Abstract
Two Ni-Cr-Mo-Nb superalloys (Alloy 625 and Alloy 718 ) were corroded in high temperature supercritical-CO2 (S-CO2) at 700 ℃ (20 MPa) for 500 h and compared in terms of oxidation and carburization behavior. A continuous chromia (Cr2O3) layer was formed on the surface of Alloy 625, whereas Ni- and Fe-rich oxide nodules were also formed with chromia on Alloy 718 . Meanwhile, the extent of carburization by formation of an amorphous C layer at the oxide/matrix interface was comparatively low for Alloy 625. This difference did not seem to stem from oxide type or underlying microstructure , and was thought to be associated with oxide properties. In terms of mechanical properties , only Alloy 625 exhibited decrease in ductility after exposure to S-CO2. This was ascribed to the microstructural evolution of the alloys during the high temperature exposure.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The supercritical-carbon dioxide (S-CO2) Brayton cycle is emerging as a cross-cutting power conversion system to replace the conventional steam Rankine cycle. It is considered to be applicable to a broad range of advanced energy applications such as next generation nuclear, fossil, and concentrated solar power plants [1,2,3,4,5]. The use of S-CO2 as a coolant allows for greater thermal efficiency especially at high temperature ranges above 650 ℃, while also allowing for a compact system.
In terms of structural materials, it is known that materials exposed to high temperature S-CO2 environments are subjected to oxidation and carburization . Generally, Fe-base alloys especially those with low Cr contents such as ferritic-martensitic steels exhibited poor oxidation and carburization resistance [6, 7]. For Fe-base austenitic alloys, the extent of oxidation and carburization was less in comparison. However, carburization at the oxide/matrix interface was seen to occur, and promote oxide delamination [8]. Also, Fe-base alloys were susceptible to carburization within the matrix by formation of carbides, exhibited decrease in ductility after exposure to S-CO2 [9]. On the other hand, Ni-base alloys are reported to show superior resistance to oxidation and carburization in S-CO2 environments, owing to formation of a continuous chromia (Cr2O3) layer and Ni-base matrix [10, 11].
In high efficiency S-CO2 systems such as direct-fired S-CO2 power cycles and concentrated solar power (CSP) plants, which operate at elevated temperatures above 700 ℃, the above issues are likely to be more pronounced. Thus, Ni-base superalloys having superior corrosion and creep resistance are anticipated to be used as structural materials. However, data on oxidation and carburization of Ni-base superalloys in S-CO2 above 700 ℃ are scarce. While some preliminary studies are being conducted [12, 13], detailed analyses of oxide layers and carburization behaviors are limited.
In this study, two Ni-Cr-Mo-Nb alloys, Alloy 718 and Alloy 625, which are candidate materials for various power conversion systems, were exposed in high temperature S-CO2 environment and evaluated of their corrosion and carburization behavior. Detailed analyses on the oxide products and microstructural changes under the oxide layer were conducted. In addition, tensile property changes of the alloys after aging in S-CO2 were also evaluated.
Experimental Procedure
Test Materials and Preparation
Solid-solution strengthened Ni-base superalloy 625 and precipitate-hardened Ni-base superalloy 718 were used in this study. Their chemical compositions were analyzed by inductively coupled plasma (ICP) spectroscopy and are listed in Table 1. The specimens were fabricated by electro-discharge machining (EDM) into coupon-type (12 mm in diameter, 1 mm in thickness) and mini-tensile specimens (16 mm in length, 5 mm in gauge length, 0.5 mm in thickness) as shown in Fig. 1. A hole of 1.5 mm in diameter was drilled on each specimen for hanging by a Pt wire in S-CO2 environment. The coupon specimens were mechanically polished by 1200 grit SiC abrasive papers to obtain a uniform surface condition prior to exposure to S-CO2 environment. The mini-sized tensile specimens were used in the as-fabricated (surface with 800 grit finish) condition. The coupon and mini-sized tensile specimens were ultrasonically cleaned in ethanol prior to corrosion testing.
