Abstract
Orthopaedic biomaterials are used in a wide variety of surgical procedures including total joint replacement, spine reconstruction, and fracture repair. Despite the development of materials with enhanced mechanical and biological properties, the attachment of an implant to the surrounding bone is still occasionally lost and revision surgery is required in some of the patients with prolonged implantation of orthopaedic biomaterials. The macrophage-associated innate immune response plays a crucial role both in the successful integration and potential rejection of the implant. Acute inflammation is essential for the successful osseointegration and bone regeneration around the implants. Chronic inflammation, on the other hand, is associated with impaired bone formation and osteolysis in the presence of excessive macrophage infiltration and pro-inflammatory cytokine secretion. Here we summarize the current development of immunomodulating strategies to improve the application of orthopaedic biomaterials. The potential drug delivery and controlled release methods that could be applied to administrate immunomodulatory biomolecules are also discussed. In summary, modulations of the innate immune response with the goal of sequential transition from a pro-inflammatory to an anti-inflammatory reaction provides a promising strategy for successful bone regeneration and implant osseointegration.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Orthopedic biomaterials
- Inflammation
- Macrophage polarization
- Immunomodulation
- Wear particles
- Osteolysis
- Bone remodeling
- Tissue engineering
- Mesenchymal stem cell
- Osteoblast
- Osteoclast
- NF-kB
1 Introduction
Musculoskeletal conditions affect millions of people and are the second leading cause of disability worldwide [1]. Through orthopedic care , patients can find pain relief, increased mobility, and improved quality of life, thus bettering the lives of individuals and improving society as a whole. Many orthopedic procedures require implants, and advances over the past half-century have allowed for the development of biomaterials with both enhanced mechanical and biological function [2]. Despite these improvements, many implants do not last forever: up to 15% of total joint implants require operative revision within 15 years of initial surgery [3, 4]. With over one million Americans undergoing total joint replacement annually, there is a need to improve the biological function and longevity of orthopedic biomaterials [5].
It is well known that inflammation is induced by implants and their resulting wear particles . On the other hand, early, transient inflammation is essential for proper bone formation and osseointegration of the implant [6]. This process is mediated through prostaglandins and macrophage-related inflammation, mimicking the natural fracture healing response beginning with acute inflammation and resolving into repair and regeneration of peri-implant tissues [7, 8]. However, if inflammation continues, the body may mount a foreign body chronic inflammatory reaction, leading to enhanced and persisting inflammation, bone resorption, osteolysis, and ultimately implant failure [3]. Wear particles produced from the bearing surface, modular connections, and motion at the interface of the implant and bone can activate the NALP inflammasome, the NF-κB pathway, and toll-like receptors (TLR)-2 and TLR-4 depending on the material, size of the particles, and surface topology, which are reviewed thoroughly by Cobelli et al., Gibon et al., and Lin et al. [4, 9, 10].
Chronic inflammation can arise from aberrant activity of the immune system to clear wear particles . If wear debris are too large for macrophages to remove, macrophages fuse to form multinucleated foreign body giant cells (FBGCs) [11]. Normally, these FBGCs are able to degrade or sequester wear particles, allowing for short-lived inflammation, and eventual resolution and repair; however, if the immune system is overwhelmed by excessive particles of appropriate size, inflammation persists [10]. This inflammation in conjunction with continued micromotion propagates wear debris formation, macrophage and T cell infiltration, and eventual osteolysis [10, 12]. As such, chronic inflammation is a vicious cycle of persistent inflammation, implant wear, and bone loss.
The longevity of an orthopedic implant is dependent upon the implant material, operative procedure, and patient-related factors [3]. Precise modulation of inflammation post-operatively has the potential to increase the lifespan of orthopedic implants. By integrating our understanding of the cellular and molecular mechanisms underlying prolonged inflammation, it is possible to develop optimized biomaterials that not only mitigate chronic inflammation, but also enhance osseointegration, vascularization, mechanical strength, and long-term healing.
2 Inflammation and Immunomodulating Strategy
2.1 Innate Immune Response and Macrophages
Cells of the innate immune system, particularly macrophages , are the main inflammatory mediators that drive successful implant integration or rejection [13, 14]. Macrophages also play a crucial role in tissue maintenance and regulation of inflammation [15, 16]. All tissues contain a specific set of macrophages known as tissue resident macrophages that remove damaged, senescent, and infected cells to maintain tissue homeostasis. These tissue resident macrophages originate from circulating, myeloid-derived, monocytes or, in some cases, are distributed among tissues during embryonic development and are maintained by local pools of precursor cells [17]. Macrophages are specialized to effectively phagocytize and remove cellular debris, microbes, and foreign substances identified as potentially dangerous or pathogenic. These foreign agents are recognized by various families of pattern recognition receptors (PRRs) that are expressed on the cell surface, endosomes, and cytoplasm of macrophages [18, 19]. Activation of PRRs initiates intracellular downstream signaling pathways that activate phagocytosis and can lead to the secretion of various cytokines and chemokines. The degree of this innate immune response depends on the nature and amount of the activating stimuli: phagocytosis of apoptotic cells induces an anti-inflammatory response to maintain tissue homeostasis and immune tolerance, while recognition of necrotic cells, damaged extracellular matrix, or pathogens initiates a pro-inflammatory response [19, 20]. Secreted inflammatory mediators then stimulate further cytokine secretion from other resident cells and recruit more immune cells to the site of inflammation. During acute or chronic inflammation, a large number of bone marrow-derived monocytes are recruited from the circulation to the inflamed tissues and undergo differentiation to inflammatory or tissue regenerative macrophages as guided by the local microenvironment [21].
