Abstract
Myopathies and muscular dystrophies are disease inherent to muscles and have the common feature of muscle weakness. Multiple sleep disorders are associated with them. Sleep disordered breathing disorder (SRBD) is the most common sleep disorder in both myopathies and muscular dystrophies. Various different factors contributed to the development of SRBD that include diaphragmatic involvement, coexisting cardiac abnormalities, reduction in lung volumes and capacities, and airway obstruction. Other sleep disorders in these conditions include insomnia, restless legs syndrome, hypersomnolence, and parasomnias.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Muscular dystrophy
- Cardiomyopathy
- Emery-Dreifuss muscular dystrophy
- Limb-girdle muscular dystrophy
- Hypersomnia
- Fascioscapulohumeral muscular dystrophy
- Sleep-related movement disorders
Introduction
Sleep disorders are frequent in patients with neuromuscular disorders but they are poorly recognized. Even with recognition of these disorders, they are sometimes wrongly attributed to be a consequence of their underlying neuromuscular diseases. With advances in sleep medicine and relatively more patients undergoing polysomnograms (PSG), the knowledge about sleep disorders in this patient population is increasing. Also, early recognition and treatment of sleep disorders have shown to improve not only the quality of life but also the life expectancy in some of these patients.
Common sleep-related complaints are fragmented sleep, breathing difficulty during sleep, abnormal limb movements during sleep, insomnia, muscle pain, early morning headaches, daytime sleepiness, etc. [1].
Common Sleep Disorders in Neuromuscular Disorders
-
1.
Sleep-related breathing disorders
-
2.
Insomnia
-
3.
Sleep-related movement disorders
-
4.
Circadian rhythm sleep-wake disorders
-
5.
Parasomnias
Sleep Disordered Breathing in Myopathies and Muscular Dystrophies
Sleep disordered breathing (SDB) are a group of disorders that occur as a result of abnormal breathing pattern during sleep. According to International Classification of Sleep Disorders (ICSD-3), these include obstructive sleep apnea syndromes, central sleep apnea syndromes, sleep-related hypoventilation disorders, and sleep-related hypoxemia disorders. The prevalence of obstructive sleep apnea (OSA) (defined as respiratory disturbance index (RDI) or apnea-hypopnea index (AHI) >5) in healthy adult population was found to be 13% in men and 6% in women [2].
The estimated prevalence of SDB in neuromuscular diseases is about 36–53% in adults [3]. The exact prevalence of SDB in myopathies and muscular dystrophies is not known. However, it is to be noted that this study [3] included patients with Duchenne’s muscular dystrophy , myotonic dystrophy along with other congenital and metabolic myopathies. In patients with muscle disease who have SDB, the presence of SDB itself could increase their morbidity and mortality. SDB could be an earlier manifestation in these patients, who eventually may develop respiratory failure [3, 4].
Normal Breathing Pattern During Sleep
Breathing normally varies significantly between wakefulness and sleep, and between supine and upright postures as well. In supine position, there is a mild reduction in inspiratory and expiratory reserve volumes and hence a reduction in the vital capacity and total lung capacity is seen.
During NREM sleep, there is a reduction in respiratory rate, tidal volume, pharyngeal dilator muscle activity, and ventilator drive of wakefulness, resulting in slight increase in the partial pressure of carbon dioxide in blood. In REM sleep, muscle atonia develops. Even though, ventilation is decreased further, due to varied involvement of muscle groups in REM (the pharyngeal and intercostal muscles are affected the most and the diaphragmatic muscles are affected the least), in a normal individual, the only muscle which is still able to maintain breathing during REM sleep is diaphragm. Hence, the role of intercostal muscles in NREM and diaphragm in REM are vital [5, 6].
Mechanisms of SDB in Patients with Muscle Disease
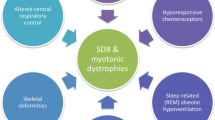
Normal functioning of respiratory muscles and diaphragm is essential to carry out breathing during sleep. Their structural integrity and functions are especially important in supine position and in REM sleep. In myopathies and muscular dystrophies, involvement of these muscles and diaphragm makes the patient susceptible for SDB. Reduction in lung volumes and capacities, diaphragmatic weakness, coexisting cardiac abnormalities, and mechanical airway obstruction could be some of the mechanisms that would contribute to the sleep disordered breathing [Fig. 4.1].
