Abstract
Iron overload in MDS results from increased intestinal iron absorption due to ineffective erythropoiesis and, more importantly, from chronic transfusion therapy. Blood transfusions are necesssary to extend the survival of patients with MDS and improve their quality of life. However, the ensuing transfusional iron overload has a dose dependent negative impact on survival. Cardiac dysfunction appears to be relevant in this context, as a consequence of chronic anemia, age-related cardiac comorbidity, and iron overload. In addition, iron-related endothelial dysfunction and a higher risk of infection should be taken into account. Iron overload may also aggravate the bone marrow failure in MDS. Clinical trials have shown that iron chelators can improve hematopoiesis in a minority of transfusion-dependent patients. Analyses of registry data suggest that iron chelation provides a survival benefit for patients with MDS, but data from a randomized clinical trial are still lacking.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
“The dose makes the poison.” This famous statement by Paracelsus is also true for iron. On the one hand, iron is essential for a variety of pivotal biological processes. On the other hand, iron can be toxic because it promotes oxidative stress. As it easily switches between its divalent (ferrous) and trivalent (ferric) form, iron can efficiently transfer single electrons and thus strongly catalyzes biochemical reactions like the Fenton reaction that generate reactive oxygen species. The latter attack macromolecules and organelles, thereby causing cellular damage that ultimately leads to tissue and organ dysfunction. This potential for toxicity is increased when too much iron is present.
2 What Are the Causes of Iron Overload in MDS?
Iron overload in MDS results from increased intestinal iron absorption and from chronic transfusion therapy. The process of iron overload starts before MDS patients become transfusion dependent because ineffective erythropoiesis suppresses hepcidin production in the liver, thereby causing unrestrained intestinal iron uptake [1].
Hepcidin normally inhibits iron uptake in the duodenum. In thalassemia major, hepcidin is suppressed by highly elevated serum levels of GDF15, secreted by maturing erythroblasts in the bone marrow [2]. In myelodysplastic syndromes, levels of GDF15 are much less elevated. Therefore, other signals from the bone marrow like TWSG1 (“twisted in gastrulation”) seem to contribute to hepcidin suppression in MDS [3]. A promising candidate is erythroferrone (ERFE), a recently discovered hormone that mediates hepcidin suppression during stress erythropoiesis in mice after bleeding or hemolysis [4]. Whether erythroferrone also suppresses hepcidin in MDS patients with expanded, ineffective erythropoiesis has not yet been confirmed. ERFE mRNA is increased [5], but protein expression data are still lacking.
While increased intestinal iron absorption clearly contributes to iron overload, the most important cause of iron overload in MDS is chronic transfusion therapy. A patient who requires four RBC units per month, which is not unusual, will receive 100 units over 2 years, equivalent to at least 20 g of iron. The normal total body iron is 3–4 g.
Amelioration of anemia is important in patients with MDS. The probability of non-leukemic death, particularly from cardiac complications, is dramatically increased in male MDS patients with a Hb <9 g/dL and female MDS patients with a hemoglobin <8 g/dL, indicating that MDS patients should be adequately transfused to maintain their hemoglobin levels at least above those critical thresholds [6]. However, while proper amelioration of anemia increases the quality of life and likelihood of survival, the concomitant development of transfusional iron overload may have an opposite effect.
3 What Are the Clinical Consequences of Iron Overload in MDS?
Iron overload, as measured by elevated serum ferritin (SF) levels, is associated with a decreased likelihood of survival in patients with lower-risk MDS. It has been shown that above a SF threshold of 1000 μg/L, iron overload has a dose-dependent impact on survival [6]. This looks like irrefutable evidence of a noxious effect, which must be abrogated by iron chelation. However, the data could also be interpreted in a different way: Higher serum ferritin levels may simply reflect greater transfusion need, which in turn reflects more severe bone marrow disease, the latter being the real cause of shorter survival. On the other hand, it was also shown that serum ferritin is an independent prognostic factor in MDS, even if transfusion burden is taken into account on multivariate analysis by including the number of transfusions per month as a covariate. Under these transfusion-adjusted conditions, there was still a 30% greater risk of death for every 500 μg/L increase in SF above the threshold of 1000 μg/L [7]. Similarly, the data from the European LeukemiaNet prospective MDS registry indicated that besides transfusion burden, which was the major prognostic factor, increasing levels of serum ferritin had independent impact on the overall survival of transfusion-dependent patients with lower-risk MDS [8].
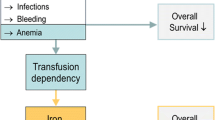
Figure 8.1 illustrates that, on the one hand, transfusion dependency is clearly linked with shortened survival because it reflects severe bone marrow disease with all its possible complications like infections, bleeding, and adverse effects of chronic anemia. On the other hand, transfusion dependency causes iron overload, thereby creating a new medical problem that has its own negative impact on survival. The relative weight of these two risk factors may vary considerably between patients. A patient with pure sideroblastic anemia (RARS) is unlikely to die from infections or bleeding. Instead, transfusional iron overload may become a clinical problem. In contrast, a patient with RAEB-I or RAEB-II, despite similar transfusion dependency, may not have the time to develop clinical complications of iron overload because survival is limited by severe bone marrow failure.
