Abstract
The adrenal glands are frequently the site of metastasis from several different types of cancers, including lung, breast, melanoma, renal cell, and colon. Traditionally, the finding of adrenal metastasis was believed to portend end-stage disease and consequently surgery was rarely performed. Since the introduction of laparoscopic adrenalectomy in 1992, resection for isolated adrenal metastases is being reported with increasing frequency, and several authors have even reported improved outcomes and survival in selected patients. Presently the evidence for this recommendation is based solely on published anecdotal reports and retrospective series. Hence prospective studies are desperately needed so that formal guidelines can be established in the decision-making process for patients with adrenal metastases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Adrenal metastases
- Oligometastases
- Stereotactic ablative body radiotherapy
- Adrenalectomy
- Laparoscopic adrenalectomy
- Radiofrequency ablation
Introduction
In the past, most if not all of the evidence reporting metastasis to the adrenal glands have come from autopsy series. A review of the literature in the first half of the twentieth century has documented the presence of metastatic lesions to the adrenal glands from almost all malignant epithelial tumors. All of these studies showed the metastatic potential of these invasive cancers and how they were able to establish a tumor “niche” in this small retroperitoneal endocrine gland [1, 2].
However, all of this did not provide any pathophysiologic dynamic that would help relate this phenomenon to the natural history of metastases to the adrenal glands [3]. The incidence of metastases to the adrenal gland is only second to the presence of non-functioning adrenal adenomas found at autopsy. Unraveling this problem had to await the imaging revolution in the last quarter of the twentieth century. The key was to be found in modern technology—the CT scan, the MRI and the PET scan [4].
With the recent emphasis on cancer surveillance, modern imaging methods have revealed the surprising fact that there is an increasing incidence of both synchronous and metachronous lesions in the adrenal gland of cancer patients. Modern radiological modalities (CT, MRI, PET scans) allow for early detection of isolated metastatic lesions to the adrenal glands. In view of the above, the surgeon is now confronted with a new clinical conundrum, what to do with the incidental adrenal nodule in a patient with a history of prior malignancy [4]?
The decision to operate on patients with disseminated metastatic disease is not an easy one. If one limits the discussion to adrenal metastasis only, whether the incidental nodule be a synchronous or metachronous lesion , it is becoming apparent that certain options are available for these patients. For patients with disseminated metastatic disease, surgery is generally not a viable option. In a subset of patients that have only a single metastatic lesion to the adrenal gland, then surgery is a viable option. Modern imaging has identified a new entity, whereby the primary cancer is in a state between locoregional extension and disseminated disease. This phase of the cancer is referred to as the stage of oligometastasis, corresponding to 1–5 macroscopic lesions. The surgical oncology literature has reported long disease-free survival following resection of isolated metastatic lesions to the adrenals [5]. Although there is no evidence that resection of isolated metastatic lesions offers any survival benefit compared to observation alone, the National Comprehensive Cancer Network (NCCN) already recommends resection of oligometastases. What data support these recommendations [6]? (Table 32.1).
Methods
Search Strategy
A systematic literature search was performed for all articles published relating to the management of adrenal gland metastases. We searched bibliographic databases (MEDLINE, EMBASE, Cochrane Collaboration, PubMed) as well as conference proceedings, using electronic search terms and keywords: adrenal gland metastases, adrenal neoplasms, catheter ablation, laparoscopy, adrenalectomy (resection, surgery, surgical). Total retrieval within each database was 78 articles in MEDLINE, 112 articles in EMBASE and 1 in the Cochrane Library. The search was limited to papers published in English, involving adult subjects (18+ years of age) and relevant articles from a 10-year period up to and including January 2015. Studies were initially screened for relevance based on title and abstract. All studies deemed relevant that met the study inclusion criteria were retained, totaling 53 articles.
Characterization of Adrenal Gland Metastasis
Incidence
The incidence of adrenal metastases is difficult to determine because most adrenal metastases are discovered at autopsy. In a retrospective series that followed patients for 30 years, 94% of adrenal metastases were discovered at autopsy (435/464), while only 4.3% (20/464) were symptomatic from their disease. Amongst those patients presenting with metastatic disease to the adrenal gland, 49% had bilateral metastases. In addition, approximately two-thirds of patients presented with synchronous disease, while one-third presented as metachronous disease, with a median time to presentation of 7 months [7].