Reduction in Lung Volumes and Capacities
Restrictive ventilatory defect in pulmonary function testing develops once respiratory muscles of the chest wall are involved in the muscle disorders. When compared to normal individuals, these patients have significantly lower inspiratory and expiratory reserve volumes and slightly higher residual volumes. These in turn cause reduction in VC, FRC, and TLC. The lung capacities are much lower in supine position during sleep [6, 7].
The decreased lung capacities are also associated with decrease in pharyngeal airway cross section, due to fall in caudal traction force, further increasing the risk of obstructive sleep apnea [8, 9]. Thus, there are severe nocturnal desaturations especially during REM sleep. The nocturnal pulse oximetry in these patients typically demonstrates a “saw-tooth” pattern. The final result is hypoventilation that leads to chronic hypercarbic respiratory failure [10]. The importance of vital capacity as an independent marker to assess disease progression has already been emphasized in patients with DMD and acid maltase deficiency [4, 15].
Involvement of the Diaphragm
Patients with muscle disease can present with diaphragmatic weakness or paralysis. Clinically, this can cause exertional dyspnea, orthopnea, paradoxical breathing, and recurrent pneumonias [11, 12]. With more understanding of the role of diaphragm in sleep, the sleep pattern in these conditions has been better studied in recent times. These patients tend to have disrupted nocturnal sleep, daytime somnolence, and fatigue. Diaphragmatic weakness has been reported in patients with rigid spine muscular dystrophy [13, 14]. In a study that included 27 subjects with acid maltase deficiency, 13 were found to have diaphragmatic weakness and 12 out of these 13 patients were diagnosed to have SDB in polysomnogram [15]. Diaphragmatic weakness can be a consequence of critical illness myopathy and inflammatory myopathies [16] as well and can pose difficulty in ventilator weaning [17].
Coexisting Cardiac Abnormalities
Cardiac abnormalities are frequently seen in some types of myopathies and muscular dystrophies and contribute significantly towards the mortality in these conditions. Some examples include Emery-Dreifuss muscular dystrophy [18], certain types of Limb-Girdle muscular dystrophy (types 1B, 1D, 2A, 2C, 2E, and 2F), Duchenne’s and Becker’s muscular dystrophies [19, 20], myotonic dystrophy [21], mitochondriopathies [22], nemaline myopathy [23], and congenital fiber type disproportion [24]. Cardiac manifestations include conduction abnormalities, systolic and diastolic dysfunctions, and intra-cardiac thrombus formation. Cheyne-Stokes breathing can be a consequence of cardiomyopathy in these patients and may be a poor prognostic factor [25].
Anatomical Airway Obstruction
SDB in patients with muscle diseases are usually related to hypoventilation [5]. However, it is also possible to see obstructive sleep pattern in some of these patients. Macroglossia and fibro-fatty replacement of tongue muscles have been reported in patients with acid maltase deficiency (Pompe’s disease) leading to obstruction in the airway and OSA [26]. In Duchenne’s muscular dystrophy, patients were found to have a bimodal pattern [5] with the increased presence of OSA, at least in the first decade and hypoventilation later on [27,28,29]. SDB can also occur in both types of myotonic dystrophy with OSA being more common in type 2 (DM2) and central sleep apnea in type 1(DM1) [30, 31].
Insomnia in Myopathies and Muscular Dystrophies
Insomnia is defined as continuing difficulty initiating or maintaining sleep despite enough chances and conditions to sleep, thereby affecting daytime functioning (ICSD-3). In patients with muscle disease, it is not uncommon to see insomnia. The frequent reasons of insomnia in these conditions are muscle pain, hypoventilation/hypercapnia, and also the side effects from medication used to treat these conditions and are listed in Fig. 4.2 below.
Pain is commonly reported in patients with muscle diseases, especially in Duchenne’s muscular dystrophy, fascioscapulohumeral muscular dystrophy (FSHD) , myotonic dystrophy, and inflammatory myopathies. It is well known that chronic pain/fatigue can cause insomnia and disrupted nocturnal sleep. This has been previously documented in patients with cancer, fibromyalgia, traumatic brain injury, and arthritis [32,33,34,35,36]. Likewise, patients with muscle diseases and muscle pain are also prone to have insomnia. In a case series of four patients with FSHD, all of them were found to have prominent muscle pain in the absence of significant muscle weakness. The muscle pain was severe enough to cause both sleep onset and maintenance insomnia in these patients. In another study which included 55 patients with FSHD, 42 had chronic pain with higher incidence of alpha delta waves during sleep (this alpha intrusion during N3 sleep has been linked with non-restorative sleep) and a reduction in N3 sleep [36, 37].
