Abstract
Salinity stress is one of the major abiotic stresses that result in significant losses in agricultural crop production across the globe. Salinity stress results in osmotic stress, ionic stress, and oxidative stress; among these, oxidative stress is considered to be the most detrimental. Oxidative stress induces the production of different reactive oxygen species (ROS) at both intracellular and extracellular locations. Plants possess redox regulatory mechanisms by employing different enzymatic and nonenzymatic antioxidants to scavenge ROS. Different antioxidants have different tissue- and organelle-specific ROS-scavenging effects. However, the causal link between the amount of antioxidants and plant salinity stress tolerance is not as straightforward as one may assume, with controversial reports available in the literature. This chapter addresses those controversies and argues that there is a need for better understanding and development of tools for targeted regulation of plant redox systems in specific cellular compartments and tissues.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Antioxidant defense system
- QTL
- Redox regulation
- ROS production
- Salt stress
- Tissue specific antioxidant activity
8.1 Introduction
Soil salinity is one of the most detrimental abiotic stresses that critically damage crops and cause major reductions in their yield (Munns et al. 2006; Tanveer and Shah 2017). Salinity is characterized by deleterious effects on plant growth, which are traditionally associated with reduced water availability under hyperosmotic saline conditions, and specific ion toxicity. In recent years, oxidative damage has been added to this list (Souza et al. 2012; Liu et al. 2017; López-Gómez et al. 2017). Like other aerobic organisms, higher plants require oxygen for efficient production of energy. During the reduction of O2 to H2O, reactive oxygen species (ROS)—namely, superoxide radicals (O2 −), H2O2, and hydroxyl radicals (OH−)—are formed (Demidchik 2015). Most cellular compartments in higher plants have the potential to become a source of ROS (Bose et al. 2014). Environmental stresses that limit CO2 fixation, including salinity, reduce nicotinamide adenine dinucleotide phosphate (NADP+) regeneration by the Calvin cycle. Consequently, the photosynthetic electron transport chain is over-reduced, producing O2 − and singlet oxygen (1O2) in chloroplasts (Wu and Tang 2004; Shao and Chu 2005). To prevent overreduction of the electron transport chain under conditions that limit CO2 fixation, higher plants have evolved the photorespiratory pathway to regenerate NADP+ (Shao and Chu 2005). As a part of the photorespiratory pathway, H2O2 is produced in the peroxisomes, where it can also be formed during the catabolism of lipids as a by-product of β-oxidation of fatty acids (Foyer and Noctor 2005; Wu et al. 2007).
Because of the highly cytotoxic and reactive nature of ROS, their accumulation in plant tissues and intracellular compartments must be tightly controlled. Higher plant metabolism must be highly regulated in order to allow effective integration of a diverse spectrum of biosynthetic pathways that are reductive in nature (Crawford 2006; Grun et al. 2006). This requires the provision that the regulation does not completely avoid photodynamic or reductive activation of molecular oxygen to produce ROS, particularly superoxide, H2O2, and 1O2 (Suzuki et al. 2012). However, in many cases, the production of ROS is genetically programmed, is induced during the course of development and by environmental fluctuations, and has complex downstream effects on both primary and secondary metabolism (Schurmann 2003; Link 2003; Rouhier et al. 2003). As a result, higher plants possess very efficient enzymatic and nonenzymatic antioxidant defense systems that allow scavenging of ROS and protection of plant cells from oxidative damage (Foyer and Noctor 2003; Anjum et al. 2016a, 2017).
For many years, the concept “the higher the antioxidant activity, the better the plant” has dominated the literature. However, in recent years it has become apparent that plants actively produce ROS as signaling molecules to control numerous physiological processes such as defense responses and cell death (Zhang et al. 2003), cross-tolerance (Bowler and Fluhr 2000), gravitropism (Mittler et al. 2004), stomatal aperture (Wang and Song 2008; Pei et al. 2000), cell expansion and polar growth (Joo et al. 2005; Pedreira et al. 2004), hormone action (Pei et al. 2000; Schopfer et al. 2002), and leaf and flower development (Sagi et al. 2004). In many cases, production of ROS is genetically programmed, and superoxide and H2O2 are used as second messengers (Foyer and Noctor 2005). A new concept of “oxidative signaling”—instead of “oxidative stress”—has been proposed (Foyer and Noctor 2005). The “positive” role of ROS has been reported at both the physiological level [e.g., regulation of ion channel activity (Foreman et al. 2003)] and the genetic level [e.g., control of gene expression (Shin and Schachtman 2004)]. This has prompted a need to rethink the above “the more, the better” concept and incorporate the signaling role of ROS and the redox state of the cell into breeding programs aimed at improving abiotic stress tolerance. Some aspects of this work are discussed in this chapter.
8.2 Antioxidant Defense System
The distinct subcellular localization and biochemical properties of antioxidant enzymes, their differential activation at the enzyme and gene expression level, and the plethora of nonenzymatic scavengers render the antioxidant system a very versatile and flexible unit that can control ROS accumulation temporally and spatially (Shao et al. 2007a, b; Anjum et al. 2015, 2016b). The sections below describe mechanisms involved in redox regulation for salinity stress tolerance in plants.
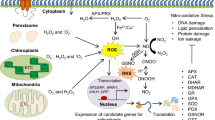
The redox state of a cell explains the ratio of the amount of oxidizing equivalents to the amount of reducing equivalents (Gabbita et al. 2000). The intracellular antioxidant systems form a powerful reducing buffer, which affects the ability of the cell to counteract the action of the prooxidant forces. The fine redox balance within a cell is thus governed by the levels of prooxidant molecules and antioxidant fluxes. Therefore, an appreciation of the different sources of oxidants and the counteracting antioxidant (or reducing) systems is necessary to understand what factors are involved in achieving a particular intracellular redox state. Salinity induces various ROS and, in response to that, plants develop complex antioxidant defense systems (Ismail et al. 2016) (Fig. 8.1). Antioxidants can be classified into three broad divisions: water-soluble compounds (reductants, ascorbate); lipid-soluble compounds (α-tocopherol, β-carotene); and enzymes (superoxide dismutase [SOD], catalase [CAT], peroxidase [POD], ascorbate peroxidase [APX], and glutathione reductase [GR]) (Chen and Dickman 2005; Gill and Tuteja 2010).