Following the recognition of threatening agents, macrophages engulf these agents to restrict any deleterious effects on adjacent tissues. This phagocytosis process triggers enzymatic degradation of the engulfed material inside macrophages in some cases and, in the case of foreign biological structures, leads to antigen presentation to activate the adaptive immune response. As regulated by macrophages , the immune system thus attempts to efficiently dispose of the harmful substance, kill the microbes encountered, and prevent further tissue injury. As an inflammatory reaction proceeds and the danger becomes resolved, macrophages begin to promote tissue healing and regeneration by secreting extra-cellular matrix precursors, a range of growth factors such as vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF), and anti-inflammatory cytokines [22]. The cytokine signaling in the local microenvironment thus coordinates the development of an immune response and macrophage function [23].
2.2 Macrophage Polarization
Macrophages are able to undergo functional changes as instructed by the surrounding cytokine milieu in tissues [16]. These phagocytes assume a distinct phenotype with divergent inflammatory, fibrotic, and regenerative properties necessary for different phases of inflammation and tissue regeneration. Initially, macrophages become activated to a pro-inflammatory phenotype following the recognition of a stimulating agent by PRRs. The specific factors promoting this classical macrophage activation, known as M1 polarization , is comprised of endogenous danger signals released from necrotic cells and pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), released from various invading micro-organisms. Activators with a similar effect also include cytokines, especially, interferon gamma (IFN-γ), granulocyte-monocyte colony stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α) [24]. Macrophages with a pro-inflammatory phenotype are essential for the early phase of repair, but prolonged inflammation by these M1 macrophages exacerbate tissue injury by actively phagocytizing potential pathogens, killing intracellular microbes by producing oxygen and nitrogen radicals, and vigorously secreting more inflammatory cytokines and chemokines.
In contrast, alternatively activated macrophages, also known as M2 polarization , have tissue regenerative, pro-fibrotic, and anti-inflammatory characteristics. Various subsets of this phenotype are induced by a combination of cytokines such as interleukin-4 (IL-4), IL-10, IL-13, transforming growth factor beta (TGF-β), glucocorticoids or macrophage colony-stimulating factor (M-CSF) [25]. For example, macrophages treated with IL-4 and IL-13 produce minimal amounts of pro-inflammatory cytokines, and their other secretory products stimulate cell growth and proliferation as well as collagen formation. Hence, M1 and M2 polarization are considered to represent the opposite ends of the continuum of macrophage phenotypes [16, 26]. Whereas M1 polarized macrophages predominate in a strong pro-inflammatory phase at an early stage of an inflammation, M2 polarization gradually takes over when the intrusive agents become cleared. The tissue under inflammatory signaling likely contains macrophages with mixed phenotypes, and crosstalk between these cells enables a proper healing process and the resolution of the inflammation.
2.3 Interaction Between Macrophages and Orthopedic Biomaterials
Tissue injury caused by surgical insertion of an orthopedic implant initially activates the immune system, but with time, the implant itself mediates inflammation as a foreign body [27]. The initial recognition of a biomaterial and the tissue trauma caused by the implantation is primarily performed by resident macrophages, which subsequently become activated to a pro-inflammatory phenotype and initiate an inflammatory response. Since many orthopedic implants, such as joint replacements, are generally designed for permanent tissue and bone integration, they are biologically non-degradable, and might provide a constant stimulus for macrophage activation. In particular, the release of particulate materials of a phagocytosable size (<10 μm in diameter) has proven to provide a constant stimulus for inflammatory macrophage activation; these phagocytosable particles have been shown to induce endosomal damage with subsequent activation of intracellular danger sensing mechanisms [28, 29]. A prolonged presence of M1 macrophages leads to an increased inflammatory status, fibrosis, and granulomatous tissue around the implant—a condition called the chronic foreign body reaction [11, 30].
The long-lasting inflammatory events at the bone-implant interface have been observed to significantly affect the bone repair process and cause implant failures via osteolysis [31, 32]. At the cellular level, pro-inflammatory mediators such as TNF-α favor bone resorption over bone formation by increasing the production of Receptor Activator of NF-κB Ligand (RANKL) and decreasing the production of osteoprotegerin (OPG) resulting in an altered RANKL/OPG ratio [33]. RANKL efficiently stimulates the activation and proliferation of osteoclast precursors whereas OPG acts as a decoy receptor inhibiting RANKL signaling. Sustained inflammation thus drives osteoclast formation and ultimately failure of the implant.
As implant-mediated inflammation closely involves adverse tissue reactions and bone regeneration, novel approaches for biomaterial engineering are being developed: a new generation of orthopedic biomaterials should be able to modulate the immune environment in order to favor osseointegration of the implant [34]. This immunomodulating strategy aims to extend the lifespan of the implant by minimizing the destructive and maximizing the regenerative effects of the immune response induced by the implant.
2.4 Modulation of Macrophage-Mediated Pro-Inflammatory Response
Macrophages are a prime target for immunomodulation in the application of orthopedic biomaterials. This is not only because these cells play an essential role in initiating and regulating the implant-mediated immune responses, but also because of their considerable heterogeneity and plasticity enable the modulation of their function [16, 30]. Methods controlling macrophage activation could discourage increases in the pro-inflammatory signaling, avoid excess fibrosis, and prevent bone loss around the implants. Thus, new therapeutic interventions are being pursued with the purpose of controlling chronic inflammation associated with implant materials by modulating macrophage polarization and thus their secretory products. Whereas continuous M1 activation impairs integration of the implant, signals that suppress the pro-inflammatory effects and support M2 polarization have emerged as an attractive means to facilitate implant integration [35, 36].