Within that conceptual framework, cardiac dysfunction may play an important role, as a consequence of chronic anemia, age-related cardiac comorbidity, and iron overload. Cardiac iron overload in MDS is detectable by MRI after 75–100 units of RBC have been transfused. A British group evaluated 43 transfused MDS patients with T2* magnetic resonance scans and found that 81% had liver and 16.8% had cardiac iron overload [9]. This is in line with a study from France looking at a relatively large series of 75 regularly transfused MDS patients [10]. The investigators found cardiac iron overload, defined by T2* ≤20 ms, in 18.2% of the patients, who mostly belonged to the IPSS low- and intermediate-1-risk groups. Severe cardiac iron overload with a T2* ≤10 ms was detected in 4% of the patients. There was a linear correlation between the T2* values and the number of RBC units transfused. Most of the patients were also studied by echocardiography. Severe cardiac dysfunction with a LVEF ≤35% was found in 3 of 11 patients (27%) with T2* ≤20 ms but only 1 of 46 patients with T2* >20 ms. The authors concluded that iron overload can be a significant aggravating factor in the pathophysiology of cardiac failure in MDS, in addition to chronic anemia and comorbidities.
Iron-related organ damage, including heart failure, may depend not only on tissue iron concentrations but also on the duration of chronic exposure to non-transferrin-bound iron and labile plasma iron, which generate oxidative stress. A patient with a serum ferritin of 2500 ng/mL may not only have more extensive iron accumulation but may also have had longer exposure to high levels of ROS than a patient with a SF level of 1500 ng/mL.
One should also be aware that cardiac function is more vulnerable to iron toxicity than liver function. It has been shown that clinically relevant cardiac dysfunction occurs at much lower tissue iron concentrations than clinically relevant liver dysfunction [11, 12].
Accordingly, the supportive care strategy for MDS patients must be twofold. On the one hand, ESAs and RBC transfusions should be used to avoid detrimental effects of chronic anemia. At the same time, iron chelation should be used to ensure that the benefits of transfusion therapy are not offset by harmful effects of iron overload.
Another potential problem, which may be underestimated, is iron-related endothelial dysfunction. This is not an entirely novel topic but one that has recently attracted renewed interest. There is some evidence that with increasing age, high circulating iron levels strongly enhance the severity of the atherosclerotic phenotype, indicating that systemic iron overload is a risk factor for atherosclerosis progression and development of cardiovascular disease [13]. According to the working hypothesis, iron can accumulate in macrophages from increased destruction of RBCs or from disturbed iron homeostasis under the influence of hepcidin. Accumulation of iron leads to increased production of ROS and decreased efflux of cholesterol from the macrophages. The resulting oxidative stress and LDL accumulation promote the formation of foam cells, inflammation, apoptosis, and eventually plaque destabilization.
Is there a beneficial role for iron chelation in that context? Fifteen years ago, it was shown that iron chelation improves endothelial function in patients with coronary artery disease [14]. Deferoxamine improved nitric oxide-mediated, endothelium-dependent vasodilation, suggesting that iron availability contributes to impaired nitric oxide action in atherosclerosis. A beneficial effect of iron chelation on arterial function has also been reported in patients with beta-thalassemia major. During 12 months of treatment, deferasirox significantly improved brachial artery flow-mediated dilation and significantly decreased the carotid arterial stiffness index. These findings were attributed to the ability of deferasirox to bind labile cell iron pools in the vascular wall, thereby diminishing reactive oxygen species formation and thus attenuating nitric oxide inactivation [15].
The connection between iron and endothelial dysfunction illustrates that iron-related complications overlap with age-related clinical problems in elderly MDS patients (Fig. 8.2). Iron overload may simultaneously aggravate several common causes of death. However, even if that adds up to a strong cumulative effect, the impact of iron overload may easily hide behind the normal, common causes of death in the elderly. Accordingly, it is difficult to determine the extent to which iron overload contributes to morbidity and mortality in elderly MDS patients.
4 Does Iron Overload Affect the Bone Marrow in MDS?
The bone marrow seems to be one of the organs that suffer from iron overload. A vicious cycle is envisaged, starting with ineffective erythropoiesis that causes anemia and transfusion dependency, thereby leading to transfusional iron overload, which in turn aggravates the bone marrow failure. Figure 8.3 illustrates where iron overload may have an aggravating effect on MDS pathophysiology. Myelodysplastic syndromes arise from primitive hematopoietic stem cells that accumulate genomic damage from various insults. One of the damaging factors is oxidative stress, which can be promoted by iron overload. If an abnormal stem cell survives and is equipped with a proliferative advantage and some degree of genomic instability, a dominant clone as well as subclones with multiple mutations can arise. The further development depends on the intrinsic qualities of the MDS clone and its interaction with the bone marrow microenvironment. Mutated clones may elicit an effective immune response and be eliminated or may elicit an ineffective immune response that may still create an inflammatory milieu. The latter may do more harm to normal hematopoietic cells than to the abnormal clone. Pathological clones may also condition the bone marrow stroma through epigenetic mechanisms, again favoring the growth of preleukemic hematopoietic cells. Abnormal selection pressure in the altered microenvironment will promote the outgrowth of maladapted clones, i.e., clones that are dysfunctional yet capable of taking advantage of the abnormal environment. Experimental evidence indicates that iron overload contributes to stromal dysfunction in MDS [16, 17]. Altogether, iron overload may exacerbate genomic instability and abnormal selection pressure, thereby hastening clonal evolution toward leukemia.
The abovementioned concept is supported by experimental and clinical data. Iron overload is known to increase oxidative stress, thereby contributing to mutagenesis. Increased oxidative stress is indeed detectable in MDS and aggravated by iron overload [18,19,20,21,22,23,24,25]. It causes oxidative DNA damage that is also made worse by iron overload [26]. The consequences of increased oxidative stress are particularly detrimental in erythroid progenitors. It has been demonstrated that iron overload impairs the proliferation of erythroid progenitor cells in patients with MDS, even at moderately elevated ferritin values, whereas the number of granulopoietic colonies is largely unaffected [27]. The negative impact was reversible through iron chelation therapy.