Prevalence
The prevalence of adrenal gland metastases is quite variable. The largest study to date is from 1950, deriving data from 1000 autopsies performed on patients diagnosed with an epithelial carcinoma. The likelihood of finding adrenal metastases was 27% in that study. However, the prevalence of adrenal metastases is difficult to define as it depends on the population of patients that are studied [1].
Etiology
Historically, autopsy series found that adrenal metastases arise most commonly from the lung, breast, kidney, gastro-intestinal tract and skin (melanoma), with lung cancer representing up to 39% of cases and breast cancer up to 35% of cases [1, 2, 7]. One thousand autopsies were performed on patients who died from a variety of epithelial malignancies. In those 1000 patients, 270 presented with metastases to the adrenal gland (27%), while other autopsy series report lower rates of adrenal gland metastases (8.6%) [2]. Out of the 270 patients, 90 were from breast cancer, 57 were from lung cancer, 25 from gastric cancer and 17 from colon cancer. It is of interest to note that adrenal metastases occurred in 57% (90/167) of breast cancer patients, 33% of lung cancer patients (57/160), 21% of gastric cancer patients (25/119) and 15% of colon cancer patients (17/117) in this autopsy series from the 1950s. The etiology of adrenal gland metastases is reflective of the era of cancer therapeutics as the rate of adrenal gland metastases from breast cancer is now rare (<3%) [8]. Potential bias may exist in studies like these, as autopsies were performed on the first consecutive 1000 patients from a cancer center, suggesting that the sample may not be representative from a general population diagnosed with cancer. Given that all of these patients died from diffuse metastatic disease, one can ask if these metastatic findings are clinically relevant. In addition, the pathological techniques for detecting metastases (like immunohistochemistry) were not as developed in the 1950s. Finally melanoma, known to be a common primary that metastasizes to the adrenal gland, was not included in this study [1]. Furthermore, a review of 2833 autopsies reported by Bullock et al., showed different results in which the overall rate of adrenal metastases was 8.6% (244/2833) compared to 27% in the Abrams study. The most noticeable differences in prevalence of metastases were related to breast and gastric cancers. Abrams reported 53% and 21%, whereas Bullock showed a prevalence of 12.8% and 4.7%, respectively. Bullock was the first to report on metastatic adrenal lesions in melanoma in 10 of 32 cases. The results of these two historical studies are quite different therefore making it difficult to establish a precise prevalence of adrenal metastases. Still, these reports set the general rule for the average probability of metastases to the adrenal glands [2]. Finally, the anatomic site of the primary cancer that leads to adrenal metastases differs depending on geographic location. In comparison to the above-mentioned North American series, a study from Hong Kong revealed that the majority of adrenal metastasis came from the lung (149/421, 35%), followed by the stomach (60/421, 14.3%), oesophagus (51/421 12.1%), liver/bile duct cancer (45/421, 10.7%) pancreas, colon, kidney and breast. Hence, the pattern of adrenal metastatic disease seems to be influenced by geographic location [7].
There are three distinct patient presentations. The first presentation is when patients with a prior history of cancer are discovered to have a metachronous lesion in the adrenal gland during the postoperative surveillance period. Lenert et al. studied this population and found that 42 of 81 patients (52%) presented with adrenal metastases related to their primary cancer [9]. The prevalence rate appears to be high, and the authors do suggest that this may represent an overestimate of the real prevalence of adrenal gland metastases due to the fact that they excluded those patients discovered to have benign lesions from the analysis. In addition, this study was conducted over a 30-year period dating back to the 1970s. This represents another confounding variable, as considerable improvements with radiological imaging have been able to distinguish a benign from a likely malignant adrenal mass. As such, benign lesions would be underrepresented, leading to an overestimation of malignant lesions in this patient population. Therefore, the overall risk of an adrenal metastasis in a patient with a proven cancer is likely below 50%.
The second presentation is when the patient with a highly suspected cancer (or a proven cancer) is found with a synchronous adrenal lesion during cancer staging. The management of these patients will depend on the extent of the metastatic burden. Resection could be proposed if the adrenal gland is the only site of metastasis [10].
Finally the third presentation can be defined as a patient with an incidentally discovered adrenal lesion in the context of an unknown primary cancer. In this population, is it useful to perform a fine needle aspiration of the adrenal mass in order to diagnose the unknown primary malignancy? Lee et al. have studied this question by analyzing 1715 cases with unknown primary cancers and found only four patients (0.2%) whereby the adrenal incidentaloma uncovered the nature of the primary cancer. However, these four cases were clinically symptomatic due to the size of the adrenal lesion (>6 cm). This suggests that for asymptomatic incidentalomas, screening for an unknown primary extra-adrenal malignancy is not necessary [11].