High-Temperature S-CO2 Corrosion Test and Analyses
Two specimens of each alloy were subjected to an isothermal corrosion test in research-grade CO2 (99.999% purity) at 700 ℃ (20 MPa) for 500 h. While a detailed description of the corrosion test facility and test procedure can be found in a previous publication by authors [9], a higher CO2 flow rate of 9.8 mL/min was used so that the test chamber would be refreshed every 2 h. For evaluation of corrosion behavior, weight change after exposure to S-CO2 was measured using an electronic microbalance (Mettler Toledo AT21 Comparator) having a resolution of 0.001 mg.
For characterization of the oxide products and microstructural changes, analytical methods such as X-ray diffraction (XRD , RIGAKU D/MAX-2500), scanning electron microscopy (SEM, FEI Magellan400) equipped with energy dispersive X-ray spectroscopy (EDS) and transmission electron microscopy (TEM , FEI Titan cubed G2 60–300) were utilized. The TEM specimens were prepared by a lift-out method via focused ion beam (FIB, FEI Helios G4). In addition, secondary ion mass spectrometry (SIMS, CAMECA IMS 7f) was employed to evaluate the extent of carburization from exposure to S-CO2 environment. Meanwhile, tensile testing was performed using mini-sized tensile specimens before and after exposure to S-CO2 for evaluation of changes in mechanical properties . Tensile testing was conducted under a strain-control condition at a rate of 3.33 × 10−4 s−1.
Results and Discussion
Corrosion Behavior in High Temperature S-CO2
The weight changes of Alloy 625 and 718 after exposure to S-CO2 at 700 ℃ (20 MPa) for 500 h are shown in Fig. 2. It can be seen that Alloy 625 exhibits greater weight gains compared to Alloy 718 . Figure 3 shows the plan-view SEM images of the oxides formed on the alloys. For Alloy 625, EDS analysis at point 1 in Fig. 3b revealed the oxide to be rich mainly in Cr and O (Table 2). In the case of Alloy 718 (Fig. 3c), large nodular oxide islands can be observed, with rest of the area being a continuous oxide similar in morphology to that formed on Alloy 625. The nodular islands were rich in Ni and O, while the continuous oxide was rich in Cr and O.
Figure 4 shows the results of grazing XRD analysis (2 theta scan) for the two alloys after S-CO2 exposure. It can be seen that for Alloy 625, the predominant peaks are from chromia (Cr2O3), as previously reported for many chromia-forming Ni-base superalloys in high temperature environments. On the other hand, for Alloy 718 , the predominant peaks are from the substrate, which may indicate a thinner oxide compared to that on Alloy 625. Oxide peaks detected on Alloy 718 were identified to be Fe3O4, NiO and chromia. In addition, for both alloys, peaks of Ni3 (Nb, Mo), also referred to as δ-phase, were identified.
TEM was utilized for detailed cross-sectional analyses of the oxide and underlying matrix (Figs. 5, 6 and 7). For Alloy 625, a continuous chromia layer (~1 μm thickness) was formed on the surface (Fig. 5), which is similar to that observed previously for other Ni-base alloys [9] and model Ni-Cr alloys [14] containing more than 14 wt% of Cr after exposure to S-CO2 environments. On the other hand, internal oxidation by Al or Ti was not observed under the chromia layer. Previous studies on oxidation behavior of Alloy 625 in S-CO2 environments at 650–750 ℃ (20–30 MPa) also reported mainly in a continuous chromia oxide formation, with little to no internal oxidation [13, 15]. Meanwhile, an enrichment of Nb and Mo was found adjacent to the oxide/metal interface, having rod-like morphology. Diffraction pattern analysis in this region confirmed it to be δ-phase [Ni3 (Nb, Mo)] (Fig. 5c). Such sub-scale formation of δ-phase has previously been observed for Alloy 625 after oxidation in high temperature environments [16, 17]. This phenomenon has been attributed to uphill diffusion of Nb to the surface due to decrease in Nb activity caused by Cr-depletion .
