Abstract
Introduction: Cardiac events in patients with Brugada syndrome (BS) typically occur at rest and mainly during sleep, suggesting that changes in autonomic modulation play an important role in the arrhythmogenesis of the disease. Moreover, sex differences in clinical manifestations of BS have been reported, identifying male patients with worse prognosis. The aim of our work was to assess and compare, according to sex, autonomic response to exercise in a clinical series including 105 BS patients.
Method: Standard 12-lead electrocardiogram recordings were collected during a physical stress test divided into four phases: warm-up, incremental exercise, active recovery, and passive recovery. Spectral non-stationary heart rate variability indicators were extracted by means of a smoothed pseudo Wigner-Ville distribution approach that adapts frequency bands to respiratory information. These indicators were then averaged in non-overlapped windows of 1 min for each patient to compare groups at each minute of the physical stress test.
Results: From the last minute of warm-up and until the third minute of incremental exercise, asymptomatic male patients presented significantly greater low-frequency (LF) values (\( \overline{{\mathrm{LF}}^{WU2}} \): p = 0.015;\( \overline{{\mathrm{LF}}^{EX1}} \): p = 0.024; \( \overline{{\mathrm{LF}}^{EX2}} \): p = 0.011; \( \overline{{\mathrm{LF}}^{EX3}} \): p = 0.002) than asymptomatic females. Conversely, asymptomatic women showed increased vagal modulation during the first minutes of incremental exercise (\( \overline{{\mathrm{HF}}^{EX1}} \): p = 0.031; \( \overline{{\mathrm{HF}}^{EX2}} \): p = 0.001). However, no significant differences were observed between symptomatic male and female patients.
Conclusion: As previously reported in healthy subjects, enhanced parasympathetic and decreased sympathetic tones appear to be not only greater in women but also defensive during cardiac stress. Based on the results, asymptomatic patients presented same-sex tendencies. However, we observed that symptomatic males developed a more female-like autonomic modulation, probably related to a more protective autonomic response to exercise. These results could be a step forward toward the understanding of the autonomic function in BS along with a potential impact on risk stratification.
 Artwork by Piet Michiels, Leuven, Belgium
Artwork by Piet Michiels, Leuven, Belgium
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Heart rate variability
- Time-frequency analysis
- Brugada syndrome
- Autonomic function
- Sex differences
- Exercise
- Arrhythmogenesis
- Parasympathetic system
- Cardioprotection
Introduction
Brugada syndrome (BS) is an inherited disease presenting a typical electrocardiographic pattern characterized by a distinct ST-segment elevation in right precordial leads (V1-V3). It is associated with a high risk for unexpected sudden cardiac death secondary to ventricular fibrillation in the absence of any apparent structural cardiopathy. Since its description in 1992 as a new cardiac disorder by Pedro and Josep Brugada [1], BS has attracted great interest because of its high incidence in some parts of the world (especially in Eastern countries), as well as its association with sudden death in young adults and, less frequently, in infants and children. It has been estimated that BS is responsible for 4%–12% of the total amount of sudden cardiac death (SCD) and for 20% of SCD in the absence of structural cardiopathy [2, 3].
Differences in phenotype and prognosis between men and women in a large population of patients with BS were reported in [4]. The analysis was based on electrocardiographic parameters extracted before and after a pharmacological test, and events during follow-up were recorded for all patients. The results clearly identify men having a greater risk clinical profile than women, although conduction disturbances could be a marker of risk in the female population.
Nevertheless, the multifactorial etiology of BS requires complex approaches capable of capturing multiple mechanisms underlying the pathology. The autonomic nervous system (ANS) plays a determinant role in the pathophysiology, arrhythmogenesis and prognosis of the disease. Major cardiac events in BS most commonly occur during parasympathetic dominance at rest or during sleep [5, 6] and studies based on positron emission tomography have confirmed autonomic dysfunction in this population [5, 7, 8]. Thus, indicators capturing changes in ANS modulation may provide useful knowledge for the analysis of sex differences in patients with BS.
Evaluation of the autonomic response can be better characterized by exciting the ANS in a controlled and repeatable fashion—by applying standardized maneuvers, such as physical-stress testing, which originates an increase in sympathetic activity and a parasympathetic withdrawal—thus resulting in higher heart rates (HR). Conversely, post-exercise cardio-deceleration is mediated by a progressive increase in parasympathetic activity [9] and a continued sympathetic recession [10].