8.2.1 Enzymatic Antioxidants
Among the different antioxidant enzymes, SOD is the most effective intracellular enzymatic antioxidant that is ubiquitous in all aerobic organisms. SOD can catalyze and reduce O2 − to H2O2 because of its dismutation. SOD also indirectly reduces the risk of OH− formation by using O2 −. SOD catalyzes the first step of the enzymatic defense mechanism: the conversion of superoxide anions to hydrogen peroxide and water. If superoxide anions are not neutralized, oxidation occurs and hydroxyl radicals are formed. Hydroxyl radicals are extremely harmful because they are very reactive. Importantly, hydroxyl radicals cannot be scavenged by enzymatic means. Hydrogen peroxide can be decomposed by the activity of CATs and several classes of PODs, which act as important antioxidants.
CAT is another important enzymatic antioxidant; it is a tetrameric heme containing enzymes with the potential to directly dismutate H2O2 into H2O and O2, and it is indispensable for ROS detoxification during stressed conditions (Garg and Manchanda 2009). CAT has the highest turnover rate: one molecule of CAT can convert about 6 million molecules of H2O2 to H2O and O2 per minute. CAT enzymes remove H2O2 from peroxisomes by oxidases involved in β-oxidation of fatty acids, photorespiration, and purine catabolism. Different CAT isozymes have been reported in different plant species: two isozymes in barley (Azevedo et al. 1998), four in sunflower (Azpilicueta et al. 2007), and 12 in Brassica (Frugoli et al. 1996). Scandalias (1990) found three CAT isozymes in maize: CAT1 and CAT2 are localized in peroxisomes and the cytosol, whereas CAT3 is mitochondrial. It has also been reported that apart from reacting with H2O2, CAT also reacts with some hydroperoxides such as methyl hydrogen peroxide (MeOOH) (Ali and Alqurainy 2006). Overexpression of CAT encoded by the katE gene in rice conferred salt-induced oxidative stress tolerance (Nagamiya et al. 2007). Similarly, increased activity of CAT has been reported in chickpea following salt stress (Eyidogan and Oz 2005; Kukreja et al. 2005). Nonetheless, Srivastava et al. (2005) reported a decrease in CAT activity in Anabaena doliolum under NaCl and Cu2+ stress. Pan et al. (2006) studied the combined effect of salt and drought stress and found that it decreased CAT activity in Glycyrrhiza uralensis seedlings.
Thioredoxin and thiol-based glutathione are also important enzymes that play a crucial role in redox regulation. Thioredoxin includes a pleiotropic reduced NADP (NADPH)–dependent disulfide oxidoreductase, which catalyzes the reduction of exposed protein S–S bridges. Because of its dithiol-to-disulfide exchange activity, thioredoxin—acting as a hydrogen donor—determines the oxidation state of protein thiols (Lu and Holmgren 2014). This small 12 kDa protein contains a characteristic conserved catalytic Trp–Cys–Gly–Pro–Cys–Lys site. The two cysteine residues within this site can be oxidized reversibly to form a disulfide bridge. A specific selenoenzyme, thioredoxin reductase, is able to reduce the disulfide bond, utilizing NADPH as a hydrogen donor. The glutaredoxin system utilizes glutathione (a cysteine-containing tripeptide) in a manner similar to that used by the thioredoxin system. The antioxidant function of glutathione is implicated through two general mechanisms of reaction with ROS.
Glutathione peroxidase (GPX) belongs to a large family of diverse isozymes that use glutathione to reduce H2O2 and organic and lipid hydroperoxides, and therefore help plant cells with oxidative stress (Noctor et al. 2002). GPX uses glutathione to eliminate H2O2 and to decrease hydroperoxidation of lipids. Thus, these enzymes regulate intracellular levels of ROS such as H2O2 and O2 − (Gabbita et al. 2000). Millar et al. (2003) identified a family of seven related proteins—named AtGPX1–AtGPX7—in the cytosol, chloroplast, mitochondria, and endoplasmic reticulum of Arabidopsis. Upregulation of the GPX gene was noted in response to 1O2, showing the involvement of GPX in quenching 1O2 (Leisinger et al. 2001). It was noted that GPX activity in transgenic cotton seedlings was 30–60% higher under normal conditions but no different from GPX activity in wild-type seedlings under salt stress conditions (Light et al. 2005).
8.2.2 Nonenzymatic Antioxidants
Ascorbic acid (ASC) is the most abundant, powerful, and water-soluble antioxidant that acts to prevent or minimize the damage caused by ROS in plants (Smirnoff 2005; Athar et al. 2008). ASC is considered a most powerful ROS scavenger because of its ability to donate electrons in a number of enzymatic and nonenzymatic reactions. It can provide protection to membranes by directly scavenging O2 − and OH−, and by regenerating α-tocopherol from the tocopheroxyl radical. In chloroplasts, ASC acts as a cofactor of violaxantin de-epoxidase, thus sustaining dissipation of excess excitation energy (Smirnoff 2005). In addition to the importance of ASC in the ascorbate–glutathione cycle, it also plays an important role in preserving the activity of enzymes that contain prosthetic transition metal ions (Noctor and Foyer 1998). The ASC redox system consists of L-ascorbic acid (L-ASC), monodehydroascorbic acid (MDHA), and dehydroascorbic acid (DHA). Both oxidized forms of ASC are relatively unstable in aqueous environments, while DHA can be chemically reduced by glutathione.
