Abstract
Skeletal growth is a key physiological function, which in the case of calcifying organisms, provides support for the general colony structure together, whilst simultaneously providing protection for internal soft tissues. Given this fundamental importance, growth patterns can therefore reflect the health status of organisms. Additionally, engineer species forming 3D structures, such as scleractinian cold-water corals, enhance local biodiversity through the provision of new structural and hydrodynamic habitats. Furthermore, cold-water corals may be used as paleoclimate indicators, and act as sources for novel pharmaceutical compounds as well as represent significant sinks for CO2 sequestration. At time of writing, cold-water coral reefs are facing several serious threats, particularly in the Mediterranean Sea, where the combined effects of climate change and other anthropogenic environmental disturbances are interacting in regions of coral colonisation. The characterisation of the Mediterranean cold-water coral growth patterns is thus a crucial step for accurate forecasting of reef resilience under environmental change and for the establishment of adequate conservation strategies. From the organisation of soft tissues to the resulting mineralogical structures formed from the polyp to the reef scale, this chapter gives an overview of the state of the art of the current understanding of scleractinian cold-water coral growth patterns. The main environmental parameters that control calcification and their influence on cold-water corals in the context of ongoing global environmental change are illustrated with examples from studies conducted with different calcifying species from the Mediterranean Sea, utilising both aquaria and in situ experimental studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Scleractinia
- Skeleton
- Calcification
- Environmental factors
- Forecast of reef growth
- Cold-water corals
- Mediterranean
36.1 Introduction
The appearance of hard structure calcifying fauna during the Cambrian was a major step in the evolution of the animal kingdom tightly linked with the global CO3 2− and CO2 budgets. The skeleton secreted by these fauna both supports the general shape of the organism as well as protects soft tissues against biotic (e.g., predation) or abiotic threats. Though a range of substances may be secreted to form hard structure skeletons, calcium carbonate based skeletal structure are the most abundant and diversified in terms of mineralogy and structure. Shells and skeletons may be made from a range of calcium carbonate compounds (e.g. aragonite, low or high-magnesium calcite, amorphous calcium carbonate), and dozens of microstructures have been identified among which the most widespread are prismatic, fibro-prismatic, nacreous and crossed-lamellar (Carter 1980).
Calcifying species build their skeletons by the periodic addition of carbonate. Both environmental conditions and metabolic activity control the rhythm and growth rates of the biomineralising fauna and deposition rate of the carbonate material. Together with respiration, reproduction and energy storage, calcification is one of the key physiological indicators of organism fitness in calcifying fauna. Biomineralisation studies offer the capacity to address research questions on both short-term (i.e., hourly to daily) and long-term (i.e., annual or multi-annual) scales, with a dual perspective: (1) to address the rapid response of organisms to biotic and/or abiotic changes in the ecosystem, and (2) to assess the resilience of calcifying species to ongoing and persistant environmental change. Additionally, calcifying organisms may be used as oceanographic and paleoceanographic environmental archives, by the analysis of geochemical proxies locked within their carbonate skeletons at the time of deposition. In particular, changes in deep-water mass dynamics can be inferred from trace metal and isotope analyses of cold-water coral (CWC) skeletons (Montero-Serrano et al. 2013).
As it is the case for their shallow water counterparts, CWCs act as ecosystem engineers, forming reefs in cold and deep waters. Indeed, scleractinian corals form three-dimensional structures, potentially accumulating over millennia following death, that provide various ecological niches for associated fauna, including feeding, spawning and nursery habitats for a variety of species (Rueda et al., this volume; D’Onghia, this volume). As a result, corals are key species that partially support the biodiversity of marine ecosystems. Hence, reef-building scleractinian corals are species of great ecological value. In the Mediterranean Sea there are several locations where well developed CWC ecosystems have been described, such as the Santa Maria di Leuca coral province in the Ionian Sea (Vertino et al. 2010; D’Onghia et al. 2011; Chimienti et al., this volume) and the submarine canyons of the Gulf of Lion (Orejas et al. 2009; Fiala-Medioni et al. 2012; Gori et al. 2013; Fourt et al., this volume; Puig and Gili, this volume) and Catalan Margin (Lastras et al. 2016, this volume; Aymà et al., this volume).
The biology of CWCs is much less understood than that of tropical corals, but during the last few decades significant research efforts have been dedicated to the characterisation of CWC physiology and ecology (Roberts et al. 2009). The analysis of CWC growth patterns to date has been based on both aquaria and in situ studies. Many studies have been conducted in the North-East Atlantic and in the Gulf of Mexico, though in recent years a substantial number of works focusing on CWCs within the world ocean have been published, including the Mediterranean. Many CWC studies conducted in the Mediterranean have been focused in the influence of temperature and acidification in these communities, being thus particularly relevant to the forecasting of CWC reef resilience to global change (see Maier et al., this volume; Movilla, this volume).
36.2 Living Tissues and Calcified Material
36.2.1 Anatomy of Corals
Corals are characterised by stinging cells (cnidocytes) that are used for prey capture and as a defence mechanism against predators. Each polyp is made of two cell layers: ectodermal and endodermal layers, separated by the mesoglea which is a collagen network (Allemand et al. 2004). The polyps are linked together by the coenosarc made by an oral (facing seawater) and aboral (facing skeleton) epithelium. Polyps and coenosarc thus cover the skeleton much like a glove (Fig. 36.1), leading to an endogenous biomineralisation.
Anatomy of two cold-water coral species from the Gulf of Lion, northwestern Mediterranean Sea. (a) Lophelia pertusa fragment showing polyp distribution along the skeleton with open polyps displaying the tentacles, (b) Lophelia polyps retracted inside the calices, (c, d) Coral tissues after decalcification showing polyp junction by both mesenterial filaments and the ceonosarc cover, (e–h) Same sequence of images as from (a) to (d) but corresponding in this case to a Madrepora oculata nubbin; note that in M oculata the coenosarc only connects the polyps. Ten tentacles, cal calix, c coenosarc, pol polyp, mf mesenterial filaments. Scale is 5 mm in (a–c) and (e–g) and 1 mm in (d) and (h). (Photographs © A.L. Meistertzheim (CNRS) and F. Lartaud (Sorbonne University))
Biomineralisation occurs in a submicrometric interface between the cells and the skeleton known as the extracellular calcifying medium, from an organic matrix composed of a mixture of proteins, glycoproteins, and polysaccharides that precisely self-assemble and control the CaCO3 crystal formation (Allemand et al. 2011). Different functions have been attributed to this organic matrix, including control of the concentration of precursor ions, constitution of a tridimensional framework, template for crystal nucleation, for determination of the calcium carbonate polymorph, control of crystal elongation, inhibition of crystal growth, determination of spatial arrangement of crystal units, and involvement with enzymatic functions and cell signalling (Marin et al. 2008).
During the calcification process, carbonic anhydrase is involved in hydration/dehydration reactions by supplying dissolved inorganic carbon to the calcification site and removing carbonic acid from the calcification site. This process is performed by the metabolic conversion of CO2 into HCO3 − and HCO3 − into CO3 2− (Bertucci et al. 2013). Finally, the nucleation – the pathfinder for crystal formation in the extracellular calcifying medium – allows the formation of an initial thermodynamically unstable mineral form, the amorphous calcium carbonate, prior to its transformation into aragonite. As an example, for the shallow-water coral Stylophora pistillata from the Gulf of Aqaba, this transient skeletal compartment has a short half-life (12.9 min) compared to the bulk of the skeleton (167 h) (Tambutté et al. 1996). The resulting structure is thus composed of self-assembled aragonite minerals with an inter- and intracrystalline organic matrix.
36.2.2 Anatomical Particularities of Scleractinian Cold-Water Corals and Their Implications for Skeletal Growth
Although reef-building CWCs exhibit many similar growth traits to other calcifying cnidarians, they also form some distinct patterns in morphology which have to be taken into account in growth studies (Lartaud et al. 2017a). This is particularly true for colonial species that form tree-like structures, such as Lophelia pertusa (recently synonymised to Desmophyllum pertusum) (Addamo et al. 2016), Madrepora oculata, Solenosmilia variabilis and Dendrophyllia cornigera.
Even though CWC colonies display the same general anatomy as many shallow-tropical corals, with polyps linked externally together by the coenosarc tissues, CWCs tend to exhibit larger polyps that extend linearly (Gass and Roberts 2011; Lartaud et al. 2017a). A branch of colonial CWC is formed by the successive addition of large calices, growing directly on tip of previous generations of polyps, which can lead to a considerable separation between polyps (Fig. 36.1a, b, e, f), in contrast to the closely packed, tiny polyps often forming the colonial structure of most tropical corals (Lartaud et al. 2017a).
The distance between polyps could also have consequences for energy distribution within a colony. Decalcification of coral skeletons shows that different species do not display the same patterns in soft tissue connection (Fig. 36.1c, d, g, h). Lophelia pertusa polyps are connected by both the coenosarc and mesenterial filaments, located within the calix. This provides different pathways for molecule transfer within the tissues. On the opposite, M. oculata grows with a clear separation between polyps, only connected by the thin coenosarc. This leads to a single route (i.e. the coenosarc) for molecule transfer inside the colony.