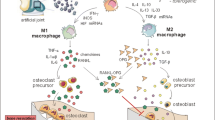
Several different strategies for macrophage-targeted immunomodulation around implants have been developed (Fig. 1) [37]. Since the degree of an implant-mediated immune reaction depends on the biomaterial characteristics, beneficial effects on macrophage function and implant integration may be achieved by modifying the physical and chemical properties of the biomaterial. For instance, the specific surface structure of the implant material and the amount of wear products accumulating in the surrounding tissues are important variables that determine the type of macrophage activation. Surface topography of the implant can be optimized for porosity, roughness, hydrophilicity, and the ability to produce wear particles in order to decrease initial monocyte adhesion and activation [34]. These micro- and nanoscale material characteristics largely determine the folding of absorbed proteins on the implant and consequent presentation of bioactive sites for macrophages. Moreover, TGF-β and PDGF directly modulate macrophage function and chemotaxis during wound healing without a foreign body and may play a similar role in peri-implant biology [38]. Implants loaded with these molecules could thus theoretically promote tissue and bone regeneration both directly and indirectly.
Strategies to modulate the innate inflammatory reactions against orthopedic biomaterials. 1. Optimize biomaterial characteristics, e.g. surface roughness, porosity, and generation of wear particles , 2. Delivery of macrophage polarizing cytokines to drive the anti-inflammatory M2 macrophage polarization, 3. Inhibition of pro-inflammatory cytokines such as TNF-α, 4. Blockade of the transcription factor NF-κB, 5. Inhibiting chemokines such as CCL2 to suppress monocyte recruitment, and 6. Coupling biomaterials with anti-inflammatory and bioactive molecules
Incorporation of immunomodulatory agents into the implant constitutes another major strategy to modulate innate immune reactions. For example, macrophage-mediated inflammation could be controlled by the local release of M2 polarizing cytokines IL-4, IL-10, or IL-13 [35]. In particular, IL-4 has shown great potential to increase implant integration to bone and mitigate wear particle-induced inflammation in animal models [39,40,41]. Delivery of IL-4 alters the function of local M1 activated macrophages towards an anti-inflammatory M2 phenotype and dramatically reduces the production of pro-inflammatory cytokines. In addition, IL-4 has anti-osteoclastogenic effects that might promote osseointegration of the implant. IL-10 and IL-13 possess similar immune-regulatory properties. These cytokines have been reported to inhibit the expression of pro-inflammatory cytokines in macrophages and drive M2 activation [25]. Several studies that used a murine subcutaneous implantation model rather than delivery of wear particles demonstrated that the release of IL-4 attracts M2 macrophages and modulates the inflammatory response to improve the implant integration [42,43,44]. Interestedly, sequential delivery of M1 and M2 polarizing factors mimicking the natural course of tissue regeneration enhanced implant vascularization. The anti-inflammatory phenotypic switch promoted implant integration also by diminishing the formation of a fibrous capsule and increasing the quality of remodeled collagen around the implant. Further studies are needed to investigate the full potential of M2 polarizing cytokines in orthopedic applications.
In addition to favoring M2 polarization, improved tissue healing around an implant could potentially be achieved by directly inhibiting pro-inflammatory signals. For example, TNF-α, one of the most potent pro-inflammatory cytokines, promotes M1 macrophage polarization, enhances fibrosis, inhibits osteoblast differentiation, induces osteoclast formation, and thus mediates osteolysis around orthopedic implants [45, 46]. Blocking these effects by anti-TNF-α therapy provides a means to modulate implant-induced immune responses. Etanercept, a decoy receptor for TNF-α, was shown to mitigate wear particle-induced cytokine production from macrophages and reduce bone resorption in animals but was not effective in a small clinical trial [47, 48]. Similar results were obtained using an antisense oligonucleotide targeting to mouse TNF-α mRNA in a murine calvarial model [49]. However, blocking the effect of only one pro-inflammatory mediator among the complicated signal network may not be enough to prevent osteolysis in the long term. The compensatory actions of other pro-inflammatory cytokines, such as IL-1β and IL-6, could maintain an inflammatory status in the peri-implant tissue in the absence of TNF-α signaling. A combination of locally delivered cytokine inhibitors might thus prove to be more effective.
Transcription factor NF-κB serves as another target for immunomodulation in the context of implant-mediated immune response [35]. This transcription factor functions as a key regulator of multiple inflammatory cytokines and chemokines in macrophages, and becomes active as a result of a relevant PRR stimulus [10, 50]. Moreover, NF-κB mediates the RANKL signaling, which is integral for osteoclast differentiation and activation. Thus, inhibition of this transcription factor offers an intriguing possibility to attenuate the biomaterial-induced inflammation and osteolysis. This inhibitory effect has been demonstrated using a decoy oligodeoxynucleotide (ODN) that competitively binds NF-κB in vitro and in vivo with polyethylene particles as the adverse inflammatory stimulus; the suppression of intracellular signaling in macrophages resulted in less cytokine expression and osteoclast activation [51, 52]. NF-κB decoy may also suppress the production of chemokines essential for monocyte recruitment.
Preventing the continued recruitment of immune cells to the bone-implant interface could mitigate the inflammatory reaction and periprosthetic bone loss and constitutes another strategy for immunomodulation. Indeed, a chemokine directed immunomodulatory method was recently established using a mutant C-C motif chemokine ligand 2 (CCL2) protein to inhibit CCL2 signaling [53, 54]. Anti-CCL2 therapy suppressed macrophage recruitment to the implant in a murine model and prevented wear particle induced inflammation and bone loss.