Results of TEM analyses of oxide layer on Alloy 625 corroded in S-CO2 at 700 ℃ (20 MPa) for 500 h; a cross-sectional STEM micrograph and EDS mapping images, b line scanning at the metal-oxide interface, c FFT pattern at point 1 shown in Fig. 4a
In the case of Alloy 718 , the TEM specimens were extracted at two locations, that is, at the boundary of the nodular oxide, and at the continuous oxide as observed in plan-view SEM images. The cross-sectional analyses of the nodular oxide can be seen in Fig. 6, where the nodule is identified to be a complex multi-layered oxide. The outer layer is rich in Ni and Fe, with Fe-rich oxide located in between the Ni-rich oxides, while chromia is formed as an inner oxide. The outer oxide nodule is about 0.8 μm in thickness, while the inner chromia is around 1 μm in depth. Meanwhile, Fig. 7 shows the TEM analyses of the continuous oxide region on Alloy 718 , and it is found to be an outer chromia layer of about 0.5 μm thickness. For both nodular and continuous oxides, internal oxides rich in Al and Ti can be seen at the oxide/matrix interface. Also, similar to Alloy 625, some Nb and Mo enriched regions can be seen under the oxide layer. Ni is seen to be also slightly enriched in these regions, and thus, these areas can also be corresponded to oxidation -induced δ-phase [Ni3 (Nb, Mo)], as also detected by XRD analysis.
Regarding the nodular oxide formation on Alloy 718 , V. Dheeradhada et al. reported some localized nodular oxide formations in S-CO2 at 550 and 750 ℃ (20 MPa) for 600 h [18]. X. Ren et al. reported layered Ni-rich and spinel oxide structures on Alloy 718 after exposure to supercritical-H2O at 500 ℃ for 500 h [19]. Also, K. A. Unoicic et al. exposed Alloy 718 in dry and wet air (air + 10% H2O) at 650–800 ℃ for up 5000 h, and reported thick outer Fe-rich oxides similar to that observed in this study (Fig. 6a) [20]. In these studies, such oxidation behavior of Alloy 718 was ascribed to its relatively less Cr content (18 wt%). However, it is interesting to note that Alloy 600 with even less Cr content (16 wt%) than Alloy 718 formed continuous chromia layer after exposure to similar S-CO2 conditions [9]. On the other hand, nodular Fe-rich oxides have been observed on austenitic stainless steels such as 316 and 347 H with around 18 wt% Cr after exposure in S-CO2 environments [8]. Considering that Alloy 718 has higher Fe content (18 wt%) than other Ni-base alloys, higher Fe content may affect the oxidation behavior . However, the exact role of Fe content and underlying cause for complex nodular oxide formation on Alloy 718 is unclear.
Meanwhile, it is generally known that Ni- and Fe-rich oxides are not as protective as chromia in high temperature oxidizing conditions, which is the reason alloys for use in such environments have an appreciable amount of Cr for formation of a continuous chromia layer. However, despite Alloy 625 having formed a continuous chromia layer, its weight gain was greater than that of Alloy 718 , which formed multilayered Ni- and Fe-rich oxide nodules along with chromia. While the nodular oxide regions on Alloy 718 are quite thicker (~1.8 μm), these nodules exist as discontinuous islands, so that their contribution to weight gain was not as great as that of the generally thicker continuous chromia formed on Alloy 625. XRD analysis supports this, as the peaks of the base metal were predominant for Alloy 718 , which would have been detected from the thinner chromia regions (Fig. 4). However, it should be noted that the test duration of 500 h may not be long enough and longer testing durations may be necessary to reflect the long-term corrosion behaviors.
Carburization Behavior in High Temperature S-CO2
Other than the oxidation issues, carburization due to exposure in high temperature S-CO2 should be addressed. While it has been reported that Ni-base alloys show superior resistance to carburization in S-CO2 environments, authors found an amorphous C layer at the oxide/metal interface for some Ni-base alloys like Alloys 600 and 690, and for Ni-Cr model alloys after exposure to high temperature S-CO2 environments [9, 14]. This was attributed to the ingress of carbonaceous species such as CO2 or CO through the oxide layer to the oxide/metal interface, and subsequent deposition of C by the Boudouard reaction. In the case of Alloy 625 however, such carburized region could not be detected by EDS line scans (Fig. 5b). On the other hand, for Alloy 718 , EDS line scan at the oxide/matrix interface revealed enrichment of C below chromia (Fig. 7b). This area could be identified as an amorphous C layer by HRTEM and diffraction pattern analysis (Fig. 7c), which is in accordance with previous observations [9, 14, 18].