Coral calcification also occurs extracellularly. This is particularly prevalent for azooxanthellate colonial corals such as L. pertusa, which produce large amounts of extracellular mucus (EMS), which can contribute to the calcification of the parchment-like tube of the symbiotic worm Eunice norvegica (Roberts 2005) (Fig. 36.2). This mucus has various functions, such as acting as an antifouling agent, protecting the coral skeleton from attacks from endolithic and boring organisms, and also as an aid in removing sediment particles. The occurrence of identical protein patterns within EMS and the newly formed aragonite suggests that EMS plays a central role in the calcification process of the skeletal parts (Reitner 2005). Mucus could also be involved in facilitating coral attachment to the substrate (e.g., rock, dead or still living corals), as it is frequently observed in situ, as well as in association with corals maintained in aquaria (Fig. 36.3).
Coral fragments where the calcification over the tube produced by the symbiotic worm Eunice norvegica can be seen. (a) E. norvegica coming out from its tube (behind the oyster shell). (b) Opposite view of the same worm showing the tube covered by Lophelia pertusa polyps. (c) Calcified tube of E. norvegica by Madrepora oculata. Those specimens were collected in the Lacaze-Duthiers canyon, northwestern Mediterranean Sea. Scale bar is 1 cm. (Photographs © F. Lartaud (Sorbonne University))
(a) Coral extra-skeleton calcification at the base of Lophelia pertusa polyp when deployed lengthened in aquaria (highlighted by a red arrow). The specimen was collected in the Lacaze-Duthiers canyon, northwestern Mediterranean Sea (b) lateral view of the polyp showing the extra-polyp cementation, (c) view from the polyp basis of extra-skeleton calcification, (d) Madrepora oculata colony from the Lacaze-Duthiers canyon covering a bivalve shell with extra-skeleton mineralisation (© UPMC-Fondation TOTAL). Scale bars are 5 mm. (Photographs © F. Lartaud (Sorbonne University))
36.3 Skeleton Microstructure and Crystallography
36.3.1 Initiation of Calcification and Growth Models
The observation of scleractinian CWC skeletal features reveals two types of microstructures. Aragonite fibres are the main skeletal components, representing the vast majority of the calcified volume. Spatially-restricted areas of micrometric scale are also present, traditionally called centers of calcification (COC) and also referred to as early mineralisation zones (EMZ) or rapid accretion deposits (RAD). These zones are characterised by granular crystals arranged in clusters (Constantz 1989). The distribution of these structures differs according to the species, with arrangements varying from discrete clusters to continuous chains (Cohen and McConnaughey 2003). In Lophelia pertusa, they are organised in a continuous line from the base of the corallite up to the calix, near the inner edge of the wall, as shown in the scanning electron microscope (SEM) image after treating the samples with an acid etching solution (Fig. 36.4a). These regions are located close to the starting point of diverting fibres and are considered to be nucleation sites for calcification (Bryan and Hill 1941). Indeed, contrary to the meaning of the traditional name (centers of calcification), these structures are not formed by a unique crystal (Fig. 36.4b), nor are they located at the physical middle of the coral wall. The COCs (or RADs) are the first biomineralised structures to appear in the wall formation (Rollion-Bard and Blamart 2014), and this term is generally used in regards to their initial support role in further crystallisation events.
(a) Microstructures in the wall of Lophelia pertusa skeleton from the Whittard canyon (NE Atlantic) under SEM, showing the Rapid Accretion Deposit (RAD) arranged in a continuous line and the Thickening Deposits (TD) layers made of aragonite fibres. Longitudinal growth direction is from left to right. Scale bar: 200 μm. ext. external part of the skeleton, int. internal part, located in the calix, (b) Detail of the bottom left part of (a) included in the red rectangle. SEM view of layers in aragonite fibres near the RAD on the internal edge of the skeleton. Scale bar: 10 μm
Since the granular crystals have a sub-micron size, it is likely that part of the calcification process is intracellular (Constantz 1989). Vesicles have been observed in the apical membrane of the epithelium which are probably responsible for providing some material to the calcifying space (Johnston 1980), as they can be a pathway for seawater. Though no mineralised structures have been observed in these vesicles, they may act as stabilisation sites for amorphous calcium carbonate (Cohen and McConnaughey 2003; Rollion-Bard et al. 2010).
It is widely agreed that the calicoblastic layer plays a role in calcification (Tambutté et al. 2011); however different models grant it a more or less important function in this process: a direct control of the calicoblastic layer (Barnes 1970; Johnston 1980), a two-steps model (Cuif and Dauphin 1998; Cuif et al. 2011), and a layered model (Stolarski 2003). In the later, COCs are called Centres of Rapid Accretion (CRA) and they are arranged into a Rapid Accretion Front (RAF). CRA and RAF form deposits composing of a mineral phase as well as an organic phase (dCRA and dRAF, respectively), themselves recovered by successive layers of mainly mineral Thickening Deposits (TD).
In the calcifying space, the supersaturation state allows spontaneous formation of nucleation clusters comprised of hydrated ions (Cölfen and Antonietti 2005). As these clusters grow, they eventually become critical crystal nuclei, putatively composed of amorphous calcium carbonate (ACC), which is thought to act as a precursor of mineralised calcium carbonate (Addadi et al. 2003). ACC is known to be present in numerous biominerals (Weiner et al. 2003), but its presence in coral skeletons is debated (Cuif and Dauphin 1998; Falini et al. 2013). Proteins and polysaccharides present in the matrix fix themselves around these nanoparticles and induce a stabilisation of the particles that can orientate themselves for association with other similar nuclei. By forming clusters and welding, they are able to form single crystals (Cartwright et al. 2012).
After putative ACC turned into nanometric crystals, aragonite fibres (located in the TD region) grow from these RADs to form sclerodermites, shaped in fans towards the external side of the skeleton and polyp to increase skeleton thickness.
36.3.2 Growth Structures in the Thickening Deposits Region
The skeleton resulting from sclerodermite formation is not homogenous. Density layers, such as the annual bands formed in tropical corals have not been observed in CWCs, but growth structures defined by opaque and translucent bands in the coral wall have been seen under transmitted light of L. pertusa from Sweden (Wainwright 1964) and Desmophyllum dianthus collected from the NW Atlantic (Lazier et al. 1999). These optically visible bands were originally interpreted as annual patterns (Risk et al. 2005). The bands were thought to have been formed by successive nucleation phases during the life of the polyp, associated with advances and retreats of the outer tissue layer that covers the corallite (Lazier et al. 1999). It has been demonstrated that the opaque and translucent bands are indeed thickening phases of the wall but their temporal meaning is probably not uniform. Indeed, Gass and Roberts (2011) found the same amount of annual banding in living polyps of different ages from the same L. pertusa colony growing on North Sea oil platforms. As a result of this analysis, it seems that these bands are not useful to establish temporal calibrations of polyp age.
Recently, new microlayers (20–100 μm width) were observed under SEM after light etching of skeletal sections from L. pertusa of the NE Atlantic and the Mediterranean Sea (Mouchi et al. 2014). General growth rates and strontium fluctuations across an orthogonal transect to these increments tend to indicate that each microlayer corresponds to a period of one lunar month of growth. Though temporal calibration of the skeleton from these microlayers is promising, it is often hampered by the apparent lateral discontinuity of these structures, particularly when occurring in opaque bands. From SEM observations of L. pertusa specimens, taken from a variety of locations in the NE Atlantic and the Mediterranean Sea, coral skeletons seem to exhibit successive growth phases in all three dimensions (Fig. 36.5) that prevents continuity of microlayers (Mouchi et al. 2014).
(a) SEM view of Thickening Deposits (TD) in the middle of the wall of a Lophelia pertusa specimen from the Porcupine Seabight (NE Atlantic), showing different packets of aragonite fibre orientations. Scale bar 100 μm, (b) interpretation of the SEM image from (a): Black lines represent subhorizontal fibres, grey lines represent oblique fibers and circles represent subvertical fibers compared to the section. Longitudinal growth is from left to right and radial growth from top to bottom
It is also worthwhile mentioning that no bands or layers have been observed in L. pertusa by Mouchi et al. (2014) which may indicate growth patterns along the longitudinal axis (i.e., in the maximum growth direction of the corallite). Still, longitudinal growth do occur as indicated by proof of crystal growth competition on solely one side of sclerodermites (i.e., the side facing the basis of the corallite with pre-existing sclerodermites), while the other side (opposite to the basis) is well ordered without disturbance (Mouchi et al. 2017). Mortensen and Rapp (1998) described white and dark lines in both the transverse and the longitudinal sections of the theca and the septa of L. pertusa from Norway, but without clear temporal pattern. Thin microlayers (<10 μm) have been observed in the septa of the cup coral D. dianthus (Lazier et al. 1999) and the authors suggested a possible relationship between these microlayers and biological clocks, such as (real or inherited) feeding cycles. Still, further chronological calibration is required to improve the understanding of skeletal growth in time and space, and facilitate the interpretations of analysed transects of geochemical proxies in terms of climatic conditions, as was the case for tropical corals and the identification of annual banding.
36.4 Synthesis of the Different Methods Used for CWC Growth Measurements
Contrary to solitary polyps such as Desmophyllum dianthus collected from the NW Atlantic, which form their skeleton along a unidirectional growth axis, the structural complexity inferred by the three-dimensional morphology of colonial species (both stony and soft corals) makes it more difficult to measure colony growth. Additionally, compared to their shallow-water counterparts, direct measurements of growth are constrained by the limited accessibility to deep-waters where CWCs live. As a result, in situ growth studies are still scarce for CWCs compared to other biogenic carbonates.