Heart rate variability (HRV) analysis is widely employed in clinical practice to characterize ANS function [11]. However, classic approaches require the signals to be stationary, which is inappropriate for studying non-stationary processes induced by physical exercise. To overcome these limitations, a time-frequency analysis could be used to evaluate the autonomic function. Thus, the aim of this study was to analyze sex differences concerning the temporal variation of different HRV features in BS patients during each phase of a standardized physical stress test by means of a time-frequency approach.
Introduction to HRV
HRV is used to describe variations of HR and respiratory rate (RR) intervals as they result from the complex mechanisms involved in HR regulation, notably the combined action of the two subsystems of the ANS : the sympathetic and parasympathetic nervous systems.
On one hand, parasympathetic neurons release acetylcholine , a cholinergic hormone that activates muscarinic M2-receptors in the heart, causing HR to decrease by slowing down nodal conduction. Because parasympathetic innervation is mainly provided by the vagus nerve, parasympathetic modulation is commonly referred to as “vagal tone .” At rest, although both sympathetic and parasympathetic tones are exerted on the heart, the latter predominates. In contrast, sympathetic neurons release norepinephrine, a catecholamine that activates the β1 receptors on the heart, causing HR to increase by increasing sinoatrial-node discharge.
Although several methods have been suggested for the study of HRV, including a recently proposed approach based on a multivariate unitary index to represent cardiac autonomic regulation [12], frequency-domain analyses are particularly popular because they correlate the autonomic mechanisms involved in RR interval fluctuations on an HR record with the analysis of two main spectral indices: the low-frequency (LF) and the high-frequency (HF) components (Fig. 12.1). These components are usually measured in absolute units of power (ms2), although they may also be calculated in normalized units (n.u.) to emphasize their balanced behavior while minimizing the effect of changes in total power (TP) under stimulated conditions.
Vagal activity is the major contributor to the HF component, whereas the interpretation of the LF component is controversial. Although some studies suggest LF as a marker for sympathetic modulation, both sympathetic and parasympathetic influences have been reported in other studies. Consequently, the LF/HF ratio has been considered to capture sympathovagal balance as well as only sympathetic modulations.
In healthy patients, spectral analyses performed during 24 h have shown that LF and HF (expressed in n.u.) present a circadian pattern and reciprocal fluctuations with greater values of LF at daytime and of HF at nighttime. Moreover, LF has been proven to increase during tilting, mental stress and exercise testing; however, increases in HF have been induced by controlled respiration tests and cold stimulations [13].
Methods
Study Population
Patients diagnosed with BS (n = 105) took part in a physical exercise stress test while continuous ECG recordings were collected. They were enrolled in eight French hospitals located in Rennes, Angers, Brest, Poitiers, Nantes, Bordeaux, La Rochelle and Tours. After approval by the ethics committee of each center, all participants provided informed consent to participate in the study, which was performed in accordance with clinical research and ethics recommendations. Participants’ ages ranged from 19 to 74 (45.17 ± 13.62) years, and 76.2% were male. Twenty-four patients had the following documented symptoms: syncope (50%), cardiac arrest (41.7%), dizziness (12.5%) and, less frequently, palpitations and nocturnal convulsions (4.2%).
Implantable cardioverter defibrillator (ICD) implantation had been performed in 18 of 81 (22.2%) asymptomatic patients based on a positive electrophysiological study test, whereas all symptomatic patients were ICD carriers. Among 76 patients in whom genetic analysis was performed (19 were symptomatic), an SCN5A mutation was found in 27 (35.5%), of whom 6 were symptomatic patients. Patients’ baseline characteristics in the symptomatic and asymptomatic groups are listed in Table 12.1.
