Abstract
Epidural spinal cord stimulation has a long history of application as a neuromodulation method for the relief of intractable pain and for improving motor control in various motor disorders. In spinal cord injury specifically, epidural stimulation of the lumbar spinal cord can effectively control severe and diffuse spasticity, without further deteriorating residual voluntary motor control. With appropriate parameters, the stimulation can also generate rhythmic activity and extension in paralyzed legs as well as enable or facilitate residual voluntary lower-limb movements. The development of transcutaneous spinal cord stimulation, a non-invasive method working through surface electrodes with similar neuromodulatory effects, allows for a wide clinical application of this technique in contemporary rehabilitation programs. While also providing background information on historical and recent developments in the field as well as on the neurophysiological mechanisms of spinal cord stimulation, this chapter provides practical information for professionals interested in using electrical neuromodulation in the rehabilitation of individuals after spinal cord injury.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Controlling Spasticity After Spinal Cord Injury: Challenges and Pathways
Severe spinal cord injury (SCI) is a devastating event, which, apart from the obvious paresis or paralysis, causes manifold secondary complications impairing vital body functions caudal to the lesion. One major cause of disability stems from spasticity as one symptom of the upper motoneuron syndrome, with about 70% of individuals being affected one year after the injury [1, 8, 92].
Academically, spasticity is rather narrowly defined as a velocity-dependent form of hypertonia resulting from hyperexcitability of tonic stretch reflexes [57] as a consequence of the lesion-induced misbalance between inhibitory and excitatory inputs to spinal circuitry below the injury [55, 89]. Clinically, associated signs like spasms, clonus, resistance to passive movements, and the clasp-knife response are also commonly subsumed under the umbrella of spasticity [89]. Together, these symptoms often present a major hindrance in rehabilitation, further deteriorate residual motor performance, and negatively impact independence and quality of life [1, 78, 93]. Yet, certain aspects associated with spasticity may as well pose some benefit by increasing trunk stability, facilitating transfers, enabling some stepping movements, reducing the risk of deep venous thrombosis, and partially maintaining muscle bulk, thereby also protecting against pressure sore formation in wheelchair-bound individuals [1, 5, 78]. Any regimen applied with the aim to reduce spasticity therefore needs to carefully balance out between the need to alleviate its detrimental effects and the maintenance of its useful facets [1, 5].
Without doubt, successful management of spasticity has remained difficult and normally requires a multimodal approach, tailored to the individual clinical picture. Treatment modalities include physical therapy, oral medication, intrathecal drug delivery, and the application of Botulinum toxin (for a review see [5, 26, 98]). Surgical neuroablative approaches are often considered as last resort in the treatment of severe, resistant forms of spasticity [78]. Yet, some of the treatments used bear the risk of undesirable side effects, particularly weakness and fatigue that may be induced by antispasticity medication, the further deterioration of residual mobility, as well as permanent lesions within (previously undamaged) neural tissue caused by surgical methods [23, 78].
Neuromodulation techniques provide for an alternative, reversible, and adjustable concept for the treatment of diffuse spasticity and work through the modification of neural signal processing by targeted circuits within the central nervous system [40]. One method to modify the altered activity in the spared neural circuitry after SCI, aside from pharmacological approaches [73], is by electrical spinal cord stimulation (SCS). This chapter will trace the first applications of this technique from its early developments in the 1960s to its recent resurgence in neurorehabilitation and motor recovery after SCI, with a focus on practical aspects and clinical applications.
2 Epidural Spinal Cord Stimulation in Spinal Spasticity: The Early Period
The pioneering work on the nature and treatment of pain by Ronald Wall and colleagues in the 1960s [60, 98]; for a current review see [61] indirectly provided the scientific breeding ground for the later developments in the field of SCS. They postulated that (peripheral) stimulation of large-diameter cutaneous sensory fibers would reduce the perception of pain through the central inhibition of small-diameter fibers in the spinal cord circuitry involved in pain transmission. To control intractable, diffuse pain, Norman Shealy and co-workers demonstrated, first in cats, the particular effectiveness of concentrating the stimulation on the posterior columns of the spinal cord white matter, where the ascending continuations of cutaneous sensory fibers related to multiple dermatomes are closely assembled [87]. Shealy also conducted the first human application of SCS for pain relief in a cancer patient via a plate electrode surgically placed over the posterior columns at T3, leading to an immediate abolition of the pain [88]. Since then, and with technological advancements, SCS for pain control has become widely used [28]. In 1989, epidural SCS gained its approval by the U.S. Food and Drug Administration for the treatment of chronic intractable pain of the trunk and limbs and since then has developed into the most common of all neuromodulation therapies [52].
