Abstract
The roots of most land plants form mycorrhizal associations with soil fungi, in which plants trade carbon for increased nutrient acquisition (e.g., N and P) under nutrient deficiency conditions. However, how nutrient enrichment affects mycorrhiza is still not well understood, in particular under future global changing scenarios such as nitrogen deposition. In this chapter, we first review the major pathways of mycorrhizal-mediated nutrient acquisition and molecular mechanisms of sensing nutrient availability for mycorrhizal fungi and roots. Next, we propose two conceptual models that may control plant C allocation to mycorrhizal fungi in response to nutrient enrichment: reciprocal reward model and root-mycorrhiza trade-off model. We also describe a plant-centric model and fungal-centric model to explain responses of the mycorrhizal fungal community to nutrient enrichment as well as examine impacts of nutrient inputs on mycorrhizas functioning.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Mycorrhizal symbiosis between plant roots and soil fungi are widely spread in terrestrial ecosystems, from forests, grasslands, and croplands to even deserts (Brundrett 2009; Smith and Read 2008). In this mutualistic symbiosis, the fungi trade nutrients, e.g., N and P, for carbon from photosynthesis of plants (Smith and Read 2008). Annually, plants might allocate ~4–20% photosynthates to their associated mycorrhizal fungi (Eissenstat et al. 1993). In the past decades, four major types of mycorrhizal associations were described based on their structure and functions, including arbuscular mycorrhiza (AM), ectomycorrhiza (EM), orchid mycorrhiza, and ericoid mycorrhiza (van der Heijden et al. 2015). Both AM and EM fungi live inside the root cortex and their hyphae function and live in the soil. However, the hyphae of AM fungi can penetrate the epidermal and cortical cell walls and form dichotomously branched arbuscules in such types of root cells (possibly the exchanging site between C and nutrients), while the mycelium of EM fungi do not penetrate into their host’s root cells but form Hartig net between epidermal and cortical root cells (Smith and Read 2008). Orchid mycorrhizas are only formed between roots in the plant family Orchidaceae and various basidiomycete fungi, while ericoid mycorrhizas are the symbiotic relationship between members of the plant family Ericaceae and several lineages of soil fungi (McCormick et al. 2012; Selosse et al. 2007). It is estimated that ~74% of land plants form arbuscular mycorrhizas with Glomeromycotan fungi, ~2% of land plants form ectomycorrhizas, and ~9% of land plants form orchid mycorrhizal associations, but only ~1% plants form ericoid mycorrhizas (Brundrett 2009).
The mycelia of arbuscular mycorrhizal fungi grow from plant roots to explore and exploit soil volume for nutrients, which are subsequently delivered to their host plants (van der Heijden et al. 2015). Mycorrhizal fungi are estimated to be responsible for a large contribution to nitrogen (N) and phosphorus (P) uptake of plant requirements in the natural ecosystems (Hobbie and Hobbie 2006; van der Heijden et al. 2006a, b), as up to 90% of P is delivered from AM hyphae/pathway to plants (Jakobsen et al. 1992; van der Heijden et al. 1998b). AM-mediated N uptake, however, is still unresolved. Several studies reported that AM fungi had no effect on plant N adsorption (van der Heijden et al. 2006a, b; Reynolds et al. 2005), while other studies found that AM fungi could facilitate N acquisition of plants under some conditions (Thirkell et al. 2016; Yang et al. 2017). Importantly, AM fungi can only adsorb and transfer inorganic forms of nutrients, e.g., ammonium, nitrates, and phosphates (van der Heijden et al. 2015). AM fungi cannot directly acquire nutrients from organic matter but must go through priming of other microbes for organic matter decomposition (Cheng et al. 2012; Hodge et al. 2001). However, EM excrete a wide range of extracellular enzymes to degrade organic matter and thus directly forage N (Tunlid et al. 2016). EM fungi may be responsible for up to ~80% of all N acquired by plants in boreal and temperate forests (Hobbie and Hobbie 2006; MacFall et al. 1992).
In this chapter, we only focus on AM fungi based on the following considerations that (1) we have already known that EM fungi are highly sensitive to nutrients (Ekblad et al. 2016; Pritchard et al. 2014; Ulm et al. 2017); (2) EM fungi are saprotrophic and can mobilize nutrients through decomposing organic matter (Koide et al. 2011); and (3) there are many known but varying results about AM fungi responses to nutrients.
17.2 Pathways of Mycorrhizal Nutrient Acquisition, Conversion, and Transfer
Mycorrhizal-mediated nutrient uptake has been gradually deciphered with the development of molecular tools. AM fungi-mediated facilitation of P uptake may be achieved through the following pathways. First, a large fraction of P is fixed in organic forms or metal complexes in soils (Javot et al. 2007), and AM colonization could induce the secretion of organic acids, e.g., oxalate or citric acids from roots (Koide and Kabir 2000; Zhang et al. 2016). AM fungi may also be able to prime P-solubilizing bacteria to release phosphatase (Zhang et al. 2014a). Soil organic P is hydrolyzed into phosphate through the abovementioned processes. The inorganic phosphate (Pi) is subsequently transferred into AM hyphae through P transporters (e.g., GvPT and GiPT), which are anchored in fungal mycelia membrane and then polymerized into poly-P by a series of polymerases (Harrison and Vanbuuren 1995; Maldonado-Mendoza et al. 2001). Poly-P is subsequently transported to the symbiotic interface, arbuscules depolarized into monomer Pi by alkaline phosphatase (Zhu et al. 2007), and finally translocated into plant root cells through phosphorus transporters (e.g., StPT5, LePT3, OsPT11, and MtPT4) (Harrison et al. 2002; Nagy et al. 2005; Paszkowski et al. 2002; Xu et al. 2007).
Mycorrhizal-mediated N adsorption occurs through several potential mechanisms as follows. AM fungi cannot directly decompose organic matter for N but can recruit other decomposing soil microbes through releasing small organic molecules (Cheng et al. 2012). The released inorganic N (e.g. NH4 + or NO3 −) or minor amounts of organic N (e.g., amino acid) will be subsequently sensed and transferred into mycorrhizal hyphae through high-affinity transporters for NO3 −, NH4 +, or amino acids (e.g., GintAMT1 or GiNT for AM fungi and HcGAP1 for EM fungi) (Lopez-Pedrosa et al. 2006; Muller et al. 2007; Tian et al. 2010). When nitrate is transported into AM hyphae, it will be transformed into ammonia by nitrate reductase and nitrite reductase (Guescini et al. 2007; Kaldorf et al. 1998). Ammonia from nitrate or directly adsorbing from soils will be transformed into arginine by GS/GOGAT enzymes in the external hyphae (Tian et al. 2010). Arginine is subsequently translocated to the internal hyphae and then broken down as ornithine and ammonia in arbuscules (Govindarajulu et al. 2005). Ammonia is finally transported into root cells through ammonia channels (e.g., AMT) (Fochi et al. 2017; Guether et al. 2009; Koegel et al. 2013).
