Abstract
This study evaluated the suitability of groundwater quality for domestic use and irrigation in Vinh Chau District, Soc Trang Province, Vietnam. Water samples from 30 domestic-use and 10 irrigation groundwater wells were collected and their physicochemical parameters were analyzed. Results indicated that, based on WHO and Vietnamese standards, the groundwater quality had good suitability, generally to the medium level, but for drinking purposes some areas were found to require water treatment. Six water quality parameters—sodium adsorption ratio, soluble sodium percentage, permeability index, sodium percentage, residual sodium carbonate, and magnesium adsorption ratio—were calculated and employed for evaluating suitability for irrigation, in addition to electric conductivity. The results showed that the six parameters stayed at the permissible level for irrigation, whereas high sodium and salinity were a concern for irrigation. Use of the Piper trilinear diagram to analyze hydrochemical facies and groundwater types showed that the groundwater samples were in the sodium-potassium-chloride-sulfate category and that sodium water type was dominant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The Mekong Delta is one of the most vulnerable regions in the world, especially under the impacts of climate change. Until now, surface water has been the primary water supply source for domestic use and agriculture in the area. However, the changing hydrological regime upstream of the Mekong Delta has caused drought in recent years, which has impacted quality and quantity of surface water (Tuan et al. 2007). Therefore, groundwater is being considered as an option for domestic use and irrigation in the Mekong Delta, especially in the coastal zone, which is the most vulnerable area due to water shortages; here the trend of groundwater use has increased strongly (Nam et al. 2017). The quality of groundwater is as important as its quantity in evaluating the suitability of water for various purposes (Schiavo et al. 2006). However, the aquifer is heavily polluted with microbial and inorganic pollutants and is considered unfit for drinking water (Danh 2008). In addition, concentrations of natural contaminants and saltwater intrusion have caused a decline in groundwater quality, and it may not be suitable either for domestic water supply or for irrigation (IUCN- International Union for Conservation of Nature 2011). The major factors driving a decline in the quality of groundwater in the Mekong Delta are poor environmental practices in the delta contributing to surface and aquifer pollution; overexploitation, inducing seawater intrusion and mixing and concentration of contaminants; and poor well construction that creates a direct pathway for inferior-quality aquifer water and surface pollutants to mix with an otherwise good-quality aquifer (IUCN- International Union for Conservation of Nature 2011). Groundwater used for domestic and irrigation purposes can vary greatly in quality, depending on the type and quantity of dissolved salts (Sarath Prasanth et al. 2012). The chemical quality of groundwater is related to the geological history of the aquifers and can reveal important information on the suitability for domestic and agricultural use (Povinec et al. 2006). Therefore, understanding hydrochemical characteristics is crucial for groundwater planning and management.
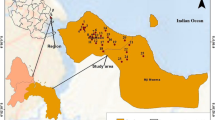
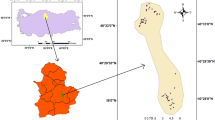
The study area, Vinh Chau District (Fig. 10.1), is located in the coastal zone of Soc Trang Province, with a mean land surface elevation of about 1.0 m above mean sea level, and land use is mainly for agricultural production. This is a former salinity-controlled area where rice farming systems dominated. From the year 2000 onward, farmers protested against the protection of the salinity control measures, leading to a diversification of local land use (Kakonen 2008). At the present, serious impacts of salinity intrusion on surface water resources and freshwater shortages are increasing (DONREs – Department of Natural Resources and Environment of SocTrang 2012). Therefore, groundwater has been accessed as the water supply resource for domestic use and agricultural production (Nam et al. 2017). In addition, the study area belongs to a vulnerable zone of climate change impacts, and sea level rise can increase salt intrusion (Nhan et al. 2008). Those issues have been recognized by the local government and people through environmental education programs (DONREs – Department of Natural Resources and Environment of SocTrang 2012). However, due to a lack of experiment and practical studies on the environment in general and on groundwater in particular, local governments are unable to make effective strategic plans for natural resource protection and management (Department of Environment in Vinh Chau 2011).