In fact, the application of epidural SCS in motor disorders is closely linked to its original use in pain conditions, as it followed from an unanticipated observation made in a patient with multiple sclerosis treated for pain [14]. In addition to relieving the pain, the stimulation, applied to the upper thoracic spinal cord, led to a considerable increase of the patient’s sensory perception and voluntary motor control over the legs. Subsequent studies including numerous individuals with multiple sclerosis in whom pain was not a main complaint reproduced the positive impact of SCS on motor performance, taking the form of reduced spasticity and a feeling of lightness when moving the legs, increased endurance during ambulation, and the enabling of some voluntary movements in otherwise paralyzed limbs under SCS [13, 20, 25, 44, 90, 100]. Yet, not all patients benefitted equally from SCS, and in some individuals, no effects were achieved at all [45, 90, 91]. These inter-individual differences were attributed to the pathophysiological complexity of the disease itself as well as to the high variability of rostro-caudal stimulation sites employed across the different studies [23]; reviewed in [67, 68].
Despite this ambiguity, the positive results obtained in the patients with multiple sclerosis soon motivated first studies in SCI individuals [7, 23, 75, 79, 80, 91], which likewise produced positive yet variable outcomes (Fig. 1a). Richardson et al. [80] reported complete alleviation of spasticity in 6 individuals with severe thoracic SCI whose spasticity could not be controlled by other treatment modalities when applying SCS via epidural electrodes placed below the injury over the lumbar and sacral spinal roots at L1–L4 vertebral levels. On the other hand, Siegfried et al. [91] found no improvements in lower-limb spasticity in any of the 15 SCI individuals studied when treated by SCS. Notably, electrodes were always placed rostral to the level of severe SCI in their study. In a cohort of 59 SCI individuals, Dimitrijevic et al. [23] found a marked or moderate effect of SCS on spasticity in 37 patients, with only a marginal or no effect in the remaining 22 patients. Reduction of spasticity was generally achieved with electrode placements caudal to the injury level in the posterior epidural space. Yet, in severe cervical spinal cord lesions and with the electrodes placed immediately caudal to the injury, SCS failed to alleviate spasticity in the lower limbs, while in incomplete SCI, stimulation from similar sites produced considerable therapeutic effects. Dimitrijevic et al. [23] concluded that the effectiveness of SCS strongly depended on the specific rostro-caudal position of the electrodes with respect to the injury site and on the severity of the spinal cord lesion. Barolat et al. [7] studied the potential of SCS to control severe spasms in 16 SCI patients. The target placement of the electrodes was always caudal to the level of the lesion, ranging from T1–T6 levels depending on the individual distribution of spasticity, and in the posterior epidural space. Such electrode placement was achieved in 14 out of the 16 individuals tested and led to marked improvements of the spasms in terms of their severity, frequency, and duration in all 14 cases [7]. Specifically, with electrode placements at or rostral to T3, also spasms in the upper limbs were controlled by the stimulation. Pinter et al. [75] showed a considerable antispasticity effect in the lower limbs of 8 individuals with severe low-cervical to mid-thoracic lesions of the spinal cord when applying SCS from the posterior epidural space at vertebral levels of T11–L1, thereby specifically targeting the lumbar spinal cord. The effect was so pronounced that antispasticity medication could be completely discontinued in 7 of the patients and substantially reduced in the remaining subject. Across the various studies, the applied stimulation frequencies were within a range of 33–120 Hz and intensities were below the level causing muscle activity in the lower extremities and produced paraesthesias in individuals with sensory incomplete SCI. The stimulation was either continuously or intermittently applied for several hours per day via plate electrodes or percutaneous leads. Barolat et al. [7] described an immediate amelioration of spasticity by the stimulation in most of the patients, but also found gradual decrease of spasticity occurring over several weeks in some of the individuals treated. In most subjects, there were carry-over effects after the stimulation had been turned off. The persistence of these effects was generally related to the duration of the stimulation, ranging from a few hours within the first weeks of stimulation to up to 5 days after several weeks of stimulation [7]. Accordingly, some patients adjusted their daily regimen of stimulation, and some could reduce its application to a few hours two or three times a week only, while maintaining the therapeutic effects [7]. Implantation as well as stimulation procedures were generally well accepted by the patients included in the various studies, and no adverse effects related to the stimulation were reported.