17.3 Why Are We Interested in Impacts of Nutrient Enrichment on Mycorrhizas?
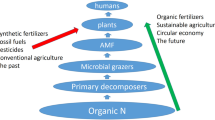
One main function for mycorrhizal symbiosis is facilitating nutrient uptake (e.g., P and N) for host plants under nutrient deficiency conditions. However, researchers are also interested in the impacts of nutrient enrichment on mycorrhizas. Increased chemical fertilizer applications are one of the core results of the Green Revolution and has contributed to increase yields of crops worldwide (Tilman et al. 2002). Since 1960, global application of N fertilizers has increased sevenfold, and P fertilizer applications increased 3.5-fold in 1995 (Tilman et al. 2002). Applications of both N and P fertilizers have been predicted to increase another threefold by 2050 (Tilman et al. 2001). However, further increased fertilizer applications are not likely to be as effective at increasing yields, and use efficiency of fertilizers declines at higher levels of addition (Tilman et al. 2002). The use efficiency of crops is estimated at only 30–50% for N fertilizers and ~45% for P fertilizers in modern intensive agricultural systems (Smil 1999, 2003). Therefore, excessive applications of chemical fertilizers may lead to N and P enrichment in agriculture, thereby negatively impacting mycorrhizas and their functioning.
Extensive fertilization is unsustainable for agricultural production from both economic and environmental perspectives. As mentioned above, the nutrient use efficiency is very low (<50%) for current crops around the world (Smil 1999, 2003). Greater than 50% of chemical fertilizers applied are not utilized by crops (Ju et al. 2009), which can accumulate in soils or lost in the environment. Such high losses of nutrients can cause severe environmental consequences, particularly for water contamination (e.g., eutrophication in lakes or bays and excessive nitrate concentration in drinking water), and cause great economic burdens for farmers (Monteagudo et al. 2012; Withers et al. 2014). Therefore, improvements in nutrient use efficiency can make crop production more sustainable, especially through tapping the potential of AM mycorrhizal symbiosis (Gosling et al. 2006).
Other human activities, such as utilization of fossil fuels for industrial production and transportation and burning of crop residues, have also greatly increased biologically reactive N entering into the atmosphere (Galloway et al. 2008; Huang et al. 2002; Zhang et al. 2014b). In particular, these activities increase the atmospheric levels of reactive nitrogen by a rate of ~25 Tg N year−1 from 1995 to 2005, which are subsequently deposited into plants and soils (Cofala et al. 2007; Galloway et al. 2008). High N deposition can result in drastic changes in ecosystems structure and functioning, altering plant community composition and reducing plant diversity (Bobbink et al. 2010; Schlesinger 2009).
17.4 Experimental Results of Nutrient Enrichment on Mycorrhizas
According to the predictions of the functional equilibrium model, carbon allocation to AM structures will be reduced when soils are sufficiently fertilized, because mycorrhizal delivery of soil resources is no longer a value to host plants (Johnson et al. 2003). In the past decades, a series of studies have been conducted to test how nutrient enrichment affects mycorrhiza in different ecosystems. Overall, nutrient enrichment is shown to be negative for mycorrhiza formation as has been synthesized in the case of N and P fertilization, leading to significant decreases in mycorrhizal abundance (Treseder 2004).
17.4.1 Forest and Shrubland Ecosystems
N and P additions can suppress arbuscular mycorrhizas in forest and shrub systems. Camenzind et al. (2014) reported that additions of N and P fertilizers significantly reduced AM fungal root colonization in a Graffenrieda emarginata-dominated tropical montane forest. AM fungal root colonization, hyphal biomass, storage, and lipid storage structures also declined in response to N addition with 30 kg ha−1 year−1 in a northern hardwood forest (van Diepen et al. 2007). Interestingly, AM fungal abundance was increased by 1.56-fold with P addition of 10 kg ha−1 year−1 but decreased by 27.45% due to N addition of 50 kg ha−1 year−1 at the elevation of 2000 m in a tropical montane forest, which might be caused by N/P co-limitation in this site (Camenzind et al. 2016). Nitrogen amendment (60 kg N ha−1 year−1) was found to significantly reduce AM fungal spore density and root colonization in a coastal sage scrub ecosystem (Egerton-Warburton and Allen 2000).
17.4.2 Grassland Ecosystems
Nutrient enrichment effects on mycorrhiza have been extensively studied in grassland ecosystems, especially for N. For example, Antoninka et al. (2011) reported that 7-year N additions (40 kg ha−1 year−1) reduced AM fungal spore abundance but did not affect the spore volume and hyphal density in a grassland ecosystem. In a semiarid grassland ecosystem, it was further found that N amendments (100 kg ha−1 year−1) significantly reduced dominant AM fungal species (Glomus intraradices and G. fasciculatum) (Porras-Alfaro et al. 2007). Saito et al. (2011) found that N additions (20 mg kg−1 soil) increased AM fungal sporulation for highly mycorrhizal-responsive plant species but inhibited it for less mycorrhizal-responsive plant species. In a semiarid steppe ecosystem, Kim et al. (2015) found that AM extraradical hyphal density was significantly decreased by N additions (100 kg ha−1 year−1). However, mycorrhizal inhibition by nitrogen enrichment might also be dependent on phosphorus availability. For example, Johnson et al. (2003) found that N enrichment (100– 170 kg ha−1 year−1) strongly decreased AM fungal structures (vesicles and coils) under the lowest soil N:P conditions but increased AM fungal structures under the highest soil N:P conditions from a wide range of grasslands. Johnson et al. (2003) found extraradical mycorrhizal structures (hyphae and spores) to be more responsive to N (100–170 kg ha−1 year−1) than P enrichment (10–200 kg ha−1 year−1). In a temperate steppe ecosystem, 6-year P fertilization (50 kg ha−1 year−1) but not N (100 kg ha−1 year−1) additions decreased AM fungal root colonization and extraradical hyphal density in Artemisia frigida-, Stipa krylovii-, and Cleistogenes squarrosa-dominated plant community (Chen et al. 2014). Nitrogen enrichment was also found to decrease mycorrhizal colonization under extraordinarily P-rich soils (120 g kg−1) (Blanke et al. 2005). Thus, soil stoichiometry between N and P might be an important determinant for mycorrhizal response to nutrient enrichment.