Tripeptide glutathione (glu–cys–gly) is one of the crucial metabolites in plants and is considered a most important intracellular component of defense against ROS-induced oxidative damage. It occurs abundantly in the reduced form in plant tissues and is localized in all cell compartments, including the cytosol, endoplasmic reticulum, vacuole, mitochondria, chloroplasts, and peroxisomes, as well as in the apoplast (Mittler and Zilinskas 1992; Jiménez et al. 1998). Glutathione provides a substrate for multiple cellular reactions that yield GSSG (i.e., two glutathione molecules linked by a disulfide bond). The balance between glutathione and GSSG is a central component in maintaining the cellular redox state (Foyer and Noctor 2005). Glutathione is necessary to maintain the normal reduced state of cells so as to counteract the inhibitory effects of ROS-induced oxidative stress (Meyer 2008). It is a potent scavenger of 1O2, H2O2, and most dangerous ROS such as OH− (Op den Camp et al. 2003).
Proline is considered a potent antioxidant and a potential inhibitor of programmed cell death (Chen and Dickman 2005). Free proline has been proposed to also act as an osmoprotectant, a protein stabilizer, and a metal chelator, and helps in scavenging ROS (Ashraf and Foolad 2007; Trovato et al. 2008). In plants, there are two different precursors for proline. The first pathway is from glutamate, which is converted to proline by two successive reductions catalyzed by pyrroline-5-carboxylate synthase (P5CS) and pyrroline-5-carboxylate reductase (P5CR), respectively. P5CS is a bifunctional enzyme catalyzing first the activation of glutamate by phosphorylation and second the reduction of the labile intermediate c-glutamyl phosphate into glutamate semialdehyde, which is in equilibrium with the P5C form (Hu et al. 1992). An alternative precursor for proline biosynthesis is ornithine, which can be transaminated to P5C by ornithine-δ-aminotransferase (Orn-δ-OAT), a mitochondrially located enzyme. The glutamate pathway is the main pathway during osmotic stress. However, in young Arabidopsis plants, the ornithine pathway also seems to contribute and δ-OAT activity is enhanced (Roosens et al. 1998). Sorbitol, mannitol, myo-inositol, and proline have been tested for OH−-scavenging capacity, and it was found that proline appeared to be an effective scavenger of OH− (Smirnoff and Cumbes 1989; Gill and Tuteja 2010). Therefore, proline is not only an important molecule in redox signaling but also an effective quencher of ROS formed under salt stress (Alia and Saradhi 1991; Tanveer and Shah 2017). Chen and Dickman (2005) showed that addition of proline to DARas mutant cells effectively quenched ROS and prevented cell death by inhibiting ROS-mediated apoptosis. Enhanced synthesis of proline under drought or salt stress has been implicated as a mechanism in alleviation of cytoplasmic acidosis and maintenance of the NADPH:NADP+ ratio at a value compatible with metabolism (Hare and Cress 1997). Moreover, proline could maintain a low NADPH:NADP+ ratio, decrease 1O2 production from photosystem I (PSI), and lessen 1O2 and OH− damage to photosystem II (PSII) under stress (Szabados and Savouré 2010). Furthermore, proline has been reported to perform various antioxidant functions including (1) reductions in OH−, H2O2, and 1O2; (2) maintenance of a low NADPH:NADP+ ratio to decrease 1O2 production; (3) assistance in preventing programmed cell death; (4) reduction of detrimental effects of ROS on PSII; and (5) stabilization of mitochondrial respiration to protect the complex II phase of the electron transport chain in mitochondria (Szabados and Savouré 2010). Exogenously applied proline reduced the leaking of K+ by reducing the production of hydroxyl radicals in Arabidopsis roots (Cuin and Shabala 2007).
Glycine betaine accumulates predominantly in chloroplasts and protects the photosynthetic apparatus during oxidative stress (Ashraf and Foolad 2007). In addition, glycine betaine stabilizes the structure and function of the oxygen-evolving complex of PSII and protects the photosynthetic apparatus under high salt stress (Papageorgiou and Murata 1995). The introduction of genes synthesizing glycine betaine into nonaccumulators of glycine betaine has been shown to be effective in increasing tolerance of various abiotic stresses (Sakamoto and Murata 2002). In addition to the direct protective roles of glycine betaine, either through positive effects on enzyme and membrane integrity or as an osmoprotectant, glycine betaine may also protect cells from environmental stresses indirectly by participating in signal transduction pathways (Subbarao et al. 2000). Even in small concentrations, glycine betaine is very effective in stress amelioration. Exogenous application of glycine betaine (even in a submillimolar concentration) alleviates OH−-generated potassium ion leakage (Cuin and Shabala 2007), improving salinity stress tolerance. Consistent with this observation, halophytes (the most salt-tolerant species on the planet) may accumulate significant amounts (up to 90 μmol on a dry weight basis) of glycine betaine and thus can better withstand oxidative damage under saline conditions (Rhodes and Hanson 1993; Flowers and Colmer 2008). Transgenic crops overexpressing halophyte betaine aldehyde dehydrogenase (BADH)—a glycine betaine–synthesizing enzyme—were shown to possess enhanced salt and drought tolerance (Fitzgerald et al. 2009). Interestingly, overexpression of a plastid BADH in the salt-sensitive carrot resulted in remarkable salt tolerance (up to 400 mM NaCl), similar to that of halophytes (Kumar et al. 2004). The above findings could be attributed to both the osmotic role of glycine betaine and its ROS-scavenging ability.
Flavonoids are usually accumulated in the plant vacuole as glycosides, but they also occur as exudates on the surfaces of leaves and other aerial plant parts. Flavonoids can be classified into flavonols, flavones, isoflavones, and anthocyanins on the basis of their structure. Flavonoids are among the most bioactive plant secondary metabolites. Flavonoids serve as ROS scavengers by locating and neutralizing radicals before they damage the cell, which is important for plants to regulate redox potential under stressful conditions (Løvdal et al. 2010). Flavonoids function by virtue of the number and arrangement of their hydroxyl groups attached to ring structures. Their ability to act as antioxidants depends on the reduction potentials of their radicals and the accessibility of the radicals. Most flavonoids outperform well-known antioxidants, such as ASC and α-tocopherol (Hernandez et al. 2009).