2 Materials and Methods
2.1 Sample Collection
Groundwater samples were collected from 10 wells for irrigation (samples VC1–VC10) and 30 wells for domestic use (samples D1–D30), ranging in depth between 90 and 120 m below ground level, during the post-monsoon season. The location of sampling points is shown in Fig. 10.2. High-density polyethylene bottles were used for sample collection. Bottles were completely filled with water, taking care that no air bubbles were trapped within the water samples. Bottles were sealed with double plastic caps, and precautions were taken to prevent evaporation and avoid sample agitation during transfer to the laboratory. Samples were then immediately transferred to the laboratory. During sample collection, standard procedures recommended by the American Public Health Association (APHA) were followed to ensure data quality and consistency.
2.2 Laboratory Measurements
Temperature, electrical conductivity (EC), pH, and dissolved oxygen were measured in situ with the use of appropriate multiparameter instruments at the same points of water sample collection. The labeled samples were analyzed in the laboratory for concentrations of major ions (Ca, Mg, Na, K, HCO3, SO4, Cl, F) using APHA standard methods (APHA – American Public Health Association 1995). Sodium (Na) and potassium (K) were determined by flame photometer. Total hardness as CaCO3, calcium (Ca2+), magnesium (Mg2+), bicarbonate (HCO3 −), and chloride (Cl) were analyzed by volumetric methods, and sulfates (SO4 2−) were estimated by using the colorimetric method.
2.3 Classification Methods
The suitability of groundwater for agricultural purposes was evaluated using the following parameters: sodium adsorption ratio (SAR), soluble sodium percentage (SSP), permeability index (PI), sodium percentage (%Na), residual sodium carbonate (RSC), and magnesium adsorption ratio (MAR). The results of the analyses were interpreted using graphical representations from the U.S. Salinity Laboratory and the Wilcox diagram. To determine the suitability of domestic water supply, we compared the values of different water quality parameters with those of the World Health Organization (WHO) (2004) and the Vietnamese standard (QCVN09) (MONRes – Ministry of Natural Resources and Environment 2015). The results of the analyses are represented using a Piper diagram of the relationships between the different cations and anions.
3 Results and Discussion
3.1 Domestic Water Supply
The following analytical results were considered to determine the suitability of groundwater in the study area for domestic water supply. The chemical analysis results of groundwater samples are presented in Table 10.1.
pH
The pH of a solution is the negative logarithm of the hydrogen ion concentration, and the pH scale commonly ranges from 0 to 14. Acids have lower pH values, from 0 to 7, and alkaline solutions have higher pH values, from 7 to 14; 7 is neutral. Drinking water pH mainly ranges from 4.4 to 8.5 (Devendra et al. 2014). The pH of water provides vital information for many types of geochemical equilibrium or solubility calculations (Hem 1985). The pH values in the study area met the standard value for drinking water, specified as 6.5–8.5 (WHO) (WHO – World Health Organization 2004) and 5.5–8.5 (QCVN09) (MONRes – Ministry of Natural Resources and Environment 2015) (see Table 10.1). Four sample points had pH <7 (6.6–6.8); most of the groundwater samples had pH >7 (7.1–8.4); thus, the groundwater in the study area is slightly alkaline. Alkalinity of groundwater may be due to the presence of one or more of a number of ions, including hydroxides, carbonates, and bicarbonates. The phenomenon may be attributed to salt intrusion, which affects pH. Alkalinity results when the salts of a strong base and a weak acid are dissolved in the water.
Sulfate
Natural water contains sulfate ions, and most of these are soluble in water. Sulfate is a combination of sulfur and oxygen and is part of naturally occurring minerals in some soil and rock formations that contain groundwater. The mineral dissolves over time and is released into groundwater. The maximum contaminant level is 400 mg/L (WHO and QCVN09) (WHO – World Health Organization 2004; MONRes – Ministry of Natural Resources and Environment 2015). The sulfate concentration in the study area ranged between 53 and 250 mg/L (Table 10.1), with an average of 146.5 mg/L, indicating that all samples fell within the desirable limit.