Defining the Extent of the Disease in the Context of Adrenal Metastasis
Solitary Metastases
A solitary metastasis represents a rare occurrence of a single and isolated metastatic lesion to the adrenal gland from an occult or known primary malignancy.
Oligometastases
Hellman and Weichselbaum defined the term oligometastases in reference to an intermediary state between locoregional and disseminated metastatic disease, defined as the existence of one to five isolated macroscopic metastases [12].
Diffuse Metastatic Disease
This situation is most commonly seen when the patient presents with metastases in multiple organs, including the adrenal gland.
Adrenal Metastases and the “Seed and Soil” Theory
Paget’s “seed and soil” hypothesis states that the interaction between the primary cancer (seed) and its organ microenvironment (soil) influences the pattern of metastases. The microenvironment of the adrenal gland appears to have the necessary components to favor metastatic growth. The adrenal gland has an extensive blood supply, exposing this endocrine organ to a significant tumor emboli transit. In addition, the adrenal gland has a vast lymphatic network throughout the cortex and medulla. Given these anatomical features, several studies have shown a predilection for metastatic deposits as they correlate with the number of capillary and lymphatic networks (3). Currently, there has been an investigation into the use of molecular markers to help predict the likelihood that a given cancer would metastasize to the adrenal gland [13].
Radiological Imaging Characterization
CT Scan
This imaging modality is most commonly used for the identification of adrenal masses. Specifically, adrenal metastases and primary adrenal cancer contain no fat as compared to benign adrenal lesions. Studies have identified cutoff values for density being 10 Hounsfield units (HU). A value below 10 HU allows for the diagnosis of an adrenal adenoma with 95% sensitivity and 80% specificity. Another useful imaging characteristic is the contrast washout behavior. Malignant lesions have an abnormal vasculature pattern, described by a high microvascular density and a high endothelial permeability resulting in slow blood flow with accumulation of contrast material within the lesion as compared to benign adrenal nodules [14]. A 50% washout value at 10 min has a sensitivity and specificity of 100% for differentiating between benign adenomas and malignant lesions [15].
MRI
MRI imaging readily identifies the lipid rich adenoma in comparison to lipid poor lesions such as primary and metastatic adrenal lesions. MRI scans achieve 89% sensitivity and 100% specificity in differentiating between benign and malignant adrenal nodules [16].
PET-CT Scan
This method is capable of detecting neoplastic lesions of the adrenal gland. When adding a low resolution CT scan with the PET scan, sensitivity and specificity are in the range of 95% [14]. This test is only useful in tumors that are FDG avid and is useful for patients with a history of prior malignancy [17].
Biopsy of an Adrenal Mass
Biopsy of the adrenal gland is generally not recommended [11, 18]. However, those patients with indeterminate adrenal lesions on imaging that prove to be non-functional, biopsy can be useful in the right setting. When an indeterminate adrenal lesion is discovered in the context of a known extra-adrenal malignancy, adrenal biopsy does have a high sensitivity and specificity (~90–95%) [4]. Therefore adrenal biopsy can be resorted to in specific clinical situations (i.e. needing a diagnosis in the setting of diffuse metastases). However, given the current advances in adrenal imaging, biopsy is rarely indicated.
Synchronous vs. Metachronous Metastases
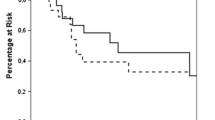
Synchronous metastases are defined as lesions appearing within 6 months of diagnosis of the primary malignancy. Metachronous metastases are lesions appearing more than 6 months following the initial diagnosis of the malignancy. There are some reports indicating that the outcomes are significantly better for patients with non-small cell lung cancer who present with resectable metachronous metastases as compared to synchronous metastatic lesions within the first 3 years. Consequently, there is some clinical relevance in determining the status of the patient with adrenal metastasis as far as non-small cell lung cancer is concerned. This is in concordance with other types of tumors such as colorectal cancer with liver metastasis, renal cell cancer with brain metastasis or non-small cell lung cancer with brain metastases. Even though some reports suggest that survival following resection of metachronous lesions is better in the short term (up to 3 years from initial diagnosis), a systematic review revealed that long-term results were similar with a 25% survival rate at 5 years for both synchronous and metachronous lesions [19, 20]. For patients presenting with bilateral adrenal metastases that undergo bilateral adrenalectomy, there is a survival benefit in a select group of patients [20]. Therefore, bilateral adrenal metastases are not an absolute contraindication to surgical resection.