Tocopherols are considered major antioxidants in biomembranes. The antioxidant ability of tocopherols against Fe2+ ascorbate–induced lipid peroxidation declined in the order of α > β ≈ γ > δ, with each single molecule of these tocopherols protecting up to 220, 120, 100, and 30 molecules of polyunsaturated fatty acids, respectively, before being consumed (Fukuzawa et al. 1982). Tocopherols have been shown to prevent the chain propagation step in lipid auto-oxidation, which makes them an effective free-radical trap. α-Tocopherol (vitamin E) detoxifies 1O2 and lipid peroxyl radicals, thus preventing lipid peroxidation under abiotic stress (Szarka et al. 2012). Among the different isoforms of tocopherols, α-tocopherol is the predominant form in plant green tissues. This isoform is synthesized in a plastid envelope and is stored in plastoglobuli of the chloroplast stroma and in thylakoid membranes, suggesting that α-tocopherol is pivotal to decreased production of ROS (primarily that of 1O2) in the chloroplast during environmental stresses (Szarka et al. 2012). Recently, it has been found that oxidative stress activates the expression of genes responsible for the synthesis of tocopherols in higher plants (Wu et al. 2007). Increased levels of α-tocopherol and ASC have been found in tomato and in A. doliolum, helping to protect membranes from oxidative damage (Hsu and Kao 2007).
α-Tocopherol can scavenge 1O2 in two ways. First, α-tocopherol quenches 1O2 physically via resonance energy transfer (Fahrenholtz et al. 1974); following this, 1O2 scavenging can happen through a chemical reaction (Falk and Munné-Bosch 2010). During the resonance energy transfer mechanism of 1O2 scavenging, α-tocopherol content is not altered significantly and one molecule of α-tocopherol can deactivate 120 molecules of 1O2, whereas the chemical scavenging mechanism implies involvement of an intermediate hydroperoxydienone that decomposes to form tocopherol quinone and tocopherol quinone epoxides, resulting in a significant decline in the α-tocopherol content (Munne-Bosch and Alegre 2002). A comparison between a halophyte (Cakile maritima) and a glycophyte (Arabidopsis thaliana) showed that C. maritima may detoxify salt-induced 1O2 production through direct quenching, because the α-tocopherol level did not change significantly during salt stress. At the same time, A. thaliana achieved the same result through a chemical reaction, showing a reduction of 50% in the production of α-tocopherol (Ellouzi et al. 2011). α-Tocopherols also function as recyclable chain reaction terminators of polyunsaturated fatty acid (PUFA) radicals generated by lipid oxidation (Hare et al. 1998). α-Tocopherols scavenge lipid peroxy radicals and yield a tocopheroxyl radical that can be recycled back into the corresponding α-tocopherol by reacting with ascorbate or other antioxidants (Igamberdiev and Hill 2004).
8.3 Targeting Redox Regulation and Antioxidant Defense Systems in Breeding Programs
The number of papers linking oxidative stress with salinity has increased exponentially over the past two decades. There have also been reports of increased antioxidant activity in plants grown under saline conditions (Hernandez et al. 2000; Sairam and Srivastava 2002). It is hardly surprising that the idea of improving salinity stress tolerance by increased antioxidant production is gaining momentum (Table 8.1). However, many other reports have questioned the validity of this approach, reporting either no correlation or a negative correlation between the activity of antioxidant enzymes and plant salinity stress tolerance (Tables 8.2 and 8.3). The possible reasons for this discrepancy most likely lie in the fact that some ROS such as H2O2 play a very important signaling role in adaptive and developmental responses, and so tampering with them may result in pleiotropic effects (De Pinto et al. 2006). It is becoming increasingly evident that considerable variations exist in the production of both enzymatic and nonenzymatic antioxidants in response to salt stress in various plant tissues and at various time points. Hence, the interspecific or intraspecific aspects of ROS production and scavenging should be taken into account. Last, but not least, the diversity of known antioxidants should be accounted for. Some supporting arguments are given below.
8.3.1 Antioxidant Activity at the Tissue/Organ Level
Activation of the antioxidant defense system occurs via enzymatic and nonenzymatic mechanisms against salt stress. The antioxidant responses, though, are different in different organs and/or tissues (Turan and Tripathy 2013). Hamada et al. (2016) found that the total antioxidant capacity and polyphenol content in maize increased with increased salinity levels in roots and mature leaves but showed no changes in young leaves. They also showed that SOD, APX, glutathione (GSH), glutathione S-transferase (GST), and ASC content increased particularly in maize roots, while total tocopherol levels increased specifically in shoot tissues. Proline content was slightly decreased in young leaves in maize plants but did not show significant changes in maize roots and mature leaves under exposure to salinity stress (Hamada et al. 2016). Other studies have also reported tissue-specific responses of some other antioxidants. For example, ASC and tocopherol content has been shown to increase in the leaves of tomato to protect them against oxidative stress under high salinity (Salama et al. 1994; Tuna 2014); however, the levels of ASC and tocopherols declined in rice leaves under salinity stress (Turan and Tripathy 2013). There was no significant change in proline content in Sorghum bicolor leaves under salinity stress, but proline content in Sorghum sudanense decreased slightly with salinity (De Oliveira et al. 2013). Redox changes estimated by the ratios of redox couples (ASC:total ascorbate and GSH:total glutathione) showed significant decreases in maize roots (Hamada et al. 2016). Tocopherol, on the other hand, appears to be a more shoot-specific antioxidant in maize seedlings (Hamada et al. 2016). In lentil, root tissues were less affected by salt stress and had higher activity of Cu/ZnSOD, APX, and GR in roots than in shoots (Bandeoğlu et al. 2004). In common bean (Phaseolus vulgaris), salinity stress led to reductions in the activity of SOD and APX in nodules but not in the bulk of the roots (Jebara et al. 2005). These conflicting results could be due to plant/tissue specificity. Moreover, plant age–related differences in antioxidant responses in plant cells, tissues, and organs could also explain the reason behind the interspecific or intraspecific aspects of ROS production and antioxidant activity. For instance, mature maize leaf cells (in the distal leaf parts) are more sensitive to high salinity than younger cells (from actively expanding leaf parts), which have higher antioxidant activity (Kravchik and Bernstein 2013). These studies have indicated possible roles of ROS in the systemic signaling from roots to leaves and activation of antioxidants for better protection against oxidative stress and/or salt stress.