Insomnia has also been reported in patients with long standing Duchenne’s muscular dystrophy who were referred for non-invasive ventilation. Insomnia was thought to be a consequence of chronic hypoventilation and hypercapnia in these patients though other unknown mechanisms are likely to contribute as well [38].
Medicines that are used in the treatment of the muscle conditions can contribute to insomnia. A 6-month prednisone trial in patients with Duchenne’s muscular dystrophy, which included 103 patients, showed that these patients had insomnia along with other behavioral symptoms like depression, irritability, and hyperactivity. Sleep-related symptoms were present in 48% of patients who were on prednisone, daily dose of 0.75 mg/kg but increased to 64%, with 1.5 mg/kg dose [39]. This indicates that insomnia could be a dose-dependent side effect of prednisone.
Sleep-Related Movement Disorders
These are disorders in which sleep disturbance is a consequence of simple commonly stereotypical movements that occur either during sleep onset or maintenance. Unlike parasomnias, these disorders do not present with abnormal complex behaviors like eating, walking, dream enactment, etc.
ICSD-3 includes restless legs syndrome (RLS), periodic limb movement disorder (PLMD), sleep-related leg cramps, bruxism, rhythmic movement disorder, and sleep-related movement disorders due to a medical condition, medication/substance use under this category. Of these, RLS is the only disorder that has been reported in patients with muscle diseases specifically in myotonic dystrophy [40].
Restless legs syndrome, also known as Willis-Ekbom disease is a disorder characterized by an uncontrollable need to move the legs, with aggravation of symptoms towards the end of the day and during inactivity. Patients also report complete or limited improvement of symptoms with activity. As a result, the sleep cycle gets disrupted in about 90% of the patients (ICSD-3). In general, RLS can be idiopathic or secondary to some predisposing factors like iron deficiency anemia, pregnancy, chronic renal failure, and medications like antihistamines, dopamine antagonists, and antidepressants except bupropion.
A study done by Mayo clinic which included 30 genetically confirmed myotonic dystrophy type 2 participants(DM2) who were compared with 43 age and sex-matched controls, six patients were found to have RLS symptoms, and three of those had low ferritin levels [40]. Dopamine dysfunction in the striatum and substantia nigra has been linked to the pathophysiology of RLS. Iron being the cofactor for dopamine synthesis, deficiency in iron and ferritin and decreased levels of these in the CSF of these patients is a key factor responsible for causing RLS symptoms [41, 42]. Similarly, in patients with myotonic dystrophy, an RNA toxicity-related abnormal functioning of dopaminergic pathway involving the hypothalamic A11 nuclei is noted [40].
RLS is a clinical diagnosis made if the above-mentioned clinical criteria are met. PSG is not required to diagnose patients with RLS. But, a coexisting finding in PSG which could be noted in patients with RLS is the presence of PLMS and PLMW (periodic limb movements of sleep and wakefulness, respectively). This has been reported in DM2 patients also [40, 42].
RLS has also been reported in a patient with Duchenne’s muscular dystrophy who had history of analgesic abuse and chronic pain after taking amitriptyline for chronic pain [43].
Disorders of Central Hypersomnolence
This includes diseases that can present with excessive daytime sleepiness (EDS) and in which the sleepiness is not a consequence of disrupted nocturnal sleep or circadian abnormalities. The disorders included under this category are narcolepsy type 1 and 2, idiopathic hypersomnia , Kleine-Levin syndrome , insufficient sleep syndrome , hypersomnia secondary to medical or psychiatric illness, and hypersomnia secondary to medication or substance use (ICSD-3).
Patients with muscle diseases can have EDS secondary to various reasons like SDB, medication use, etc. But in certain group of patients like myotonic dystrophy, excessive sleepiness can occur in the absence of other factors. The prevalence of EDS could vary between 50% in childhood onset DM and about 70–80% in adult onset patients [44, 45]. A possible “central” etiology wherein structural abnormalities of the cerebral gray and white matter areas have been identified in these patients resulting in daytime sleepiness, cognitive abnormalities, and even depression. Loss of dorsal raphe serotonin producing neurons, which play a vital role in sleep-wake modulation, has also been described in neuropathological analysis. Even though EDS was also noted in DM 2 patients, it was less common than in DM 1. DM 2 patients had higher frequency of depression due to atrophy of limbic region [46].