17.4.3 Agroecosystems
A large quantity of studies showed that increasing N or P availability could reduce crop responses to AM fungi. For example, Azcón et al. (2003) reported that high N and P availability in the soil reduced macro- and micronutrients in mycorrhizal lettuce (with a rate of 9 mM vs. 1 mM for N and 0.5 mM vs. 0.1 mM for P). Al-Karaki and Clark (1999) reported that high P level (1.6 m mol kg−1) significantly decreased seed dry weight and seed P content by AM fungi when compared to no P addition. At high N or P conditions, crops might reduce C allocation to their associated AM fungal symbionts and thus decreasing mycorrhizal abundance (Nagy et al. 2009). For example, an application of 90 kg N ha−1 year−1 as ammonium nitrate reduced AM fungal spore density in the rhizosphere of Zea mays and Medicago sativa in a Mediterranean agroecosystem (Avio et al. 2013). Wang et al. (2017) found that increasing P inputs to a level of 75–100 kg ha−1 year−1 significantly reduced AM colonization of Z. mays in an experimental field (a plot size of 35 m2). Kahiluoto et al. (2001) reported that 20 years of P fertilization (45 kg ha−1 year−1) significantly reduced mycorrhizal root colonization and spore density of Linum usitatissimum L., Trifolium pratense L., and Hordeum vulgare L., respectively. However, it is very likely that more complicated factors might impact AM response to nutrient enrichment, such as the background soil nutrient availability (N or P limited when added), the fertilizer type (organic or mineral), the species diversity of AM fungi inoculated, as well as the host crop varieties. All of these might change the outcome of fertilizer enrichment on AM fungi. For example, Gryndler et al. (2006) found that mineral fertilization showed reversed effects on AM fungal external biomass when compared with manure application. Some other studies also reported that modern wheat cultivar was shown to be less dependent and responsive to AM fungi than their ancestors (Hetrick et al. 1993; Zhu et al. 2001).
17.5 Potential Mechanisms that Underlie Mycorrhizal Responses to Nutrient Enrichment
Mycorrhizal responses to nutrient enrichment conforms to the predictions of functional equilibrium model that high nutrient availability might reduce carbon allocation from plants to AM fungi, which thus declines the fungal abundance (van Diepen et al. 2007). However, the underlying mechanisms are still not well-known, particularly about how plants precisely control carbon allocation to different AM fungal species. Here, we will discuss some conceptual models and the underlying molecular mechanisms.
17.5.1 Plant Control of C Allocation to AMF: Conceptual Models
A long-standing controversy is that how plants may control their AM fungal partners as well as how to maintain fair trade for resources (e.g., N and P) between both partners (Kiers and van der Heijden 2006). In a split root experiment, Bever et al. (2009) demonstrated that plant could prefer to allocate carbon to more beneficial AM symbionts. However, they did not provide evidence to explain how plants achieved such goals. Based on results from more precisely controlled experiments (including isotope-labeling and triple split-plate system), Kiers et al. (2011) proposed a reciprocal reward model for stabilizing cooperation between mycorrhizal partners (Fig.17.1) in which plants can detect, discriminate, and reward the best fungal partners with more carbohydrates, and in turn their fungal partners enforce mutual cooperation by increasing nutrient transfer only to those roots providing more carbohydrates.
Conceptual model of reciprocal reward strategy for mycorrhizal symbiosis (modified according to Kiers et al. 2011). The black arrows represent the carbon/nutrient flow between host and less beneficial AMF, while the gray arrows stand for the more beneficial interactions between AMF and their hosts
Mycorrhizal symbiosis is primarily asymmetric, namely, most plants can complete their life cycle without mycorrhizal fungi but the fungi cannot (Werner and Kiers 2015). So, there might be an active pathway for which plants need to evaluate how to allocate carbon to AM fungi (Nagy et al. 2009). Fellbaum et al. (2012) further provided experimental evidence supporting such predictions that carbon flux was an activator of mycorrhizal pathway for nitrogen. Mycorrhizal-mediated nitrogen transfer would be triggered only when carbon was delivered by plants across the mycorrhizal interface. This finding suggests that plants may actively control carbon allocation or at least play a role in the initial priming for carbon consumption. Host plants may be capable of evaluating nutrient requirements and decide to whether activate the mycorrhizal pathway. When nutrients are limited and the mycorrhizal pathway is required, more photosynthesis-fixed carbon will be allocated to mycorrhizal interface than to root cells, thus stimulating mycorrhizal nutrient uptake through exploring more extensive soil volumes. When nutrients are sufficient, carbon allocation to mycorrhizas might be reduced and thus reducing AM abundance.
The root system and its associated mycorrhizal fungi are two key components in nutrient adsorption for plants (Smith and Read 2008). However, both root turnover and mycorrhizal proliferation are carbon-consuming processes, with an estimated ~30–50% of photosynthesized carbon allocated to root systems in forests and even more in grasses (Gill and Jackson 2000), and mycorrhizal carbon may account for ~20% of total net primary production (van der Heijden et al. 2008). Thus, how plants balance root development and mycorrhizal proliferation is a central question in root biology. Koide (2000) proposed a functional complementarity model in which AM fungi possess functions that complement those of roots. This model described that root hairs and fine roots were capable of adsorbing nutrients from soils close to the root surface (direct nutrient uptake pathway), while mycorrhizal fungi could transport nutrients from beyond the reach of root systems (in direct nutrient uptake pathway). Based on this model, we further constructed a conceptual model and argue that host plants could trade off carbon allocation between fine root development and mycorrhizal formation, but this trade-off depends on nutrient availability (Fig.17.2; also see Unger et al. 2016).
Under nutrient-sufficient conditions, nutrients are available for fine roots, and nutrient requirements for plant growth can be easily satisfied with root nutrient uptake. Thus C allocation is only needed to sustain fine root turnover. However, when availability of nutrients close to fine roots is low, such as with P, plants have to take up two possible strategies: one is to elongate fine roots, and the other is to activate the mycorrhizal pathway. Here, a trade-off might occur for plants. If the plant invests C in fine roots but not receiving as much nutrients back as investing the same C in mycorrhizas, then the selection might be for C allocation to mycorrhizas.
17.6 Molecular Evidence of Mycorrhizal Responses to Nutrient Enrichments
Nitrogen and phosphorus are indispensable macroelements for plant growth. For mycorrhizal plants, a crucial process is that how both partners sense the availability of nutrients surrounding root hairs or fungal hyphae in the soil. Generally, two types of proteins anchored in cell membrane are functioned as nutrient sensors and transporters in plant root or fungal mycelium (Amtmann et al. 2006; Scheible and Rojas-Triana 2015). However, some proteins exhibited a dual role of sensing and transporting for nutrients, which is named as transceptor (transporter–receptor) (Scheible and Rojas-Triana 2015).