Surgical and Ablative Therapies in the Treatment of Adrenal Gland Metastases
Surgical Resection
Who Are the Candidates?
First and foremost, patients must be fit for surgery in order to undergo a major abdominal organ resection under general anesthesia. Contraindications to surgery include cardiac and pulmonary comorbidities, local invasion of other organs by the tumor and disseminated metastases. Optimal control of the primary malignancy is also a prerequisite for enrolling patients for an adrenal resection [4, 10].
Solitary vs. Oligometastases vs. Diffuse Metastatic Disease
As defined earlier, a solitary adrenal metastasis corresponds to the adrenal gland being the only site of metastasis. Oligometastases is an intermediate state between loco-regional and disseminated metastatic disease, usually defined as the existence of 1–5 isolated macroscopic metastases. Diffuse metastases are when the adrenal gland is part of multiple metastatic sites. There is no report that differentiates between all these subgroups primarily because the diffuse state is always seen as a contraindication to surgery except for symptomatic palliation.
The goal of surgery depends on the differences with respect to the biology of the primary lesions as discussed in the review by Sancho et al. An illustration of resecting oligometastases can be found in non-small cell lung cancer where there is anecdotal evidence of a short-term survival benefit from adrenalectomy following resection of brain metastases [21]. On the other hand, no benefit was seen for performing adrenalectomy in the setting of metastatic melanoma if complete surgical control of the primary tumor was not possible [22]. Yet, others suggest that resection of oligometastases seem to benefit from adrenalectomy if all other metastatic sites are potentially resectable [23].
Synchronous vs. Metachronous Adrenal Metastases
The 6-month cut-off that distinguishes synchronous from metachronous metastatic lesions is important for establishing prognosis as some studies report the metachronous group fares better when compared to the synchronous group. Earlier series from MSKCC found that a disease-free interval of greater than 6 months was a predictor for improved survival [24]. However, when the MSKCC group analyzed a larger cohort of patients with metastatic adrenal lesions, the disease-free interval was no longer considered a significant predictor of survival [25]. The authors explained this discrepancy due to a short follow-up period in their initial publication. Despite this finding, many other studies found a significant difference between the synchronous and metachronous groups [19, 26]. Although there is controversy surrounding the prognostic significance of the disease-free interval, subgroup analysis of the individual primary malignancies may reveal a more accurate prognosis when accounting for tumor biology [26]. Yet a greater disease-free interval may still be viewed as a surrogate marker for a primary tumor that is less aggressive [5].
Outcomes: Morbidity of Surgery, Local Control, Overall Survival
Morbidity is inconsistently reported in the different surgical retrospective studies. An exhaustive meta-analysis reviewed 30 surgical cohorts of patients. Only 60% (18/30) reported complications in their series. From the 18 studies totaling 491 patients, there were six reported deaths. The total reported complication rate was 17% and the major complication rate was 7.5% [5].
To add to the difficulties in interpreting these studies, the local control rate was reported even less frequently. The local control rate ranged from 82.6% to 100% for a 2-year period, this being reported in only 11 studies out of the 30 cohorts included in the Gunjur meta-analysis [5].
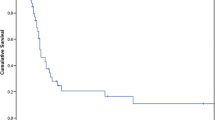
Survival rates (overall survival) varied widely between studies and the one-year survival rate was reported to range from 55% to 100%. Not surprisingly, the one-year survival rate is the lowest for non-small cell lung cancer patients and highest in the renal cell cancer patients. The rate at 5 years had similar variability, ranging from 10 to 45% [5]. Although some series report up to a 60% rate of survival at 5 years, these studies included patients who had different primary malignancies at variable stages of disease progression [10]. Therefore, it is difficult to obtain a realistic estimate of survival at 5 years. Still, it is important to note that there is the possibility of long-term survivorship. The major issue lies in patient selection. (Table 32.2).
Non Invasive Options: Curative vs Palliative
Stereotactic Ablative Body Radiotherapy and Percutaneous Catheter Ablation
The non-surgical options for treating adrenal gland metastases include ablative techniques in the form of stereotactic ablative body radiotherapy and percutaneous catheter ablation. In general, ablative techniques in metastatic disease are feasible and can be offered to a carefully select group of patients, usually after consultation with a local interdisciplinary tumor board.