In addition, SIMS analysis covering a comparatively greater interactive area (~100 μm2) was performed and the results are shown in Fig. 8. Oxygen was detected deeper for Alloy 625 than Alloy 718 , which is in agreement with cross-sectional analysis. In the case of C, a high C peak is clearly detected for Alloy 718 where the O profile decreases near the matrix, corresponding to the bottom of chromia layer. For Alloy 625, a C peak is also seen at the corresponding location, while the intensity is somewhat lower than that of Alloy 718 . Thus, SIMS results also suggest that the degree of carburization for Alloy 625 is less than that of Alloy 718 .
For this difference, the effect of sub-surface δ-phase on carburization could be considered. However, while C enrichment could not be detected at the oxide/δ-phase interface for Alloy 625 (Fig. 5b), EDS line scanning of Alloy 718 in Fig. 7b shows C enrichment above δ-phase. For Alloy 718 , such C enrichment is also found at adjacent matrix/oxide interface without δ-phase. This suggests that sub-surface δ-phase has little effect on amorphous C layer formation.
On the other hand, it should be mentioned that while a continuous chromia layer was formed on Alloy 625, Fe- and Ni-rich oxides were also formed on Alloy 718 . As it is known that chromia provides better protection against carburization compared to Fe-rich oxides, it may be thought that this is the cause of the difference in carburization behavior. Thus, in order to discern whether the different oxide products played a role, additional SIMS analysis was conducted on Alloy 600 and 690, which formed outer chromia layer after exposure in the same S-CO2 conditions (Fig. 9). It can be seen that the C enrichment of Alloy 600 (16 wt% Cr) and 690 (28 wt% Cr) is quite greater than that of Alloy 625 (22 wt% Cr). In addition, the Cr contents of the alloys do not seem to have a clear relationship to the extent of carburization . Moreover, comparing the SIMS results of Alloy 625 and Alloy 690, it is interesting that even with the similar thickness of chromia layer, the extent of carburization is quite less for Alloy 625.
From the above observations, it can be inferred that there would be some other reasons for less carburization of Alloy 625 than sub-surface δ-phase or chromia formation. Regarding this, fundamental chromia properties and underlying growth mechanism for different alloys in high temperature S-CO2 should be examined.
Tensile Property Evaluation
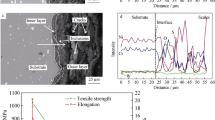
Room temperature tensile testing was performed for both alloys before and after exposure to S-CO2 (Fig. 10). For Alloy 625, it can be seen that there is a significant increase in ultimate tensile strength (UTS) by 26% and decrease in elongation by 38% compared to the as-received condition. On the other hand, for Alloy 718 , there is a slight reduction in UTS by 4% while there is hardly any change in elongation after S-CO2 exposure.
The difference in tensile property changes despite both alloys having formed δ-phase precipitates under the oxide layer suggest that such sub-surface δ-phase may not significantly affect tensile properties of the alloys. In addition, in order to isolate the effect of oxidation and sub-surface δ-phase on tensile property changes, tensile properties of Alloy 625 after exposure to varying oxidizing environments (air, steam , atmospheric-CO2, S-CO2) at 650 ℃ for 1000 h were compared to those after thermal aging also at 650 ℃ for 1000 h (not shown in this paper) [21]. It was found that thermal aging resulted in significant increase in UTS and reduction in elongation. On the other hand, exposure to oxidizing environments resulted in slight additional embrittlement compared to the thermally aged condition. Thus, it could be said that while oxidation and sub-surface δ-phase formation somewhat affects tensile properties , bulk aging has a greater effect on tensile property changes for Alloy 625.
Thus, the above observation suggests that the difference between the two alloys may be more likely due to the initial heat treatment conditions and subsequent aging effect of the alloys. Alloy 625 is solid-solution strengthened, while Alloy 718 is precipitation -hardened mainly by γ″ [Ni3Nb]. For Alloy 625, it is reported that prolonged exposures in temperatures ranging from 550–760 ℃ results in reduction of ductility [22,23,24]. This is attributed to the formation of γ″ and δ-phase in the matrix, which would have occurred after aging in 700 ℃ for 500 h.