8.3.2 Antioxidant Activity at the Organelle Level
Besides tissue-specific and/or organ-specific responses, organelle-specific responses of antioxidants under salt stress have been observed. The activity of different antioxidants within different compartments of a cell, tissue, or organ plays a role in protection against oxidative stress caused by salt stress. Hernandez et al. (2000) showed that salt stress tolerance in pea was associated with high SOD activity in the apoplast and high activity of dehydroascorbate reductase (DHAR), GR, and monodehydroascorbate reductase (MDHAR) in the symplast. Moreover, Mittova et al. (2004) showed that the mitochondria and peroxisomes of salt-treated roots of wild tomato had increased levels of lipid peroxidation and H2O2, coupled with decreased activity of SOD, POD, ASC, and GSH, suggesting that improved endogenous production of antioxidants in the mitochondria and/or peroxisome could improve salt stress tolerance. In another study, higher activity of SOD, APX, and GR was noted in the chloroplastic fraction as compared with the mitochondrial fraction and cytosolic fraction (Sairam and Srivastava 2002).
8.3.3 Antioxidant Activity at Different Time Points
Antioxidant activity also shows a pronounced time dependence and thus may be different at various time points. Mhadhbi et al. (2011) reported increased activity of CAT, SOD, and POD in salt-treated Medicago truncatula roots at 24 h; however, this activity was lost after 48 h of salt stress. In pea, SOD activity increased after 48 h of salt stress, while GR showed an increase after 24 h of salt stress Hernandez et al. (2000). These authors also found no change in APX during salt stress at any time point. Shalata et al. (2001) showed that under long-term salt stress conditions, the activity of SOD, APX, CAT, and MDHAR was increased and reached a maximum 16 days after the beginning of salinization, and then decreased, while GR activity decreased from the start of salt stress. All of these studies reported highly varied responses and production of antioxidants at various time points under salt stress, and suggested that the variations could have been due to differences in experimental conditions, data collection, and plant species.
8.3.4 Targeting Quantitative Trait Loci for Antioxidant Activity
The practical aspect of targeting antioxidant activity in breeding programs should also be considered. Jiang et al. (2013) conducted quantitative trait locus (QTL) analysis of the activity of antioxidant enzymes and malondialdehyde content in wheat seeds during germination. These authors discovered 22 unconditional QTLs on 1A, 1B, 1D, 2B, 2D, 3A, 4A, 6B, 7A, and 7B, scattered on nine chromosomes. Eight of these QTLs were for SOD activity, five for POD, and six for CAT. In rice, two QTLs for malondialdehyde (MDA) content in rice leaves were detected on chromosome 1, with additive effects from maternal and paternal parents accounting for 4.33% and 4.62% of phenotype variations, respectively. Rousseaux et al. (2005) found 20 QTLs in tomato; of these, five were for total antioxidant activity, five for ASC, and nine for total phenolics. Frary et al. (2010) carried out QTL analysis and found nine QTLs for phenolic compounds and 14 for flavonoids in tomato. Given these numbers, it will be very difficult—if it is at all possible—to make a valid recommendation to breeders as to which of these QTLs play bigger role(s) and thus should be transferred into high-yielding varieties to increase their stress tolerance.
It has also been argued (Bose et al. 2014) that truly salt-tolerant plants do not allow formation of harmful ROS in the first instance and, as such, require no higher antioxidant activity. This can be achieved by increasing the rate of sodium exclusion from the cytosol, either into vacuoles (via mechanisms including NHX tonoplast Na+/H+ exchange) (Blumwald 2000) or into the apoplast (via SOS1-mediated Na+ exclusion from the cell) (Shi et al. 2000). Halophytes show high levels of salt stress tolerance and possess high levels of antioxidant production at an intrinsic level. As described in Sects. 8.1 and 8.2.1, SOD rapidly converts O2 – to H2O2 at the initial level of salt stress and the latter plays an important role as a second messenger, triggering cascades of different adaptive responses at the genetic and physiological levels; thus, rapid conversion of O2 – to H2O2 could be essential for early defense in halophytes. Nonetheless, it still remains to be established how the stress-induced increase in H2O2 production is ultimately converted into plant adaptive responses (Miller et al. 2010). Because of similarities between Ca2+- and H2O2-induced signatures, the roles of other enzymatic antioxidants may be attributed to the need to decrease the basal levels of H2O2 once the signaling has been processed. In this context, the roles of CAT and APX in shaping the H2O2 signature may be similar to those in Ca2+ efflux systems (Bose et al. 2011).
8.4 Concluding Remarks
ROS are produced as a result of a large number of metabolic processes, occurring at both intracellular and extracellular locations. Plants possess different enzymatic and nonenzymatic antioxidants to scavenge ROS, regarded as redox regulatory mechanisms in plants. Different antioxidants have different ROS-scavenging effects (Fig. 8.1), and their effects are highly tissue specific and organelle specific. A significant variability exists among different plant species and/or among different genotypes of the same plant species in terms of the kinetics of antioxidant production and activity. Different reports have described controversial results regarding increases or decreases in the activity of different antioxidants in response to salinity. In the light of this, it appears not to be highly fruitful to try and improve plant stress tolerance by increasing the activity of some specific antioxidants via either genetic engineering or a MAS-based approach, without accounting for the tissue and time dependence of this process. There is a need for better understanding of the role of ROS as signaling components of plant adaptive mechanisms, and development of tools for targeted regulation of plant redox systems in specific cellular compartments and tissues.