Epidural spinal cord stimulation (SCS) for spasticity control after spinal cord injury (SCI). a Studies conducted by various groups starting from the 1970s produced ambiguous results on the effectiveness of epidural SCS to reduce spasticity. The sketch on the left presents vertebral relative to spinal cord levels, white bars on the right depict ranges of SCI levels of the patients studied in the different studies, and black bars the respective rostro-caudal ranges of electrode positions. Studies are arranged from left to right according to the reported effectiveness of SCS to alleviate spasticity. Numbers in brackets are numbers of responders relative to the total numbers of SCI individuals included. b SCS can electrically activate large-to-medium-diameter sensory posterior root fibers or, given their functional integrity, their ascending continuations in the posterior columns of the spinal cord white matter, depending on the rostro-caudal electrode position. c The effectiveness of SCS to reduce lower-limb spasticity strongly depends on the rostro-caudal placement of the epidural electrode, the severity of SCI, and the neural mechanisms set into action by the stimulation. (i) SCS applied to the thoracic spinal cord caudal to a severe SCI activates the lumbar segmental circuitry via antidromic activation of posterior column fibers. (ii) In incomplete SCI, the stimulation may likely work through spinal-brainstem-spinal loops set into action by orthodromic conduction evoked within the posterior columns as well as through segmental spinal mechanisms following the antidromic posterior column activation. (iii) SCS over the lumbar spinal cord activates the local circuitry transsynaptically through the electrical stimulation of afferent fibers within the lumbar posterior roots
As suggested by Dimitrijevic et al. [23], the variability in the results produced by the different studies must be discussed in the light of the respective rostro-caudal stimulation sites employed, leading to the electrical activation of distinct neural structures, and in conjunction with the severity of the spinal cord lesions. At therapeutic intensities for the management of spasticity (see subsect. 3 of this chapter), the neural structures electrically stimulated through electrodes placed in the posterior epidural space are afferent fibers within the posterior roots or their rostral continuations within the posterior columns of the spinal cord white matter [41], also depending on the specific segmental electrode position. SCS targeted to the lumbar spinal cord predominantly activates large-to-medium-diameter afferents within the posterior roots [76] (Fig. 1b). Notably, of the afferent fibers originating from muscles, tendons, joints, and cutaneous tissues of the hip and lower limbs that enter the spinal cord via the lumbar and upper sacral posterior roots, only the ascending continuations of the cutaneous fibers are present also within the posterior columns with increasing distance to the lumbar spinal cord, since the other fiber types leave the posterior columns to ascend via alternative systems [19]. All other spinal neural structures are transsynaptically recruited through the SCS-induced sensory input [10, 62].
Following this line, three potential neural pathways by which the activity produced by SCS may reach (and modulate) the lumbar spinal circuitry involved in gating afferent input and regulating motoneuronal excitability associated with the lower limbs were suggested: first, via antidromic activation of the posterior column fibers when stimulation is directed to the thoracic spinal cord (Fig. 1c(i); [43]); second, via orthodromic conduction evoked within the posterior columns, leading to increased descending activation of spinal inhibitory circuitry through brainstem-spinal cord loops in incomplete SCI (Fig. 1c(ii); [23, 86]); and third, with SCS over the lumbar spinal cord, via orthodromic activation of afferent fibers within the lumbar and upper sacral posterior roots (Fig. 1c(iii); [62, 64, 71, 75, 76]).
These variable neural mechanisms set into action by the stimulation also provide a likely explanation for the lack of effectiveness in some patients versus the good results obtained in others (Fig. 1c). In the individuals with complete cervical SCI and the electrodes placed just caudal to the lesion zone, the functional integrity of the posterior columns at the stimulation site may too have been compromised by the injury or the effects would have required the stimulation of fiber types arising in the legs that are not present in the posterior columns at such distance from the lumbar spinal cord [68, 69]. Satisfactory results, on the other hand, were obtained with stimulation applied from same sites but in individuals with incomplete SCI. Apart from acting on lumbar spinal segmental circuity via antidromic posterior-column activation in these cases [23, 86], the stimulation likely also increased the descending activation of inhibitory spinal mechanisms through brainstem-spinal loops [23]; cf. [83].