Why should erythropoiesis be particularly vulnerable to the toxic effects of iron overload? Researchers in Israel, using flow cytometry techniques, found that both the TfR-deficient mature RBC and their TfR-containing precursors at all stages of maturation can take up non-Tf iron that accumulates as redox-active labile iron and generates reactive oxygen species [28]. This transferrin-independent pathway is operative in pathological iron overload situations in the presence of non-Tf iron in the serum. Unfortunately, the incoming non-Tf iron does not participate in heme synthesis and Hb production but induces ROS generation that results in cytotoxicity and a decrease in the erythroid cell yield. The effect of iron overload and chelation on erythroid differentiation has also been investigated [29]. Iron overload significantly suppressed the formation of BFU-E and their differentiation to mature erythroblasts; these effects were canceled by iron chelation with deferoxamine (DFO). Moreover, excessive iron burden promoted apoptosis in immature erythroblasts by elevating intracellular reactive oxygen species (ROS).
Iron overload also causes stromal dysfunction, as shown in a mouse model [16]. BM transplantation from normal donors to IO recipients resulted in delayed hematopoietic reconstitution, indicating that excess iron has an unfavorable impact on the hematopoietic microenvironment. MSC showed markedly reduced expression of surface molecules known to be involved in stem cell homing.
Also in mice, IOL impaired the proliferation of mouse BM mesenchymal cells, and free iron catalyzed in vitro oxidative damage to mesenchymal cells, thereby attenuating hematopoiesis [17].
When the effects of iron overload on genomic instability in MDS were investigated, the results supported the assumption that iron overload might be causally related to genetic instability [30]. The data also suggested that SF levels not only above 1000, but also between upper limit of normal value but below 1000, adversely affect genetic stability. Therefore, iron chelation might be relevant for patients with MDS at lower SF levels than previously thought.
The fact that hereditary hemochromatosis and thalassemia major are not associated with an increased incidence of MDS and AML suggests that iron overload by itself does not transform normal hematopoietic stem cells into preleukemic stem cells. However, hematopoietic stem cells that have already acquired genomic instability from other causes and are also subject to continuous proliferative stress seem to be vulnerable to the additional genotoxic stress from iron overload.
Apparently, the abovementioned vicious cycle between iron overload and bone marrow failure can be interrupted by iron chelation therapy. A number of case reports, small patient series, and larger studies found that the iron chelator deferasirox can improve hematopoiesis in MDS. Table 8.1 shows the largest studies conducted so far. Erythroid response rates range between 11 and 22%.
Using strict criteria for erythroid response, a prospective Italian multicenter study found that during 12 months of treatment with DFX, the RBC transfusion requirement declined significantly from a median of 3 per month to a median of 1 per month [34]. The cumulative incidence of transfusion independence (the latter defined as more than 3 months without transfusions and a hemoglobin maintained above 9 g/dL) increased from 2.6% at 6 months to 12.3% at 9 months and 15.5% at 12 months.
This is not a novel phenomenon. About 20 years ago, Jensen et al. [35] achieved a remarkable effect of iron chelation on hemopoiesis in MDS patients with transfusional iron overload when they followed 11 MDS patients for up to 60 months during and after treatment with deferoxamine. They observed a greater than 50% reduction in transfusion requirement in 7 of 11 patients, and 5 patients even became transfusion independent. All patients in whom iron chelation was highly effective showed improvement of erythropoietic output. As this was achieved with deferoxamine (rather than deferasirox), hematological responses do not seem to be restricted to a particular iron chelator. It is probably more important to keep patients well chelated over a long time. Long-term suppression of oxidative stress may improve conditions in the bone marrow microenvironment and may slow down the pace of genetic evolution.
5 Is There a Survival Benefit from Chelation Therapy?
Numerous studies suggest that iron chelation improves survival in transfusion-dependent MDS (Table 8.2). However, the main problem with these studies is that patient populations were usually well characterized regarding disease-related risk factors, but not characterized and stratified according to overall performance status. This introduces a possible bias because patients with a better overall performance status may have been more likely to receive iron chelation, thus shifting survival curves in favor of this treatment.
This problem has been addressed by a recent study from the Canadian MDS Registry, which appears to be the only MDS registry that carefully documents performance status and comorbidities. The investigators examined the outcomes of 70 patients with low/Int-1-risk MDS who received ICT in comparison with 149 who did not [45]. There was no significant difference in the frailty and comorbidity scores between the iron-chelated group and the non-iron-chelated group. On multivariate analysis, ICT retained significance for overall survival, with a hazard ratio of 1.8 and p value of 0.0152. However, despite showing no significant bias in terms of general fitness, MDS patients receiving iron chelation were significantly younger and had a more favorable distribution among MDS risk groups. Therefore, this study only partly alleviates the concerns regarding selection bias.
Age and risk group distribution were not problematic in a matched pair analysis performed in the Düsseldorf MDS Registry [38]. Here, cohorts were carefully matched for age, gender, MDS type, and MDS risk groups. Again, there was a significant survival advantage for the patients receiving iron chelation therapy. The disadvantage of this study was that the matching did not include performance score and comorbidities caused by a lack of data. Assuming that hematologists in Germany acted similar to their Canadian colleagues and did not restrict iron chelation to fitter patients, this potential bias may be negligible, and the Düsseldorf data may reflect the true survival advantage of iron chelation therapy.
Probably, the best data available so far are those from the European LeukemiaNet MDS (EUMDS) Registry [46]. Overall survival of 192 chelated patients was shown to be significantly better when compared with a large control group of 573 non-chelated patients, even after adjustment for all relevant prognostic factors, i.e., age, sex, comorbidity, performance status, and number of RBC units transfused prior to the start of chelation. Another advantage of this study is that it looked at survival from the point in time when patients reached the eligibility criteria for iron chelation. Therefore, long-lasting stable intervals between diagnosis and onset of transfusion dependency were not counted and thus not misinterpreted as prolonged survival due to iron chelation. Short of a randomized prospective trial, these data may come as close as possible to reflecting the true survival benefit of iron chelation in lower-risk MDS.