Lastly, orthopedic biomaterials can be coupled with anti-inflammatory drugs such as glucocorticoids. These drugs elicit an alternative macrophage phenotype with an increased ability to recognize and scavenge dying cells. These macrophages suppress the production of numerous inflammatory mediators such as pro-inflammatory cytokines, chemokines, prostaglandins, leukotrienes, and proteolytic enzymes, whereas an enhanced expression of anti-inflammatory cytokine IL-10 has been reported. Other bioactive molecules that can also be considered as immunomodulatory, include TGF-β, VEGF, and PDGF [27]. These growth factors tightly regulate the healing process by targeting fibroblasts and endothelial cells, rather than macrophages. Moreover, TGF-β and PDGF directly modulate macrophage function and chemotaxis at least during wound healing without a foreign body [38]. Implants loaded with these molecules could thus potentially also promote bone regeneration and implant integration either directly or indirectly.
3 Sequential Modulation of Inflammatory Response for Optimal Bone Regeneration/Osseointegration
3.1 Essential Role of Acute Inflammation in Bone Regeneration
Determination of the appropriate timeframe of immunomodulation is critical for optimizing their application as orthopedic biomaterials. Acute phase inflammation is crucial for proper bone repair after trauma. Impairing early inflammatory conditions in a murine fracture model resulted in diminished stem cell recruitment and differentiation, fracture callus formation, and overall bone growth [55,56,57,58]. The inflammatory phase sparks the repair cascade by initiating angiogenesis, recruiting and stimulating the differentiation of mesenchymal stem cells (MSCs) , and encouraging extracellular matrix synthesis [59,60,61].
Specific cytokines appear to be tied to the inflammatory phase of bone repair, namely TNF-α and IL-1 [62]. TNF-α and IL-1 are more commonly known for mediating foreign body reactions that can result in impaired tissue function and rejection of prosthetic implants [63]. Gerstenfeld et al. showed that a reduction in TNF-α signaling results in improper formation of fracture callus and delayed endochondral and intramembranous bone formation [56]. The key difference between pathological and therapeutic inflammation is that the latter is highly regulated, both in intensity, duration, and timing to provide a foundation for healing [62].
3.2 Transition of Macrophage Polarization Status for Optimal Bone Formation
Macrophage polarization status also plays a critical role in bone regeneration. M1 macrophages, despite releasing inflammatory cytokines, are highly angiogenic, stimulate early mineralization by MSCs, and support overall bone healing [64,65,66,67]. M2 macrophages, on the other hand, secrete anti-inflammatory cytokines such as IL-10 and IL-1Ra and have been associated with enhanced bone formation [68,69,70]. This proves to be a delicate balance that can result in failed bone regeneration if tipped too far one way or another. As such, the interplay between M1- and M2-dominated microenvironments is one that provides interesting avenues through which to pursue new immune-modulatory therapies.
After an injury, the acute inflammatory phase has been shown to last from 3–7 days before the anti-inflammatory phase begins to exert its longer-lasting influence [43, 71, 72]. Proper timing of the transition between the two phases is crucial to optimal bone regeneration. Indeed, Loi et al. showed that transition from M1 to M2-like macrophages at 72 hours resulted in significantly increased osteogenesis by MC3T3 osteoprogenitors in a co-culture model. Further studies exploring the mechanisms and temporal modulation of the M1 to M2 transition are warranted, as this could provide a prime early target for improved bone repair and implant integration.
The task of stimulating M1 macrophages to transition to M2 macrophages to enhance bone regeneration is one that is currently under investigation. One possible method is to utilize a controlled release system to maintain a short period of M1, followed by a transition to M2 polarization via cytokines such as IFN-γ, IL-4, and IL-10. Kumar et al. reported the development of a multi-domain peptide hydrogel that delivered IL-4 and CCL2 in a biphasic manner. This biphasic, sustained delivery was able to modulate both non-polarized (M0) and M1 macrophages towards an M2-like phenotype [73]. Finally, Spiller et al. utilized a decellularized bone scaffold to release IFN- γ over the first 3 days of repair, along with release of IL-4 over the first 6 days. The bone scaffolds were able to spur polarization towards an M2 phenotype in vitro and led to enhanced angiogenesis in an in vivo subcutaneous murine model [43]. The modulation of M1 to M2 is not limited to cytokine release systems; Rostam et al. has shown that physical and chemical modifications to biomaterial surfaces alone can shift the macrophage polarization towards M1 or M2 [74, 75].
4 Application of Immunomodulating Reagents on Orthopedic Biomaterials
The delivery method of various immunomodulating reagents to enhance the performance of orthopedic biomaterials is dependent on the physical and biological characteristics of the agent. The therapeutic molecules with different biological features including molecular size, hydrophilic/hydrophobic, stability (degradation rate), effective dose, and the optimal administration time points determine the optimal strategy for drug delivery. Different materials used for orthopedic implants can also influence the drug delivery efficiency. For example, the absorption of small peptides on the metal surface is ineffective compared to the application on a polymeric surface.
Surface coating and drug releasing materials are an interesting strategy to modulate the tissue environment surrounding orthopedic implants. The various strategies to apply these bioactive coating on orthopedic implants including hydrogel, layer-by-layer, and immobilization have been summarized comprehensively in other reviews [76, 77]. Agarwal et al. summarized strategies to enhance osseointegration of orthopedic implants by biomolecules such as growth factors, with similar delivery methods being potentially applicable for the delivery of immunomodulating reagents [78]. In the following section, the immunomodulating candidates are classified into four categories including: (1) protein, (2) nucleic acid, (3) small molecule, and (4) cell-based therapies (Table 1). The current development of administration strategies and the therapeutic effects in the application of orthopedic biomaterials are discussed.