Nitrate
Nitrogen is present in raw water, mainly in the form of HNO3 (in its oxidized state). Levels of nitrates in groundwater in the study area ranged between 0.0 and 5.4 mg/L (Table 10.1), implying that all samples were within safe levels proposed by WHO (50 mg/L) and QCVN09 (15 mg/L) (WHO – World Health Organization 2004; MONRes – Ministry of Natural Resources and Environment 2015).
Chloride
Chloride is a negative ion of the element chlorine (Cl) and is widely distributed in the environment. Chloride is found naturally in groundwater through the weathering and leaching of sedimentary rocks and soils and the dissolution of salt deposits. Chloride concentrations in some groundwater wells of the study area were slightly higher than the WHO and QCVN09 standard of 250 mg/L (WHO – World Health Organization 2004; MONRes – Ministry of Natural Resources and Environment 2015) (Table 10.1), which may be caused by saltwater intrusion. The average chloride concentration was about the 205 mg/L, with a maximum of 480 mg/L and minimum of 113 mg/L.
Iron
Iron (Fe) is a metal that occurs naturally in soils, rocks, and minerals. In the aquifer, groundwater comes in contact with these solid materials, dissolving them, releasing their constituents, including Fe, to the water. Samples at D1, D3, and D17 had very high Fe values. At concentrations approaching 0.2 mg/L Fe (WHO – World Health Organization 2004), water use efficiency may become seriously affected. However, most of sample points had safe values based on WHO and QCVN09 standards (WHO – World Health Organization 2004; MONRes – Ministry of Natural Resources and Environment 2015).
Chemical Oxygen Demand (COD)
COD is a measure of the oxygen required for the chemical oxidation of organic matter with the help of a strong chemical oxidant. In the study area, the COD of most groundwater samples indicated organic contamination (Table 10.1). This clearly demonstrates that the contamination generated from the surface is affecting groundwater quality in the adjacent areas through percolation in the subsoil.
Total Coliform
Normally, groundwater does not contain this contaminant, and most samples in the study area were negative. However, coliform was found in wells D8, D12, D19, and D30 at very low concentrations. The presence of COD may indicate recent contamination of the groundwater by human sewage in the study area.
3.2 Irrigation Suitability
To assess overall water quality for irrigation, we assessed the water quality parameters SAR, SSP, PI, %Na, RSC, and MAR. In addition, EC is a good measure of salinity hazard to crops, and its relationship with water quality parameters also reflects the groundwater classification. Their corresponding values are presented in Table 10.2.
3.2.1 Sodium Adsorption Ratio (SAR)
SAR is a measure of the sodicity of the soil determined through quantitative chemical analysis of water in contact with it. Excess HCO3 − and CO3 2− ions in water react with Na+ in soil, resulting in a sodium hazard (Subramani et al. 2005). SAR values are plotted against EC values (in μmhos/cm) over the U.S. Salinity diagram to categorize analyzed water samples according to their irrigational suitability quotient. SAR was calculated as
where concentrations of all ions are expressed in meq/L.
In the study area, the SAR values ranged from 15.90 to 31.73. Based on the SAR values, all samples have high sodium hazard. Plotting the results on the U.S. Salinity diagram (Fig. 10.3) shows that 80% of groundwater samples fall in the C3-S4 category, and 20% fall in the C4-S4 category, which means very high sodium content and high salinity in the sampled groundwater. For irrigation purposes, salinity should be controlled and plants with good salt tolerance should be proposed in the study area.
3.2.2 Permeability Index (PI)
Based on the PI, a water suitability classification for irrigation water was developed (Doneen 1964). The PI was calculated as
where all the ions are expressed in meq/l.
The PI values vary from 56.72 to 79.25, with the average value of about 65.00. A classification based on PI as recommended by the WHO for assessing suitability of irrigation (WHO – World Health Organization 2008) reveals that all samples belong to class 2, with PI ranging between 25% and 75%.