It is difficult to pinpoint why iron chelation provides a survival advantage to MDS patients. Data from a US registry showed that the rate of cardiac causes of death was somewhat lower, infections occurred less frequently, and other malignancies were very rare [43]. However, the differences were not statistically significant. The IRON2 study from Spain, which found a significantly longer overall survival and leukemia-free survival in chelated patients with lower-risk MDS, also demonstrated a highly significant difference regarding the median event-free survival related to cardiac complications [44]. As already alluded to, it may be important to pay attention to iron overload not only as a cause of cardiomyopathy but also as an aggravating factor of atherosclerosis.
Clinical data regarding a beneficial effect of iron chelation on AML transformation are controversial. While the data from the Düsseldorf MDS Registry did not suggest a tangible effect [38], the abovementioned US registry found that iron chelation delays AML transformation [43].
6 How Is Iron Overload Diagnosed in MDS?
A suitable and widely used method to measure iron overload is to measure serum ferritin (SF), which generally shows good correlation with liver iron content as well as total body iron. However, serum ferritin can be influenced by inflammatory conditions. Therefore, it is important not to place too much emphasis on single measurements. More important is the trend of SF over time, which should be monitored monthly. Measurement of transferrin saturation (TfS) may also be useful because values above 70–80% are associated with the appearance of non-transferrin-bound iron (NTBI) and labile plasma iron (LPI), leading to increased iron uptake and oxidative stress in parenchymal cells of various organs.
Liver iron concentration can be assessed using magnetic resonance imaging (MRI). Although this is a very useful method that avoids the sampling errors and risk of complications associated with liver biopsy, MRI is not readily available everywhere.
It should be noted that there may be substantial discordance between liver and cardiac iron content, prior to and during iron chelation therapy. Iron accumulates in the liver first but later goes to the heart as well. During iron chelation therapy, removal of iron from the liver is much faster than from the heart. Furthermore, it is easier to improve cardiac iron overload if the liver is not grossly iron overloaded. A high liver iron content seems to prevent iron chelators from suppressing LPI and removing much iron from the heart [47, 48]. If cardiac iron overload is suspected, cardiac MRI should be performed. If this is not available, serial monitoring of cardiac function by echocardiography can be useful. In order to detect iron-related organ damage, laboratory tests of liver function and endocrine function should be done.
7 When and How to Treat Iron Overload in Patients with MDS?
Iron chelation therapy in patients with transfusion-dependent MDS has been shown to be safe and effective [31, 49, 50]. Numerous national and international guidelines have been written on the use of iron chelation therapy in MDS. They are often included in guidelines on MDS treatment in general. For example, the European LeukemiaNet guideline on the diagnosis and treatment of primary myelodysplastic syndromes in adults includes a short segment on ICT [51]. The expert panel agreed that iron chelation should be considered in transfusion-dependent patients with RA, RARS, or MDS with isolated 5q deletion and a serum ferritin level higher than 1000 ng/mL after approximately 25 units of red cells. In addition, MDS patients who are potentially candidates for allo-SCT can be considered for appropriate iron chelation therapy prior to the conditioning regimen for transplantation. Guidelines on ICT in MDS are similar in different countries. They usually recommend chelation therapy for patients who have a certain transfusion history (usually at least 20 or 25 pRBC units), whose serum ferritin levels exceed a certain threshold (1000 or 1500 ng/μl) and who have lower-risk MDS with a reasonable life expectancy because such patients often receive long-term transfusion therapy that puts them at risk of developing clinical complications of iron overload.
Nowadays, the most commonly used iron chelator is deferasirox. Most physicians experienced in the field of iron chelation have adopted a gentle approach when starting the treatment [52]. Instead of using the recommended dose of 20 mg/kg/day, it is advisable to start at a lower dose, i.e., a fixed dose of 500 mg/day (nowadays 360 mg/day with the new film-coated tablets). Patients are monitored regarding drug tolerability, and the dose is increased in weekly increments of 5 mg/kg, with a target dose of 20 mg/kg in patients with a low transfusion frequency of <2 pRBC per 4 weeks, 30 mg/kg in patients with an intermediate transfusions frequency, and 30–40 mg/kg in patients with a transfusion frequency of >2 pRBC. This approach helps to avoid unpleasant gastrointestinal adverse events after treatment initiation and is intended to strengthen compliance, which is known to decay as a result of AEs and the lack of symptoms from iron overload.
In order to strengthen compliance, patients should be well informed about possible GI adverse events. Diarrhea is the most common side effect of DFX and occurs much more frequently in elderly MDS patients than in young thalassemia patients. To manage this side effect, it is useful to discontinue any laxatives (which are commonly used by elderly patients), consider the use of loperamide, maintain hydration, and try to administer DFX at night [53].
For moderate diarrhea, it is recommended to reduce the DFX dose to 10 mg/kg/day and, for severe diarrhea, to hold the DFX dose until the diarrhea has resolved, and then try to reinitiate DFX at dose of 10 mg/day and adjust in increments of 5 mg/day each week. If that does not help, DFX should be discontinued. It is important that patients try to keep themselves hydrated (by drinking small, steady amounts of clear liquids, e.g., electrolyte solutions), because it helps to avoid additional renal problems.