3 Epidural Stimulation of the Lumbar Spinal Cord for the Control of Spasticity: Current Practice and Clinical Considerations
The various studies starting from the 1970s have taught that refractory forms of lower-limb spasticity may be alleviated by activating the lumbar spinal segmental circuitry involved in the regulation of afferent inputs and of the motoneuronal excitability associated with the legs and that this circuitry can—largely independently from the specific site and severity of SCI—be accessed with SCS specifically directed to the lumbar spinal cord [75]. Notably, despite the promising therapeutic outcomes achieved with epidural SCS in numerous patients suffering from various conditions [99], its application in motor disorders has remained off-label.
Practically, the stimulation is applied via a thin cylindrical lead with several electrodes on the distal end that is placed percutaneously and thus minimally invasive under fluoroscopic control into the posterior epidural space over the lumbar spinal cord. Alternatively, the stimulation may be delivered via a surgical paddle lead with electrodes arranged in arrays that require laminotomy or laminectomy, but at the same time allow for a more flexible control over the stimulation site employed [54]. On average, the rostro-caudal position of the electrodes corresponds to the T11 and T12 vertebral levels, but may be as caudal as the L1 vertebral level, as well [62, 71] (Fig. 2a). Stimulation from the targeted site allows for the activation of posterior-root afferents of several lumbar and upper sacral spinal cord levels bilaterally at the same time [62] (Fig. 2b). Consequently, at low stimulation frequencies (e.g., 2 or 5 Hz) and with adequate stimulation intensity, each stimulus pulse evokes twitch-contractions in multiple muscles of both legs [71] (Fig. 2c), so-called posterior root-muscle (PRM) reflexes termed according to their initiation and recording sites [62, 65]. With other words, from the targeted stimulation site over the lumbar spinal cord, PRM reflexes will be elicited in muscle groups with distinct segmental innervation (cf. Fig. 2b), and intraoperative surface-electromyographic recordings of such reflex responses hence serve as a physiological marker guiding the correct rostro-caudal placement of the epidural electrodes over the lumbar spinal cord [32, 38, 64, 71, 75]. In individuals with sensory incomplete SCI, the placement can be also guided by the elicitation of paraesthesias in the lower-limb dermatomes, when stimulation is applied at higher frequencies (e.g., 30 or 50 Hz), like in epidural SCS for pain control [54].
Epidural stimulation of the lumbar spinal cord. a X-ray and schematic sketch illustrate the placement of the epidural electrodes in the epidural space, inside the vertebral canal and outside the meninges covering the spinal cord, over the lumbar spinal cord corresponding on average to T11 and T12 vertebral levels. b Epidural stimulation of the lumbar spinal cord synchronously activates large-to-medium-diameter afferent fibers within the lumbar and upper sacral posterior roots bilaterally that are associated with muscle groups of the lower limbs. Sketch on the right depicts segmental innervation of quadriceps (Q), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS). c Stimulation of the lumbar spinal cord with above motor-threshold intensity elicits short-latency posterior root-muscle reflexes, i.e., reflexes initiated within posterior-root afferents and recorded via surface-electromyography (EMG), in multiple lower-limb muscle groups bilaterally. Stimulus-triggered, superimposed representation of 10 consecutive PRM reflexes elicited at 2 Hz in right (R) and left (L) Q, Ham, TA, and TS, black arrows indicate times of stimulus application
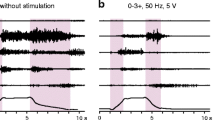
After its implantation in the lumbar epidural space, the electrode lead is normally externalized and connected to a test stimulator for a trial period of 1–2 weeks. During this period, various combinations of SCS parameter settings are systematically tested for their effectiveness in controlling lower-limb spasticity. The epidural lead carries several independent electrodes that can be set to “+”, “–”, and “off”, allowing for different bi- (and multi-) polar electrode combinations. The selection of the active cathode also allows for shifting the active stimulation site along the extent of the multiple electrodes. Stimulation frequencies normally used for spasticity control are within a range of 50–100 Hz [75]. Therapeutic stimulation intensities are below the level evoking muscle twitches in the trunk, hip, or lower limbs and are generally within a range of 0.5–5 V with an impedance of 300–1000 Ω for a bipolar electrode configuration [75]. Individuals with incomplete SCI may perceive a non-painful tingling sensation (paraesthesias) in the lower-limb dermatomes during the stimulation. With the designated parameter settings, the effects of SCS on the patients’ spasticity and residual motor control are thoroughly assessed clinically and neurophysiologically, also tailored to the patients’ individual clinical picture of spasticity and needs, and comparing them to the corresponding assessments conducted before the implantation and with the stimulation turned off (Fig. 3). This trial procedure is necessary since there are still no generally accepted clinical or physiological markers to clearly identify in advance those patients who will benefit from epidural SCS. A more recently developed transcutaneous version of SCS may develop into an easy-to-apply and useful procedure, which could serve this purpose in the future (see Sect. 5 of this chapter). Given a positive evaluation by the patient, the attending neurologist, and the involved physiotherapists after the trial period, a programmable implantable pulse generator (IPG) is eventually placed subcutaneously in the abdominal wall [54] and connected to the epidural electrode lead, forming a closed system for chronic stimulation. The IPG is then set to run continuously with the determined frequency and electrode combination, which are typically only altered should the effect change over time, e.g. because of migration of the electrode lead [75] or carry-over effects emerging over time, allowing the patient to (temporarily) withdraw the stimulation or reduce the stimulation amplitudes [7]. The stimulation intensity is manually adjustable using a patient programmer. At the Neurological Center, Otto-Wagner-Hospital, Vienna, roughly 30 individuals with chronic SCI have received devices for epidural lumbar SCS for spasticity control within the past 15–20 years.