Conversely, while Alloy 718 is also susceptible to δ-phase formation due to thermal aging [25], it is reported that long-term exposures at such temperature ranges result in decrease in strength [26]. In addition, Y. Desvallees et al. directly compared the mechanical properties of Alloy 718 and a variant having greater δ-phase (less γ″) content in the matrix [27]. It was found that the latter exhibited lower yield strength and increased elongation compared to the former. In the above studies, this behavior was attributed to the formation of δ-phase due to thermal aging . It is well reported that thermal aging of Alloy 718 results in transformation of metastable tetragonal γ″ into equilibrium orthorhombic δ-phase [28, 29]. As γ″ is coherent and δ-phase is not, the depletion of γ″ in the matrix by this transformation could be associated with loss of strength of Alloy 718 .
Conclusion
Two Ni-Cr-Mo-Nb Alloys (625 and 718 ) were evaluated of their corrosion and carburization behavior after exposure to S-CO2 at 700 ℃ (20 MPa) for 500 h. For Alloy 625, a continuous outer chromia (Cr2O3) was formed, while internal oxidation was not observed. On the other hand, for Alloy 718 , complex multi-layered oxide nodules having outer NiO and Fe3O4 and inner chromia could be observed along with continuous chromia where nodules were not formed. Both alloys formed oxidation -induced δ-phase [Ni3 (Nb, Mo)] under the oxide layer as a result of decreased Nb activity in the Cr-depleted region. Meanwhile, it was found that the extent of carburization was comparatively lower for Alloy 625 than other Ni-base alloys. In addition, the oxide product and sub-surface δ-phase formation did not seem to affect the formation of amorphous C layer. Thus, it is thought that carburization behavior may be associated with oxide properties and transport mechanisms that require fundamental investigations.
In terms of tensile properties , Alloy 625 exhibited a significant increase in UTS and decrease in elongation after corrosion in S-CO2. On the other hand, only a slight decrease in UTS was measured for Alloy 718 . This difference was suggested to be related to changes in the bulk matrix in the alloys due to thermal aging , rather than surface oxidation or oxidation -induced sub-surface δ-phase formation.
References
Dostal V, Hejzlar P, Driscoll MJ (2006) High-performance supercritical carbon dioxide cycle for next-generation nuclear reactors. Nucl Technol 154:265–282
Ahn Y, Bae SJ, Kim M, Cho SK, Baik S, Lee JI, Cha JE (2015) Review of supercritical CO2 power cycle technology and current status of research and development. Nucl Eng Technol 47:647–661
Maio DVD, Boccitto A, Caruso G (2015) Supercritical carbon dioxide applications for energy conversion systems. Energy Proced 82:819–824
Mecheri M, Moullec YL (2016) Supercritical CO2 Brayton cycles for coal-fired power plants. Energy 103:758–771
Iverson BD, Conboy TM, Pasch JJ, Kruizenga AM (2013) Supercritical CO2 Brayton cycles for solar-thermal energy. Appl Energy 111:957–970
Tan L, Anderson M, Taylor D, Allen TR (2011) Corrosion of austenitic and ferritic-martensitic steels exposed to supercritical carbon dioxide. Corros Sci 53:3273–3280
Rouillard F, Furukawa T (2016) Corrosion of 9-12Cr ferritic-martensitic steels in high-temperature CO2. Corros Sci 105:120–132
Cao G, Firouzdor V, Sridharan K, Anderson M, Allen TR (2012) Corrosion of austenitic alloys in high temperature supercritical carbon dioxide. Corros Sci 60:246–255
Lee HJ, Kim H, Kim SH, Jang C (2015) Corrosion and carburization behavior of chromia-forming heat resistant alloys in high-temperature supercritical-carbon dioxide environment. Corros Sci 99:227–239
Olivares RI, Young DJ, Marvig P, Stein W (2015) Alloys SS316 and Hastelloy-C276 in supercritical CO2 at high temperature. Oxid Met 84:585–606