The mean creatinine concentrations in MDS patients during the EPIC trial and other clinical studies showed that an initial 20% increase in serum creatinine values should be expected. However, with proper dose adjustment of DFX, this is usually followed by a new steady state rather than progressive elevation [54, 55]. According to the drug label in the USA, iron chelation with deferasirox can be used in patients with a creatinine clearance above 40 mL/min. Nevertheless, close monitoring of renal function is required in elderly MDS patients with preexisting chronic renal insufficiency, diabetes, hypertension, and congestive heart failure.
Liver toxicity is not a problem of DFX treatment in daily clinical practice. Transaminase levels actually decrease with decreasing serum ferritin levels, indicating that iron chelation is beneficial rather than toxic for the liver of iron-overloaded patients [56]. Hepatotoxicity is a rare exception, apparently confined to a few patients with preexisting liver problems.
Treating iron overload has recently become more convenient because an improved deferasirox formulation in the form of film-coated tablets is now available. The new formulation is well tolerated and can be taken with or without a meal [57, 58]. Anything that makes iron chelation easier to handle may help to improve patient adherence and thus carries the potential to improve survival.
8 Iron Overload in the Context of Allogeneic Stem Cell Transplantation
The incidence of iron overload in the context of allogeneic hematopoietic stem cell transplantation (HSCT) for MDS varies according to both time and method of assessment. Table 8.3 summarizes relevant data from posttransplant assessments in a series of patients undergoing allo-HSCT (in 10–20% for MDS). The incidence of iron overload ranges from 54 to 100% [59, 60, 61, 63]. There is generally a good correlation between iron overload assessed by serum ferritin, liver iron content (LIC) assessed by magnetic resonance imaging (MRI), and the number of packed red blood cell units (pRBC) transfused. However, this is true only when iron overload is assessed late after transplant in long-term survivors, and confounding factors that can influence SF levels are excluded (hepatitis, alcoholism, immunosuppressive therapy, hepatotoxic drugs, GVHD, veno-occlusive disease, inflammation, other cancers, or relapse) [63]. Iron overload appears to be more frequent in the early posttransplant phase and to decrease over time, suggesting that the confounding factors mentioned above become less prevalent over time [64].
Retrospective studies found that transfusion burden and/or ferritin levels >1000 ng/mL, assessed prior to allo-HSCT (Table 8.4), were associated with a higher risk for non-relapse mortality (NRM), acute GVHD, and severe infections [66, 67]. Specifically in MDS patients, increased risk of infection with iron overload after HSCT was seen in retrospective studies [65, 69, 62,62,]. High NTBI levels in the early phase after HSCT have been reported to predict grade III or IV toxicity [71]. However, as ferritin is an acute-phase reactant, elevation of SF may reflect inflammatory conditions including active infection or more advanced bone marrow disease, which are expected to confer an adverse prognosis in HSCT independent of iron overload. The same caveat applies to hepcidin levels which are also influenced by iron overload as well as inflammation. In a series of 166 patients undergoing allo-HSCT, patients in the high-hepcidin group had a significantly shorter overall survival than those in the low-hepcidin group (49.2 vs. 69.0%, respectively, p = 0.006) [72].
In the context of myeloablative conditioning (MAC) regimens, LIC and SF levels have only been assessed regarding overall survival, showing no significant impact [64, 66,67,, 74]. A subgroup analysis restricted to patients with MDS-AML (25%) also failed to yield significant results.
Prospective studies produced conflicting results regarding the impact of iron overload on posttransplant non-relapse mortality. Armand et al. published a meta-analysis on the four prospective studies investigating the impact of iron overload on posttransplant outcome [69]. This meta-analysis showed that SF levels >1000 ng/L were a risk factor for shorter survival in the entire cohort but did not specifically impact the non-relapse mortality. Liver iron content (>5 or >7 mg/g) had no statistically significant impact on outcome. The authors concluded that “the results should not be interpreted to imply that iron is irrelevant in HSCT.” The trends suggest a possible prognostic effect, although it does not appear as strong as suspected based on the ferritin-related literature, and it may be restricted to certain subgroups. Moreover, iron may be related to disease pathology in ways that are just beginning to be understood.
In summary, MDS patients who are candidates for allo-HSCT should be considered for iron chelation therapy prior to transplantation. The indication for iron chelation therapy during conditioning and during the posttransplant remains to be defined [51, 69,76,77].
9 Future Directions and Ongoing High-Impact Patient-Centered Clinical Trials
Altogether, the side effects of oral iron chelation therapy in MDS appear to be outweighed by the survival benefit that was consistently observed in several retrospective analyses. Whether the survival benefit was solely due to iron removal or also influenced by confounding factors or biases must be settled by a prospective randomized trial. The only such trial is TELESTO, a prospective multicenter study to investigate the clinical benefit of chelation therapy with deferasirox in MDS patients (ClinicalTrials.gov Identifier: NCT00940602). It turned out to be difficult to recruit patients for this trial in countries where DFX is licensed and reimbursed, and after much discussion with the FDA, the number of patients to be recruited was reduced from 630 to 210. Meanwhile, patient accrual has been completed. While the statistical power of the trial may no longer be sufficient to provide unequivocal evidence of improved overall survival, there might still be a strong signal indicating that transfusion-dependent patients with lower-risk MDS benefit from iron chelation therapy.
References
Santini V, Girelli D, Sanna A, Martinelli N, Duca L, Campostrini N, et al. Hepcidin levels and their determinants in different types of myelodysplastic syndromes. PLoS ONE. 2011;6(8):e23109.
Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–101.
Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells of the iron regulatory protein hepcidin. Blood. 2009;114:181–6.
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84.
Mossner M, Stoehr A, Jann J-C, Nolte F, Nowak V, Oblaender J, et al. Erythroferrone (ERFE) is selectively expressed in human CD71+ erythroprogenitor cells and deregulated overexpression is associated with a favorable outcome in low risk myelodysplastic syndrome (MDS). Blood. 2015;126(23):2859.