Methods to gauge the skeletal growth patterns of colonial CWCs are mainly derived from those developed for the investigation of shallow-water scleractinians. They include both non-destructive methods and those which require the sacrifice of live coral fragments. Some methods can only be used in aquaria experiments, whereas others can be implemented under in situ conditions. All of these methods provide useful complementary information but with several restrictions. The selection of the method is determined by the scientific objectives of the studies and the time available to conduct the study. In situ direct observations are suitable for the study at colony level, but the specific role of various parameters influencing growth can be difficult to interpret. Regarding aquaria experiments, the complexity of the natural habitat is difficult to mimic, but the advantage of this kind of experiments is that the environmental conditions can be manipulated, with results of experimental studies therefore providing insight into the understanding of the potential drivers and environmental controls on CWC growth (see Orejas et al., this volume). Whenever possible, in situ experiments are recommended, in order to investigate the influence of environmental variability and the combination of abiotic factors in their natural habitats on coral growth processes (e.g., Brooke and Young 2009 for Lophelia pertusa in the Gulf of Mexico and Lartaud et al. 2014 for Mediterranean L. pertusa and Madrepora oculata).
The main methods for the study of CWC growth can be summarised into different sets of techniques, each providing different types of information.
The first group relates to techniques that measure calcification rates (generally expressed as g CaCO3 g−1 skeleton day−1 or % day−1). These techniques are mainly used in aquaria experiments to test the causal relationship between physicochemical changes in water chemistry and the biomineralisation response of the CWCs (e.g., Orejas et al. 2011a, b; Naumann et al. 2014; Gori et al. 2014; Rodolfo Metalpa et al. 2015 for L. pertusa, M. oculata, Dendrophyllia cornigera and D. dianthus from the Mediterranean Sea). The techniques most frequently used to measure calcification rates are: the alkalinity anomaly, the buoyant weight or the radioisotope techniques (Maier et al. 2009; Form and Riebesell 2012).
The second group of techniques quantify linear or surface growth rates (expressed in mm year−1 or cm2 year−1). These methods can be used in aquaria and in situ (see Orejas et al. 2011a; Lartaud et al. 2017b for L. pertusa, M. oculata, D. cornigera and D. dianthus, in both cases with specimens from the Mediterranean Sea). The surface and linear growth extension of coral fragments can be measured at the colony level, or more accurately at polyp level using sclerochronological or sclerochemical tools. Radiometric dating is also used to determine the rates of extension.
The third group of methods investigates the renewal rate of the colony, by determining the budding rate (i.e. new polyp addition over a period of time) (see Mortensen 2001; Lartaud et al. 2014). Due to logistical constraints and the long life cycle of many CWC species, the modelling approach (Galli et al. 2016) is a further promising technique for the study of CWC growth patterns.
36.4.1 Alkalinity Anomaly
Alkalinity anomaly is a commonly used technique to estimate skeletal growth. Measures take place in closed chambers where coral fragments are incubated; water samples are collected from the chambers at the beginning and at the end of the experimental period. The water volume of the chambers needs to be small enough to allow an accurate measure of potential changes in the total alkalinity (A T). Calcification rates use the 2:1 stoichiometric relationship between the decrease of A T and CaCO3 precipitation (Chisholm and Gattuso 1991). A correction must be applied for the changes of A T due to the significant release of inorganic nutrients during incubation, as has been documented for L. pertusa and M. oculata collected from the Lacaze-Duthiers canyon in the Gulf of Lion (Maier et al. 2013). The alkalinity anomaly technique is however not always applicable for long-term experiments due to several side-effects on the water chemistry (e.g. microbial activity of biofilters, water exchanges, feeding) which are indistinguishable from the corals’ calcification activity (Form and Riebesell 2012 in L. pertusa from the Norwegian coast).
36.4.2 Buoyant Weight
The buoyant weight technique is a common method used for CWC growth experiments in aquaria. Calcification is inferred from changes in weight of the living organism in seawater of known density. The method is based on the Archimedes’ Principle and a correction factor is applied as it has been observed that tissue weight accounts for 3–4% of the skeletal buoyant weight for M. oculata from Cap de Creus canyon in the NW Mediterranean and L. pertusa from the Island of Malta (Orejas et al. 2011a) and 14 ± 4% for D. cornigera corals from Cap de Creus canyon and D. dianthus from the Island of Malta (Movilla et al. 2014b). The net buoyant weight values of corals is converted into dry weight (DW) using the density of the pure aragonite (2.94 g.cm−3) (Rodolfo Metalpa et al. 2015 on D. dianthus from Malta and D. cornigera from the Ionian Sea). Although a negative influence of handling cannot be excluded, this method is suitable for measuring calcification over monthly intervals or longer and it can be recommended for long-term rather than short-term experiments. Results however need to be taken with caution as potential artefacts of coral response can be associated to long-term aquaria studies.
36.4.3 Radioisotopes
The radioisotope method involves the incubation of a fresh coral fragment in a seawater volume containing radioactive elements (45Ca or 14C). After an incubation period of several minutes to hours, the coral pieces are rinsed in filtered seawater, the tissue removed, the skeleton dried and dissolved with a strong acid and the incorporated radioactivity measured using a scintillation counter (e.g. Hennige et al. 2014b for L. pertusa from the Mingulay Reef Complex, NE Atlantic). The radioisotope method is highly accurate for measurements at short timescales (periods can be minutes), but this method requires sacrificing the organism for analyses and is restricted to small volumes (e.g., 50 mL Falcon tubes).
36.4.4 Surface and Linear Growth Extension
This is a useful technique, both for in situ and aquaria measurements. The growth measures are performed by means of image analysis of photographs taken at different periods. The surface extension provides information on the colony size changes (in cm2) (see Larcom et al. 2014 on L. pertusa from the Gulf of Mexico), and the linear growth extension highlights the growth rate (in mm year−1) of coral branches along the main growth axis (Orejas et al. 2008 for L. pertusa and M. oculata from Cap de Creus canyon, northwestern Mediterranean Sea; Orejas et al. 2011a for M. oculata and L. pertusa from the Cap de Creus canyon and L. pertusa and D. dianthus from the Island of Malta; Lartaud et al. 2017b for L. pertusa and M. oculata from the Lacaze-Duthiers canyon, NW Mediterranean Sea). Key benefits of this method are the “low cost” of the approach and that the technique is non-destructive and particularly suitable for aquaria studies.
36.4.5 Sclerochronology and Sclerochemistry
The analysis of growth increments (sclerochronology) or fluctuations of geochemical signals throughout the growth (sclerochemistry) requires sacrificing the organisms under study. These analyses however provide an accurate measure of growth rates in coral stem and branches and/or time allowing a better characterisation of the temporal dynamics in CWC growth.
Sclerochronology is the “biomineral” equivalent to dendrochronology used on trees. The concept is based on the assumption that each growth increment has been formed by the organism in successive equivalent time intervals whatever their thickness (Knutson et al. 1972). The production of carbonate structures by calcifying species periodically decelerates and eventually ceases, although environmental conditions still remain favourable for skeletal growth. Growth increments (also called growth bands or growth rings) are formed according to different periodicities, from infra-daily to annual rates. The observations are made on skeletal sections (radial or longitudinal, depending on the species) under optical microscopy, scanning electron microscopy or X-ray fluorescence. The method is currently used for deep-water octocorals and antipatharians (e.g., Sherwood et al. 2005 on Primnoa resedaeformis collected offshore Nova Scotia, Canada) and for shallow-water stony corals (Le Tissier et al. 1994 on Porites lutea from Indo-Pacific and Porites porites from Caribbean Sea), but it is still rarely applied to scleractinian CWCs due to limited knowledge on the growth periodicity increments formation (Lartaud et al. 2017a and references in Sect. 36.3.2 of this chapter).
The use of chemical markers is very useful, as they allow a “time 0” point to be marked within the coral (i.e. the date of dye use), and the growth of the coral after this point can then be measured. The incorporation of fluorochromes into the skeleton produces an internal fluorescent mark and subsequently the growth increment can be readily estimated. Calcein (Hassenrück et al. 2013 for D. dianthus from Patagonia; Lartaud et al. 2013 for L. pertusa and M. oculata from the Gulf of Lion) and alizarin red (Brooke and Young 2009 for L. pertusa from the Gulf of Mexico; Form and Riebesell 2012 for L. pertusa from Norwegian coast) are classically used in mark and recapture experiments with CWCs. However, recent comparison between calcein and alizarin red stainings have shown that calcein appears more suitable than alizarin red, as the last has been shown to strongly limit growth of NW Mediterranean L. pertusa and M. oculata by delaying coral recovery following the treatment (Lartaud et al. 2017b).
Geochemical proxies (e.g., stable isotopes, minor and trace metals) can also be analysed along the growth profile to determine growth patterns. Sclerochemistry methods were developed for shallow-water species, particularly for scleractinian corals (Pätzold 1984). However, the use of the sclerochemistry is limited with CWCs due to important metabolically-induced skeleton geochemical changes (so called “vital effects”), leading to an uptake of isotopes and elements during the ion transfer between seawater and skeleton (Adkins et al. (2003) for L. pertusa and D. dianthus from the NW Atlantic; López-Correa et al. (2010) for L. pertusa from the Santa Maria di Leuca coral province in the Ionian Sea; Marali et al. (2013) for D. dianthus from the Azores; Raddatz et al. (2013) for L. pertusa from various locations from the European continental margin).
36.4.6 Radiometric Dating
Radiometric or radioactive dating compares the abundance of a naturally occurring radioactive isotope within the skeleton to the abundance of its decay products, which form at a constant rate. Different techniques are used for CWC species, including 14C (Roark et al. 2006 for Corallium secundum, Gerardia sp. and Leiopathes glaberrima off Hawaii), 230Th-U (Cheng et al. 2000 for D. dianthus from the Pacific, Atlantic and Southern Oceans) and 210Pb-226Ra (Sabatier et al. 2012 for L. pertusa and M. oculata from Røst Reef, off Norway). Initially applied for ageing fossil-reef structures, radiogenic isotopes are also useful to determine the life span of long-lived species when ring counting is not possible.