Signal Acquisition During Physical Stress Test
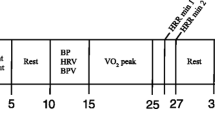
Patients underwent a clinical protocol recommended by the American Heart Association [14] described as a triangular stress test because the load was increased until it reached the patient’s submaximal potential. It was performed on a cyclo-ergometer (Ergoline 900 Egamed, Piestany, Slovakia) and it was divided in the following phases, as represented in Fig. 12.2, where the non-stationarity of the RR series can be noted:
-
Warm-up phase: for men, initial load of 50 watts (W); for women, initial load of 30 W; both for 2 min
-
Incremental exercise phase : for men, initial load of 80 W for 2 min and then incrementing load by 20 W every 2 min; for women, initial load of 50 W and then incrementing load 20 W every 2 min
-
Active recovery phase: for men, fixed load of 50 W; for women, fixed load of 30 W; both for 3 min
-
Passive recovery phase: total cessation of effort for 3 min
Exercise testing was divided into four phases : warm-up, incremental exercise, active recovery and passive recovery. HRV time series estimated from the RR series were averaged in the following 1-min windows: both mins of warm-up (WU1 and WU2), first 3 min of incremental exercise (EX1, EX2, EX3), last min of exercise before peak effort (PE), 3 min of active recovery (AR1, AR2, AR3) and 3 min of passive recovery (PR1, PR2, PR3)
The purpose of the test was to reach at least the 80% of the theoretical maximum HR of each patient as defined by the formula MHR = 220−age [15]. The 12-lead ECG recordings were collected and analyzed by the central board (Centre Hospitalier Universitaire de Rennes) using a Holter monitor (ELA Medical, Sorin Group, Le Plessis Robinsson, France) at a sampling frequency of 1000 Hz. Signals were continuously recorded during the warm-up (2 min), incremental exercise (11.86 ± 3.24 min), and recovery (6 min) phases.
As illustrated in Fig. 12.1, RR interval and R-peak amplitude series were obtained using a noise-robust wavelet-based algorithm for QRS complex detection and subsequent R-wave peak location [16]. After performing manual corrections to the obtained series when necessary, HRV parameters were obtained for the different test phases.
Time–Frequency Signal Analysis
Given that signals on a physical stress test are typically non-stationary, spectral characteristics associated with HRV were analyzed using a time–frequency approach. After cubic splines interpolation and regular resampling at 10 Hz, RR series were high-pass filtered at 0.03 Hz with a fourth-order Butterworth filter applied in both forward and backward directions to remove phase distortion.
Then, a smoothed pseudo Wigner-Ville distribution (SPWVD) transform was employed because it has proved its usefulness for the analysis of cardiovascular signals [17]. The Wigner-Ville distribution is a quadratic time−frequency technique defined as the Fourier transform of the instantaneous auto-correlation/cross-correlation function [18]. Because it is affected by significant interference terms, the SPWVD introduces a smoothing kernel function, ψ(τ, v), defined in Costa and Boudreau-Bartels GF [19], that attenuates interferences while maintaining a suitable time–frequency resolution . Because ARR(τ, v), the ambiguity function of the RR series, was xRR(t), the SPWVD is defined as follows:
The parameters vo and τo, associated with time and frequency resolution, were defined from [19]. HRV was measured as the TP in the LF and HF bands (noted as LFb and HFb), obtained from the SPWVD :
Assuming that sympathetic activity always lies within the standard LF band, this band was fixed between 0.04 and 0.15 Hz for the whole stress test. However, the TP in the HF band captures parasympathetic activity , which is closely related to respiratory sinus arrhythmia. Because respiratory frequency during ANS stimulation , especially during exertion, may not be restricted to the classic HF band (0.15–0.4 Hz) and can increase up to 0.7 Hz, HRV analysis within the standard frequency band would lead to unreliable measures of the parasympathetic activity. To overcome this limitation, a time-varying HF band, based on an estimation of the respiration activity captured by the ECG-derived respiration (EDR) series, has been defined [20].
The applied EDR method estimates respiratory information from the amplitude modulation of R-wave peaks [21]. The estimated respiration signal was then band-pass filtered by a fourth-order Butterworth filter between 0.15 and 0.7 Hz applied in both forward and backward directions to remove frequencies from the respiratory range. The same SPWVD transform used for the RR series was then applied to the EDR-filtered signals to estimate the instantaneous respiratory frequency, fr (t). Once the estimated respiratory frequency series fr (t) was obtained, the time-varying HF band for HRV analysis was defined as HFb(t) = [fr (t) − 0.125, fr (t) + 0.125] Hz with t covering the whole test.