For host plants, Pi transporters have been identified in different plant species, all of which belong to PHT gene family, e.g., OsPht1;1~OsPht1;13 and OsPht2;1 in Oryza sativa L., TaPht1;1~TaPht1;11 in Triticum aestivum L., and GmPht1;1~ GmPht1;14 in Glycine max (Linn.) Merr. (Goff et al. 2002; Qin et al. 2012; Teng et al. 2017). However, Pi sensors/transceptors have not yet been reported or identified in plants, despite evidence provided for Pi sensing in root tips (Scheible and Rojas-Triana 2015; Svistoonoff et al. 2007). For plant-sensing ammonium (NH4 +), an ammonium transceptorAM1;1 was identified in Arabidopsis thaliana (Lanquar et al. 2009). The activation of AM1;1 requires effective interactions between a trimmer of subunits. Conformational change accompanies ammonium transport with AM1;1 transceptor. The allosteric regulation is mediated by a cytosolic C-terminal trans-activation domain, which carries a conserved Thr (T460) in a critical position. Phosphorylation of T460 can lead to inactivation of the trimmeric complex, but this process is dependent on NH4 + concentrations. Higher NH4 + concentrations can trigger phosphorylation of T460 in AM1,1, which functions to prevent ammonium from accumulating at toxic levels in root cells. NO3 sensors have also been found in plant root cells, e.g., NTR1,1 (Munos et al. 2004). NTR1,1 is a transporting transceptor, which is not only a NO3 − sensing receptor but also can transport NO3 − and facilitate the uptake of auxin (Krouk et al. 2010).
For mycorrhizal fungal partners, some high-affinity phosphate transporters have been identified, e.g., GiPT, GvPT, and GmosPT (Benedetto et al. 2005; Harrison and Vanbuuren 1995; Maldonado-Mendoza et al. 2001). However, how AM fungi sense P and whether these phosphate transporters act as sensors remain unclear in the past decades. Recently, researchers found that such transporters resemble Pho84 from yeast, which might have a dual role of sensing and transporting for phosphate (Tisserant et al. 2012). Xie et al. (2016) confirmed such predictions and found that GigmPT functions as a transceptor in Gigaspora margarita, which can activate the phosphate-signaling pathway and protein kinase A (PKA) signaling cascade. GigmPT showed similar DNA sequences and protein structure with Pho84 sensor in yeast (Popova et al. 2010), and the potential mechanisms might be as follows: GigmPT is induced under Pi-deficient conditions; however, when Pi becomes available, Pi transport will cause conformational changes of this protein and activates PKA, which, in turn, might phosphorylate GigmPT. Phosphorylated GigmPT will be ubiquitinated and finally degraded. Mycorrhizal fungi also facilitate N uptake, and some N transporters have been identified, e.g., GintAMT1, GiNT, and HcGAP1 (Lopez-Pedrosa et al. 2006; Muller et al. 2007; Tian et al. 2010). However, how AM fungi sense N signal is still not well characterized. In yeast, Mep2 protein has been found to function as an ammonium sensor under ammonium-limiting conditions (Lorenz and Heitman 1998). Javelle et al. (2003) argued that high-affinity ammonium transporters from AM fungi could act in a similar manner as yeast to sense N availability in the soil environment.
17.7 Responses of the Mycorrhizal Fungal Community to Nutrient Enrichment
The mycorrhizal community structure (e.g., diversity and composition) may determine a series of cascading functions of plant systems. For example, AM fungal diversity might mitigate plant–plant competition, promote plant diversity, reduce ecosystem variability, and increase productivity (van der Heijden et al. 1998b; Wagg et al. 2011). Thus, such importance of mycorrhizal functioning has attracted researchers to decipher how nutrient enrichment affects AM fungal community structure in the past decades. To date, many studies have described AM community responses to various nutrient inputs across many ecosystems and attempted to decipher the underlying mechanisms as described below.
17.7.1 Forests and Shrublands
In a northern hardwood forest, a 12-year continuous N additions (NaNO3 with a rate of 30 kg ha−1 year−1) significantly altered the AMF community composition, but the AMF diversity was unaffected (van Diepen et al. 2011). In a coastal sage scrub ecosystem, N enrichment (60 kg ha−1 year−1) was shown to shift AMF composition through a displacement of Gigasporaceae by Glomeraceae, which thus led to a reduction in species richness and diversity (Egerton-Warburton and Allen 2000). In a tropical montane forest, AMF species richness was significantly reduced by N (50 kg ha−1 year−1) and P (10 kg ha−1 year−1) additions as well as their combinations, mainly through replacing rare AM fungal species (Camenzind et al. 2014). More importantly, this study further elucidated that Diversiporales richness was mainly reduced by N amendment while Glomerales was more sensitive to P additions after 2 years.
17.7.2 Grasslands
Positive, neutral, and negative effects have all been reported for responses of AMF community to N and P enrichment in grassland ecosystems, but the outcome or direction of the response depends on the beginning nutrient availability/limitation and the specific nutrient. For the positive response, Kim et al. (2015) reported that N additions (100 kg ha−1 year−1) significantly increased AMF diversity but not species richness, as well as altered species composition through shifts in plant community in a semiarid steppe ecosystem. Xiang et al. (2016) additionally reported positive responses of AMF species richness and phylogenetic diversity to N and P additions in the nutrient-limited Qinghai–Tibet Plateau alpine grasslands. Porras-Alfaro et al. (2007) also found that N amendments decreased dominant AMF species, but may have reduced suppression to subdominant or rare species, and thus increased total diversity in semiarid grassland. For negative effects, Chen et al. (2014) found that N fertilization (100 kg ha−1 year−1) in a temperate steppe ecosystem significantly reduced AM fungal species richness, but did not affect the diversity, while P fertilization (50 kg ha−1 year−1) had no effect on either of two parameters, leading to alterations of AMF community composition by N, but not by P fertilization. From a cross-site grassland experiment, Egerton-Warburton et al. (2007) found that N fertilization (>100 kg ha−1 year−1) reduced AM fungal species richness and diversity namely because the abundance of Glomeraceae was higher in P-rich soils while there was more Gigasporaceae abundant species in P-poor soils. However, Antoninka et al. (2011) found that long-term N fertilization (40 kg ha−1 year−1) did not affect AMF spore species richness in a grassland ecosystem. Such large variation might determine by the background N or P availability; thus the baseline value of nutrient is necessary for explaining the response of AM fungal community to nutrient enrichment.