Malcovati L, Della Porta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica. 2011;96(10):1433–40.
Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91:1588–90.
de Swart L, Smith A, Fenaux P, Bowen D, Sanz G, Hellström-Lindberg E, et al. Transfusion-dependency Is the most important prognostic factor for survival in 1000 newly diagnosed MDS patients with low- and intermediate-1 risk MDS in the European LeukemiaNet MDS registry. Blood. 2011;118(21):2775.
Roy NB, Myerson S, Schuh AH, Bignell P, Patel R, Wainscoat JS, et al. Cardiac iron overload in transfusion-dependent patients with myelodysplastic syndromes. Br J Haematol. 2011;154(4):521–4.
Pascal L, Beyne-Rauzy O, Brechignac S, Marechaux S, Vassilieff D, Ernst O, et al. Cardiac iron overload assessed by T2* magnetic resonance imaging and cardiac function in regularly transfused myelodysplastic syndrome patients. Br J Haematol. 2013;162(3):413–5.
Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson MD, Coates TD, et al. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of cardiomyopathy. Circulation. 2005;112:535–43.
Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, et al. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123(14):1519–28.
Vinchi F, Muckenthaler MU, Da Silva MC, Balla G, Balla J, Jeney V. Atherogenesis and iron: from epidemiology to cellular level. Front Pharmacol. 2014;5:94.
Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF Jr, et al. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103(23):2799–804.
Cheung YF, Chan GCF, Ha SY. Effect of deferasirox (ICL670) on arterial function in patients with beta-thalassaemia major. Br J Haematol. 2008;141:728–33.
Okabe H, Suzuki T, Uehara E, Ueda M, Nagai T, Ozawa K. The bone marrow hematopoietic microenvironment is impaired in iron-overloaded mice. Eur J Haematol. 2014;93(2):118–28.
Zhang Y, Zhai W, Zhao M, Li D, Chai X, Cao X, et al. Effects of iron overload on the bone marrow microenvironment in mice. PLoS One. 2015;10(3):e0120219.
Bowen D, Wang L, Frew M, Kerr R, Groves M. Antioxidant enzyme expression in myelodysplastic and acute myeloid leukemia bone marrow: further evidence of a pathogenetic role for oxidative stress? Haematologica. 2003;88:1070–2.
Thompson JE, Conlon JP, Yang X, Sanchez PV, Carroll M. Enhanced growth of myelodysplastic colonies in hypoxic conditions. Exp Hematol. 2007;35(1):21–31.
Ghoti H, Amer J, Winder A, Rachmilewitz E, Fibach E. Oxidative stress in red blood cells, platelets, and polymorphonuclear leukocytes from patients with myelodysplastic syndromes. Eur J Haematol. 2007;79:463–7.
Novotna B, Bagryantseva Y, Siskova M, Neuwirtova R. Oxidative damage in bone marrow cells of patients with low-risk myelodysplastic syndrome. Leuk Res. 2009;33:340–3.
Chung YJ, Robert C, Gough SM, Rassool FV, Aplan PD. Oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome. Leuk Res. 2014;38(1):95–102.
Saigo K, Takenokuchi M, Hiramatsu Y, Tada H, Hishita T, Takata M, et al. Oxidative stress levels in myelodysplastic syndrome patients: their relationship to serum ferritin and haemoglobin values. J Int Med Res. 2011;39(5):1941–5.
de Souza GF, Barbosa MC, Santos TE, Carvalho TM, de Freitas RM, Martins MR, et al. Increased parameters of oxidative stress and its relation to transfusion iron overload in patients with myelodysplastic syndromes. J Clin Pathol. 2013;66(11):996–8.
Lu W, Zhao M, Rajbhandary S, Xie F, Chai X, Mu J, et al. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol. 2013;91(3):249–61.
Kikuchi S, Kobune M, Iyama S, Sato T, Murase K, Kawano Y, et al. Improvement of iron-mediated oxidative DNA damage in patients with transfusion-dependent myelodysplastic syndrome by treatment with deferasirox. Free Radic Biol Med. 2012;53(4):643–8.
Hartmann J, Braulke F, Sinzig U, Wulf G, Maas JH, Konietschke F, et al. Iron overload impairs proliferation of erythroid progenitors cells (BFU-E) from patients with myelodysplastic syndromes. Leuk Res. 2013;37(3):327–32.
Prus E, Fibach E. Uptake of non-transferrin iron by erythroid cells. Anemia. 2011;2011:8.
Taoka K, Kumano K, Nakamura F, Hosoi M, Goyama S, Imai Y, et al. The effect of iron overload and chelation on erythroid differentiation. Int J Hematol. 2012;95(2):149–59.
Westhofen G, Ganster C, Beier F, Rassaf T, Al-Ali HF, Stuhlmann R, et al. Comprehensive genomic analysis provides further evidence that iron overload can induce genetic instability in myelodysplastic syndromes. Blood. 2015;126(23):2842.
List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez-Lopez N, et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol. 2012;30(17):2134–9.
Gattermann N, Finelli C, Della Porta M, Fenaux P, Stadler M, Guerci-Bresler A, et al. Hematologic responses to deferasirox therapy in transfusion-dependent patients with myelodysplastic syndromes. Haematologica. 2012;97(9):1364–71.
Nolte F, Höchsmann B, Giagounidis A, Lübbert M, Platzbecker U, Haase D, Lück A, Gattermann N, Taupitz M, Baier M, Leismann O, Junkes A, Schumann C, Hofmann WK, Schrezenmeier H. Results from a 1-year, openlabel, single arm, multi-center trial evaluating the efficacy and safety of oral Deferasirox in patients diagnosed with low and int-1 risk myelodysplastic syndrome (MDS) and transfusion-dependent iron overload. Ann Hematol. 2013;92(2):191–8.