Abbreviations
- 1O2 :
-
Singlet oxygen
- AKR1:
-
NADPH-dependent aldo-ketoreductase
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbic acid
- BADH:
-
Betaine aldehyde dehydrogenase
- CAT:
-
Catalase
- codA:
-
Choline dehydrogenase gene
- DHA:
-
Dehydroascorbic acid
- DHAR:
-
Dehydroascorbate reductase
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- GST:
-
Glutathione S-transferase
- L-ASC:
-
L-Ascorbic acid
- MAPK 1:
-
Mitogen-activated protein kinase phosphatase
- MDA:
-
Malondialdehyde
- MDHA:
-
Monodehydroascorbic acid
- MDHAR:
-
Monodehydroascorbate reductase
- MeOOH:
-
Methyl hydrogen peroxide
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
- NADPH:
-
Reduced NADP
- O2 − :
-
Superoxide radical
- OH− :
-
Hydroxyl radical
- Orn-δ-OAT:
-
Ornithine-δ-aminotransferase
- P5CR:
-
Pyrroline-5-carboxylate reductase
- P5CS:
-
Pyrroline-5-carboxylate synthase
- POD:
-
Peroxidase
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- PUFA:
-
Polyunsaturated fatty acid
- QTL:
-
Quantitative trait locus
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Agarwal S, Pandey V (2004) Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol Plant 48:555–560
Ahanger MA, Agarwal RM (2017) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460
Ali AA, Alqurainy F (2006) Activities of antioxidants in plants under environmental stress. In: Motohasci A (ed) The lutein-prevention and treatment for diseases. Transworld Research Network, New Delhi, pp 187–256
Alia P, Saradhi P (1991) Proline accumulation under heavy metal stress. J Plant Physiol 138:554–558
Anjum SA, Tanveer M et al (2015) Cadmium toxicity in maize: consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22:17022–17030
Anjum SA, Tanveer M, Hussain S et al (2016a) Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ Sci Pollut Res 23:11864–11875
Anjum SA, Tanveer M et al (2016b) Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ Sci Pollut Res 23:17132–17141
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Abbas F, Saleem MF, Ali I, Wang LC (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci 8:69. https://doi.org/10.3389/fpls.2017.00069
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.) Environ Exp Bot 63:266–273
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Athar HR, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot 63:224–231
Azevedo RA, Alas RM, Smith RJ, Lea PA (1998) Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in leaves and roots of wild-type and catalase-deficient mutant of barley. Physiol Plant 104:280–292
Azpilicueta CE, Benavides MP, Tomaro ML, Gallego SM (2007) Mechanism of CATA3 induction by cadmium in sunflower leaves. Plant Physiol Biochem 45:589–595
Bandeoğlu E, Eyidoğan F, Yücel M, Öktem HA (2004) Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul 42:69–77
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Bose J, Pottosin I, Shabala SS, Palmgren MG, Shabala S (2011) Calcium efflux systems in stress signaling and adaptation in plants. Front Plant Sci 2:1–17
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Bowler C, Fluhr R (2000) The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci 5:241–246
Chen C, Dickman MB (2005) Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. PNAS 102:3459–3464
Chutipaijit S, Cha-Um S, Sompornpailin K (2009) Differential accumulations of proline and flavonoids in Indica rice varieties against salinity. Pak J Bot 41:2497–2506
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
Cuin TA, Shabala S (2007) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30:875–885
Daneshmand F, Arvin MJ, Kalantari KM (2010) Physiological responses to NaCl stress in three wild species of potato in vitro. Acta Physiol Plant 32:91–101
de Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
de Oliveira VP, Marques EC, de Lacerda CF, Prisco JT, Gomes Filho E (2013) Physiological and biochemical characteristics of Sorghum bicolor and Sorghum sudanense subjected to salt stress in two stages of development. Afr J Agric Res 8:660–670
De Pinto MC, Paradiso A, Leonetti P, De Gara L (2006) Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J 48:784–795
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Demiral T, Türkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Desingh R, Kanagaraj G (2007) Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. Gen Appl Plant Physiol 33:221–234
Di Martino C, Delfine S, Pizzuto R, Loreto F, Fuggi A (2003) Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol 158:455–463
Ellouzi H, Ben Hamed K, Cela J, Munne-Bosch S, Abdelly C (2011) Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol Plant 142:128–143
Eyidogan F, Oz MT (2005) Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol Plant 29:485–493
Fahrenholtz S, Doleiden F, Trozzolo A, Lamola A (1974) On the quenching of singlet oxygen by α-tocopherol. Photochem Photobiol 20:505–509
Falk J, Munné-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61:1549–1566
Fitzgerald TL, Waters DL, Henry RJ (2009) Betaine aldehyde dehydrogenase in plants. Plant Biol 11(2):119–30
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422(6930):442–446
Foyer CH, Noctor G (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Frary A, Göl D, Keleş D, Ökmen B, Pınar H et al (2010) Salt tolerance in Solanum pennellii: antioxidant response and related QTL. BMC Plant Biol 10:58–73
Frugoli JA, Zhong HH, Nuccio ML, McCourt P, McPeek MZ, Thomas TL, McClung CR (1996) Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Plant Physiol 112:327–336
Fukuzawa K, Tokumura A, Ouchi S, Tsukatani H (1982) Antioxidant activities of tocopherols on Fe2+-ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids 17:511–513
Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K (2000) Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys 376:1–13
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:8–96
Gengmao Z, Yu H, Xing S, Shihui L, Quanmei S, Changhai W (2015) Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crop Prod 64:175–181
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gossett DR, Millhollon EP, Lucas M (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Grun S, Lindermayr C, Sell S (2006) Nitric oxide and gene regulation in plants. J Exp Bot 57:507–516
Hamada AbdElgawad GZ, Hegab MM, Pandey R, Asard H, Abuelsoud W (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276–287
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553