Control of lower-limb spasticity by epidural stimulation targeting the lumbar spinal cord. Electromyographic (EMG) activity recorded in an individual with spinal spasticity during passive flexion and extension movements at hip and knee in the supine position (i) without stimulation and (ii) under continuous 50 Hz stimulation with sub-motor threshold intensity of 5 V using a bipolar electrode configuration with the cathode targeting the upper lumbar spinal cord segments, which corresponded in this subject to the 12th thoracic vertebral level as identified by X-ray. The stimulation suppresses lower-limb spasticity as reflected by complete attenuation of the EMG activity associated with the tonic stretch reflex recorded from quadriceps (Q), adductors (Add), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS). Shaded backgrounds mark the passive movement, shown are two repetitions of the same maneuver. Data derived from an individual with chronic motor complete spinal cord injury, American Spinal Injury Association Impairment Scale (AIS) grade B, neurological level of injury: C5–C6
4 The Recent Resurgence of Epidural Spinal Cord Stimulation: Inducing and Enabling Movement After Spinal Cord Injury
Apart from controlling severe forms of spasticity, which, by itself, allows the expression of some voluntary mobility in many cases, lumbar SCS with certain stimulation parameter settings may also induce [17, 24, 47, 62, 64, 77] or enable [4, 6, 32, 68, 69] movements in otherwise paralyzed legs, as well as facilitate the activity produced during assisted treadmill training [32, 33, 42, 63].
Specifically, epidural stimulation of the lumbar spinal cord at 25–50 Hz can induce rhythmic contraction-relaxation patterns across multiple lower-limb muscle groups in motor complete SCI individuals lying supine, and some of these patterns have the appropriate coordination to result in synergistic flexion-extension movements over several leg joints [17, 24, 47, 62]. When applied in conjunction with assisted treadmill stepping with body weight support in patients with severe SCI [32, 63], epidural SCS within such frequency range and with intensities close to or slightly above the level eliciting PRM reflexes in the lower limb muscle groups has an immediate augmentative effect on the electromyographic activity as produced by the gait-phase related proprioceptive feedback input alone [21, 59, 102], and can recruit additional lower-limb muscle groups that are not responding to the guided stepping motions alone. It should be noted, however, that independent stepping movements were not yet achieved in these patients. In wheel-chair dependent individuals with incomplete lesions but sub-functional motor strength in the lower limbs, on the other hand, the addition of SCS may increase the outcome of intensive locomotor training and lead to improved overground ambulation, walking speed, step length, and endurance [33, 42].
When applied at 5–16 Hz, epidural SCS can induce bilateral extension in the lower-limbs of (motor) complete SCI individuals [47]. In a recent study, SCS could induce full-weight bearing standing in four patients with (motor) complete SCI and after intensive training standing could be maintained for several minutes with minimal self-assistance for balance control under ongoing SCS [32, 77].