Angelucci E, Santini V, Di Tucci AA, Quaresmini G, Finelli C, Volpe A, et al. Deferasirox for transfusion-dependent patients with myelodysplastic syndromes: safety, efficacy, and beyond (GIMEMA MDS0306 Trial). Eur J Haematol. 2014;92(6):527–36.
Jensen PD, Heickendorff L, Pedersen B, Bendix-Hansen K, Jensen FT, Christensen T, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996;94:288–99.
Leitch HA, Leger CS, Goodman TA, Wong KK, Wong DHC, Ramadan KM, et al. Improved survival in patients with myelodysplastic syndrome receiving iron chelation therapy. Clin Leuk. 2008;2:205–11.
Rose C, Brechignac S, Vassilief D, Pascal L, Stamatoullas A, Guerci A, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDs patients? A multicenter study by the GFM. Leuk Res. 2010;34:864–70.
Neukirchen J, Fox F, Kundgen A, Nachtkamp K, Strupp C, Haas R, et al. Improved survival in MDS patients receiving iron chelation therapy - a matched pair analysis of 188 patients from the Dusseldorf MDS registry. Leuk Res. 2012a;36(8):1067–70.
Neukirchen J, Germing U, Fox F, Glaser S, Gattermann N. The impact of iron chelation therapy on clinical outcomes in real-world lower-risk patients with myelodysplastic syndromes (MDS): results from the Düsseldorf registry. Haematologica. 2012b;97(s1):144.
Komrokji RS, Al Ali NH, Padron E, Lancet JE, List A. Impact of iron chelation therapy on overall survival and AML transformation in lower-risk MDS patients treated at the Moffitt Cancer Center. Blood. 2011;118(21):2776.
Zeidan AM, Hendrick F, Friedman E, Gore SD, Baer MR, Sasane M, et al. Deferasirox is associated with reduced mortality risk in a Medicare population with myelodysplastic syndromes. Blood. 2012;120(21):426.
Delforge M, Selleslag D, Beguin Y, Triffet A, Mineur P, Theunissen K, et al. Adequate iron chelation therapy for at least six months improves survival in transfusion-dependent patients with lower risk myelodysplastic syndromes. Leuk Res. 2014;38(5):557–63.
Lyons RM, Marek BJ, Paley C, Esposito J, McNamara K, Garbo L, et al. Relationship between chelation and clinical outcomes in lower-risk patients with myelodysplastic syndrome (MDS): registry analysis at 5 years. Blood. 2014;124(21):1350.
Remacha AF, Arrizabalaga B, Villegas A, Duran MS, Hermosin L, de Paz R, et al. Evolution of iron overload in patients with low-risk myelodysplastic syndrome: iron chelation therapy and organ complications. Ann Hematol. 2015;94(5):779–87.
Parmar A, Leitch HA, Wells RA, Nevill TJ, Zhu NY, Yee KWL, et al. Iron chelation is associated with improved survival adjusting for disease and patient related characteristics in low/int-1 risk MDS at the time of first transfusion dependence: A MDS-CAN study. Blood. 2015;126(23):1701.
Langemeijer S, de Swart L, Yu G, Smith A, Crouch S, Johnston T, et al. Impact of treatment with Iron chelators in lower-risk MDS patients participating in the European LeukemiaNet MDS (EUMDS) Registry. Blood. 2016;128(22):3186.
Wood JC, Glynos T, Thompson A, Giardina P, Harmatz P, Kang BP, et al. Follow-up report on the 2-year cardiac data from a deferasirox monotherapy trial. Am J Hematol. 2010;85(10):818–9.
Wood JC, Glynos T, Thompson A, Giardina P, Harmatz P, Kang BP, et al. Relationship between labile plasma iron, liver iron concentration and cardiac response in a deferasirox monotherapy trial. Haematologica. 2011;96(7):1055–8.
Cappellini MD, Porter J, El-Beshlawy A, Li CK, Seymour JF, Elalfy M, et al. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010;95(4):557–66.
Gattermann N, Finelli C, Della Porta M, Fenaux P, Ganser A, Guerci-Bresler A, et al. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk Res. 2010;34:1143–50.
Malcovati L, Hellstrom-Lindberg E, Bowen D, Ades L, Cermak J, Del Canizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943–64.
Nolte F, Angelucci E, Breccia M, Gattermann N, Santini V, Vey N, et al. Updated recommendations on the management of gastrointestinal disturbances during iron chelation therapy with deferasirox in transfusion dependent patients with myelodysplastic syndrome - emphasis on optimized dosing schedules and new formulations. Leuk Res. 2015;39(10):1028–33.
Nolte F, Angelucci E, Beris P, Macwhannell A, Selleslag D, Schumann C, et al. Clinical management of gastrointestinal disturbances in patients with myelodysplastic syndromes receiving iron chelation treatment with deferasirox. Leuk Res. 2011;35(9):1131–5.
Gattermann N, Schmid M, Della Porta M, Taylor K, Seymour JF, Habr D, et al. Efficacy and safety of deferasirox (Exjade) during 1 year of treatment in transfusion-dependent patients with myelodysplastic syndromes: results from EPIC trial. Blood. 2008;112(11):633.
Schmid M, Cappellini MD, Porter JB, Greenberg PL, Lawniczek T, Glaser S, et al. Safety of Deferasirox (Exjade®) in myelodysplastic syndromes (MDS) and non-MDS patients with transfusional iron overload: a pooled analysis focusing on renal function. Blood. 2009;114(22):1768.