The principle of stereotactic ablative body radiotherapy (SABR) is to deliver a form of external beam radiotherapy with accuracy and precision using a high dose of radiation to a given target in one or few treatment fractions [27]. This technique uses a multiple number of beams that each deliver a small dose of irradiation, but when combined will result in a much larger dose at a given focal area of treatment [5].
The percutaneous ablation techniques include radiofrequency ablation (RFA) and microwave ablation (MWA). Through image guidance, these thermal ablative techniques to the adrenal gland, can deliver thermal energy of greater than 50 °C thereby exerting cytotoxic effects by denaturing intra- and extracellular proteins leading to cell dessication and coagulative necrosis.
Nine trials were evaluated in the Gunjur meta-analysis, which totaled 178 patients. The majority of patients had lung cancer primaries (68%) [5]. Fractioned doses of radiotherapy were quite different ranging from 10 to 60 Gy with body equivalent dosing of 28 to 110 Gy. The local control rate ranged from 55% to 100% at 1 year. Overall survival was quite low, with a reported rate of 55% at 1 year to 14% at 2 years. In these studies no serious adverse events were reported. Only grade one and two toxicities were reported at a rate of 6%. It has been suggested that a total body equivalent dosage greater or equal to 100 Gy is necessary to get local control of non-small cell lung cancer [28]. Due to the lower dose of radiation used in these studies, this can explain the low complication rate as well as the low overall survival rate. The lower dose of radiation given in the majority of these studies reflects a palliative dose, thus providing an explanation for the poor local control and overall survival rates.
Outcomes: Local Control Versus Overall Survival from Surgical and Ablative Treatments
There is a paucity of data concerning the newer ablative techniques regarding local control and overall survival. The majority of outcome data were derived from surgical series that examined disease control. In a recent review of 30 retrospective studies, a total of 818 patients were evaluated [5]. The three most common malignancies were lung (non-small cell), renal cell carcinoma and melanoma. 75% of these patients presented with isolated adrenal metastases. A third of the patients underwent laparoscopic surgery despite the debate between open and minimally invasive techniques. Local control was rarely reported in these studies. The compilation of the local control data, representing a total of 93 patients (11% of the total patients), gave a local control rate of 84% at 2 years. The overall survival rate, which is the more frequently reported value, was 46% at 2 years. Of note, the follow up period for the majority of these studies was less than 2 years [5].
The data for stereotactic ablative body radiotherapy (SABR) was not as robust when compared to surgery as a treatment for adrenal metastases. A total of 178 patients from nine different studies were examined. The majority of adrenal metastases treated by SABR were from a lung cancer primary (68%) while 4% were of renal origin. Local control was reported in eight out of nine studies, with a local control rate of 63% at 2 years. The overall survival at 2 years was 19%. Although the overall survival was much lower in the SABR series, the surgery treatment group could not really be compared to the SABR group, as the clinical characteristics of these populations were not equivalent [5].
Percutaneous radiofrequency ablation or microwave ablation are other methods of local control. Only six studies with a total of 51 patients were identified. Adrenal metastasis from renal cell carcinoma was the most common primary malignancy treated (45%), while lung cancer was the second most common metastatic lesion treated (27%). Local control was only reported in one of six studies, examining only five patients. Of this small cohort, a local control rate of 80% was achieved. The overall survival rate was not reported in any of these studies [5].
Even if the populations are difficult to compare, the local control and overall survival rates seem to be highest in the surgical cohort which could be partially explained by the better overall health and performance of the surgically-treated patients (Table 32.2).
Outcomes: Morbidity from Surgery Compared with SABR and Percutaneous Ablation
Complications from each of the different modalities are inconsistently reported in the literature. The systematic review by Gunjur et al. looked at a total of 30 studies but the complications were not systematically reported. Of the studies reporting complications in the surgery cohort, a wide range of major and minor complications were recorded. Major complications included: 4 bowel perforations (0.84%) (1 gastric, 1 duodenal and 2 small bowel), 1 vena cava laceration (0.2%), 1 bronchopleural fistula (0.2%), 1 evisceration (0.2%) and 1 diaphragmatic tear (0.2%). Minor complications included 1 surgical site infection but the majority of the studies did not specify minor complications [5].
For the SABR group, complications were categorized as either acute or late toxicity. These complications were reported for all nine studies. Five studies reported no acute complications. Combining the remaining four studies, GI toxicity (grade 2) was reported in 4.5% of patients. For complications regarding late toxicity, there were 1.7% of patients with GI toxicity (grade 2), 0.5% reported fatigue (grade 2) and another 0.5% reported adrenal insufficiency (grade 2).