Firouzdor V, Sridharan K, Cao G, Allen TR (2013) Corrosion of a stainless steel and nickel-based alloys in high temperature supercritical carbon dioxide environment. Corros Sci 69:281–291
Pint BA, Brese RG, Keiser JR (2017) Effect of pressure on supercritical CO2 compatibility of structural alloys at 750 ℃. Mater Corros 68:151–158
Pint A, Unocic KA, Brese RG, Keiser JR (2017) Characterization of chromia scales formed in supercritical carbon dioxide. Mater High Temp 1–11
Subramanian GO, Lee HJ, Kim SH, Jang C (2017) Corrosion and carburization behavior of Ni-xCr binary alloys in a high-temperature supercritical-carbon dioxide environment. Oxid Met (in press)
Holcomb GR, Carney C, Dogan ON (2016) Oxidation of alloys for energy applications in supercritical CO2 and H2O. Corros Sci 109:22–35
Chyrkin A, Huczkowski P, Shemet V, Singheiser L, Quadakkers WJ (2011) Sub-scale depletion and enrichment processes during high temperature oxidation of the nickel base alloy 625 in the temperature range 900–1000 ℃. Oxid Met 75:143–166
Garcia-Fresnillo L, Chyrkin A, Böhme C, Barnikel J, Schmitz F, Quadakkers WJ (2014) Oxidation behavior and microstructural stability of alloy 625 during long-term exposure in steam. J Mater Sci 49:6127–6142
Dheeradhada V, Thatte A, Karadge M, Drobnjak M (Oct 2015 ) Corrosion of supercritical CO2 turbomachinary components. In: EPRI international conference on corrosion in power plants. San Diego, CA, USA
Ren X, Sridharan K, Allen TR (2007) Corrosion behavior of alloys 625 and 718 in supercritical water. Corrosion 63:603–612
Unocic KA, Pint BA (2014) Effect of environment on the high temperature oxidation behavior of 718 and 718plus. In: TMS superalloy 718 and derivatives. pp 667–677
Lee HJ, Kim SH, Jang C Oxidation and microstructure evolution of a Ni-Cr-Mo-Nb superalloy during high temperature oxidation (Unpublished raw data)
Köhler M (1991) Effect of the elevated-temperature-precipitation in alloy 625 on properties and microstructure. In: TMS superalloys 718, 625 and various derivatives. pp 363–374
Tawancy HM (1992) Thermal stability of an Ni-Cr-Mo-Nb alloy. Mater Charact 28:221–240
Suave LM, Bertheau D, Cormier J, Villechaise P, Soula A, Hervier Z, Laigo J (2014) Impact of microstructural evolutions during thermal aging of alloy 625 on its monotonic mechanical properties. MATEC Web Conf 14:21001
Sundararaman M, Mukhopadhyay P, Banerjee S (1988) Precipitation of the δ-Ni3Nb phase in two nickel base superalloys. Metall Mater Trans A 19:453–465
Korth GE, Trybus CL (1991) Tensile properties and microstructure of Alloy 718 thermally aged to 50,000 h. In: TMS superalloys 718, 625 and various derivatives. pp 437–446
Desvallees Y, Bouzidi M, Bois F, Beaude N (1994) Delta phase in Inconel 718: mechanical properties and forging process requirements. In: TMS superalloys 718, 625, 706 and various derivatives. pp 281–291
Azadian S, Wei L-Y, Warren R (2004) Delta phase precipitation in Inconel 718. Mater Charact 53:7–16
Sundararaman M, Nalawade S, Singh JB, Verma A, Paul B, Ramaswamy K (2010) Evolution of δ phase microstructure in alloy 718. In: TMS superalloy 718 and derivatives. pp 737–750
Acknowledgements
This work was supported by the MOTIE/KETEP of the Republic of Korea (No. 20151520101050). Financial support for two of the authors is provided by the BK-Plus Program of the MSIP/NRF of the Rep. of Korea.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Kim, S.H., Kim, C., Subramanian, G.O., Jang, C. (2018). Corrosion and Carburization Behaviour of Ni-Cr-Mo-Nb Superalloys in a High Temperature Supercritical-CO2 Environment. In: Ott, E., et al. Proceedings of the 9th International Symposium on Superalloy 718 & Derivatives: Energy, Aerospace, and Industrial Applications. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-89480-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-89480-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89479-9
Online ISBN: 978-3-319-89480-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)