4.1 Protein-Based Biomolecules
The size of the immunomodulating protein determines the biomaterial coating strategy and release pattern. Large proteins such as antibodies or fusion protein inhibitors (~150 kDa) targeting pro-inflammatory cytokines or the associated receptors can be coated with hydrogels with larger pore sizes. Anti-TNFα antibodies conjugated with a hyaluronic acid hydrogel was applied to a burn wound and demonstrated an inhibitory effect on inflammation [79]. Direct treatment of a soluble TNFα inhibitor (Etanercept) mitigated wear particle-induced osteolysis [47]. However, no significant difference was observed between Etanercept and placebo-treated patients with acetabular loosening [48]. The results may be due to the limited number of patients, or the compensatory effects of other pro-inflammatory cytokines.
Small proteins including anti-inflammatory cytokines IL-4, IL-10, and IL-13 (ranging from 15 to 21 kDa) can be applied via a hydrogel with smaller pore size or layer-by-layer coating. A nanometer thickness IL-4 eluting layer-by-layer coated polypropylene mesh showed improved implant integration and enhanced M2 macrophage polarization in a subcutaneous implantation murine model [44]. Further validation is required to demonstrate the potential to improve osseointegration of IL-4 eluting bone implants. Another example of this protein delivery approach demonstrated that titanium rods coated with mutant CCL2 protein (7ND) with a layer-by-layer technique mitigated polyethylene wear particle-induced osteolysis in a murine femoral infusion model (See Sect. 2.4 for details) [53].
Small peptides with anti-microbial and immunomodulating activity have recently been identified [80, 81]. Compared to whole protein biomolecules, a higher concentration of small peptides could be potentially applied onto or within biomaterials and thus increase the immunomodulating efficiency [76]. Inhibition of NF-κB activation by a small peptide termed NEMO-binding domain peptide suppressed poly(methyl methacrylate) (PMMA) induced osteoclastogenesis and osteolysis in a murine calvarial model, yet the modulation of an inflammatory response was not characterized [82].
4.2 Nucleic Acid
Gene therapy is mediated through the delivery of nucleic acid-based biomolecules, including plasmid DNA, RNA interference (RNAi), micro-RNA, and ODN, to express proteins or modulate gene expression in the target cells. Delivery of naked nucleic acid is inefficient due to low cell attachment/uptake and rapid nuclease-mediated degradation. Therefore, viral and non-viral vectors are utilized to mediate the delivery of anti-inflammatory genes or silence pro-inflammatory gene expression in vivo. Viral vectors are efficient in transducing target gene expression ex vivo and thus are effective tools to induce gene expression in cell-based therapy (see Sect. 4.4). In contrast, the immunogenicity and potential cytotoxicity effects of viral vectors may limit their direct translational use in vivo. Non-viral vectors, including calcium phosphate, liposomes, nano-hydroxyapatite [83], chitosan [84, 85], polyethyleneimine [86], and dendrimer [87], have lower immunogenicity and cytotoxicity but also lower transfection efficiency in vivo. Raftery et al. summarized the current development of delivering nucleic acid-based biomolecules in orthopedic biomaterials [88].
The combination of scaffolds and gene delivery vectors is a highly promising strategy for prolonged immunomodulation and controlled released of nucleic acid-based biomolecules. Previous studies showed that a combination of collagen or poly-lactic-co-glycolic acid (PLGA) scaffolds with viral or non-viral vectors delivered plasmid DNA or RNAi enhances tissue regeneration [89,90,91,92,93,94]. The therapeutic potential of immunomodulation using this strategy remains to be investigated in inflammatory bone disorders. Decoy ODN can be taken up by the cellular receptor in a sequence-specific manner [95]. The administration of decoy ODN without delivery vectors via local infusion was shown to mitigate orthopedic wear particle-induced osteolysis [51].
4.3 Small Molecules
Small molecule drugs have several advantages in clinical applications including the efficient administration and relative low cost for large-scale production. Steroids and molecular kinase inhibitors are potent anti-inflammatory small molecules that could be applied to orthopedic biomaterials. Signal transduction pathways including NF-κB and MAP kinase are crucial for the regulation of inflammatory responses [50, 96] and periprosthetic osteolysis [10, 97]. Titanium particles have induced VEGF expression and increased macrophage chemotactic activity in primary human macrophages, which was inhibited by MAPK kinase inhibitor PD98059 [98].
A daily injection of N- (2-hydroxypropyl) methacrylamide copolymer-dexamethasone conjugate mitigated osteolysis in the murine femur infused with PMMA particles [99]. Systemic bone loss was not observed in the conjugated dexamethasone injected mice. Several advanced drug-delivery strategies have been developed to apply dexamethasone in pre-clinical inflammatory disease models [100,101,102]. An inflammation-targeting hydrogel generated from ascorbyl palmitate was developed to deliver dexamethasone in an inflammatory bowel disease model [103]. While these drug delivery strategies have shown promise for the treatment of inflammatory disorders, the application in orthopedic biomaterials remains to be examined.
4.4 Cell-Based Therapy
MSCs-based therapy has been applied to bone tissue engineering and inflammatory disorders. The ability to modulate innate [104, 105] and adaptive immune responses [106] further underscored its translational potential to modulate inflammation associated with orthopedic biomaterials. Moreover, MSCs can serve as gene expression carriers to secrete immunomodulating cytokines such as IL-4 or IL-10 [107, 108]. The applications of MSC-based therapy in bone regeneration and immunomodulation are discussed in other reviews [109,110,111]. The following section focuses on scaffold and delivery strategies in MSC-based therapy.