Overall complications were minimal when patients underwent percutaneous radiofrequency ablation. Amongst the reported complications, there were 8% hypertensive crises, 8% back pain, 4% retroperitoneal hematomas, 2% abscesses, 2% pleural effusions and 2% myocardial infarctions.
As expected, the complication rate was higher in the surgery group. The only deaths reported were in the surgical cohort representing a 1.25% mortality rate. There were more major complications in the surgical group as compared to the SABR and percutaneous ablation groups. When comparing non-surgical local control techniques, the complication rate was higher in the percutaneous ablation group as compared to the SABR group [5] (Table 32.2).
Summary Recommendations
The choice between an invasive or non-invasive approach in the treatment algorithm for adrenal gland metastases remains a challenge. This is due primarily to the lack of strong evidence in support of either surgical resection, focused ablative techniques or systemic therapies. With the majority of evidence composed of retrospective reviews and meta-analyses, it appears that surgical resection offers the best chance of improved survival when compared to other therapeutic modalities. Selection criteria for any type of adrenal-directed therapy for metastasis must ensure that the patient is fit to undergo a particular treatment. The literature suggests that surgical resection should be considered when faced with the single metachronous lesion or isolated adrenal metastasis, and for patients with resectable oligometastases. If the metastases are synchronous, unresectable or diffuse, palliative treatments should be considered, including ablative or systemic therapies, based on the origin of the primary malignancy. The best therapeutic strategy for the treatment of adrenal gland metastases is yet to be determined. Hopefully this will be based on information forthcoming from prospective trials thereby providing evidence-based guidelines for the treatment of this problem.
References
Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74–85.
Bullock WK, Hirst AE. Metastatic carcinoma of the adrenal. Am J Med Sci. 1953;226(5):521–4.
Onuigbo WI. Lymphangiogenesis may explain adrenal selectivity in lung cancer metastases. Med Hypotheses. 2010;75(2):185–6.
McLean K, Lilienfeld H, Caracciolo JT, Hoffe S, Tourtelot JB, Carter WB. Management of isolated adrenal lesions in cancer patients. Cancer Control. 2011;18(2):113–26.
Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’ - a systematic review. Cancer Treat Rev. 2014;40(7):838–46.
Kulke MH, Shah MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Cancer Netw. 2015;13(1):78–108.
Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol. 2002;56(1):95–101.
Liu XJ, Shen P, Wang XF, Sun K, Sun FF. Solitary adrenal metastasis from invasive ductal breast cancer: an uncommon finding. World J Surg Oncol. 2010;8:7.
Lenert JT, Barnett CC, Kudelka AP, Sellin RV, Gagel RF, Prieto VG, et al. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery. 2001;130(6):1060–7.
Sancho JJ, Triponez F, Montet X, Sitges-Serra A. Surgical management of adrenal metastases. Langenbeck’s Arch Surg. 2012;397(2):179–94.
Lee JE, Evans DB, Hickey RC, Sherman SI, Gagel RF, Abbruzzese MC, Abbruzzese JL. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124(6):1115–22.
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10.
Raynaud CM, Mercier O, Dartevelle P, Commo F, Olaussen KA, de Montpreville V, et al. Expression of chemokine receptor CCR6 as a molecular determinant of adrenal metastatic relapse in patients with primary lung cancer. Clin Lung Cancer. 2010;11(3):187–91.
Blake MA, Holalkere NS, Boland GW. Imaging techniques for adrenal lesion characterization. Radiol Clin N Am. 2008;46(1):65–78. vi.
Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, Raghupathi KI. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222(3):629–33.
Haider MA, Ghai S, Jhaveri K, Lockwood G. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231(3):711–6.
Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47(1):32–7.
Lee JE, Evans DB, Sherman SI, Gagel RF. Evaluation of the incidental adrenal mass. Am J Med. 1997;103(3):249–50.
Mercier O, Fadel E, de Perrot M, Mussot S, Stella F, Chapelier A, Dartevelle P. Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130(1):136–40.
Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, Bepler G. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26(7):1142–7.
Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83(2):409–17. discussion 417-8.
Haigh PI, Essner R, Wardlaw JC, Stern SL, Morton DL. Long-term survival after complete resection of melanoma metastatic to the adrenal gland. Ann Surg Oncol. 1999;6(7):633–9.