17.7.3 Agroecosystems
A 55-year experiment in Skåne, Sweden, with continuous N×P full-factorial treatments showed that N fertilization altered AM fungal species composition and reduced diversity, but P fertilization showed no effect in a crop rotation of spring barley (Hordeum vulgare L.)–white mustard (Sinapis alba L.) or spring oilseed rape (Brassica napus L.)–winter wheat (Triticum aestivum L.)–sugar beet (Beta vulgaris L.) (Williams et al. 2017). N fertilization increased the abundance of Funneliformis sp.1 and Rhizophagus irregularis sp.2 but decreased the abundance of Claroideoglomus sp.3, C. glomus sp.5 and Funneliformis mosseae (Williams et al. 2017). A 90-year experiment with continuous N and P fertilizer treatments since 1914 at Hokkaido University (Sapporo, Japan) showed that both P (100 kg ha−1 year−1) and N (100 kg ha−1 year−1) fertilization altered AM fungal composition and reduced diversity, but P fertilization led to a larger reduction in the AM fungal diversity than N fertilization (Cheng et al. 2013). A 21-year experiment in a wheat (Triticum aestivum L)–maize (Zea mays L.) rotation system showed that long-term balanced NP fertilization decreased AM fungal species richness and diversity, as well as altered community composition by increasing Glomeraceae species but reducing the number of the dominant Gigasporaceae species (Lin et al. 2012). Thus, continuous fertilization might provide a selective pressure to AM fungal species, and the N- or P-tolerating ones will be maintained, but others might be discarded in the cropping systems.
17.8 Responses of Mycorrhizal Functioning to Nutrient Enrichment
Nutrient enrichment exerts profound effects on the intra- and extraradical abundance, diversity, and species composition of AM fungi. Such changes in mycorrhizas might first induce plant responses at individual levels, e.g., stress tolerance, and then cause cascading ecosystem responses, such as changes to plant productivity and diversity, soil aggregation, and carbon storage.
Nutrient enrichment may decrease the stress tolerance effects on individual hosts mediated by AM fungi. For some abiotic stresses, such as drought, salt, or heavy metal, mycorrhizal colonization might activate genes responsible for such tolerance, i.e., aquaporins, proline, or metallothionein (Reddy et al. 2016; Ruiz-Lozano et al. 2006). For some biotic stresses, mycorrhizal colonization might induce systematic resistance in host plants, such as releasing volatiles to repel insect herbivores (Bennett and Bever 2009), activating chitinase expression to inhibit nematode growth (Li et al. 2006), or upregulating pathogenesis-related genes (Campos-Soriano et al. 2012). In addition, competitive inhibition for carbon resource or colonization site is another mechanism for mycorrhizal-mediated resistance to biotic stresses (Borowicz 2001). However, as shown in some cases, increased nutrient inputs can reduce mycorrhizal root colonization, which may weaken the induction and activation of systematic resistance or tolerance toward stresses under nutrient-rich conditions.
Shifts in the mycorrhizal fungal community in response to nutrient enrichment can also lead to changes in plant community. Plant species has been shown to differ in their responsiveness to AM fungi, and different fungal species have distinct effects on plant growth variables (van der Heijden et al. 1998a, 2006a, b). Thus, shifts in mycorrhizal species composition might potentially drive changes in plant community composition through modifying plant competition. For example, distinct competitive outcome was shown between mycorrhizal and non-mycorrhizal plants when inoculated with Gigaspora margarita and Glomus intraradices (Facelli et al. 2010). Mycorrhizal fungi markedly increased competitiveness of a pioneer tree (Rhus chinensis) on a late-pioneer (Celtis sinensis) or mid-successional tree (Cinnamomum camphora), but the competitive strength was dependent on fungal identity (Shi et al. 2016). Mycorrhizal fungal identity was shown to have a large impact on competitive interactions between a grass and a legume by favoring the latter (Wagg et al. 2011). These experiments implied that nutrient enrichment-induced community shifts in mycorrhizal fungi might contribute to plant–plant interactions and subsequently regulate plant community structure and functions.
Reduction in mycorrhizas as a result of nutrient enrichment may negatively affect soil structure and reduce soil carbon storage. Mycorrhizal fungal hyphae can excrete a long carbon chain glycoprotein, glomalin, which can promote adhesion of microaggregates (Rillig et al. 2015). At the same time, AM fungal extraradical hyphae can also enmesh microaggregates into macroaggregates (Peng et al. 2013). Additionally, glomalin has been shown to be difficult to degrade in soils, and thus glomalin-C would be sequestrated in the soils for a long time (Wilson et al. 2009). Mycorrhizal-mediated soil aggregation might also provide physical protection for organic carbon from microbial decomposition (Rillig 2004).
17.9 What Controls Mycorrhizal Community Composition in Roots and Soils?
Changes in AM fungal composition can be explained through responses to nutrient enrichment of both partners. Here, we proposed two models: a plant-centric model and a fungal-centric model.
17.9.1 Plant-Centric Model
This model predicts that N or P enrichment first induces shifts in plant community composition and subsequently drives changes in their associated mycorrhizal fungal community. Many studies have shown that nutrient enrichment, in particular N, can significantly alter plant community composition and reduced plant diversity (Bobbink et al. 2010; Clark and Tilman 2008; Stevens et al. 2004). However, host preference has also been shown for mycorrhizal fungi to some extent. For example, distinctive mycorrhizal species composition was found to be among co-existing trees, forbs, or grasses, respectively (Husband et al. 2002; Vandenkoornhuyse et al. 2002, 2003). Plant composition was also shown to greatly affect mycorrhizal fungal diversity and led to distinct community composition (Johnson et al. 2004). Thus, alteration in plant composition induced by nutrient enrichment might drive changes in mycorrhizal fungal community because of the asymmetric nature of mycorrhiza, which we define as driving effect.
17.9.2 Fungal-Centric Model
This model can be derived by two hypotheses. One is that nutrient enrichment promotes AM fungal competition for plant photosynthate (here we defined it as competitive effect); the other is that different mycorrhizal fungal species have different sensitivities and uptake capacity for different nutrients (here we define it as selective effect). For the competitive effect, plants might reduce carbon allocation to their selective AM fungi because nutrient enrichment decreases the value of mycorrhiza for nutrient uptake (Johnson et al. 2003). Consequently, if host carbon becomes a scarce resource for AM fungi, competitive exclusion might reduce or eliminate rare species but maintain dominant ones (Knegt et al. 2016). Such competitive exclusion for carbon could lead to reduced AM fungal species richness and diversity over time (Liu et al. 2015). For the selective effect, experimental evidence has shown that Diversiporales is sensitive to N enrichment but tolerant to P, while Glomerales is sensitive to P but not to N (Lin et al. 2012; Williams et al. 2017). Thus, nutrient enrichment might select some N- or P-loving mycorrhizal fungal species and exclude some sensitive taxa, the idea which is especially supported by farmlands, e.g., dominance and persistence of some specific AM fungal species in high N or P availability (Cheng et al. 2013; Lin et al. 2012).