36.4.7 Budding Rate
Colonial CWCs (such as L. pertusa and M. oculata) produce large polyps compared to octocorals and most tropical scleractinians. Colony growth is primarily driven by the addition of new polyps (Gass and Roberts 2011). Quantifying the new polyp additions over a period of time (budding rate) is a useful and non-destructive technique to measure colony-growth. Comparison between studies is however difficult as different works start with different polyp number. A standardised method was proposed by Lartaud et al. (2014) for L. pertusa and M. oculata from the Lacaze-Duthiers canyon, NW Mediterranean Sea, using the rate of new polyp addition per polyp initially present per year (expressed in %).
36.4.8 Growth Modelling
A complementary approach to the direct measurement of growth is CWC modelling. Numerical simulation techniques for marine fauna have been developed across different scales, ranging from small-scale physiological processes to whole ecosystem dynamics. Organism and population growth models are based on the “bioenergetics approach”, calibrated with experimental observations. A bioenergetic growth model describes growth as the evolution of a quantity (energy, biomass or mass of a specific compound) over time through the balance of input and output fluxes, through the system boundary and between compartments within the system. The simplest formula considers one single compartment for biomass (or energy), one input and one output flux that depend on the system biomass/energy usage according to allometric laws (Glazier 2005). The resulting estimations typically allow forecasting of the number of polyps and colony masses over time (see more details in Lartaud et al. 2017a). Those models appear particularly relevant for the study of ecological properties and their impacts on coral growth under changing environmental conditions, such as illustrated by Galli et al. (2016) on Corallium rubrum, an endemic species to the Mediterranean Sea.
36.5 Growth: From the Colony to the Reef
36.5.1 Extension of the Colony
For scleractinian corals, colony-growth rate measurements refer mainly to the linear extension (increase in length), whereas growth rate estimates for soft corals (gorgonian, zoanthid and antipatharian species) mostly derive from radial thickening analysis. As they form the largest reef structures across many regions of the North and South Atlantic, as well as the Mediterranean, growth studies were principally dedicated to Lophelia pertusa. In contrast, little effort has thus far been dedicated at determining growth rates of other Mediterranean scleractinians such as Madrepora oculata, Dendrophyllia cornigera or Dianthus dianthus.
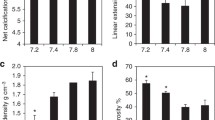
Lophelia pertusa exhibits the highest growth rates from all CWC known species thus far investigated, ranging from 0.01 to 38.1 mm year−1 (see Table 36.1 and Roberts et al. 2009). The reason for this large inter-individual variability is unknown. At present, no clear influence of bathymetry has been detected in CWC growth rates. However different environmental conditions can contribute to partially explain the high variability recorded in L. pertusa growth rates. Environmental variability can be considered at different scales (e.g. between oceans or at the habitat scale) and local topography or current flow exposition may explain the large growth fluctuations observed within the same geographic area. An example of these local variations are the growth rates recorded for corals from two different locations in the western Mediterranean: (1) 2.2–17 mm year−1, for specimens collected at ~200 m in the Cap de Creus canyon (Orejas et al. 2008, 2011a) and (2) 0.01–38.1 mm year−1 for specimens growing at ~500 m in the Lacaze-Duthiers canyon (Lartaud et al. 2014, 2017b). This example also shows that Mediterranean CWC specimens of L. pertusa can reach similar maximum growth rates to those observed to date in the North Sea and in the Gulf of Mexico (maximum observed of 34 mm year−1 in the North Sea (Gass and Roberts 2006) and 32.3 mm year−1 in the Gulf of Mexico (Larcom et al. 2014)). Finally, in situ tag and recapture techniques have shown a decrease in colony growth rate over time (Fig. 36.6). This may reflect faster growth in younger colonies (Larcom et al. 2014) and suggest that the age of a colony could also play an important role, partially explaining differences between growth rate measurements (Table 36.1).
Madrepora oculata from the NW Mediterranean Sea shows mean growth rates ranging from 0.01 to 18 mm year−1 (Orejas et al. 2008, 2011a; Lartaud et al. 2013, 2014, 2017b). These values are within the same range as those measured in the Norwegian Sea for this species and elsewhere for other colonial species, except for Solenosmilia variabilis which has been observed to have very slow growth rates (~1 mm year−1; Table 35.1). As for L. pertusa, a decrease in colony growth rate over time for M. oculata is observed (Fig. 36.6).
Currently, no data exist for the rates of extension of D. cornigera in its habitat. However, several studies have been conducted with this species in aquaria, using the buoyant weight technique. D. cornigera displays growth rates of 7–30% per year (Orejas et al. 2011a; Gori et al. 2014), which are similar to the calcification rates of the long-life span D. dianthus (>200 years, Risk et al. 2002), with calcification rates of 11–33% (Orejas et al. 2011a; Rodolfo Metalpa et al. 2015). This last species exhibits growth rates lower than 3 mm year−1 (Table 36.1).
36.5.2 Growth Dynamics
In addition to long-term changes, seasonal differences in growth rates have been identified. In the Lacaze-Duthiers canyon differences have been observed over the year for M. oculata growth patterns (budding and polyp growth rates) (Lartaud et al. 2014); these differences may be owed to the seasonal change in hydrodynamic conditions that occurs in the canyon heads of the Gulf of Lion (NW Mediterranean). For instance, the observed increase in budding rate during the winter/spring period may be promoted by organic particle supply induced by episodes of dense water shelf cascades and associated resuspension events (Canals et al. 2006; Heussner et al. 2006; Palanques et al. 2006). The response to environmental changes seems however species-specific as L. pertusa specimens living in the same habitat display the same growth rates throughout the year (Lartaud et al. 2014). This behaviour suggests more plasticity in the energetic requirement of this species, likely associated with different feeding strategies (Kiriakoulakis et al. 2005) and bacterial-host associations (Meistertzheim et al. 2016). This growth pattern contrasts with the seasonal reproductive cycle described for L. pertusa but not for M. oculata (Waller and Tyler 2005).
Differences in growth patterns also occur at a long term scale. L. pertusa from the NE Atlantic produces micro-layers according to monthly lunar cycle (28 days) and the Sr/Ca composition inside the micro-layers revealed an additional cycle related to semi-lunar oscillations (14 days) (Mouchi et al. 2014). Drivers of those growth rate changes seem to be related to the hydrological context, as in the studied region of the N Atlantic, rapid downwelling of surface waters are caused by hydraulic control of tidal flow, associated with advection onto deep bottom water reefs during peak tides, which periodically increase the availability of organic particles for coral colonies (Davies et al. 2009). As deep as 900 m water depth, tidal influence induces substantial temperature changes (1.5–2 °C) and input of organic matter in canyon ecosystems (van Haren et al. 2014). The thickness of carbonate micro-layers varies according to growth rate changes, showing two slow phases during each year: one in winter months likely caused by a decrease in food availability, with the second possibly correlated with the gametogenesis period (Mouchi et al. 2014).
Similar micro-layers have been detected but poorly revealed in Mediterranean L. pertusa (Mouchi et al. 2014). To date, no chronological calibration has been done and more investigations of these structures in an ecosystem free of strong tidal control would be of great interest to better determine the role of environmental or endogenous rhythms on the biomineralisation of scleractinians.
36.5.3 Main Environmental Parameters Governing CWC Growth
A large number of environmental factors (e.g. temperature, salinity, nutrient concentration, currents) and/or biotic factors (symbiosis, predation, competition) can affect coral calcification (Miller 1995). The direct impact and relative importance of these various parameters on CWC growth remains to be defined, and most probably they will be different for the different species. Laboratory studies suggest that an increase in food supply (considering quality and quantity), rather than temperature, may be more important in determining growth rates of scleractinian CWCs (as seen for L. pertusa from Norway by Mortensen 2001 and M. oculata from Cap de Creus canyon, NW Mediterranean by Orejas et al. 2011b). Growth rates observed in aquaria for NW Mediterranean M. oculata are higher when corals are fed five times a week with a mixed diet of Mysidacea, frozen Cyclops and Artemia salina nauplii (Orejas et al. 2011a) compared to growth rates of corals fed three times a week with A. salina nauplii only (Lartaud et al. 2013). Temperature and pH are also important parameters that can affect growth rates, with distinct responses depending on the species or local habitat conditions (cf. details from Sect. 36.6 of this chapter, as well as chapters by Reynaud and Ferrier-Pagès, this volume; Maier et al., this volume; and Movilla, this volume).
Sediment accumulation stress slows coral growth, as seen for L. pertusa from south Norway (Larsson et al. 2013). The authors suggest that lower growth is likely due to the additional energy for polyps to clean themselves which decrease the energy available for feeding, or to a lower polyp extension to reduce abrasion risk by sediment particles, limiting ion exchanges from the environment to extracellular medium (i.e., the area where mineralisation occurs). Anthropogenic pollutants such as drill cuttings can create a similar stressor to sedimentation (Larsson et al. 2013 on L. pertusa from south Norway).