MSC based therapy can be administrated through local implantation or systemic delivery. Natural and synthetic scaffolds are crucial for the local administration of MSC-based therapy by providing the appropriate mechanical strength and cell viability [112]. Commonly used natural scaffolds in bone tissue engineering include collagen hyaluronic acid fibrin and poly(ε-caprolactone)/poly(vinyl alcohol)/chitosan-associated hybrid scaffolds. However purity issues and poor mechanical properties limit the application of natural scaffolds. Synthetic scaffolds including PLGA polyglycolic acid (PGA) and poly-l-lactic acid (PLA) enable the precise control of mechanical properties and stability of the scaffold to further enhance therapeutic efficiency. For example a macroporous and highly flexible gelatin-based scaffold with a microribbon-like structure has recently been demonstrated to increase MSC proliferation and bone regeneration [113]. However the biocompatibility of the synthetic scaffold remains a concern since degradation products could initiate inflammatory responses [114]. The systemic delivery of MSCs provides an alternative strategy of minimally invasive procedures to patients with orthopedic implants. Though MSCs can naturally migrate into inflammatory sites conjugating with antibodies targeting bone or inflammation-associated molecules can further enhance their homing efficiency [115, 116].
5 Conclusion
Transient acute inflammation is closely associated with successful osseointegration and bone regeneration in orthopedic biomaterial implantation. The transition between the pro-inflammatory M1 and anti-inflammatory M2 macrophage phenotypes has been shown to be a key step in bone regeneration. Alternatively, chronic inflammatory bone diseases associated with implants often exhibit excessive pro-inflammatory macrophage infiltration and the generation of wear particles . The combination of bone regenerating scaffolds and controlled drug releasing systems has great potential for advancing clinical applications of orthopedic biomaterials for a variety of conditions including aseptic loosening, osteonecrosis, and fracture nonunion. Taken together, optimizing the timing and efficacy of the innate immune reaction provide a promising approach to harness the inflammatory response for therapeutic applications of orthopedic biomaterials.
Abbreviations
- CCL2:
-
C-C motif chemokine ligand 2
- FBGC:
-
Foreign-body giant cell
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- M-CSF:
-
Macrophage colony-stimulating factor
- MSC:
-
Mesenchymal stem cell
- ODN:
-
Oligodeoxynucleotide
- OPG:
-
Osteoprotegerin
- PAMP:
-
Pathogen-associated molecular pattern
- PDGF:
-
Platelet-derived growth factor
- PGA:
-
Poly-glycolic-acid
- PLA:
-
Poly-lactic-acid
- PLGA:
-
Poly-lactic-glycolic-acid
- PRR:
-
Pattern-recognition receptor
- RANKL:
-
Receptor-activator of NF-κB ligand
- RNAi:
-
RNA interference
- TGF-β:
-
Transforming growth factor-β
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor
References
Global Alliance for Musculoskeletal Health. Key facts from the global burden of disease. 2012, http://bjdonline.org/key-facts-and-figures/.
Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface. 2008;5(27):1137–58.
Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol. 2007;3(3):165–71.
Cobelli N, Scharf B, Crisi GM, Hardin J, Santambrogio L. Mediators of the inflammatory response to joint replacement devices. Nat Rev Rheumatol. 2011;7(10):600–8.
LBHN Service. Joint replacements in U.S. exceed 1 million a year, Pittsburgh Post-Gazette, Pittburgh Post-Gazette. 2013. http://www.post-gazette.com/news/health/2013/03/04/Joint-replacements-in-U-S-exceed.
Lewallen EA, Riester SM, Bonin CA, Kremers HM, Dudakovic A, Kakar S, Cohen RC, Westendorf JJ, Lewallen DG, van Wijnen AJ. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng Part B Rev. 2015;21(2):218–30.
Vitkov L, Hartl D, Hannig M. Is osseointegration inflammation-triggered? Med Hypotheses. 2016;93:1–4.
Ma QL, Zhao LZ, Liu RR, Jin BQ, Song W, Wang Y, Zhang YS, Chen LH, Zhang YM. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials. 2014;35(37):9853–67.
Gibon E, Amanatullah DF, Loi F, Pajarinen J, Nabeshima A, Yao Z, Hamadouche M, Goodman SB. The biological response to orthopaedic implants for joint replacement: part I: metals. J Biomed Mater Res B Appl Biomater. 2017;105(7):2162–73.
Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, Goodman SB. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10(1):1–10.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100.
Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28(34):5044–8.
Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, Konttinen YT, Goodman SB, Gallo J. Macrophages-key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101:3033.
Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271–86.
Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–6.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69.
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–95.
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–73.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373–84.
Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–89.
Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74.
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37.
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–62.
Lech M, Anders HJ. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989–97.
Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol. 2014;5:614.
Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32(28):6692–709.
Maitra R, Clement CC, Scharf B, Crisi GM, Chitta S, Paget D, Purdue PE, Cobelli N, Santambrogio L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol Immunol. 2009;47(2–3):175–84.
Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res. 2009;27(7):847–54.
Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015;18(6):313–25.
Goodman SB, Gibon E, Yao Z. The basic science of periprosthetic osteolysis. Instr Course Lect. 2013;62:201–6.
Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–61.
Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292(4):490–5.
Chen Z, Klein T, Murray RZ, Crawford R, Chang J, Wu C, Xiao Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19(6):304–21.
Goodman SB, Gibon E, Pajarinen J, Lin TH, Keeney M, Ren PG, Nich C, Yao Z, Egashira K, Yang F, Konttinen YT. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J R Soc Interface. 2014;11(93):20130962.
Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–802.
Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12(2):188–96.