Hernandez JA, Jimerez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum) to long term salt stress is associated with induction of antioxidant defences. Plant Cell Biol 23:853–862
Hernandez I, Chacón O, Rodriguez R, Portieles R, López Y, Pujol M, Borrás-Hidalgo O (2009) Black shank resistant tobacco by silencing of glutathione S-transferase. Biochem Biophys Res Commun 387:300–304
Hichem H, Mounir D (2009) Differential responses of two maize (Zea mays L.) varieties to salt stress: changes on polyphenols composition of foliage and oxidative damages. Ind Crop Prod 30:144–151
Hsu YT, Kao CH (2007) Heat shock-mediated H2O2 accumulation and protection against Cd toxicity in rice seedlings. Plant Soil 300:137–147
Hu CA, Delauney AJ, Verma DPS (1992) A bifunctional D1-enzymepyrroline-5-carboxylate synthetase catalyzes the first two steps in proline biosynthesis in plants. PNAS 89:9354–9358
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Ismail H, Maksimović JD, Maksimović V, Shabala L, Živanović BD, Tian Y, Jacobsen S, Shabala S (2016) Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct Plant Biol 43(1):75–86
Jaleel CA, Gopi R, Manivannan P, Panneerselvam R (2007a) Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta Physiol Plant 29:205–209
Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekumar A, Sridharan R, Panneerselvam R (2007b) Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. S Afr J Bot 73:190–195
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936
Jiang P, Wan Z, Wang Z, Li S, Sun Q (2013) Dynamic QTL analysis for activity of antioxidant enzymes and malondialdehyde content in wheat seed during germination. Euphytica 190:75–85
Jiménez A, Hernández JA, Pastori G, del Rıo LA, Sevilla F (1998) Role of the ascorbate–glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol 118:1327–1335
Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS (2005) Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett 579:1243–1248
Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89
Koca H, Bor M, Özdemir F, Türkan I (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Kravchik M, Bernstein N (2013) Effects of salinity on the transcriptome of growing maize leaf cells point at cell-age specificity in the involvement of the antioxidative response in cell growth restriction. BMC Genomics 14:24. https://doi.org/10.1186/1471-2164-14-24
Kukreja S, Nandval AS, Kumar N, Sharma SK, Sharma SK, Unvi V, Sharma PK (2005) Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant 49:305–308
Kumar S, Dhingra A, Daniell H (2004) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol 136:2843–2854
Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD, Kwak SS (2002) Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen mediated oxidative stress. Plant Cell Environ 25:873–882
Lee DH, Kim YS, Lee CB (2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.) J Plant Physiol 158:737–745
Leisinger U, Rüfenacht K, Fischer B, Pesaro M, Spengler A, Zehnder AJB, Eggen RIL (2001) The glutathione peroxidase homologous gene from Chlamydomonas reinhardtii is transcriptionally up-regulated by singlet oxygen. Plant Mol Biol 46:395–408
Light GG, Mahan JR, Roxas VP, Allen RD (2005) Transgenic cotton (Gossypium hirsutum L.) seedlings expressing a tobacco glutathione S-transferase fail to provide improved stress tolerance. Planta 222:346–354
Link G (2003) Redox regulation of chloroplast transcription. Antioxid Redox Signal 5:79–87
Liu CG, Wang QW, Jin YQ, Pan KW, Wang YJ (2017) Photoprotective and antioxidative mechanisms against oxidative damage in Fargesia rufa subjected to drought and salinity. Funct Plant Biol 44:302–311
López-Gómez M, Hidalgo-Castellanos J, Muñoz-Sánchez JR, Marín-Peña AJ, Lluch C, Herrera-Cervera JA (2017) Polyamines contribute to salinity tolerance in the symbiosis Medicago truncatula–Sinorhizobium meliloti by preventing oxidative damage. Plant Physiol Biochem 116:9–17
Løvdal T, Olsen KM, Slimestad R, Verheul M, Lillo C (2010) Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 71:605–613
Lu J, Holmgren A (2014) The thioredoxin antioxidant system. Free Radic Biol Med 66:75–87
Lutts S, Kinet JM, Bouharmont J (1996) Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regul 19:207–218
Mandhania S, Madan S, Sawhney V (2006) Antioxidant defense mechanism under salt stress in wheat seedlings. Biol Plant 50:227–231
Meloni DA, Gulotta MR, Martínez CA, Oliva MA (2004) The effects of salt stress on growth, nitrate reduction and proline and glycine betaine accumulation in Prosopis alba. Braz J Plant Physiol 16:39–46
Meyer AJ (2008) The integration of glutathione homeostasis and redox signaling. J Plant Physiol 165:1390–1403
Mhadhbi H, Fotopoulos V, Mylona PV, Jebara M, Elarbi Aouani M, Polidoros AN (2011) Antioxidant gene–enzyme responses in Medicago truncatula genotypes with different degree of sensitivity to salinity. Physiol Plant 141:201–214
Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH (2003) Control of ascorbate synthesis by respiration and its implication for stress responses. Plant Physiol 133:443–447
Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler RON (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Misra N, Gupta AK (2005) Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Sci 169:331–339
Mittler R, Zilinskas BA (1992) Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem 267:21802–21807
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55:1105–1113
Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK (2002) Transgenics of an elite Indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor Appl Genet 106:51–57
Munne-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Munns R, James RA, Launchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Nagamiya K, Motohasci T, Nakao K, Prodhan SH, Hattori E, Hirose S, Ozawa K, Ohkawa Y, Takabe T, Takabe T, Komamine A (2007) Enhancement of salt tolerance in transgenic rice expressing an Escherichia coli catalase gene, katE. Plant Biotechnol Rep 1:49–55
Noctor G, Foyer CH (1998) A re-evaluation of the ATP:NADPH budget during C3 photosynthesis. A contribution from nitrate assimilation and its associated respiratory activity? J Exp Bot 49:1895–1908
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation, and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Noreen Z, Ashraf M (2009) Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol 166:1764–1774
op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49:157–165
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem-II complex. Photosynth Res 44:243–252