17.10 Future Directions
While a number of studies have examined mycorrhizal responses to nutrient enrichment in various settings, significant knowledge gaps still persist. This limits our capacity to predict the impact of nutrient inputs on mycorrhizas and potentially to manage nutrient inputs to maximize mycorrhizal benefits to plants. First, it is still unclear whether there are some unifying mechanisms/patterns that underlie arbuscular mycorrhizal responses to nutrient enrichment, including C and nutrient trade-offs, thresholds, and molecular controlling mechanisms. Secondly, although increasing experimental evidence has shown that nutrient enrichment alters AM fungal community composition (Kim et al. 2015; van Diepen et al. 2011; Williams et al. 2017), much less is known about the identity of fungal species under impact. There is limited evidence showing that Glomerales taxa are sensitive to P but not to N additions, while Diversisporales seemed to be the opposite. Yet, many questions remain: Does this represent a general pattern? What about other AM taxa responses? Third, AM fungal diversity showed positive, neutral, or negative responses to nutrient enrichment. We do not know what factors lead to such variations and how initial soil conditions contribute to this variability. Fourth, many previous studies so far have focused on additions of single nutrient with one high rate. This raises questions to whether there is a nutrient threshold for mycorrhizal responses. If yes, does the threshold vary with the fungal species or taxa? Experiments that examine mycorrhizal responses across a gradient nutrient input are needed in the future, especially in field conditions and under different N and P limitations. Moreover, how AM fungal response to one nutrient depends on the availability of other nutrients warrants further study. These knowledge gaps highlight the need for factorial experiments. Lastly, how other environmental factors, such as water availability and temperature, may modulate arbuscular mycorrhizal responses to nutrient enrichment requires examination. This knowledge is essential for us to understand mycorrhizal functions in a globe with multifaceted changes.
References
Al-Karaki GN, Clark R (1999) Mycorrhizal influence on protein and lipid of durum wheat grown at different soil phosphorus levels. Mycorrhiza 9:97–101
Amtmann A, Hammond JP, Armengaud P, White PJ (2006) Nutrient sensing and signalling in plants: potassium and phosphorus. In: Callow JA (ed) Advances in botanical research. Incorporating advances in plant pathology, vol 43. Academic, London, pp 209–257
Antoninka A, Reich PB, Johnson NC (2011) Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol 192:200–214
Avio L, Castaldini M, Fabiani A, Bedini S, Sbrana C, Turrini A, Giovannetti M (2013) Impact of nitrogen fertilization and soil tillage on arbuscular mycorrhizal fungal communities in a Mediterranean agroecosystem. Soil Biol Biochem 67:285–294
Azcón R, Ambrosano E, Charest C (2003) Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci 165:1137–1145
Benedetto A, Magurno F, Bonfante P, Lanfranco L (2005) Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 15:620–627
Bennett AE, Bever JD (2009) Trade-offs between arbuscular mycorrhizal fungal competitive ability and host growth promotion in Plantago lanceolata. Oecologia 160:807–816
Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M (2009) Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol Lett 12:13–21
Blanke V, Renker C, Wagner M, Fullner K, Held M, Kuhn AJ, Buscot F (2005) Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol 166:981–992
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman JW, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057–3068
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Camenzind T, Hempel S, Homeier J, Horn S, Velescu A, Wilcke W, Rillig MC (2014) Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob Chang Biol 20:3646–3659
Camenzind T, Homeier J, Dietrich K, Hempel S, Hertel D, Krohn A, Leuschner C, Oelmann Y, Olsson PA, Suarez JP, Rillig MC (2016) Opposing effects of nitrogen versus phosphorus additions on mycorrhizal fungal abundance along an elevational gradient in tropical montane forests. Soil Biol Biochem 94:37–47
Campos-Soriano L, Garcia-Martinez J, Segundo BS (2012) The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol Plant Pathol 13:579–592
Chen YL, Zhang X, Ye JS, Han HY, Wan SQ, Chen BD (2014) Six-year fertilization modifies the biodiversity of arbuscular mycorrhizal fungi in a temperate steppe in Inner Mongolia. Soil Biol Biochem 69:371–381
Cheng L, Booker F, Tu C, Burkey K, Zhou L, Shew H, Rufty T, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084
Cheng Y, Ishimoto K, Kuriyama Y, Osaki M, Ezawa T (2013) Ninety-year-, but not single, application of phosphorus fertilizer has a major impact on arbuscular mycorrhizal fungal communities. Plant Soil 365:397–407
Clark CM, Tilman D (2008) Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451:712–715
Cofala J, Amann M, Klimont Z, Kupiainen K, Höglund-Isaksson L (2007) Scenarios of global anthropogenic emissions of air pollutants and methane until 2030. Atmos Environ 41:8486–8499
Egerton-Warburton LM, Allen EB (2000) Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10:484–496
Egerton-Warburton LM, Johnson NC, Allen EB (2007) Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecol Monogr 77:527–544
Eissenstat DM, Graham JH, Syvertsen JP, Drouillard DL (1993) Carbon economy of sour orange in relation to mycorrhizal colonization and phosphorus status. Ann Bot 71:1–10
Ekblad A, Mikusinska A, Ågren GI, Menichetti L, Wallander H, Vilgalys R, Bahr A, Eriksson U (2016) Production and turnover of ectomycorrhizal extramatrical mycelial biomass and necromass under elevated CO2 and nitrogen fertilization. New Phytol 211:874–885
Facelli E, Smith SE, Facelli JM, Christophersen HM, Smith FA (2010) Underground friends or enemies: model plants help to unravel direct and indirect effects of arbuscular mycorrhizal fungi on plant competition. New Phytol 185:1050–1061
Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Buecking H (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 109:2666–2671
Fochi V, Chitarra W, Kohler A, Voyron S, Singan VR, Lindquist EA, Barry KW, Girlanda M, Grigoriev I, Martin F, Balestrini R, Perotto S (2017) Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytol 213:365–379
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Goff SA, Ricke D, Lan TH, Presting G, Wang RL, Dunn M et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp japonica). Science 296:92–100
Gosling P, Hodge A, Goodlass G, Bending GD (2006) Arbuscular mycorrhizal fungi and organic farming. Agr Ecosyst Environ 113:17–35
Govindarajulu M, Pfeffer P, Jin H, Abubaker J, Douds D, Allen J, Bücking H, Lammers P, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gryndler M, Larsen J, Hršelová H, Řezáčová V, Gryndlerová H, Kubát J (2006) Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 16:159–166