Unlike classical HRV parameters, SPWVD leads to time–frequency HRV estimators that are indeed time series that vary during the application of the stress test. These markers, accounting for the sympathetic and parasympathetic influences of the ANS on HR, were normalized by the TP, which was defined as the sum of both spectral bands (TP(t) = LF(t) + HF(t)), leading to the time series LFnu (t) and HFnu (t). LF/HF(t) was also obtained from dividing LF(t) by HF(t) in the time–frequency domain to obtain the global sympathovagal balance.
Analysis of Effort Testing
HRV time series were extracted from the time–frequency analysis based on the SPWVD of the RR series, which adapts frequency bands to respiratory information resulting from EDR signals. These HRV time series were averaged in temporal non-overlapped windows of 1 min for each patient, leading to \( \overline{{\mathrm{LF}}^i} \), \( \overline{{\mathrm{LF}}_{nu}^i} \), \( \overline{{\mathrm{HF}}^i} \), \( \overline{{\mathrm{HF}}_{nu}^i} \), \( \overline{\mathrm{LF}/{\mathrm{HF}}^i} \), which stand for each time series intra-patient mean for the following time periods: i ∈ {WU1, WU2, EX1, EX2, EX3, PE, AR1, AR2, AR3, PR1, PR2, PR3}.
Because each test differed in the duration of the incremental exercise phase and the shortest case in our clinical series lasted less than 5 min, only the first 3 min of incremental exertion (EX1, EX2 and EX3) and the last minute before peak effort (PE) were assessed. In addition, the entire warm-up (WU1 and WU2) and active (AR1, AR2 and AR3) and passive recovery (PR1, PR2 and PR3) phases were compared between male and female patients. Figure 12.2 displays the analyzed periods for different phases of the exercise test, indicating the peak effort instant.
Finally, HRV features extracted during exercise and recovery were compared between male and female groups using Mann-Whitney U non-parametric tests. To compare the last minute of exertion and recovery, all patients had to be synchronized with respect to the peak-effort instant. The analysis was made using the commercially available software MatLab (Mathworks Inc., MI, USA) and setting the level of significance at p <0.05.
Results
Figure 12.3 displays an exemplifying RR series and its associated SPWVD spectral power for the same patient; the LF and respiration-centered HF bands are represented by dashed white lines (second panel). The third panel shows the time series LF(t) and HF(t) extracted from time–frequency analysis , where the 1-min windows used to calculate the mean value of HF during the first minute of active recovery (\( \overline{{\mathrm{HF}}^{AR1}} \)) and the mean value of LF at the peak effort (\( \overline{{\mathrm{LF}}^{PE}} \)) are indicated.
From the RR series, the normalized SPWVD spectral power is calculated. The second panel indicates the LF and HF bands in dashed white lines. In the third panel, the TP in LF (blue) and HF (black) bands at each time instant are represented. Then, two examples of averaged time series (\( \overline{{\mathrm{LF}}^{PE}} \) and \( \overline{{\mathrm{HF}}^{AR1}} \)) account for the sympathetic and parasympathetic contributions along the whole exercise test. A vertical dashed line in all panels refers to the peak effort instant
Table 12.2 lists the evolution of significant HRV features and their associated p-values obtained when comparing male and female asymptomatic patients for different segments of physical stress and recovery.
From the last minute of warm-up and until the third minute of incremental exercise , asymptomatic male patients presented significantly greater LF values (\( \overline{{\mathrm{LF}}^{WU2}} \): p = 0.015; \( \overline{{\mathrm{LF}}^{EX1}} \): p = 0.024; \( \overline{{\mathrm{LF}}^{EX2}} \): p = 0.011; \( \overline{{\mathrm{LF}}^{EX3}} \): p = 0.002) compared with asymptomatic females. Conversely, based on HF values, asymptomatic women showed increased vagal modulation during the first minutes of incremental exercise \( \Big(\overline{{\mathrm{HF}}^{EX1}} \): p = 0.031; \( \overline{{\mathrm{HF}}^{EX2}} \): p = 0.001). However, no significant differences were observed regarding normalized variables or between symptomatic male and female patients.