3.2.3 Sodium Percentage (%Na)
The %Na in the groundwater samples collected varied from 53.73 to 76.57 (Table 10.2). For rating irrigation water, the Wilcox diagram was used, in which the %Na is plotted against EC. Figure 10.4 shows the diagram plot, which indicates that 80% of the groundwater samples fell in the “Permissible to doubtful” range and 20% in the “Doubtful to unsuitable” range. The source of Na+ in the groundwater has been attributed to the weathering of feldspar and to overexploitation of groundwater.
3.2.4 Soluble Sodium Percentage (SSP)
SSP values preferably should be less than 60% for water to be suitable for irrigation purposes. In the present study SSP values ranged between 53.73% and 76.57%, with 50% of the water samples <60% and 50% exceeding the permissible level (U.S. Salinity Laboratory 1954) (Table 10.3).
3.2.5 Residual Sodium Carbonate (RSC)
The RSC index signifies the alkalinity hazard posed by water and indicates suitability of water for irrigation of clay soils (Raju 2007). The RSC values in the present study ranged from −122.43 to −35.75. Based on the U.S. Salinity Laboratory’s classification level (U.S. Salinity Laboratory 1954), 100% of water samples were at suitable levels (Table 10.4).
3.2.6 Magnesium Hazard Ratio
MAR categorizes water into two broad classes: MAR <50 is considered suitable for irrigation, whereas MAR >50 is considered high magnesium hazard and has an adverse effect on crop yield. In this study 50% of groundwater samples were in safe level and 50% indicated water sources unsuitable for irrigation (Table 10.2).
3.3 Hydrochemical Facies
Figure 10.5 is a modification of the Piper diagram with a view to extend its applicability in representing water analysis in the simplest possible way. A Piper diagram is a graphical representation classifying water based on the dominant presence of cations and anions and has widespread use in assessing water type. The results are plotted on the proposed diagram to test its applicability for geochemical classification of groundwater and hydrochemical facies. The values obtained for groundwater types in the study area plotted on the Piper diagrams reveal that the major groundwater type is Na – K – Cl – SO4 and sodium.
4 Conclusions
This study examined groundwater quality by assessing samples from 30 domestic water supply sources and 10 irrigation sources in the coastal zone of the Mekong Delta (Figs. 10.1 and 10.2). Based on our results, conclusions can be summarized as follows:
-
1.
Chemical analyses showed that, based on WHO and Vietnamese standards, the groundwater quality had good suitability, generally to the medium level, but for drinking purposes some areas were found to require water treatment.
-
2.
According to six computed groundwater quality indicators—sodium adsorption ratio (SAR), soluble sodium percentage (SSP), permeability index (PI), sodium percentage (%Na), residual sodium carbonate (RSC), and magnesium adsorption ratio (MAR)—the groundwater quality was found to be suitable to permissible for irrigation. However, in terms of sodium and salinity hazards, most testing locations were unsuitable for irrigation.
-
3.
Regarding hydrochemical facies of groundwater, the groundwater samples fell under the sodium-potassium-chloride-sulfate category, and there is a dominance of sodium water type.
References
APHA – American Public Health Association. (1995). Standard methods for the examination for water and wastewater (19th ed.pp. 211–281). Washington, DC: Byrd Prepress Springfield.
Danh V. T. (2008). Household switching behavior in the use of groundwater in the Mekong Delta (p. 115), Economy and Environment Program for Southeast Asia (EEPSEA) Research Report rr2008121.
Department of Environment in Vinh Chau. (2011). Annual environment report, pp 100–184 (in Vietnamese).
Devendra, D., Shriram, D., & Atul, K. (2014). Analysis of ground water quality parameters: A review. Research Journal of Engineering Sciences, 3(5), 26–31. ISSN 2278-9472.
Doneen, L. D. (1964). Notes on water quality in agriculture (p. 400). Davis: Department of Water Science and Engineering, University of California, Water Science and Engineering.