A narcolepsy-like state with reduced CSF orexin levels was noted in these patients. Unlike narcolepsy with cataplexy, these patients were found to be negative for HLA-DQB1*0602, (a genetic association seen in almost all patients of narcolepsy with cataplexy when compared to general population) and instead patients with DM1 and EDS showed a higher incidence of HLA haplotype DRW6-DQW1 when compared to controls in few studies [47]. In PSG, there was evidence of decreased sleep onset latency and MSLT showed >2 sleep onset REM periods (SOREMPs ) which denotes the presence of REM within 15 min of sleep onset (ICSD-3). These findings which are typically seen in patients with narcolepsy were also found in these DM1 patients with narcolepsy-like state. They also showed good treatment response to modafinil [47].
Parasomnias
These are abnormal complex movements associated with behavioral changes, dream enactment, perceptions, and autonomic changes that occur either during sleep onset, maintenance, or during arousal. These disorders can result in physical injuries to the patients or their bed partners. These are classified as NREM and REM parasomnias. NREM parasomnias include confusional arousals, sleepwalking, sleep terrors, and sleep-related eating disorders. REM parasomnias include REM sleep behavior disorder, recurrent isolated sleep paralysis, and nightmare disorder. Figure 4.3 highlights the various different parasomnias.
Even though there are no reports of patients who had history suggestive of any parasomnias, few patients with myotonic dystrophy were found to have increased EMG activity during REM sleep suspicious for REM behavioral disorder. This finding in PSG during REM sleep is called REM sleep without atonia (RSWA) [45, 48].
Circadian Rhythm Disorders
These are disorders that occur due to mismatch between our internal circadian rhythm and outside environment resulting in difficulties with patient’s personal and social life. Under this category, some of the disorders included are delayed sleep-wake phase disorder, advanced sleep-wake phase disorder, irregular sleep-wake rhythm disorder, free running disorder or non-24 h sleep-wake rhythm disorder, jet lag disorder, and shift work disorder. To the best of our knowledge, so far there have been no reports of Circadian rhythm disorders in patients with myopathy and muscular dystrophy.
Conclusion
Having understood about the different types of sleep disorders that can occur in patients with muscular dystrophies and myopathies, special attention needs to be given to obtain sleep history in these patients. Appropriate investigations including polysomnograms, MSLT, or even referral to sleep clinics can be considered depending on what type of sleep disorder is suspected.
Key Points
-
1.
Breathing normally varies significantly between wakefulness and sleep, and between supine and upright postures as well.
-
2.
In a normal individual, the only muscle which is still able to maintain breathing during REM sleep is diaphragm.
-
3.
When compared to normal individuals, patients with muscle disease have significantly lower inspiratory and expiratory reserve volumes and slightly higher residual volumes. These in turn cause reduction in VC, FRC, and TLC.
-
4.
The decreased lung capacities are also associated with decrease in pharyngeal airway cross section, due to fall in caudal traction force, further increasing the risk of obstructive sleep apnea.
-
5.
The frequent reasons of insomnia in patients with muscular dystrophy and myopathy are muscle pain, hypoventilation/hypercapnia, and also the side effects from steroid use.
-
6.
A narcolepsy-like state with reduced CSF orexin levels was noted in myotonic dystrophy patients.
-
7.
Few patients with myotonic dystrophy were found to have increased EMG activity during REM sleep suspicious for REM behavioral disorder. This finding in PSG during REM sleep is called REM sleep without atonia (RSWA).
-
8.
In patients with myotonic dystrophy, an RNA toxicity-related abnormal functioning of dopaminergic pathway involving the hypothalamic A11 nuclei is noted, which might contribute to RLS symptoms.
-
9.
A possible “central” etiology wherein structural abnormalities of the cerebral gray and white matter areas have been identified in myotonic dystrophy patients resulting in daytime sleepiness, cognitive abnormalities, and even depression.
-
10.
Appropriate investigations including polysomnogram MSLT or even referral to sleep clinics can be considered depending on what type of sleep disorder is suspected.
References
Fermin AM, Afzal U, Culebras A. Sleep in neuromuscular diseases. Sleep Med Clin. 2016;11(1):53–64. https://doi.org/10.1016/j.jsmc.2015.10.005.Epub2016Jan9.
Peppard PE, Young T, Hla KM. Increased prevalence of sleep-disorderd breathing in adults. Am J Epidemiol. 2013;177(9):1006–14.