Discussion
HRV is recognized as a relevant measure of autonomic function . The major findings of this study are as follows: (1) females showed greater HRV-HF values after exercise; (2) asymptomatic male patients presented significantly greater LF values than asymptomatic females at the end of warm-up and during the beginning of incremental exercise ; and (3) sex differences seem to disappear for symptomatic male and female patients. Overall, the results suggest an evolution of sex differences with symptomatic status. Asymptomatic females presented greater vagal modulation after exercise, whereas males displayed increased sympathetic dominance. These results are supported by the results of previous works [22] showing that females exhibit a cardio-protective autonomic profile compared with males and thus show a different autonomic response to exercise. In Koenig and Thayer [23], women showed a greater mean HR while presenting a greater vagal contribution captured by HF power of the HRV.
Main differences in autonomic responses were found during the end of warm-up and the beginning of exercise, which is associated with rapid parasympathetic decrease, gradual sympathetic activation and circulating catecholamines [24]. Asymptomatic women presented increased vagal modulation during the first minutes of incremental exercise compared with men. This difference in response to exercise may be due to ovarian hormones [25], which have been shown to have a clear influence on autonomic response [26] through the preservation of sympathovagal balance [27] and baroreflex sensitivity [28]. Moreover, estrogen was shown to improve vasomotor tone, vascular integrity, lipid profiles and cholesterol metabolism and decrease blood pressure [29].
Concerning BS, Benito et al. [4] reported that men were predominant and displayed a higher risk profile compared with women, which could be explained by sex-related intrinsic differences in ionic currents and hormonal influences. In fact, Di Diego et al. [30] proved that transient outward potassium currents were significantly greater in male than in female right-ventricle epicardia of arterially perfused canine heart preparations. Hormones also have important influences in the phenotypic manifestations of BS [31], which could explain the significant levels of testosterone reported in male BS patients compared with control subjects [32]. Moreover, results of experimental studies [33, 34] have demonstrated that hormones could induce ionic membrane current variations, which might explain sex-related ionic current differences in patients with BS.
According to the current results, asymptomatic males and females presented significant differences in the autonomic response to exercise, which concurs with observations obtained in healthy subjects [22]. Increased parasympathetic and decreased sympathetic modulation were found in asymptomatic women because it is associated with defensive behavior during cardiac stress. No significant differences were observed between symptomatic male and female patients. Consequently, the results suggest that the ANS response evolves with symptomatology because symptomatic males seem to develop a female-like autonomic modulation, probably related to the emergence of cardio-protective behavior .
Conclusion
As previously reported in healthy subjects, increased parasympathetic and decreased sympathetic modulation appears to be not only greater in women but also defensive during cardiac stress. Based on the present results, asymptomatic patients presented the same-sex tendencies. However, we observed that symptomatic males developed a more female-like autonomic modulation, probably related to a more protective autonomic response to exercise. Although the results are based on approximations of ANS modulation and conclusions on neural activity cannot be inferred, this work presents a step forward toward the understanding of the autonomic function in BS patients and thus a potential impact on risk stratification. The differences found between male and female patients suggest that risk stratification should consider sex when evaluating indicators of risk based on autonomic information.
References
Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20(6):1391–6.
Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3 : a marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998;97(5):457–60.
Antzelevitch C. Heart Rhythm Society; European Heart Rhythm Association. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111(5):659–70.
Benito B, Sarkozy A, Mont L, Henkens S, Berruezo A, Tamborero D, et al. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol. 2008;52(19):1567–73.
Kies P, Wichter T, Schäfers M, Paul M, Schäfers KP, Eckardt L, et al. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome. Circulation. 2004;110(19):3017–22.
Matsuo K, Kurita T, Inagaki M, Kakishita M, Aihara N, Shimizu W, et al. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J. 1999;20(6):465–70.
Paul M, Meyborg M, Boknik P, Gergs U, Schmitz W, Breithardt G, et al. Autonomic dysfunction in patients with Brugada syndrome: further biochemical evidence of altered signaling pathways. Pacing Clin Electrophysiol. 2011;34(9):1147–53.
Wichter T, Matheja P, Eckardt L, Kies P, Schäfers K, Schulze-Bahr E, et al. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105(6):702–6.
Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24(6):1529–35.
Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol. 1982;53(6):1572–5.
Camm AJ, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, et al. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65.
Sala R, Malacarne M, Solaro N, Pagani M, Lucini D. A composite autonomic index as unitary metric for heart rate variability: a proof of concept. Eur J Clin Investig. 2017;47(3):241–9.
Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation [Internet]. 1991;84(2):482–92. Available from: http://circ.ahajournals.org/content/84/2/482.abstract
Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40(8):1531–40.
Fox SM 3rd, Haskell WL. Physical activity and the prevention of coronary heart disease. Bull N Y Acad Med. 1968;44(8):950–65.
Dumont J, Hernandez AI, Carrault G. Improving ECG beats delineation with an evolutionary optimization process. IEEE Trans Biomed Eng. 2010;57(3):607–15.
Orini M, Mainardi LT, Gil E, Laguna P, Bailón R. Dynamic assessment of spontaneous baroreflex sensitivity by means of time-frequency analysis using either RR or pulse interval variability. In: 2010 Annual international conference of the IEEE engineering in medicine and biology. 2010. pp 1630–1633.
Hlawatsch F, Boudreaux-Bartels GF. Linear and quadratic time-frequency signal representations. IEEE Signal Process Mag. 1992;9(2):21–67.
Costa AH, Boudreau-Bartels GF. Design of time-frequency representations using a multiform, tiltable exponential kernel. IEEE Trans Signal Process. 1995;43(10):2283–301.
Bailón R, Laguna P, Mainardi L, Sornmo L. Analysis of heart rate variability using time-varying frequency bands based on respiratory frequency. In: Engineering in medicine and biology society, 2007 EMBS 2007 29th Annual international conference of the IEEE. 2007. pp 6674–6677.
Moody GB, Mark RG, Bump MA, Weinstein JS, Berman AD, Mietus JE, et al. Clinical validation of the ECG-derived respiration (EDR) technique. Comput Cardiol. 1986;13:507–10.
Kappus RM, Ranadive SM, Yan H, Lane-Cordova AD, Cook MD, Sun P, et al. Sex differences in autonomic function following maximal exercise. Biol Sex Differ [Internet]. 2015;6(1):28. Available from: http://www.bsd-journal.com/content/6/1/28
Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310.
Breuer HW, Skyschally A, Schulz R, Martin C, Wehr M, Heusch G. Heart rate variability and circulating catecholamine concentrations during steady state exercise in healthy volunteers. Heart [Internet]. 1993;70(2):144–9. Available from: http://heart.bmj.com/cgi/doi/10.1136/hrt.70.2.144
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation. 2016;133:e38–48.
Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862.
Saleh TM, Connell BJ. Estrogen-induced autonomic effects are mediated by NMDA and GABAA receptors in the parabrachial nucleus. Brain Res. 2003;973(2):161–70.
Mohamed MK, El-Mas MM. Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Phys. 1999;276(4 Pt 2):R1030–7.
Benjamin IJ, Christians E. Exercise, estrogen, and ischemic cardioprotection by heat shock protein 70. Circ Res. 2002;90(8):833–5.
Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Pérez GJ, et al. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation. 2002;106(15):2004–11.
Antzelevitch C. Androgens and male predominance of the Brugada syndrome phenotype. Pacing Clin Electrophysiol. 2003;26(7p1):1429–31.
Shimizu W, Matsuo K, Kokubo Y, Satomi K, Kurita T, Noda T, et al. Sex hormone and gender difference – role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18(4):415–21.
Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, et al. Remodeling of Kv4.3 Potassium Channel gene expression under the control of sex hormones. J Biol Chem. 2001;276(34):31883–90.
Bai CX, Kurokawa J, Tamagawa M, Nakaya H, Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112(12):1701–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Calvo, M. et al. (2018). Sex Differences in Autonomic Response to Exercise Testing in Patients with Brugada Syndrome. In: Kerkhof, P., Miller, V. (eds) Sex-Specific Analysis of Cardiovascular Function. Advances in Experimental Medicine and Biology, vol 1065. Springer, Cham. https://doi.org/10.1007/978-3-319-77932-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-77932-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77931-7
Online ISBN: 978-3-319-77932-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)