36.5.4 Reef Formation
After settlement of a coral larva, asexual formation of new polyps drives extension of the colony in three dimensions. Self-recognition of adjacent coral colonies, as well as skeletal fusion in areas with low levels of aragonite crystal organisation and strong molecular bonding, facilitate ecosystem engineering, as described for L. pertusa from Norway (Hennige et al. 2014a). Morevover, coral skeleton cementation can also occur between different species, such as for L. pertusa and M. oculata from the NW Mediterranean (Fig. 36.7), inducing spatial complexity of the living area. Polyp death rates at the base of colonies increase with the colony lifespan, and hence the proportion of dead coral framework increases, leading to zonation of the habitat (Buhl-Mortensen et al. 2010). Other organisms actively promote reef aggregation as well, such as the symbiotic worm Eunice norvegica, which stimulates excessive calcification of Norwegian L. pertusa (Roberts 2005; Mueller et al. 2013). Coral calcification over the tube produced by worms living within the coral branches strengthens the framework by thickening and connecting coral branches (Fig. 36.2). Sponges and fungi that attack dead coral parts at the base of the reef tend to form coral rubble by bioerosion. Rubble also contributes in reef formation by providing a substratum for new larvae settlement (Roberts et al. 2009).
Colony extension occurs over the whole lifespan of the coral, and it is supported by the long lifespan of some reef-building species. Radioisotope measurements reveal that the age of a 40 cm high L. pertusa colony from the NE Atlantic can be up to 250 years (Pons-Branchu et al. 2005). This means that a 2–3 m high colony needed several centuries to reach this size. However, lifespan differs depending on the species and/or the environment as a 45 cm high M. oculata colony from Norway has been estimated to be ~40 years old (Sabatier et al. 2012). Nevertheless if sedimentation takes place at a slower rate than coral growth, colony growth and colonisation by additional coral larvae in the dead or living part of the reef contributes to maintain reef formation over very long periods. The oldest L. pertusa reef found to date is estimated in 8600 years old and it is located in Norwegian waters (Hovland and Mortensen 1999).
Reef growth is divided into five stages, corresponding to: (1) reef initiation, (2) framework expansion, (3) framework collapse, (4) partial burial of the coral rubble by superficial sediments, and (5) finally possible larval recruitment on the coral rubble depending on the environmental conditions, allowing the formation of a geological structure induced by cyclic alternation of these different phases (Douarin et al. 2014). The cyclic stages are facilitated by climatic evolution (e.g., glacial/interglacial cycles) allowing formation of coral carbonate mounds over thousands of years (Roberts et al. 2006). The present-day CWC reefs in the Mediterranean Sea were initiated at the Late Pliocene/Early Pleistocene (Taviani et al. 2005, this volume). Depending on the area, reef formation may have been interrupted for long periods inducing temporary extinction of CWCs in the eastern Mediterranean Sea during the early to middle Holocene from 11.4 to 5.9 kyr BP linked to low oxygen conditions (Fink et al. 2012).
36.6 Growth in the Future Mediterranean Sea
36.6.1 Potential Influence of Climate Change on the Mediterranean CWCs
It is estimated that >80% of global warming over the last 40 years has been taken up by the oceans and penetrated to depths of at least 700 m (Barnett et al. 2005). Among other anthropogenic stressors, the imprint of climate change on deep and intermediate Mediterranean waters is expected to generate specific threats to CWC habitats, such as in canyons or seamount flanks (see Ramirez-Llodra et al. 2010; Levin and Le Bris 2015). The combination of warming and acidification of mediterranean intermediate waters combined with change in nutrient supply and deoxygenation could affect the capacity of CWCs to maintain growth rates (Cordes et al. 2016). Predictive habitat modelling approaches and global datasets of key environmental parameters (e.g. temperature, salinity, dissolved O2 and nutrients), are suggesting changes in the distribution of suitable habitats for CWC taxa in future climate scenarios (IPCC 2014), though there are still large uncertainties in these approaches (Davies and Guinotte 2011). The spatial resolution and accuracy of these models are still largely insufficient to anticipate responses due to short-term changes in local conditions (e.g. recovery times after physical disturbance, fluctuations of environmental factors and feeding constraints). According to future predicted climate IPCC-A2 scenario (IPCC 2014), the average temperature for the deep Mediterranean Sea may increase by 1.5 °C by the end of the twenty-first century with an increase of 0.23 for salinity (Somot et al. 2006). Together with the increase of temperature, pH will decrease as a result of anthropogenic CO2 absorbed by seawater. In the future (year 2100) Mediterranean deep waters, the pH change is estimated to be between −0.005 and −0.06 units (Palmiéri et al. 2015). The local enhancement of acidification (e.g. due to an increase in organic matter remineralisation) and associated cumulative stressors yet constitute potential threats to CWCs.
Consequences of climate change for Mediterranean CWCs and their associated communities have to be evaluated. Details on this topic are given by Maier et al. (this volume) and Movilla (this volume).
36.6.2 Impact of Temperature
Physiological behaviour of scleractinian CWCs have been monitored in aquaria under different thermal conditions. Long-term (12 months) aquaria experiments on Lophelia pertusa from the Atlantic Ocean do not show absolute changes in calcification rates under seawater temperatures of 9 °C and 12 °C (Hennige et al. 2015). Lophelia pertusa and M. oculata from the Mediterranean Sea however revealed species-specific growth rate responses with similar calcification rates at 9 °C and 12 °C for L. pertusa, but lower rates at 9 °C for M. oculata. At 6 °C, both species show low calcification rates (Naumann et al. 2014). Similarly, calcification of Dendrophyllia cornigera from the Menorca Channel decreases from 12 to 8 °C (Gori et al. 2014).
Thermal acclimation of calcification processes is well established for a number of temperate and tropical scleractinian coral species and the optimal temperatures for several species have been determined. However, for scleractinian CWCs the thermal optimum and the potential for adaptation to temperature changes are still unknown. In situ and aquaria experiments suggested that the upper lethal temperature for L. pertusa from the Gulf of Mexico is near 15 °C (Brooke et al. 2013). Freiwald et al. (2004) suggested that scleractinian CWCs, which occur less frequently in the Mediterranean Sea than in Atlantic and Pacific oceans, are at the uppermost of their thermal tolerance range in the Mediterranean (14 °C). Considering this optimal range, it would be expected that growth experiments conducted at temperatures higher than 13 °C, should display a decline in the calcification rate for CWCs. However, not all CWC species follow this rule as the calcification of D. cornigera from the shallow part of the Menorca Channel (~200 m) increases when temperature rises from 12 to 16 °C (Gori et al. 2014). Desmophyllum dianthus from a deeper location in the Adriatic Sea (430 m) shows significant decreases in calcification rates when exposed for a long period (8 months) to 15 °C waters (Gori et al. 2016); this might be linked to the decline in activity of enzymes involved in calcification (e.g., carbonic anhydrase). Reef formation in the future Mediterranean may be dramatically affected by global warming or, assuming the different thermal tolerances of species (e.g. D. cornigera exhibits a metabolism more efficient at higher temperature, Gori et al. 2014), an increase in temperature may contribute to a shift in Mediterranean CWC community composition. Analyses of fossils (Wienberg et al. 2009) and recent corals from the Atlantic (Keller and Os’kina 2008) suggest a higher temperature tolerance of M. oculata compared to L. pertusa. Additionally, scleractinian deep-water corals (Dendrophyllia sp. and Eguchipsammia fistula) have been found in the Red Sea at temperatures exceeding 20 °C, leading to a revisiting on the main persistence and resilience concepts for CWCs (Roder et al. 2013). If the future baseline temperature changes in the deep Mediterranean provinces, questions regarding acclimation of Mediterranean corals have to be addressed. D. cornigera from Cap de Creus canyon, NW Mediterranean, and D. dianthus from south of Malta, tolerate elevated temperatures (17.5 °C) during 3 months in aquaria, showing higher growth rates for D. cornigera when temperature increases, which suggests that those species may be more capable of surviving in warmer environments than previously thought (Naumann et al. 2013).
36.6.3 Ocean Acidification
Acidification is known to alter growth rate of calcifying organisms as global ocean composition becomes undersaturated in calcium carbonate. However, for scleractinian CWCs, long-term aquaria studies generally suggest that calcification of L. pertusa, M. oculata and D. cornigera colonies and D. dianthus solitary corals from the Mediterranean Sea are not affected by the pCO2 level projected at the end of the century (Maier et al. 2013; Rodolfo Metalpa et al. 2015; Gori et al. 2016), although some studies report an expected higher sensititvity of L. pertusa and D. cornigera species (Movilla et al. 2014b). McCulloch et al. (2012) estimated that the energetic cost associated with pH up-regulation was ~10% per 0.1 pH unit decreases in seawater. A recent study suggests that a small fraction (<3%) of the total energy demand is required for M. oculata calcification, allowing corals from the Adriatic Sea to maintain growth rates in more acidic waters even under low feeding conditions (Maier et al. 2016).
The synergic effect of temperature and ocean acidification has been recently tested and the first results suggest that crystallographic and molecular-scale bonding organisation rather than calcification rate of L. pertusa from NE Atlantic are affected by thermal and pCO2 changes (Hennige et al. 2015). Nevertheless, additional studies providing information on the main sources of energy metabolised are required, as highlighted by results from Gori et al. (2016) on D. dianthus from the Adriatic Sea.
As this volume includes two chapters (already mentioned in this text) dealing with effects of ocean acidification in CWCs, no more details on this topic will be included in this section.