DONREs – Department of Natural Resources and Environment of SocTrang. (2012). Annual report of natural resource and environment. pp. 10–184 (in Vietnamese).
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural water (p. 2254, 3rd ed.). US Geological Survey Water-supply Paper.
IUCN- International Union for Conservation of Nature. (2011). Groundwater in the Mekong Delta (Discussion paper) (online). https://cmsdata.iucn.org/downloads/iucn_groundwater_in_mekong_delta_25_may_11_w.pdf
Kakonen, M. (2008). Mekong Delta at the crossroads: More control or adaptation? Ambio, 37(3), 205–212.
MONRes – Ministry of Natural Resources and Environment. (2015). Groundwater quality standard of Vietnam (pp. 34–84) (in Vietnamese).
Nam, N. D. G., Goto, A., & Osawa, K. (2017). Groundwater modeling for groundwater management in the coastal area of Mekong Delta. Journal of JSIDRE, Japan, 85, 93–103 Online ISSN: 1884-7242, Print ISSN: 1882-2789.
Nhan, D. K., Be, N. V., & Trung, N. H. (2008). Chapter 4: Water use and competition in the Mekong Delta, Vietnam (pp. 10–18).
Povinec, P. P., Aggarwal, P. K., Aureli, A., Burnett, W. C., Kontar, E. A., Kulkarni, K. M., Moore, W. S., Rajar, R., Taniguchi, M., Comanducci, J. F., Cusimano, G., Dulaiova, H., Gatto, L., Groening, M., Hausser, S., Levy Palomo, I., Oregioni, B., Ozorovich, Y. R., Privitera, A. M. G., & Schiavo, M. A. (2006). Characterisation of submarine groundwater discharge offshore south-eastern Sicily. Journal of Environmental Radioactivity, 89(1), 81–101.
Raju, N. J. (2007). Hydro-geochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Andhara Pradesh, South India, Environmental Geology. p. 8
Sarath Prasanth, S. V., Magesh, N. S., Jitheshlal, K. V., Chandrasekar, N., & Gangadhar, K. (2012). Apply Water Science, 2, 165–175.
Schiavo, M. A., Havser, S., Gusimano, G., & Gatto, L. (2006). Geochemical characterization of groundwater and submarine discharge in the south-eastern Sicily. Continental Shelf Research, 26(7), 826–834.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agriculture use in Chithar River Basin, Tamil Nadu India. Environmental Geology, 47(8), 1099–1110.
Tuan, L. A., Hoanh, C. T., Miller, F., & Sinh, B. T. (2007) Chapter 1: Flood and salinity management in the Mekong Delta, challenges to sustainable development in the Mekong Delta: Regional and national policy issues and research needs. pp. 6–25.
U.S. Salinity Laboratory. (1954). Diagnosis and improvement of saline and alkali soils (Agricultural handbook, USDA, No. 60, p. 160). Washington, DC: U.S. Government Printing Office.
World Health Organization. (2004). Guidelines for drinking-water quality (Vol. 1, 3rd ed.p. 547). Geneva: World Health Organization (WHO) Press.
World Health Organization. (2008). Guidelines for drinking-water qualiy: Recommendations Incorporating first and second addenda (Vol. 1, 3rd ed.p. 668). Geneva: World Health Organization (WHO) Press.
Acknowledgments
The authors would like to acknowledge the Division of Natural Resource and Environment of Vinh Chau District, Department of Natural Resource and Environment of Soc Trang Province, and the Can Tho University College of the Environment and Natural Resources Laboratory for providing data and support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Nam, N.D.G., Akira, G., Kazutoshi, O., Trung, N.H., Ngan, N.V.C. (2019). Assessment of Groundwater Quality and Its Suitability for Domestic and Irrigation Use in the Coastal Zone of the Mekong Delta, Vietnam. In: Stewart, M., Coclanis, P. (eds) Water and Power. Advances in Global Change Research, vol 64. Springer, Cham. https://doi.org/10.1007/978-3-319-90400-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-90400-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90399-6
Online ISBN: 978-3-319-90400-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)