Mittendorf EA, Lim SJ, Schacherer CW, Lucci A, Cormier JN, Mansfield PF, et al. Melanoma adrenal metastasis: natural history and surgical management. Am J Surg. 2008;195(3):363–8. discussion 368-9.
Sarela AI, Murphy I, Coit DG, Conlon KC. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol. 2003;10(10):1191–6.
Strong VE, D'Angelica M, Tang L, Prete F, Gönen M, Coit D, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14(12):3392–400.
Moreno P, de la Quintana BA, Musholt TJ, Paunovic I, Puccini M, Vidal O, et al. Adrenalectomy for solid tumor metastases: results of a multicenter european study. Surgery. 2013;154(6):1215–22. discussion 1222-3.
Guckenberger M, Andratschke N, Alheit H, Holy R, Moustakis C, Nestle U, et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol. 2014;190(1):26–33.
Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a japanese multiinstitutional study. Cancer. 2004;101(7):1623–31.
Branum GD, Epstein RE, Leight GS, Seigler HF. The role of resection in the management of melanoma metastatic to the adrenal gland. Surgery. 1991;109(2):127–31.
Lo CY, van Heerden JA, Soreide JA, Grant CS, Thompson GB, Lloyd RV, Harmsen WS. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83(4):528–31.
Wade TP, Longo WE, Virgo KS, Johnson FE. A comparison of adrenalectomy with other resections for metastatic cancers. Am J Surg. 1998;175(3):183–6.
Heniford BT, Arca MJ, Walsh RM, Gill IS. Laparoscopic adrenalectomy for cancer. Semin Surg Oncol. 1999;16(4):293–306.
Harrison J, Ali A, Bonomi P, Prinz R. The role of positron emission tomography in selecting patients with metastatic cancer for adrenalectomy. Am Surg. 2000;66(5):432–6. discussion 436-7.
Bretcha-Boix P, Rami-Porta R, Mateu-Navarro M, Hoyuela-Alonso C, Marco-Molina C. Surgical treatment of lung cancer with adrenal metastasis. Lung Cancer. 2000;27(2):101–5.
Porte H, Siat J, Guibert B, Lepimpec-Barthes F, Jancovici R, Bernard A, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg. 2001;71(3):981–5.
Momoi H, Shimahara Y, Terajima H, Iimuro Y, Yamamoto N, Yamamoto Y, et al. Management of adrenal metastasis from hepatocellular carcinoma. Surg Today. 2002;32(12):1035–41.
Pfannschmidt J, Schlolaut B, Muley T, Hoffmann H, Dienemann H. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer. 2005;49(2):203–7.
Lucchi M, Dini P, Ambrogi MC, Berti P, Materazzi G, Miccoli P, Mussi A. Metachronous adrenal masses in resected non-small cell lung cancer patients: therapeutic implications of laparoscopic adrenalectomy. Eur J Cardiothorac Surg. 2005;27(5):753–6.
Sebag F, Calzolari F, Harding J, Sierra M, Palazzo FF, Henry JF. Isolated adrenal metastasis: the role of laparoscopic surgery. World J Surg. 2006;30(5):888–92.
Kita M, Tamaki G, Okuyama M, Saga Y, Kakizaki H. Adrenalectomy for metastatic adrenal tumors. Hinyokika Kiyo. 2007;53(11):761–6.
Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, Kim BR. What is the best treatment modality for adrenal metastasis from hepatocellular carcinoma? J Surg Oncol. 2007;96(1):32–6.
Adler JT, Mack E, Chen H. Equal oncologic results for laparoscopic and open resection of adrenal metastases. J Surg Res. 2007;140(2):159–64.
Collinson FJ, Lam TK, Bruijn WM, de Wilt JH, Lamont M, Thompson JF, Kefford RF. Long-term survival and occasional regression of distant melanoma metastases after adrenal metastasectomy. Ann Surg Oncol. 2008;15(6):1741–9.
Bonnet S, Gaujoux S, Leconte M, Thillois JM, Tissier F, Dousset B. Laparoscopic adrenalectomy for metachronous metastasis from renal cell carcinoma. World J Surg. 2008;32(8):1809–14.
Mourra N, Hoeffel C, Duvillard P, Guettier C, Flejou JF, Tiret E. Adrenalectomy for clinically isolated metastasis from colorectal carcinoma: report of eight cases. Dis Colon Rectum. 2008;51(12):1846–9.