36.6.4 Impact of Oceanographic Conditions on the Growth of CWCs: The Case Study of Dense Water Shelf Cascades in the Mediterranean Sea
In the Mediterranean Sea, different areas (Aegean Sea, Adriatic Sea, Catalan Margin, Creta Island, Gulf of Lion) are influenced by episodic dense water shelf cascades driven by wind-induced conditions (Canals et al. 2006). These dense water plumes that overflow the shelf edge are associated with significant decreases in temperature, significant increases in the current speed and they may transport of large amounts of coarse sediment and organic matter (Palanques et al. 2006; Heussner et al. 2006; Canals et al. 2006). The precise effects of cascading on CWC communities are not well documented (but see Puig and Gili, this volume). As mentioned before, in situ experiments showed a seasonal difference in the growth patterns of M. oculata, which are suspected to result from differences in the food availability induced by winter cascading events in the Lacaze-Duthiers canyon (Lartaud et al. 2014). The expected scenario for the end of the twenty-first century by the IPCC suggests a stronger stratification of the water column, which would result in a decrease of cascading events of at least 60%, compared to the present climate conditions (Hermann et al. 2008). Considering these preliminary results, the effects of climate change could result in additional strong threats to the resilience of CWC reefs in the Mediterranean Sea.
36.7 Conclusion
The study of growth patterns is a basic approach to determine the age of corals and to better understand the ecological features of CWCs. The evolution of methodologies and techniques to determine growth rates of CWCs provides increasily more precise approaches to measure growth, and also improvements in the standardisation to compare results obtained from CWCs and tropical and temperate corals, as well as among juvenile and adult individuals. Unexpected results have been highlighted by recent research, for instance the observation that some CWC species can reach growth rates similar to those of some tropical species. Still, growth rates differ depending on location, age of colony or the period of the year. Differences between species, including those living in the same habitat, have also been identified, raising the possibility that specific ecological niches may be preferencially occupied by specific species. These observed differences point out that further work is needed to determine the most effective conservation strategies for the CWC fauna which display diverse ecological performance.
Further, more studies are necessary to elucidate the key environmental and physiological drivers of growth, from the integration of inter-disciplinary studies to take into consideration skeletal growth as a part of the response of the whole organism. For example, a detailed understanding of the biology of the main CWC species still lacks, including knowledge of the role of reproductive cycle, the associated microbiome or the energy invested in growth by the calcifying species. Understanding critical aspects of CWC growth will require studies with substantial multi-parameter monitoring – both biotic and abiotic – focusing on several temporal scales. Within this framework, the Mediterranean Sea is a perfect fieldwork environment as numerous CWC species have been identified in the area, and the effects of global environmental changes on these could be particularly remarkable in this semi-enclosed sea. For the survival of the associated biodiversity, the growth and survival of CWC species in this small ocean is critical.
References
Addadi L, Raz S, Weiner S (2003) Taking advantage of disorder: amorphous calcium carbonate and its roles in biomineralization. Adv Mater 15:959–970
Addamo AA, Vertino A, Stolarski J, et al (2016) Merging scleractinian genera: the overhelming genetic similarity between solitary Desmophyllum and colonial Lophelia. BMC Evol Biol 16:108. https://doi.org/10.1186/s12862-016-0654-8
Adkins JF, Boyle EA, Curry WB, et al (2003) Stable isotopes in deep-sea corals and a new mechanisms for “vital effects”. Geochim Cosmochim Acta 67:1129–1143
Adkins JF, Henderson GM, Wang SL, et al (2004) Growth rates of the deep-sea scleractinia Desmophyllum cristagalli and Enallopsammia rostrata. Earth Planet Sci Lett 227:481–490
Allemand D, Ferrier-Pagès C, Furla P, et al (2004) Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. CR Palevol 3:453–467
Allemand D, Tambutté É, Zoccola D, et al (2011) Coral calcification, cells to reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 119–150
Barnes DJ (1970) Coral skeletons: an explanation of their growth and structure. Science 170:1305–1308
Barnett TP, Pierce DW, AchutaRao KM, et al (2005) Penetration of human-induced warming into the world’s oceans. Science 309:284–287
Bell N, Smith J (1999) Coral growing on North Sea oil rigs. Nature 402:601
Bertucci A, Moya A, Tambutté S, et al (2013) Carbonic anhydrases in anthozoan corals—a review. Bioorg Med Chem 21:1437–1450
Brooke S, Young CM (2009) In situ measurement of survival and growth of Lophelia pertusa in the northern Gulf of Mexico. Mar Ecol Progr Ser 397:153–161
Brooke S, Ross SW, Bane JM, et al (2013) Temperature tolerance of the deep-sea coral Lophelia pertusa from the southeastern United States. Deep-Sea Res Part 2 Top Stud Oceanogr 92:240–248
Bryan WB, Hill D (1941) Spherulitic crystallization as a mechanism of skeletal growth in the hexacorals. Proc R Soc Qld 52:78–91
Buhl-Mortensen L, Vanreusel A, Gooday A, et al (2010) Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar Ecol 31:21–50
Canals M, Puig P, de Durrieu de Madron X, et al (2006) Flushing submarine canyons. Nature 444:354–357
Carter JG (1980) Guide to bivalve shell microstructures. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms. Plenum Press, New York, pp 645–673
Cartwright JH, Checa AG, Gale JD, et al (2012) Calcium carbonate polyamorphism and its role in biomineralization: how many amorphous calcium carbonates are there? Angew Chem Int Ed 51:11960–11970
Cheng H, Adkins JF, Edwards RL, et al (2000) U-Th dating of deep-sea corals. Geochim Cosmochim Acta 64:2401–2416
Chisholm JRM, Gattuso JP (1991) Validation of the alkalinity anomaly technique for investigating calcification of photosynthesis in coral reef communities. Limnol Oceanogr 36:1232–1239
Cohen AL, McConnaughey TA (2003) Geochemical perspectives on coral mineralization. In: Dove PM, De Yoreo JJ, Weiner S (eds) Biomineralization, reviews in mineralogy and geochemistry. Mineralogical Society of America and the Geochemical Society, Washington, DC, 54:151–187
Cölfen H, Antonietti M (2005) Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew Chem Int Ed 44:5576–5591
Constantz BR (1989) Skeletal organisation in Acropora. In: Crick RE (ed) Origin, evolution and modern aspects of biomineralization in plants and animals. Plenum Press, New York, pp 175–200
Cordes E, Arnaud-Haond S, Bergstad OA, et al (2016) Cold-water corals. In: Inniss L, Simcock A (eds) The first global integrated marine assessment: World Ocean Assessment I. United Nations regular process for global reporting and assessment of the state of the marine environment, including socioeconomic aspects. United Nations, New York, 28 p
Cuif JP, Dauphin Y (1998) Microstructural and physicochemical characterization of centers of calcification in septa of some recent Scleractinian corals. Paläontol Z 72:257–270
Cuif JP, Dauphin Y, Sorauf J (2011) Biominerals and fossils through time. Cambridge University Press, Cambridge, p 490
D’Onghia G, Indennidate A, Giove A, et al (2011) Distribution and behaviour of deep-sea benthopelagic fauna observed using towed cameras in the Santa Maria di Leuca cold-water coral province. Mar Ecol Progr Ser 443:95–110
Davies AJ, Guinotte JM (2011) Global habitat suitability for framework-forming cold-water corals. PLoS One 6:e18483
Davies AJ, Duineveld GCA, Lavaleye MSS, et al (2009) Downwelling and deep-water bottom currents as food supply mechanisms to the cold-water coral Lophelia pertusa (Scleractinia) at the Mingulay Reef complex. Limnol Oceanogr 54:620–629
Douarin M, Sinclair DJ, Elliot M, et al (2014) Changes in fossil assemblage in sediment cores from Mingulay Reef Complex (NE Atlantic): implications for coral reef buid-up. Deep-Sea Res Part 2 Top Stud Oceanogr 99:286–296
Duncan PM (1877) On the rapidity of growth and variability of some Madreporaria on an Atlantic Cable, with remarks upon the rate of accumulation of foraminiferal deposits. Proc R Soc Lond 26:133–137
Falini G, Reggi M, Fermani S, et al (2013) Control of aragonite deposition in colonial corals by intra-skeletal macromolecules. J Struct Biol 183:226–238
Fallon SJ, Thresher RE, Adkins J (2014) Age and growth of the cold-water scleractinian Solenosmilia variabilis and its reef on SW Pacific seamounts. Coral Reefs 33:31–38
Fiala-Medioni A, Madurell T, Romans P, et al (2012) Rov and submersible surveys on faunal assemblages in a deep-sea canyon (rech Lacaze-Duthiers, western Mediterranean Sea). Vie Milieu 62:173–190
Fink HG, Wienberg C, Hebbeln D, et al (2012) Oxygen control on Holocene cold-water coral development in the eastern Mediterranean Sea. Deep-Sea Res Part 1 Oceanogr Res Pap 62:89–96