Arens R, Muzumdar H. Sleep, Sleep disordered breathing, and nocturnal hypoventilation in children with Neuromuscular diseases. Pediatr Respir Rev. 2010;11(1):24.
Phillips MF, Smith PE, Carroll N, Calverley PM. Nocturnal oxygenation and prognosis in Duchenne muscular dystrophy. Am J Respir Crit Care Med. 1999;160(1):198–202.
Aboussouan LS. Sleep-disordered breathing in neuromuscular disease. Am J Respir Crit Care Med. 2015;191(9):979–89.
Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol. 1984;57:1319–22.
Ibanez J, Raurich JM. Normal values of functional residual capacity in the sitting and supine positions. Intensive Care Med. 1982;8:173–7.
Appleberg J, Nordahl G, Janson C. Lung volume and its correlation to nocturnal apnoea and desaturation. Respir Med. 2000;94:233–9.
Van de Graff WB. Thoracic influence on upper airway patency. J Appl Physiol (1985). 1988;65:2124–31.
White JE, Drinnan MJ, Smithson AJ, Griffiths CJ, Gibson GJ. Respiratory muscle activity and oxygenation during sleep in patients with muscular weakness. Eur Respir J. 1989;2:26–30.
Smith PE, Edwards RH, Claverly PM. Mechanisms of sleep-disordered breathing in chronic neuromuscular disease: implications for management. Q J Med. 1991;81:961–73.
McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med. 2012;366:932–42.
Shahrizaila N, Kinnear WJM, Wills AJ. Respiratory involvement in inherited primary muscle conditions. J Neurol Neurosurg Psychiatry. 2006;77(10):1108–15.
Ferrerio A, Quijano-Roy S, Piche C. Mutations of the Selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet. 2002;71(4):739–49. https://doi.org/10.1086/342719.
Mellies U, Ragette R, Schwake C. Sleep disordered breathing and respiratory failure in acid maltase deficiency. Neurology. 2001;57(7):1290–5.
Teixeira A, Cherin P, Similowski T. Diaphragmatic dysfunction in patients with idiopathic inflammatory myopathies. Neuromuscul Disord. 2005;15(1):32–9. https://doi.org/10.1016/j.nmd.2004.09.006.
Latronico N, Bolton C. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–41. https://doi.org/10.1016/S1474-4422(11)70178-8.
Madej-Pilarczyk A, Kochanski A. Emery-Dreifuss muscular dystrophy: the most recognizable laminopathy. Folia Neuopathol. 2016;54(1):1–8.
Sveen M-L, Thune JJ, Keber L. Cardiac involvement in patients with Limb-Girdle Muscular Dystrophy Type 2 and Becker muscular dystrophy. Arch Neurol. 2008;65(9):1196–201. https://doi.org/10.1001/arch-neur.65.9.1196.
van der Kooi AJ, Van Meegen M, Bolhuis PA. Genetic localization of a newly recognized autosomal dominant limb-girdle muscular dystrophy with cardiac involvement (LGMD1B) to chromosome 1q11-21. Am J Hum Genet. 1997;60:891–5.
Perloff JK, Stevenson WG, Weiss J. Cardiac involvement in myotonic muscular dystrophy: a prospective study of 25 patients. Am J Cardiol. 1984;54(8):1074–81. https://doi.org/10.1016/S0002-9149(84)80147-2.
Zhang LH, Fang LG, Cheng ZW, Fang Q. Cardiac manifestations of patients with mitochondrial disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(10):892–5.
Muller-Hocker J, Schafer S, Mendel B, Lochmuller H, Pongratz D. Nemaline cardiomyopathy in a young adult: an ultraimmunohistochemical study and review of literature. Ultrastruct Pathol. 2000;24(6):407–16.
Banwell BL, Becker LE, Jay V, et al. Cardiac manifestations of congenital fiber-type disproportion myopathy. J Child Neurol. 1999;14(2):83–7.
Lanfranchi PA, Braghiroli A, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–40.
Muller C, Jones H, O’Grady G, Suzrez A, Heller J, Kishnani P. Language and speech function in children with infantile Pompe disease. J Pediatr Neurol. 2009;7:147–56.
Renard D, Humbertclaude V, Labauge P. Macroglossia in adult Duchenne muscular dystrophy. Acta Neurol Belg. 2010;110:288.