Hosgood G. Wound healing. The role of platelet-derived growth factor and transforming growth factor beta. Vet Surg. 1993;22(6):490–5.
Wang Y, Wu NN, Mou YQ, Chen L, Deng ZL. Inhibitory effects of recombinant IL-4 and recombinant IL-13 on UHMWPE-induced bone destruction in the murine air pouch model. J Surg Res. 2013;180(2):e73–81.
Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, Smith RL, Goodman SB. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res A. 2013;101((7):1926–34.
Sato T, Pajarinen J, Behn A, Jiang X, Lin TH, Loi F, Yao Z, Egashira K, Yang F, Goodman SB. The effect of local IL-4 delivery or CCL2 blockade on implant fixation and bone structural properties in a mouse model of wear particle induced osteolysis. J Biomed Mater Res A. 2016;104(9):2255–62.
Minardi S, Corradetti B, Taraballi F, Byun JH, Cabrera F, Liu X, Ferrari M, Weiner BK, Tasciotti E. IL-4 release from a biomimetic scaffold for the temporally controlled modulation of macrophage response. Ann Biomed Eng. 2016;44(6):2008–19.
Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207.
Hachim D, LoPresti ST, Yates CC, Brown BN. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107.
Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45(2):367–76.
Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141(11):3956–64.
Childs LM, Goater JJ, O'Keefe RJ, Schwarz EM. Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res. 2001;16(2):338–47.
Schwarz EM, Campbell D, Totterman S, Boyd A, O'Keefe RJ, Looney RJ. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. J Orthop Res. 2003;21(6):1049–55.
Dong L, Wang R, Zhu YA, Wang C, Diao H, Zhang C, Zhao J, Zhang J. Antisense oligonucleotide targeting TNF-alpha can suppress Co-Cr-Mo particle-induced osteolysis. J Orthop Res. 2008;26(8):1114–20.
Lin TH, Pajarinen J, Lu L, Nabeshima A, Cordova LA, Yao Z, Goodman SB. NF-kappaB as a therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol. 2017;107:117–54.
Lin TH, Pajarinen J, Sato T, Loi F, Fan C, Cordova LA, Nabeshima A, Gibon E, Zhang R, Yao Z, Goodman SB. NF-kappaB decoy oligodeoxynucleotide mitigates wear particle-associated bone loss in the murine continuous infusion model. Acta Biomater. 2016;41:273–81.
Sato T, Pajarinen J, Lin TH, Tamaki Y, Loi F, Egashira K, Yao Z, Goodman SB. NF-kappaB decoy oligodeoxynucleotide inhibits wear particle-induced inflammation in a murine calvarial model. J Biomed Mater Res A. 2015;103(12):3872–8.
Nabeshima A, Pajarinen J, Lin TH, Jiang X, Gibon E, Cordova LA, Loi F, Lu L, Jamsen E, Egashira K, Yang F, Yao Z, Goodman SB. Mutant CCL2 protein coating mitigates wear particle-induced bone loss in a murine continuous polyethylene infusion model. Biomaterials. 2017;117:1–9.
Keeney M, Waters H, Barcay K, Jiang X, Yao Z, Pajarinen J, Egashira K, Goodman SB, Yang F. Mutant MCP-1 protein delivery from layer-by-layer coatings on orthopedic implants to modulate inflammatory response. Biomaterials. 2013;34(38):10287–95.
Gerstenfeld L, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes G, Graves D, Einhorn T. Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J Bone Miner Res. 2003;18(9):1584–92.
Gerstenfeld L, Cho T-J, Kon T, Aizawa T, Cruceta J, Graves B, Einhorn T. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169(3):285–94.
Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Camacho NP, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41(6):928–36.
Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci. 2011;108(4):1585–90.
Xing Z, Lu C, Hu D, Yu Y-y, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3(7–8):451–8.
Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–84.
Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, Gerstenfeld LC, Einhorn TA. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004–14.
Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14(2):179–86.
Luttikhuizen DT, Harmsen MC, Luyn MJV. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12(7):1955–70.
Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, Cope AP, Horwood NJ. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7(7):e39871.
Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, Richards CD, Chevalier S, Rédini F, Heymann D. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30(4):762–72.
Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88.
Lu LY, Loi F, Nathan K, Lin Th, Pajarinen J, Gibon E, Nabeshima A, Cordova L, Jämsen E, Yao Z. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. 2017;35:2378.
Raggatt LJ, Wullschleger ME, Alexander KA, Wu ACK, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early Endochondral ossification. Am J Pathol. 2014;184(12):3192–204.
Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78–89.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55.
Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5.
Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133–43.
Kumar VA, Taylor NL, Shi S, Wickremasinghe NC, D'Souza RN, Hartgerink JD. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 2015;52:71–8.
Rostam H, Singh S, Vrana N, Alexander M, Ghaemmaghami A. Impact of surface chemistry and topography on the function of antigen presenting cells. Biomater Sci. 2015;3(3):424–41.
Rostam HM, Singh S, Salazar F, Magennis P, Hook A, Singh T, Vrana NE, Alexander MR, Ghaemmaghami AM. The impact of surface chemistry modification on macrophage polarisation. Immunobiology. 2016;221(11):1237–46.
Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34(13):3174–83.
Tobin EJ. Recent coating developments for combination devices in orthopedic and dental applications: a literature review. Adv Drug Deliv Rev. 2017;112:88.
Agarwal R, Garcia AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev. 2015;94:53–62.
Friedrich EE, Sun LT, Natesan S, Zamora DO, Christy RJ, Washburn NR. Effects of hyaluronic acid conjugation on anti-TNF-alpha inhibition of inflammation in burns. J Biomed Mater Res A. 2014;102(5):1527–36.