Form AU, Riebesell U (2012) Acclimatation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob Chang Biol 18:843–853
Försterra G, Häussermann V (2003) First report on large scleractinian (Cnidaria: Anthozoa) accumulations in cold-temperate shallow water of south Chilean fjords. Zool Verh Leiden 345:117–128
Freiwald A, Henrich R, Pätzold J (1997) Anatomy of a deep-water coral reef mound from Stjernsund, West Finnmark. North Nor SEPM Spec Publ 56:141–162
Freiwald A, Fossa JH, Grehan A, et al (2004) Cold-water coral reefs: out of sight – no longer out of mind. UNEP-WCMC, Cambridge, 86 p
Galli G, Bramanti L, Priori C, et al (2016) Modelling red coral (Corallium rubrum) growth in response to temperature and nutrition. Ecol Model 337:137–148
Gass SE, Roberts JM (2006) The occurrence of the cold-water coral Lophelia pertusa (Scleractinia) on oil and gas platforms in the North Sea: colony growth, recruitment and environmental controls on distribution. Mar Pollut Bull 52:549–559
Gass SE, Roberts JM (2011) Growth and branching patterns of Lophelia pertusa (Scleractinia) from the North Sea. J Mar Biol Assoc UK 91:831–835
Glazier DS (2005) Beyond the: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev 80:611
Gori A, Orejas C, Madurell T, et al (2013) Bathymetrical distribution and size structure of cold-water coral populations in the Cap de Creus and Lacaze-Duthiers canyons (northwestern Mediterranean). Biogeosciences 10:2049–2060
Gori A, Reynaud S, Orejas C, et al (2014) Physiological performance of the cold-water coral Dendrophyllia cornigera reveals its preference for temperate environments. Coral Reefs 33:665–674
Gori A, Ferrier-Pagès C, Hennige S, et al (2016) Physiological response of the cold-water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ 4:e1606
Hassenrück C, Jantzen C, Försterra G, et al (2013) Rates of apical septal extension of Desmophyllum dianthus: effect of association with endolithic photo-autotrophs. Mar Biol 160:2919–2927
Hennige SJ, Morrison CL, Form AU, et al (2014a) Self-recognition in corals facilites deep-sea habitat engineering. Sci Rep 4:6782
Hennige SJ, Wicks LC, Kamenos NA, et al (2014b) Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to ocean acidification. Deep-Sea Res Part 2 Top Stud Oceanogr 99:27–35
Hennige SJ, Wicks LC, Kamenos NA, et al (2015) Hidden impacts of ocean acidification to live and dead coral framework. Proc R Soc B 282:20150990
Hermann M, Estournel C, Déqué M, et al (2008) Dense water formation in the Gulf of Lions shelf: impact of atmospheric interannual variability and climate change. Cont Shelf Res 28:2092–2112
Heussner S, Durrieu de Madron X, Calafat A, et al (2006) Spatial and temporal variability of download particle fluxes on a continental slope: lessons from an 8-yr experiment in the Gulf of Lions (NW Mediterranean). Mar Geol 234:63–92
Hovland M, Mortensen PB (1999) Norske korallrev og prosesser i havbunnen (Norwegian coral reefs and seebed processes). J Grieg, Bergen, p 167
IPCC (2014) In: Pachauri RK, Meyer LA (eds) Climate change 2014: synthesis report. Contribution of working groups I, II and III tot the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, 151 p
Johnston IS (1980) The ultrastructure of skeletogenesis in hermatypic corals. Int Rev Cytol 67:171–214
Keller NB, Os’kina NS (2008) Habitat temperature ranges of azooxantellate scleractinian corals in the world ocean. Oceanology 48:77–84
Kiriakoulakis K, Fisher E, Wolff GA, et al (2005) Lipids and nitrogen isotopes of two deep-water corals from the North-East Atlantic: initial results and implications. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, Heidelberg, pp 715–729
Knutson DW, Buddemeier RW, Smith SV (1972) Coral chronometers – seasonal growth bands in corals. Science 177:270–272
Larcom AA, McKean DL, Brooks JM, et al (2014) Growth rates, densities, and distribution of Lophelia pertusa on artificial structures in the Gulf of Mexico. Deep-Sea Res Part 1 Oceanogr Res Pap 85:101–109
Larsson AI, van Oevelen D, Purser A, et al (2013) Tolerance to long-term exposure of suspended benthic sediments ad drill cuttings in the cold-water coral Lophelia pertusa. Mar Pollut Bull 70:176–188
Lartaud F, Pareige S, de Rafelis M, et al (2013) A new approach for assessing cold-water coral growth in situ using fluorescent calcein staining. Aquat Living Resour 26:187–196
Lartaud F, Pareige S, de Rafelis M, et al (2014) Temporal changes in the growth of two Mediterranean cold-water coral species, in situ and in aquaria. Deep-Sea Res Part 2 Top Stud Oceanogr 99:64–70
Lartaud F, Galli G, Raza A, et al (2017a) Growth patterns in long-lived coral species. In: Rossi S, Bramanti L, Gori A, et al (eds) Marine animal forests: the ecology of benthic biodiversity hotspots. Springer, Cham, pp 595–626
Lartaud F, Meistertzheim AL, Peru E, et al (2017b) In situ growth experiments of reef-building cold-water corals: the good, the bad and the ugly. Deep-Sea Res Part 1 Oceanogr Res Pap 121:70–78
Lastras G, Canals M, Ballesteros E, et al (2016) Cold-water corals and anthropogenic impacts in La Fonera submarine canyon head, Northwestern Mediterranean Sea. PLoS One 11:e0155729
Lazier AV, Smith JE, Risk MJ, et al (1999) The skeletal structure of Desmophyllum cristagalli: the use of deep-water corals in sclerochronology. Lethaia 32:119–130
Le Tissier MDA, Clayton B, Brown BE, et al (1994) Skeletal correlates of coral density banding and an evaluation of radiography as used in sclerochronology. Mar Ecol Progr Ser 110:29–44
Levin LA, Le Bris N (2015) The deep ocean under climate change. Science 350:766–768
Lopez-Correa M, Montagna P, Vendrell-Simon B, et al (2010) Stable isotopes (δ18O and δ13C), trace and minor element compositions of Recent scleractinians and Last Glacial bivalves at the Santa Maria di Leuca deep-water coral province, Ionian sea. Deep-Sea Res Part 2 Top Stud Oceanogr 57:471–486
Maier C, Hegeman J, Weinbauer MG, et al (2009) Calcification of the cold-water coral Lophelia pertusa under ambient and reduced pH. Biogeosciences 6:1671–1680
Maier C, Schubert A, Berzunza Sànchez MM, et al (2013) End of the century pCO2 levels do not impact calcification in Mediterranean cold-water corals. PLoS One 8:e62655
Maier C, Popp P, Sollfrank N, et al (2016) Effects of elevated pCO2 and feeding on net calcification and energy budget of the Mediterranean cold-water coral Madrepora oculata. J Exp Biol 219:3208–3217
Marali S, Wisshak M, Lopez-Correa M, et al (2013) Skeletal microstructure and stable isotope signature of three bathyal solitary cold-water corals from the Azores. Palaeogeogr Palaeoclimatol Palaeoecol 373:25–38
Marin F, Luquet G, Marie B, et al (2008) Molluscan shell proteins: primary structure, origin, and evolution. Curr Top Dev Biol 80:209–276
McCulloch M, Trotter J, Montagna P, et al (2012) Resilience of cold- water scleractinian corals to ocean acidification: boron isotopic systematics of pH and saturation state up-regulation. Geochim Cosmochim Acta 87:21–34
Meistertzheim AL, Lartaud F, Arnaud-Haond S, et al (2016) Patterns of bacteria- host associations suggest different ecological strategies between two reef building cold-water coral species. Deep-Sea Res Part 1 Oceanogr Res Pap 114:12–22
Mikkelsen N, Erlenkeuser H, Killingley JS, et al (1982) Norwegian corals: radiocarbon and stable isotopes in Lophelia pertusa. Boreas 11:163–171
Miller MW (1995) Growth of a temperate coral: effects of temperature, light, depth, and heterotrophy. Mar Ecol Progr Ser 122:217–225
Montero-Serrano JC, Frank N, Tisnérat-Laborde N, et al (2013) Decadal changes in the mid-depth water mass dynamic of the Northeastern Atlantic margin (Bay of Biscay). Earth Planet Sci Lett 364:134–144
Mortensen PB (2001) Aquarium observations on the deep-water coral Lophelia pertusa (L., 1958) (scleractinia) and selected associated invertebrates. Ophelia 54:83–104
Mortensen PB, Rapp HT (1998) Oxygen and carbon isotope ratios related to growth line patterns in skeletons of Lophelia pertusa (L.) (Anthozoa, Scleractinia): implications for determining of linear extension rates. Sarsia 83:433–446
Mouchi V, Crowley QG, Jackson AL, et al (2014) Potential seasonal calibration for palaeoenvironmental reconstruction using skeletal microstructures and strontium measurements from the cold-water coral Lophelia pertusa. J Quat Sci 29:803–814
Mouchi V, Vonlanthen P, Verrecchia EP, et al (2017) Multi-scale crystallographic ordering in the cold-water coral Lophelia pertusa. Sci Rep 7:8987
Movilla J, Gori A, Calvo E, et al (2014a) Resistance of two Mediterranean cold-water coral species to low-pH conditions. Water 5:59–67
Movilla J, Orejas C, Calvo E, et al (2014b) Differential response of two Mediterranean cold-water coral species to ocean acidification. Coral Reefs 33:675–686
Mueller CE, Lundälv T, Middelburg JJ, et al (2013) The symbiosis between Lophelia pertusa and Eunice norvegica stimulates coral calcification and worm assimilation. PLoS One 8:e58660
Naumann MS, Orejas C, Ferrier-Pagès C (2013) High thermal tolerance of two Mediterranean cold-water coral species maintained in aquaria. Coral Reefs 32:749–754
Naumann MK, Orejas C, Ferrier-Pagès C (2014) Species-specific physiological response by the cold-water corals Lophelia pertusa and Madrepora oculata to variations within their natural temperature range. Deep-Sea Res Part 2 Top Stud Oceanogr 99:36–41