Marangos IP, Kazaryan AM, Rosseland AR, Røsok BI, Carlsen HS, Kromann-Andersen B, et al. Should we use laparoscopic adrenalectomy for metastases? Scandinavian multicenter study. J Surg Oncol. 2009;100(1):43–7.
de Haas RJ, Rahy Martin AC, Wicherts DA, Azoulay D, Castaing D, Adam R. Long-term outcome in patients with adrenal metastases following resection of colorectal liver metastases. Br J Surg. 2009;96(8):935–40.
Muth A, Persson F, Jansson S, Johanson V, Ahlman H, Wängberg B. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol. 2010;36(7):699–704.
Pascual Piédrola JI, Rincón Mayans A, Tolosa Eizaguirre E, Barba Abad J, Romero Vargas L, Rosell Costa D. Laparoscopic adrenalectomy for metachronous metastasis. Experience in 12 cases. Actas Urol Esp. 2010;34(2):201–5.
Wu HY, Yu Y, Xu LW, Li XD, Yu DM, Zhang ZG, Li GH. Transperitoneal laparoscopic adrenalectomy for adrenal metastasis. Surg Laparosc Endosc Percutan Tech. 2011;21(4):271–4.
Raz DJ, Lanuti M, Gaissert HC, Wright CD, Mathisen DJ, Wain JC. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg. 2011;92(5):1788–92. discussion 1793.
Crenn G, Delaunay B, Salloum A, Vezzosi D, Bellec L, Thoulouzan M, et al. Carcinological results of laparoscopic adrenalectomy for adrenal metastasis. Prog Urol. 2011;21(9):607–14.
Zerrweck C, Caiazzo R, Clerquin B, Donatini G, Lamblin A, El Khatib Z, et al. Renal origin and size are independent predictors of survival after surgery for adrenal metastasis. Ann Surg Oncol. 2012;19(11):3621–6.
Katoh N, Onimaru R, Sakuhara Y, Abo D, Shimizu S, Taguchi H, et al. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol. 2008;87(3):418–24.
Chawla S, Chen Y, Katz AW, Muhs AG, Philip A, Okunieff P, Milano MT. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75(1):71–5.
Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: a retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011;7(1):145–51.
Oshiro Y, Takeda Y, Hirano S, Ito H, Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol. 2011;34(3):249–53.
Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187(4):245–51.
Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82(2):919–23.
Guiou M, Mayr NA, Kim EY, Williams T, Lo SS. Stereotactic body radiotherapy for adrenal metastases from lung cancer. J Radiat Oncol. 2012;1(2):155–63.
Ahmed KA, Barney BM, Macdonald OK, Miller RC, Garces YI, Laack NN, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol. 2013;36(5):509–13.
Scorsetti M, Alongi F, Filippi AR, Pentimalli S, Navarria P, Clerici E, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: a retrospective analysis of 34 patients. Acta Oncol. 2012;51(5):618–23.
Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablationpreliminary results. Radiology. 2004;231(1):225–30.
Carrafiello G, Laganà D, Recaldini C, Giorgianni A, Ianniello A, Lumia D, et al. Imaging-guided percutaneous radiofrequency ablation of adrenal metastases: preliminary results at a single institution with a single device. Cardiovasc Intervent Radiol. 2008;31(4):762–7.
Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperth. 2009;25(6):455–61.
Mouracade P, Dettloff H, Schneider M, Debras B, Jung JL. Radio-frequency ablation of solitary adrenal gland metastasis from renal cell carcinoma. Urology. 2009;74(6):1341–3.
Yamakado K, Anai H, Takaki H, Sakaguchi H, Tanaka T, Kichikawa K, Takeda K. Adrenal metastasis from hepatocellular carcinoma: radiofrequency ablation combined with adrenal arterial chemoembolization in six patients. AJR Am J Roentgenol. 2009;192(6):W300–5.
Wolf FJ, Dupuy DE, Machan JT, Mayo-Smith WW. Adrenal neoplasms: effectiveness and safety of ct-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81(8):1717–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Mercier, F., Feldman, L.S., Mitmaker, E.J. (2018). Resection Versus Observation for Adrenal Gland Metastasis. In: Angelos, P., Grogan, R. (eds) Difficult Decisions in Endocrine Surgery. Difficult Decisions in Surgery: An Evidence-Based Approach. Springer, Cham. https://doi.org/10.1007/978-3-319-92860-9_32
Download citation
DOI: https://doi.org/10.1007/978-3-319-92860-9_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92858-6
Online ISBN: 978-3-319-92860-9
eBook Packages: MedicineMedicine (R0)