Much of the current resurgence of interest in epidural SCS in the rehabilitation of SCI is most probably attributable to the rediscovery of its enabling effects on otherwise ‘clinically silent’ translesional volitional motor control. Even in an SCI clinically classified as complete, some residual white matter tracts through the injury zone or propriospinal system bridging the lesion [27, 72] are generally still present [22, 48,49,50]. These surviving connections may provide for some—subclinical—excitatory [22] or inhibitory [12] brain/brainstem influence over the lumbar spinal circuitry despite the otherwise clearly perturbed neural signal transmission [68]. The SCS-evoked ‘tonic’ driving input increases the excitability of the lumbar spinal motor circuitry and thereby enhances its responsiveness to this otherwise insufficient supraspinal input, allowing for rudimentary volitional motor control over otherwise paralyzed legs (cf. [68]). First reported in the 1980s [6, 7], this therapeutic potential of SCS was recently revisited [4, 32]. Under SCS at 25 Hz or 30 Hz, four patients with clinically classified (motor) complete SCI could volitionally induce hip and knee flexion, dorsiflexion, and toe extension. After intense training, one patient maintained the regained voluntary control over leg flexion after SCS was turned off [4].
These recent studies on epidural SCS applied to augment residual motor control have fueled ambitious expectations on the level of functional recovery that may be achieved even after clinically complete SCI. In ensemble with current technological [11, 101] and pharmacological [29,30,31, 94] advancements, as well as the introduction of new training paradigms pursuing the principles of activity-dependent neuroplasticity [4, 46], epidural SCS may indeed be considered as high priority for imminent translation to individuals with severe SCI (cf. [68, 69]).
5 Transcutaneous Spinal Cord Stimulation: A Non-invasive Method to Activate the Lumbar Spinal Circuitry
The bilateral and synchronous activation of afferent fibers within multiple posterior roots and the resulting multisegmental driving input to the lumbar spinal circuitry produced with tonic stimulation was previously suggested to be the key to the observed neuromodulation effects of epidural lumbar SCS in SCI individuals [17, 38, 62, 64, 75]. With the development of a non-invasive, transcutaneous version of SCS, the stimulation of posterior root afferents has become possible from the body surface [65, 66]. The set-up originally described by Minassian et al. [65] utilizes self-adhesive transcutaneous electrical neural stimulation (TENS) electrodes placed over the T11 and T12 spinous processes, manually identified by palpation, as well as larger indifferent electrodes placed paraumbilically on the abdomen (Fig. 4a). Other electrode set-ups have been used as well [15, 18, 53, 84], and the exact dimensions and shapes of the surface electrodes are not decisive [66]. When using a stimulator delivering biphasic stimulus pulses, the electrodes are connected to the stimulator such that the paravertebral electrodes act as anode for the first and as cathode for the second pulse phase [36, 39]. In case of monophasic stimulus pulses, the paraspinal electrodes are connected to the negative output of the stimulator, and the abdominal electrodes to the positive output [70].
Transcutaneous stimulation of the lumbar spinal cord. a Schematic sketch illustrates the placement of the paraspinal stimulating electrodes on the back at the level of the lumbar spinal cord corresponding on average to T11 and T12 vertebral levels and of the indifferent abdominal electrodes. Sketch in the middle depicts stimulation (stim.) through the better conductive elements (ligaments and discs) in-between the bony structures of the spine, along with a computer simulation of the current flow produced in a mid-sagittal plane. b Transcutaneous stimulation of the lumbar spinal cord elicits posterior root-muscle (PRM) reflexes in multiple lower-limb muscle groups bilaterally. Stimulus-triggered, superimposed representation of 3 consecutive PRM reflexes elicited in right (R) and left (L) quadriceps (Q), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS) of an individual with chronic incomplete spinal cord injury, American Spinal Injury Association Impairment Scale (AIS) grade C, neurological level of injury: C6. Black arrows indicate times of stimulus application (cf. Fig. 2c). c The stimulation of afferent fibers can be verified by testing the recovery cycle of the evoked responses using double-stimuli at varying interstimulus intervals. Shown are exemplary results of left triceps surae (LTS) at interstimulus intervals of 30, 50, and 100 ms derived from an individual with chronic incomplete spinal cord injury, AIS grade D, neurological level of injury: C5
Despite the relatively distant stimulation and the non-focused electrical field produced, transcutaneous SCS indeed allows for the selective activation of large-to-medium-diameter afferent fibers within the lumbar and upper sacral posterior roots bilaterally [16, 56, 65]. This is possible because of tissue heterogeneities between the paraspinal and abdominal electrodes as well as along the neural pathways of the roots [56]. First, at the level of the lower thoracic and upper lumbar spine, the posterior aspect of the vertebral canal is only partially shielded by bony structures. The transversal electrical resistance is substantially reduced by the ligaments and intervertebral discs that have considerably better electrical conductivities than bony structures, which allow the current flow produced by transcutaneous SCS to cross the vertebral canal and thecal sac [96]. Second, fibers within the lumbar and upper sacral posterior roots have particularly low excitation thresholds when entering the spinal cord inter alia due to the considerable change in electrical conductivities at the interface of the cerebrospinal fluid and the spinal cord [56, 76]. Further, myelinated afferent fibers with larger diameters corresponding to groups I [56, 65] and II [36, 39] have the lowest thresholds for electrical stimulation [16, 76], while thresholds considerably increase with decreasing fiber diameters [95, 97].