Guescini M, Zeppa S, Pierleoni R, Sisti D, Stocchi L, Stocchi V (2007) The expression profile of the Tuber borchii nitrite reductase suggests its positive contribution to host plant nitrogen nutrition. Curr Genet 51:31–41
Guether M, Neuhauser B, Balestrini R, Dynowski M, Ludewig U, Bonfante P (2009) A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol 150:73
Harrison MJ, Vanbuuren ML (1995) A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378:626–629
Harrison MJ, Dewbre GR, Liu J (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14:2413
Hetrick B, Wilson G, Cox T (1993) Mycorrhizal dependence of modern wheat cultivars and ancestors: a synthesis. Can J Bot 71:512–518
Hobbie JE, Hobbie EA (2006) N-15 in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology 87:816
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Huang RJ, Zhang YL, Bozzetti C, Ho KF, Cao JJ, Han YM et al (2002) Molecular diversity of arbuscular mycorrhizal fungi and patterns of host association over time and space in a tropical forest. Mol Ecol 11:2669–2678
Husband R, Herre EA, Turner SL, Gallery R, Young JPW (2002) Molecular diversity of arbuscular mycorrhizal fungi and patterns of host association over time and space in a tropical forest. Mol Ecol 11:2669–2678
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. I: spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380
Javelle A, Andre B, Marini AM, Chalot M (2003) High-affinity ammonium transporters and nitrogen sensing in mycorrhizas. Trends Microbiol 11:53–55
Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 104:1720–1725
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908
Johnson D, Vandenkoornhuyse PJ, Leake JR, Gilbert L, Booth RE, Grime JP, Young JPW, Read DJ (2004) Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol 161:503–515
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci 106:3041–3046
Kahiluoto H, Ketoja E, Vestberg M, Saarela I (2001) Promotion of AM utilization through reduced P fertilization 2. Field studies. Plant Soil 231:65–79
Kaldorf M, Schmelzer E, Bothe H (1998) Expression of maize and fungal nitrate reductase genes in arbuscular mycorrhiza. Mol Plant Microbe Interact 11:439
Kiers ET, van der Heijden MGA (2006) Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology 87:1627–1636
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bucking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Kim YC, Gao C, Zheng Y, He XH, Yang W, Chen L, Wan SQ, Guo LD (2015) Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 25:267–276
Knegt B, Jansa J, Franken O, Engelmoer DJ, Werner GD, Bücking H, Kiers ET (2016) Host plant quality mediates competition between arbuscular mycorrhizal fungi. Fungal Ecol 20:233–240
Koegel S, Lahmidi NA, Arnould C, Chatagnier O, Walder F, Ineichen K, Boller T, Wipf D, Wiemken A, Courty P-E (2013) The family of ammonium transporters (AMT) in Sorghum bicolor: two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol 198:853–865
Koide RT (2000) Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol 147:223–235
Koide RT, Kabir Z (2000) Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol 148:511–517
Koide RT, Fernandez CW, Peoples MS (2011) Can ectomycorrhizal colonization of Pinus resinosa roots affect their decomposition? New Phytol 191:508–514
Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, Zazimalova E, Benkova E, Nacry P, Gojon A (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18:927–937
Lanquar V, Loque D, Hormann F, Yuan LX, Bohner A, Engelsberger WR, Lalonde S, Schulze WX, von Wiren N, Frommer WB (2009) Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell 21:3610–3622
Li HY, Yang GD, Shu HR, Yang YT, Ye BX, Nishida I, Zheng CC (2006) Colonization by the arbuscular mycorrhizal fungus Glomus versiforme induces a defense response against the root-knot nematode Meloidogyne incognita in the grapevine (Vitis amurensis Rupr.), which includes transcriptional activation of the class III chitinase gene VCH3. Plant Cell Physiol 47:154–163
Lin XG, Feng YZ, Zhang HY, Chen RR, Wang JH, Zhang JB, Chu HY (2012) Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing. Environ Sci Technol 46:5764–5771
Liu YJ, Mao L, Li JY, Shi GX, Jiang SJ, Ma XJ, An LZ, Du GZ, Feng HY (2015) Resource availability differentially drives community assemblages of plants and their root-associated arbuscular mycorrhizal fungi. Plant Soil 386:341–355
Lopez-Pedrosa A, Gonzalez-Guerrero M, Valderas A, Azcon-Aguilar C, Ferrol N (2006) GintAMT1 encodes a functional high-affinity ammonium transporter that is expressed in the extraradical mycelium of Glomus intraradices. Fungal Genet Biol 43:102–110
Lorenz MC, Heitman J (1998) The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J 17:1236–1247
MacFall JS, Slack SA, Wehrli S (1992) Phosphorus distribution in red pine roots and the ectomycorrhizal fungus Hebeloma arenosa. Plant Physiol 100:713–717
Maldonado-Mendoza I, Dewbre G, Harrison M (2001) A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol Plant Microbe Interact 14:1140–1148
McCormick MK, Taylor DL, Juhaszova K, Burnett RK, Whigham DF, O'Neill JP (2012) Limitations on orchid recruitment: not a simple picture. Mol Ecol 21:1511–1523
Monteagudo L, Luis Moreno J, Picazo F (2012) River eutrophication: irrigated vs. non-irrigated agriculture through different spatial scales. Water Res 46:2759–2771
Muller T, Avolio M, Olivi M, Benjdia M, Rikirsch E, Kasaras A, Fitz M, Chalot M, Wipf D (2007) Nitrogen transport in the ectomycorrhiza association: the Hebeloma cylindrosporum-Pinus pinaster model. Phytochemistry 68:41–51
Munos S, Cazettes C, Fizames C, Gaymard F, Tillard P, Lepetit M, Lejay L, Gojon A (2004) Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. Plant Cell 16:2433–2447
Nagy R, Karandashov V, Chague V, Kalinkevich K, Tamasloukht M, Xu G, Jakobsen I, Levy A, Amrhein N, Bucher M (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J 42:236
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181:950–959
Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 99:13324
Peng SL, Guo T, Liu GC (2013) The effects of arbuscular mycorrhizal hyphal networks on soil aggregations of purple soil in southwest China. Soil Biol Biochem 57:411–417
Popova Y, Thayumanavan P, Lonati E, Agrochao M, Thevelein JM (2010) Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc Natl Acad Sci USA 107:2890–2895
Porras-Alfaro A, Herrera J, Natvig DO, Sinsabaugh RL (2007) Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 296:65–75
Pritchard SG, Taylor BN, Cooper ER, Beidler KV, Strand AE, McCormack ML, Zhang S (2014) Long-term dynamics of mycorrhizal root tips in a loblolly pine forest grown with free-air CO2 enrichment and soil N fertilization for 6 years. Glob Chang Biol 20:1313–1326
Qin L, Zhao J, Tian J, Chen LY, Sun ZA, Guo YX, Lu X, Gu MA, Xu GH, Liao H (2012) The high-affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol 159:1634–1643