Khan Y, Heckmatt JZ. Obstructive apnoeas in Duchenne muscular dystrophy. Thorax. 1994;49:157–61.
Suresh S, Wales P, Cooper DG. Sleep-related breathing disorder in Duchenne muscular dystrophy: disease spectrum in pediatricpopulation. J Paediatr Child Health. 2005;41:500–3.
Leonardis L, Blague R, Dolenc Groselj L. Sleep and breathing disorders in myotonic dystrophy type 2. Acta Neurol Scand. 2015;132:42–8.
Bianchi MLE, Losurdo A, Silvestri G. Prevalence and clinical correlates of sleep disordered breathing in myotonic dystrophy types 1 and 2. Sleep Breath. 2014;18:579–89. https://doi.org/10.1007/s11325-013-0921-5.
Beetar JT, Guilmette TJ, Sparadeo FR. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch Phys Med Rehabil. 1996;77(12):1298–302.
Hoffman AJ, Given BA, Von Eye A. Relationships among pain, fatigue, insomnia and gender in persons with lung cancer. Oncol Nurs Forum. 2007;34(4):785–92.
Jennum P, Drewes AM, Andreasen A, Nielsen KD. Sleep and other symptoms in primary fibromyalgia and in healthy controls. J Rheumatol. 1993;20(10):1756–9.
Power JD, Perrucio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53(6):911–9.
Della Marca G, Frusciante R, Vollono C, Ricci E. Pain and the alpha-sleep anomaly: a mechanism of sleep disruption in fascioscapulohumeral muscular dystrophy. Pain Med. 2013;14(4):487–97. https://doi.org/10.1111/pme.12054.
Bushby KM, Pollitt C, Johnson MA, Chinnery PF. Muscle pain as a prominent feature of FSHD: four illustrative case reports. Neuromuscul Disord. 1998;8(8):574–9.
Vianello A, Bevilacqua M, Vincenti E. Long-term nasal intermittent positive pressure ventilation in advanced Duchenne’s muscular dystrophy. Chest. 1994;105(2):445–8.
Mendell JR, Moxley RT, Florence J. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320(24):1592–7.
Lam EM, Shepard PW, Milone M. Restless legs syndrome and daytime sleepiness are prominent in myotonic dystrophy type 2. Neurology. 2013;81(2):157–64. https://doi.org/10.1212/WNL.0b013e31829a340f.
Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med. 2009;266(5):419–31. https://doi.org/10.1111/j.1365-2796.2009.02159.
Salas RE, Gamaldo CE, Allen RP. Update in restless legs syndrome. Curr Opin Neurol. 2010;23(4):401–6. https://doi.org/10.1097/WCO.0b013e32833bcdd8.
Akamine RT, Grossklauss LF, Tufik S. Restless leg syndrome exacerbated by amytriptiline in a patient with Duchenne muscular dystrophy. Sleep Sci. 2014;7(3):178–80. https://doi.org/10.1016/j.slsci.2014.09.010. Epub 2014 Sep 27
Dauvilliers YA, Laberge L. Myotonic dystrophy type 1, daytime sleepiness and REM sleep dysregulation. Sleep Med Rev. 2012;16(6):539–45. https://doi.org/10.1016/j.smrv.2012.01.001. Epub 2012 Mar 31
Yu H, Laberge L, Dauvilliers Y. Daytime sleepiness and REM sleep characteristics in myotonic dystrophy: a case-control study. Sleep. 2011;34(2):165–70.
Schneider-Gold C, Bellenberg B, Lukas C. Cortical and subcortical grey and white matter atrophy in myotonic dystrophies type 1 and 2 is associated with cognitive impairment, depression and daytime sleepiness. PLoS One. 2015;10(6):e0130352.
Romigi A, Albanese M, Massa R. Sleep-wake cycle and daytime sleepiness in the myotonic dystrophies. J Neurodegener Dis. 2013;2013:692026.
Chokroverty S, Bhat S, Rosen D, Farheen A. REM behavior disorder in myotonic dystrophy type 2. Neurology. 2012;78(24):2004. https://doi.org/10.1212/WNL.0b013e318259e28c.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Joseph, V., Devasahayam, J., Goyal, M. (2018). Sleep Issues in Myopathic Disorders and Muscular Dystrophies. In: Govindarajan, R., Bollu, P. (eds) Sleep Issues in Neuromuscular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-73068-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-73068-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73067-7
Online ISBN: 978-3-319-73068-4
eBook Packages: MedicineMedicine (R0)