Like epidural SCS, transcutaneous stimulation of the lumbar spinal cord evokes PRM reflexes in multiple lower-limb muscles bilaterally [34, 56, 65] (Fig. 4b), which can serve as a means to neurophysiologically monitor the placement of the paraspinal electrodes over the lumbar spinal cord and to identify the immediately, electrically stimulated neural structures [36, 39]. The stimulation of afferent input structures to the lumbar spinal cord circuitry can be tested by applying double-stimuli at varying interstimulus intervals of e.g. 30, 50, and 100 ms to assess the recovery cycle of the evoked responses [36, 65, 81] (Fig. 4c). The presence of post-activation depression [74], as reflected by attenuated responses to the second stimulus pulse, verifies the transsynaptic and hence the reflex nature of the evoked responses [65, 81]. The stimulation of motor fibers in the anterior roots, on the other hand, would lead to the elicitation of two responses of similar amplitude even at such short interstimulus intervals.
Given the activation of the same neural input structures to the spinal cord as by epidural SCS, the transcutaneous technique may as well be used as a neuromodulation tool to modify altered activity of spinal circuits after SCI when used to apply ‘tonic’ stimulation [35,36,37, 39, 63, 66]. Additionally, as a non-invasive method, transcutaneous SCS can be employed to evoke ‘test’ PRM reflexes in neurophysiological studies of the organization of motor control and sensorimotor transmission at the level of the spinal cord, both in individuals with intact or altered central nervous system [2, 3, 15, 34, 65, 66, 81, 82], very similarly as in classical conditioning-test paradigms utilizing the H reflex [51, 85].
When applied for neuromodulation purposes, one has to consider though that unlike epidural stimulation, transcutaneous SCS is not suitable for permanent or chronic use. To be of therapeutic value, the induced effects therefore need to outlast the stimulation application or must stem from the intensification of the outcome obtained by other treatment modalities with which transcutaneous SCS is combined.
In the control of spinal spasticity specifically, a recent proof of concept study has demonstrated that a single 30 min session of transcutaneous SCS at 50 Hz and with an intensity producing paraesthesias but no muscle activity in the lower limbs temporarily alleviated various clinical signs of spasticity and enhanced voluntary motor control of three individuals with incomplete SCI [36] (Fig. 5a). Preliminary results obtained in seven subjects with SCI of various severity further suggest the temporary persistence of these antispasticity effects for at least two hours after the stimulation [37]. In one of the patients, the effects of repetitive exposure to transcutaneous SCS over a period of six weeks was tested [37]. It was found that the stimulation-induced effects outlasted each stimulation session for at least 24 h and were progressively increasing over the six weeks. The effects could still be detected seven days after the last application of transcutaneous SCS [37]. The subject was later selected for implantation of an epidural system with which effective spasticity control was also achieved, suggesting that transcutaneous SCS may serve as a non-invasive trial procedure to identify responders to epidural SCS.