Reddy MS, Kour M, Aggarwal S, Ahuja S, Marmeisse R, Fraissinet-Tachet L (2016) Metal induction of a Pisolithus albus metallothionein and its potential involvement in heavy metal tolerance during mycorrhizal symbiosis. Environ Microbiol 18:2446–2454
Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA (2005) Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol 167:869
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2015) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388
Ruiz-Lozano JM, Porcel R, Aroca R (2006) Does the enhanced tolerance of arbuscular mycorrhizal plants to water deficit involve modulation of drought-induced plant genes? New Phytol 171:693–698
Saito M, Oba H, Kojima T (2011) Effect of nitrogen on the sporulation of arbuscular mycorrhizal fungi colonizing several gramineous plant species. Soil Sci Plant Nutr 57:29–34
Scheible W-R, Rojas-Triana M (2015) Sensing, signalling, and control of phosphate starvation in plants: molecular players and applications. In: Plaxton WC, Lambers H (eds) Phosphorus metabolism in plants, vol 48. Wiley, Hoboken, pp 25–63
Schlesinger WH (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci USA 106:203–208
Selosse MA, Setaro S, Glatard F, Richard F, Urcelay C, Weiss M (2007) Sebacinales are common mycorrhizal associates of Ericaceae. New Phytol 174:864–878
Shi NN, Gao C, Zheng Y, Guo LD (2016) Arbuscular mycorrhizal fungus identity and diversity influence subtropical tree competition. Fungal Ecol 20:115–123
Smil V (1999) Nitrogen in crop production: an account of global flows. Global Biogeochem Cycles 13:647–662
Smil V (2003) Phosphorus in the environment: natural flows and human interferences. Annu Rev Energy Environ 25:53–88
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, San Diego
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796
Teng W, Zhao YY, Zhao XQ, He X, Ma WY, Deng Y, Chen XP, Tong YP (2017) Genome-wide identification, characterization, and expression analysis of PHT1 phosphate transporters in wheat. Front Plant Sci 8:543
Thirkell TJ, Cameron DD, Hodge A (2016) Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ 39:1683
Tian C, Kasiborski B, Koul R, Lammers PJ, Bücking H, Shachar-Hill Y (2010) Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: gene characterization and the coordination of expression with nitrogen flux. Plant Physiol 153:1175–1187
Tilman D, Fargione J, Wolff B, D'Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677
Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A et al (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol 193:755–769
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Tunlid A, Floudas D, Koide RT, Martin F (2016) Molecular mycorrhizal symbiosis: 15. Soil organic matter decomposition mechanisms in ectomycorrhizal fungi. Wiley, Hoboken
Ulm F, Gouveia C, Dias T, Cruz C (2017) N fertilization in a Mediterranean ecosystem alters N and P turnover in soil, roots and the ectomycorrhizal community. Soil Biol Biochem 113:60–70
Unger S, Friede M, Hundacker J, Volkmar K, Beyschlag W (2016) Allocation trade-off between root and mycorrhizal surface defines nitrogen and phosphorus relations in 13 grassland species. Plant Soil 407:279–292
van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998a) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998b) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006a) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006b) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
van der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423
van Diepen LTA, Lilleskov EA, Pregitzer KS, Miller RM (2007) Decline of arbuscular mycorrhizal fungi in northern hardwood forests exposed to chronic nitrogen additions. New Phytol 176:175–183
van Diepen LTA, Lilleskov EA, Pregitzer KS (2011) Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. Mol Ecol 20:799–811
Vandenkoornhuyse P, Husband R, Daniell TJ, Watson IJ, Duck JM, Fitter AH, Young JPW (2002) Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol Ecol 11:1555–1564
Vandenkoornhuyse P, Ridgway KP, Watson IJ, Fitter AH, Young JPW (2003) Co-existing grass species have distinctive arbuscular mycorrhizal communities. Mol Ecol 12:3085–3095
Wagg C, Jansa J, Stadler M, Schmid B, van der Heijden MGA (2011) Mycorrhizal fungal identity and diversity relaxes plant-plant competition. Ecology 92:1303–1313
Wang C, White PJ, Li C (2017) Colonization and community structure of arbuscular mycorrhizal fungi in maize roots at different depths in the soil profile respond differently to phosphorus inputs on a long-term experimental site. Mycorrhiza 27:369–381
Werner GDA, Kiers ET (2015) Partner selection in the mycorrhizal mutualism. New Phytol 205:1437–1442
Williams A, Manoharan L, Rosenstock NP, Olsson PA, Hedlund K (2017) Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol 213:874–885
Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461
Withers PJA, Neal C, Jarvie HP, Doody DG (2014) Agriculture and eutrophication: where do we go from here? Sustainability 6:5853–5875
Xiang X, Gibbons SM, He J-S, Wang C, He D, Li Q, Ni Y, Chu H (2016) Rapid response of arbuscular mycorrhizal fungal communities to short-term fertilization in an alpine grassland on the Qinghai-Tibet Plateau. Peerj 4:e2226
Xie XA, Lin H, Peng XW, Xu CR, Sun ZF, Jiang KX, Huang A, Wu XH, Tang NW, Salvioli A, Bonfante P, Zhao B (2016) Arbuscular mycorrhizal symbiosis requires a phosphate transceptor in the Gigaspora margarita fungal symbiont. Mol Plant 9:1583–1608
Xu GH, Chague V, Melamed-Bessudo C, Kapulnik Y, Jain A, Raghothama KG, Levy AA, Silber A (2007) Functional characterization of LePT4: a phosphate transporter in tomato with mycorrhiza-enhanced expression. J Exp Bot 58:2491
Yang HS, Zhang Q, Koide RT, Hoeksema JD, Tang J, Bian X, Hu SJ, Chen X (2017) Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. J Ecol 105:219–228
Zhang L, Fan J, Ding X, He X, Zhang F, Feng G (2014a) Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol Biochem 74:177–183
Zhang Y, Zang GQ, Tang ZH, Chen XH, Yu YS (2014b) Burning straw, air pollution, and respiratory infections in China. Am J Infect Control 42:815–815
Zhang L, Xu M, Liu Y, Zhang F, Hodge A, Feng G (2016) Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol 210:1022–1032
Zhu Y, Smith S, Barritt A, Smith F (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Zhu HH, Yao Q, Sun XT, Hu YL (2007) Colonization, ALP activity and plant growth promotion of native and exotic arbuscular mycorrhizal fungi at low pH. Soil Biol Biochem 39:942–950
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Yang, H., Schroeder-Moreno, M., Giri, B., Hu, S. (2018). Arbuscular Mycorrhizal Fungi and Their Responses to Nutrient Enrichment. In: Giri, B., Prasad, R., Varma, A. (eds) Root Biology. Soil Biology, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-319-75910-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-75910-4_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75909-8
Online ISBN: 978-3-319-75910-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)