Gattermann N, Schmid M, Guerci-Bresler A, Della Porta M, Taylor K, Habr D, et al. Correlation between decreased serum ferritin and improved liver transaminases during Deferasirox (Exjade®) treatment in iron-overloaded patients with myelodysplastic syndromes. Blood. 2009;114(22):3803.
Taher AT, Origa R, Perrotta S, Kouraklis A, Ruffo GB, Kattamis A, et al. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or MDS: results of the randomized, phase II E.C.L.I.P.S.E. study. Blood. 2016;128(22):1285.
Taher AT, Origa R, Perrotta S, Kouraklis A, Ruffo GB, Kattamis A, et al. Improved patient-reported outcomes with a film-coated versus dispersible tablet formulation of deferasirox: results from the randomized, phase II E.C.L.I.P.S.E. Study. Blood. 2016;128(22):850.
McKay PJ, Murphy JA, Cameron S, Burnett AK, Campbell M, Tansey P, et al. Iron overload and liver dysfunction after allogeneic or autologous bonemarrow transplantation. Bone Marrow Transplant. 1996;17(1):63–6.
Strasser SI, Kowdley KV, Sale GE, McDonald GB. Iron overload in bone marrow transplant recipients. Bone Marrow Transplant. 1998;22(2):167–73.
Strasser SI, Sullivan KM, Myerson D, Spurgeon CL, Storer B, Schoch HG, Murakami CS, McDonald GB. Cirrhosis of the liver in long-term marrow transplant survivors. Blood. 1999;93(10):3259–66.
Tomas JF, Pinilla I, Garcia-Buey ML, Garcia A, Figuera A, Gomez-Garcia deSoria VGG, et al. Long-term liver dysfunction after allogeneic bonemarrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant. 2000;26(6):649–55.
Rose C, Ernst O, Hecquet B, Maboudou P, Renom P, Noel MP, et al. Quantification by magnetic resonance imaging and liver consequences of post-transfusional iron overload alone in long term survivors after allogeneic hematopoietic stem cell transplantation (HSCT). Haematologica. 2007;92(6):850–3.
Meyer SC, O’Meara A, Buser AS, Tichelli A, Passweg JR, Stern M. Prognostic impact of posttransplantation iron overload after allogeneic stemcell transplantation. Biol Blood Marrow Transplant. 2013;19(3):440–4.
Armand P, Kim HT, Cutler C, Ho VT, Koreth J, Alyea EP, et al. Prognostic Impact of elevated pre-transplant serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;108:595. https://doi.org/10.1181/blood-2006-10-054924.
Platzbecker U, Bornhauser M, Germing U, Stumpf J, Scott BL, Kroger N, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS). Biol Blood Marrow Transplant. 2008;14(11):1217–25.
Alessandrino EP, Della Porta MG, Bacigalupo A, Malcovati L, Angelucci E, Van Lint MT. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–84.
Trottier BJ, Burns LJ, DeFor TE, Cooley S, Majhail NS. Association ofiron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122(9):1678–84.
Armand P, Kim HT, Virtanen JM, Parkkola RK, Itala-Remes MA, Majhail NS, et al. Iron overload in allogeneic hematopoietic cell transplantation outcome: a meta-analysis. Biol Blood Marrow Transplant. 2014;20(8):1248–51.
Tachibana T, Takasaki H, Tanaka M, Maruta A, Hyo R, Ishigatsubo Y, et al. Serum ferritin and disease status at transplantation predict the outcome of allo-SCT in patients with AML or myelodysplastic syndrome. Bone Marrow Transplant. 2011;46(1):150–1.
Naoum FA, Esposito BP, Ruiz LP, Ruiz MA, Tanaka PY, Sobreira JT, et al. Assessment of labile plasma iron in patients who undergo hematopoietic stem cell transplantation. Acta Haematol. 2014;131(4):222–6.
Sakamoto S, Kawabata H, Kanda J, Uchiyama T, Mizumoto C, Kitano T, et al. High pretransplant hepcidin levels are associated with poor overall survival and delayed platelet engraftment after allogeneic hematopoietic stem cell transplantation. Cancer Med. 2017;6(1):120–8.
Bazuaye GN, Buser A, Gerull S, Tichelli A, Stern M. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2012;47(1):60–4.
Wermke M, Schmidt A, Middeke JM, Sockel K, von Bonin M, Schonefeldt C, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Cancer Res. 2012;18(23):6460–8.
Lee JW, Kang HJ, Kim EK, Kim H, Shin HY, Ahn HS. Effect of iron overload and iron-chelating therapy on allogeneic hematopoietic SCT in children. Bone Marrow Transplant. 2009;44(12):793–7.
Sivgin S, Baldane S, Kaynar L, Kurnaz F, Pala C, Ozturk A, et al. Pretransplant serum ferritin level may be a predictive marker for outcomes in patients having undergone allogeneic hematopoietic stem cell transplantation. Neoplasma. 2012;59(2):183–90.
Sivgin S, Baldane S, Kaynar L, Kurnaz F, Pala C, Sivgin H, et al. Pretransplant iron overload may be associated with increased risk of invasive fungal pneumonia (IFP) in patients that underwent allogeneic hematopoietic stem cell transplantation (alloHSCT). Transfus Apher Sci. 2013;48(1):103–8.
Langemeijer S, De Swart L, Yu G, Smith A, Crouch S, Johnston T, Fenaux P, et al. Impact of treatment with iron chelators in lower-risk MDS patients participating in the European Leukemianet MDS (EUMDS) Registry. Blood. 2016;128:3186.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Gattermann, N., Rose, C. (2018). Iron Chelation. In: Platzbecker, U., Fenaux, P. (eds) Myelodysplastic Syndromes . Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-319-76879-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-76879-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76878-6
Online ISBN: 978-3-319-76879-3
eBook Packages: MedicineMedicine (R0)