little J, Elam K, Bierlan K, Burch MD, Brookes JD (2013) Mixing generated by Solarbee mixer and its impact on the phytoplankton. Ecol Eng 61:245–250
US Department of Agriculture (1999) A procedure to estimate the response of aquatic systems to changes in phosphorus and nitrogen inputs. United States Department of Agriculture, Natural Resources Conservation Service, Washington, DC
Visser PM, Ibelings BW, van der Veer B, Koedoods J, Mur LR (1996) Artificial mixing prevents nuisance blooms of the cyanobacterium Microcystis in Lake Nieuwe Meer, The Netherlands. Freshwater Biol 36:435–450
Visser PM, Ibelings BW, Bormans M, Huisman J (2015) Artificial mixing to control cyanobacterial blooms: a review. Aquat Ecol 50:423–441
WDC (2009) Virginia Lake management plan: vision, objectives and policies. Wanganui District Council, Wanganui, New Zealand
Wedderburn EM (1912) Temperature observations in Loch Earn with a further contribution to the hydrodynamical theory of temperature seiches. Trans R Soc Edin 48:629–695
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Gibbs, M.M., Howard-Williams, C. (2018). Physical Processes for In-Lake Restoration: Destratification and Mixing. In: Hamilton, D., Collier, K., Quinn, J., Howard-Williams, C. (eds) Lake Restoration Handbook. Springer, Cham. https://doi.org/10.1007/978-3-319-93043-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-93043-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93042-8
Online ISBN: 978-3-319-93043-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)