Orejas C, Gori A, Gili JM (2008) Growth rates of live Lophelia pertusa and Madrepora oculata from the Mediterranean Sea maintained in aquaria. Coral Reefs 27:255
Orejas C, Gori A, Lo Iacono C, et al (2009) Cold-water corals in the Cap de Creus canyon, northwestern Mediterranean: spatial distribution, density and anthropogenic impact. Mar Ecol Progr Ser 397:37–51
Orejas C, Ferrier-Pagès C, Reynaud S, et al (2011a) Long-term growth rates of four Mediterranean cold-water coral species maintained in aquaria. Mar Ecol Progr Ser 429:57–65
Orejas C, Ferrier-Pagès C, Reynaud S, et al (2011b) Experimental comparison of skeletal growth rates in the cold-water coral Madrepora oculata Linnaeus, 1758 and three tropical sceractinian corals. J Exp Mar Biol Ecol 405:1–5
Palanques A, Durrieu de Madron X, Puig P, et al (2006) Suspended sediment fluxes and transport processes in the Gulf of Lions submarine canyons. The role of storms and dense water cascading. Mar Geol 234:43–61
Palmiéri J, Orr JC, Dutay JC, et al (2015) Simulated anthropogenic CO2 storage and acidification of the Mediterranean Sea. Biogeosciences 12:781–802
Pätzold J (1984) Growth rythms recorded in stable isotopes and density bands in the reef coral Porites lobata (Cebu, Philippines). Coral Reefs 3:87–90
Pons-Branchu E, Hillaire-Marcel C, Deschamps P, et al (2005) Early diagenesis impact on precise U-series dating of deep-sea corals: example of a 100–200 year old Lophelia pertusa sample from the Northeast Atlantic. Geochim Cosmochim Acta 69:4865–4879
Raddatz J, Liebetrau V, Rüggeberg A, et al (2013) Stable Sr-isotope, Sr/Ca, Mg/Ca, Li/Ca and Mg/Li ratios in the scleractinian cold-water coral Lophelia pertusa. Chem Geol 352:143–152
Ramirez-Llodra E, Brandt A, Danovaro R, et al (2010) Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7:2851–2899
Reed JK (2002) Deep-water Oculina coral reefs of Florida: biology, impacts, and management. Hydrobiologia 471:43–55
Reitner J (2005) Calcifying extracellular mucus substances (EMS) of Madrepora oculata – a first geobiological approach. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, Heidelberg, pp 731–744
Risk MJ, Heikoop JM, Snow MG, et al (2002) Lifespans and growth patterns of two deep-sea corals: Primnoa resedaeformis and Desmophyllum cristagalli. Hydrobiologia 471:125–131
Risk MJ, Hall-Spencer J, Williams B (2005) Climate records from the Faroe-Shetland Channel using Lophelia pertusa (Linnaeus, 1758). In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, Heidelberg, pp 1097–1108
Roark EB, Guilderson TP, Dunbar RB, et al (2006) Radiocarbon-based ages and growth rates of Hawaiian deep-sea corals. Mar Ecol Progr Ser 327:1–14
Roberts JM (2002) The occurrence of the coral Lophelia pertusa and other conspicuous epifauna around an oil platform in the North Sea. J Soc Underw Technol 25:83–91
Roberts JM (2005) Reef-aggregating behaviour by symbiotic eunicid polychaetes from cold-water corals: do worms assemble reefs? J Mar Biol Assoc UK 85:813–819
Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–547
Roberts JM, Wheeler A, Freiwald A, et al (2009) Cold-water corals: the biology and geology of deep-sea coral habitats. Cambridge University Press, New York, p 334. https://doi.org/10.1017/CBO9780511581588
Roder C, Berumen ML, Bouwmeester J, et al (2013) First biological measurements of deep-sea corals from the Red Sea. Sci Rep 3:2802
Rodolfo Metalpa R, Montagna P, Aliani S, et al (2015) Calcification is not the Achilles’ heel of cold-water corals in an acidifying ocean. Glob Chang Biol 21:2238–2248
Rollion-Bard C, Blamart D (2014) SIMS method and examples of applications in coral biomineralization. In: DiMasi E, Gower LB (eds) Biomineralization sourcebook: characterization of biominerals and biomimetic materials. Taylor & Francis Group, London, pp 249–258
Rollion-Bard C, Blamart D, Cuif JP, et al (2010) In situ measurements of oxygen isotopic composition in deep-sea coral, Lophelia pertusa: re-examination of the current geochemical models of biomineralization. Geochim Cosmochim Acta 74:1338–1349
Sabatier P, Reyss JL, Hall-Spencer J, et al (2012) 210Pb-226Ra chronology reveals rapid growth rate of Madrepora oculata and Lophelia pertusa on world’s largest cold-water coral reef. Biogeosciences 9:1253–1265
Sherwood O, Scott D, Risk M, et al (2005) Radiocarbon evidence for annual growth rings in the deep-sea octocoral Primnoa resedaeformis. Mar Ecol Progr Ser 301:129–134
Somot S, Sevault F, Deque M (2006) Transient climate change scenario simulation of the Mediterranean Sea for the 21st century using a high-resolution ocean circulation model. Clim Dyn 27:851–879
Stolarski J (2003) Three-dimensional micro- and nanostructural characteristics of the scleractinian coral skeleton: a biocalcification proxy. Acta Palaeontol Pol 48:497–530
Tambutté E, Allemand D, Mueller E, et al (1996) A compartmental approach to mechanisms of calcification in hermatypic corals. J Exp Biol 199:1029–1041
Tambutté S, Holcomb M, Ferrier-Pagès C, et al (2011) Coral biomineralization: from the gene to the environment. J Exp Mar Biol Ecol 408:58–78
Taviani M, Freiwald A, Zibrowius H (2005) Deep coral growth in the Mediterranean Sea: an overview. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, Heidelberg, pp 137–156
van Haren H, Mienis F, Duineveld GCA, et al (2014) High-resolution temperature observations of a trapped nonlinear diurnal tide influencing cold-water corals on the Logachev mounds. Progr Oceanogr 125:16–25
Vertino A, Savini A, Rosso A, et al (2010) Benthic habitat characterization and distribution from two representative sites of the deep-water SML Coral Province (Mediterranean). Deep-Sea Res Part 2 Top Stud Oceanogr 57:380–396
Wainwright SA (1964) Studies of the mineral phase of coral skeleton. Exp Cell Res 34:213–230
Waller RG, Tyler PA (2005) The reproductive biology of two deep-water, reef-building scleractinians from the NE Atlantic Ocean. Coral Reefs 24:514–522
Weiner S, Levi-Kalisman Y, Raz S, et al (2003) Biologically formed amorphous calcium carbonate. Connect Tissue Res 44:214–218
Wienberg C, Hebbeln D, Fink HG, et al (2009) Scleractinian cold-water corals in the Gulf of Cádiz – first clues about their spatial and temporal distribution. Deep-Sea Res Part 1 Oceanogr Res Pap 56:1873–1893
Wilson JB (1979) Patch’ development of the deep-water coral Lophelia pertusa (L.) on Rockall Bank. J Mar Biol Assoc UK 59:165–177
Cross-References
Aymà A, Aguzzi J, Canals M, et al (this volume) Occurrence of living cold-water corals at large depths within submarine canyons of the Northwestern Mediterranean Sea
Chimienti G, Bo M, Taviani M, et al (this volume) Occurrence and biogeography of Mediterranean cold-water corals
D’Onghia G (this volume) Cold-water coral as shelter, feeding and life-history critical habitats for fish species: ecological interactions and fishing impact
Fourt M, Goujard A, Chevaldonné P (this volume) Working with visual methods, comparison among the french deep-sea Canyons
Lastras G, Sanchez-Vidal A, Canals M (this volume) A cold-water coral habitat in La Fonera submarine Canyon, Northwestern Mediterranean Sea
Maier C, Weinbauer MG, Gattuso JP (this volume) Fate of Mediterranean scleractinian cold-water corals as a result of global climate change. A synthesis
Movilla J (this volume) A case study: variability in the calcification response of Mediterranean cold-water corals to ocean acidification
Orejas C, Taviani M, Ambroso S, et al (this volume) Cold-water coral in aquaria: advances and challenges. A focus on the Mediterranean
Puig P, Gili JM (this volume) Submarine Canyons in the Mediterranean: a shelter for cold-water corals
Reynaud S, Ferrier-Pagès C (this volume) Biology and ecophysiology of Mediterranean cold-water corals
Rueda JL, Urra J, Aguilar R, et al (this volume) Cold-water coral associated fauna in the Mediterranean sea and adjacent areas
Taviani M, Vertino A, Angeletti L, et al (this volume) Paleoecology of Mediterranean cold-water corals
Acknowledgements
FL, LC and NLB works on CWC have been supported under the chair ‘Biodiversity, extreme marine environments and global change’ of UPMC supported by Fondation Total. LC doctoral contract is supported by UPMC (ED129 Doctoral school of environmental sciences). VM work presented in this paper was made possible by the ENS Ph.D. programme. The Earth and Natural Sciences Doctoral Studies Programme is funded under the Programme for Research in Third-Level Institutions Cycle-5 and co-funded under the European Regional Development Fund. Autun Purser is acknowledged for his support in editing the English language of the manuscript. We also thank the guest editor for his advices and inviting us to contribute to this chapter, and two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Lartaud, F., Mouchi, V., Chapron, L., Meistertzheim, AL., Le Bris, N. (2019). 36 Growth Patterns of Mediterranean Calcifying Cold-Water Corals. In: Orejas, C., Jiménez, C. (eds) Mediterranean Cold-Water Corals: Past, Present and Future. Coral Reefs of the World, vol 9. Springer, Cham. https://doi.org/10.1007/978-3-319-91608-8_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-91608-8_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91607-1
Online ISBN: 978-3-319-91608-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)