Pedreira J, Sanz N, Pena MJ, Sanchez M, Queijeiro E, Revilla G, Zarra I (2004) Role of apoplastic ascorbate and hydrogen peroxide in the control of cell growth in pine hypocotyls. Plant Cell Physiol 45:530–534
Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Hydrogen peroxide-activated Ca2+ channels mediate guard cell abscisic acid signaling. Nature 406:731–734
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Roosens NH, Thu TT, Iskandar HM, Jacobs M (1998) Isolation of the ornithine-δ-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol 117:263–271
Rouhier N, Vlamis-Gardicas A, Lilling CH (2003) Characerization of redox properties of poplar glutaredoxin. Antioxid Redox Signal 5:15–22
Rousseaux MC, Jones CM, Adams D, Chetelat R, Bennett A, Powell A (2005) QTL analysis of fruit antioxidants in tomato using Lycopersicon pennellii introgression lines. Theor Appl Genet 111:1396–1408
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16:616–628
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Salama S, Trivedi S, Busheva M, Arafa AA, Garab G, Erdei L (1994) Effects of NaCl salinity on growth, cation accumulation, chloroplast structure and function in wheat cultivars differing in salt tolerance. J Plant Physiol 144:241–247
Scandalias JG (1990) Response of plant antioxidant defense genes to environmental stress. Adv Genet 28:1–41
Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828
Schurmann P (2003) Redox signaling in the chloroplast: the ferredoxin/thioredoxin system. Antioxid Redox Signal 5:69–78
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plant 112:487–494
Shao HB, Chu LY (2005) Plant molecular biology in China: opportunities and challenges. Plant Mol Biol Report 23:345–358
Shao HB, Jiang SY, Li FM, Chu LY, Zhao CX, Shao MA, Zhao XN, Li F (2007a) Some advances in plant stress physiology and their implications in the systems biology era. Biointerphases 54:33–36
Shao HB, Guo QJ, Chu LY et al (2007b) Understanding molecular mechanism of higher plant plasticity under abiotic stress. Biointerphases 54:37–45
Shi HZ, Ishitani M, Kim CS, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. PNAS 97:6896–6901
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. PNAS 101:8827–8832
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28(4):1057–1060
Smirnoff N (2005) Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Smirnoff N (ed) Antioxidants and reactive oxygen species in plants. Blackwell, Oxford, pp 53–86
Souza ER, Freire MBGS, Cunha KPV, Nascimento CWA, Ruiz HA et al (2012) Biomass, anatomical changes and osmotic potential in Atriplex nummularia Lindl. cultivated in sodic saline soil under water stress. Environ Exp Bot 82:20–27
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol Plant 109:435–442
Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in antioxidative defense system of Anabaena doliolum. Microb Biotechnol 22:1291–1298
Subbarao GV, Nam NH, Chauhan YS, Johansen C (2000) Osmotic adjustment, water relations and carbohydrate remobilization in pigeon pea under water deficits. J Plant Physiol 157:651–659
Sunkar R, Bartels D, Kirch HH (2003) Overexpression of a stress inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35:452–464
Suzuki N, Koussevitzky S, Mittler RON, Miller GAD (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Szarka A, Tomasskovics B, Bánhegyi G (2012) The ascorbate–glutathione–α-tocopherol triad in abiotic stress response. Int J Mol Sci 13:4458–4483
Tanaka Y, Hibin T, HayASCi Y, Tanaka A, Kishitani S, Takabe T, Yokota S, Takabe T (1999) Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci 148:131–138
Tanveer M, Shah AN (2017) An insight into salt stress tolerance mechanisms of Chenopodium album. Environ Sci Pollut Res 24:16531–16535
Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rend Lincei 19:325–346
Tuna AL (2014) Influence of foliarly applied different triazole compounds on growth, nutrition, and antioxidant enzyme activities in tomato (‘Solanum lycopersicum’ L.) under salt stress. Aust J Crop Sci 8:71–79
Turan S, Tripathy BC (2013) Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma 250:209–222
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)—differential response in salt-tolerant and sensitive varieties. Plant Sci 165:1411–1418
Vemanna RS, Babitha KC, Solanki JK, Reddy VA, Sarangi SK, Udayakumar M (2017) Aldo-keto reductase-1 (AKR1) protect cellular enzymes from salt stress by detoxifying reactive cytotoxic compounds. Plant Physiol Biochem 113:177–186
Wang P, Song CP (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178:703–718
Wang Y, Ying Y, Chen J, Wang X (2004) Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci 167:671–677
Wang Y, Wisniewski M, Meilan R, Cui M, Webb R, Fuchigami L (2005) Overexpression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. J Am Soc Hortic Sci 130:167–173
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Wu YS, Tang KX (2004) MAP kinase cascades responding to environmental stress in plants. Acta Bot Sin 46:127–136
Wu G, Wei ZK, Shao HB (2007) The mutual responses of higher plants to environment: physiological and microbiological aspects. Biointerphases 59:113–119
Yan H, Li Q, Park SC, Wang X, Liu YJ, Zhang YG et al (2016) Overexpression of CuZnSOD and APX enhance salt stress tolerance in sweet potato. Plant Physiol Biochem 109:20–27
Zaidi I, Ebel C, Belgaroui N, Ghorbel M, Amara I, Hanin M (2016) The wheat MAP kinase phosphatase 1 alleviates salt stress and increases antioxidant activities in Arabidopsis. J Plant Physiol 193:12–21
Zhang W, Wang C, Qin C, Wood T, Olafsdottir G, Welti R, Wang X (2003) The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 15:2285–2295
Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in Thellungiella and Arabidopsis callus. Plant Cell Tissue Org Cult 98:97–103
Acknowledgements
This work was supported by the Australian Research Council and Qatar National Science Foundation (NPRP-8-126-1-024) grants to Sergey Shabala.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Tanveer, M., Shabala, S. (2018). Targeting Redox Regulatory Mechanisms for Salinity Stress Tolerance in Crops. In: Kumar, V., Wani, S., Suprasanna, P., Tran, LS. (eds) Salinity Responses and Tolerance in Plants, Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-319-75671-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-75671-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75670-7
Online ISBN: 978-3-319-75671-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)