Haney EF, Hancock RE. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers. 2013;100(6):572–83.
Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–8.
Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. J Orthop Res. 2004;22(1):13–20.
Matsiko A, Levingstone TJ, O'Brien FJ, Gleeson JP. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater. 2012;11:41–52.
Raftery R, O'Brien FJ, Cryan SA. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules. 2013;18(5):5611–47.
Raftery RM, Tierney EG, Curtin CM, Cryan SA, O'Brien FJ. Development of a gene-activated scaffold platform for tissue engineering applications using chitosan-pDNA nanoparticles on collagen-based scaffolds. J Control Release. 2015;210:84–94.
Tierney EG, Duffy GP, Hibbitts AJ, Cryan SA, O'Brien FJ. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J Control Release. 2012;158(2):304–11.
Schatzlein AG, Zinselmeyer BH, Elouzi A, Dufes C, Chim YT, Roberts CJ, Davies MC, Munro A, Gray AI, Uchegbu IF. Preferential liver gene expression with polypropylenimine dendrimers. J Control Release. 2005;101(1–3):247–58.
Raftery RM, Walsh DP, Castano IM, Heise A, Duffy GP, Cryan SA, O'Brien FJ. Delivering nucleic-acid based Nanomedicines on biomaterial scaffolds for orthopedic tissue repair: challenges, progress and future perspectives. Adv Mater. 2016;28(27):5447–69.
Huang CL, Leblond AL, Turner EC, Kumar AH, Martin K, Whelan D, O'Sullivan DM, Caplice NM. Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol Pharm. 2015;12(3):991–6.
Deng Y, Bi X, Zhou H, You Z, Wang Y, Gu P, Fan X. Repair of critical-sized bone defects with anti-miR-31-expressing bone marrow stromal stem cells and poly(glycerol sebacate) scaffolds. Eur Cell Mater. 2014;27:13–24. discussion 24-5.
Zhang M, Gao Y, Caja K, Zhao B, Kim JA. Non-viral nanoparticle delivers small interfering RNA to macrophages in vitro and in vivo. PLoS One. 2015;10(3):e0118472.
Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6:19.
Boehler R, Kuo R, Shin S, Goodman A, Pilecki M, Leonard J, Shea L. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol Bioeng. 2014;111(6):1210–21.
Li Y, Fan L, Liu S, Liu W, Zhang H, Zhou T, Wu D, Yang P, Shen L, Chen J, Jin Y. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials. 2013;34(21):5048–58.
Benimetskaya L, Loike JD, Khaled Z, Loike G, Silverstein SC, Cao L, el Khoury J, Cai TQ, Stein CA. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997;3(4):414–20.
Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754(1–2):253–62.
Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102–13.
Miyanishi K, Trindade MC, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. J Bone Miner Res. 2003;18(9):1573–83.
Ren K, Dusad A, Yuan F, Yuan H, Purdue PE, Fehringer EV, Garvin KL, Goldring SR, Wang D. Macromolecular prodrug of dexamethasone prevents particle-induced peri-implant osteolysis with reduced systemic side effects. J Control Release. 2014;175:1–9.
Urbanska J, Karewicz A, Nowakowska M. Polymeric delivery systems for dexamethasone. Life Sci. 2014;96(1–2):1–6.
Webber MJ, Matson JB, Tamboli VK, Stupp SI. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33(28):6823–32.
Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J Control Release. 2006;110(3):531–41.
Zhang S, Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao B, Korzenik JR, Glickman JN, Vemula PK, Glimcher LH, Traverso G, Langer R, Karp JM. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Transl Med. 2015;7(300):300ra128.
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–95.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50.
Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, Cho CS. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153(2):269–76.
Tan CQ, Gao X, Guo L, Huang H. Exogenous IL-4-expressing bone marrow mesenchymal stem cells for the treatment of autoimmune sensorineural hearing loss in a Guinea pig model. Biomed Res Int. 2014;2014:856019.
Pajarinen J, Lin TH, Nabeshima A, Jamsen E, Lu L, Nathan K, Yao Z, Goodman SB. Mesenchymal stem cells in the aseptic loosening of total joint replacements. J Biomed Mater Res A. 2017;105(4):1195–207.
Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96.
Rosenbaum AJ, Grande DA, Dines JS. The use of mesenchymal stem cells in tissue engineering: a global assessment. Organogenesis. 2008;4(1):23–7.
Han LH, Conrad B, Chung MT, Deveza L, Jiang X, Wang A, Butte MJ, Longaker MT, Wan D, Yang F. Winner of the young investigator award of the Society for Biomaterials at the 10th world biomaterials congress, may 17-22, 2016, Montreal QC, Canada: microribbon-based hydrogels accelerate stem cell-based bone regeneration in a mouse critical-size cranial defect model. J Biomed Mater Res A. 2016;104(6):1321–31.
Uebersax L, Hagenmuller H, Hofmann S, Gruenblatt E, Muller R, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006;12(12):3417–29.
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16.
Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18(3):456–62.
Acknowledgement
This work was supported by NIH grants 2R01AR055650, 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J. P. was supported by a grant from the Jane and Aatos Erkko foundation.
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Lin, T., Jämsen, E., Lu, L., Nathan, K., Pajarinen, J., Goodman, S.B. (2018). Modulating Innate Inflammatory Reactions in the Application of Orthopedic Biomaterials. In: Li, B., Webster, T. (eds) Orthopedic Biomaterials . Springer, Cham. https://doi.org/10.1007/978-3-319-89542-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-89542-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89541-3
Online ISBN: 978-3-319-89542-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)