Applications of transcutaneous stimulation of the lumbar spinal cord in rehabilitation after spinal cord injury. a Control of lower-limb spasticity by transcutaneous stimulation of the lumbar spinal cord. Electromyographic (EMG) activity elicited by tonic stretch reflex in an individual with spinal spasticity during passive flexion and extension movements at hip and knee (i) before stimulation and (ii) after a 30 min session of tonic 50 Hz stimulation. The stimulation led to almost complete suppression of the EMG activity recorded from quadriceps (Q), adductors (Add), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS) and this effect outlasted the application of transcutaneous SCS for several hours. Shaded backgrounds mark the passive movement in the supine position, shown are two repetitions of the same maneuver. Data derived from an individual with chronic incomplete spinal cord injury (SCI), American Spinal Injury Association Impairment Scale (AIS) grade C, neurological level of injury: C6. b Transcutaneous stimulation at around 30 Hz and sub-motor threshold intensity can immediately modulate the walking capability of individuals with motor incomplete SCI. Displayed stick-figures were calculated on the basis of hip and knee goniometric data and averaged from 10 consecutive gait cycles during ongoing spinal cord stimulation (SCS on, left) and without stimulation (SCS off, right). The stimulation enhanced movement during swing and increased joint stability during stance. Note that all stepping movements were volitionally initiated and maintained by the subject, no EMG activity was produced by the stimulation in the absence of the voluntary attempt to step. Subject with incomplete SCI classified as AIS D, neurological level of injury: T9, stepping without manual assistance or body weight support, treadmill belt speed: 1.6 km/h. c Stimulation at around 15 Hz with an intensity above the motor threshold can generate standing-up and upright standing in individuals with severe SCI. Shown are stick-figures of one leg along with corresponding ground reaction forces. Starting from a supported sitting position in an overhead harness, the stimulation induced bilateral lower-limb extension, leading to an upright standing position with ground reaction forces of up to 40 kg per leg. Note that with increased loading of the legs, additional proprioceptive feedback input to the spinal cord was produced that further supported extension. The standing position was maintained until the stimulation was turned off (SCS off). Data derived from an individual with complete SCI classified as AIS A, neurological level of injury: T9
Transcutaneous SCS at around 30 Hz, i.e., within frequency ranges found to be effective in epidural SCS to promote locomotor-like activity, and with intensities below motor threshold for the lower limbs was found to facilitate residual voluntary locomotor control in ambulatory, motor incomplete SCI individuals actively stepping on a treadmill [35, 39] (Fig. 5b). The effects included the step-phase appropriate augmentation of electromyographic activity in the lower limbs and changes in the gait kinematics as assessed by goniometric recordings from the hip and knee joints, mainly an augmented flexion movement during swing phase. Notably, the step-phase appropriate modulations occurred despite continuous administration of transcutaneous SCS during stepping with unchanged parameters throughout the gait cycles. Further, as soon as the treadmill belt was stopped and the subject stopped the active stepping, i.e., without the subjects’ voluntary contribution, no electromyographic activity was produced in the lower limbs by the stimulation alone. It was hypothesized that the stimulation elevated the state of excitability of the lumbar locomotor circuitry, which in turn became more responsive to the voluntary commands to step through the surviving descending axons [39]. Considering the incomplete nature of the injuries, the stimulation could have modulated the activity of neural circuits rostral to the lesion via the partially functional posterior-column tracts as well. In individuals with (motor) complete SCI passively stepping on a treadmill using a robotic-driven gait orthosis, transcutaneous SCS at 30 Hz and with intensities above the motor threshold for the lower extremities considerably enhanced the motor output produced by the proprioceptive feedback input and recruited additional muscle groups [70] a finding reminiscent of that obtained with epidural SCS [32, 63].
Finally, transcutaneous SCS at around 15 Hz and with intensities above the lower-limb motor thresholds can induce standing in individuals with motor complete SCI (Fig. 5c). Two mechanisms thereby facilitate the extension movements of the legs generated by SCS: first, the progressive increase in lower-limb load when initiating the standing-up movement from a sitting position by manipulating body position leads to an increase in the proprioceptive feedback input to the spinal cord, which likely adds to the activation of the spinal circuitry; second, due to the rich connectivity of each group Ia muscle spindle fiber to a large proportion of its homonymous (and partially also heteronymous) motoneuron pools [9, 58], the activation of even a portion of the afferents within the posterior roots by transcutaneous SCS can effectively increase the motoneuronal excitability and recruitment.
6 Conclusions
Electrical SCS has been employed for the rehabilitation of various motor disorders for more than 40 years, but has not yet gained general acceptance and has been used in a few interested and specialized centers only. Recent high-profile studies that rediscovered the use of SCS as a neuro-augmentative tool have fueled a resurgence of interest in electrical neuromodulation of the spinal cord. Not only can SCS be tuned to effectively control diffuse and severe forms of spinal spasticity without further negatively impacting residual motor control in SCI individuals, it may indeed improve functional motor recovery even in patients with severe SCI. A wide spread use and eventual acceptance of SCS in clinical practice will essentially depend on a better understanding of its interaction with the neurophysiology of the targeted neural networks as well as the identification of markers that can distinguish responders from non-responders before implantation of an SCS system. The availability of transcutaneous SCS may facilitate these processes and by itself develop into a useful clinical tool for neuromodulation of altered motor control.
References
Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–86.
Andrews JC, Stein RB, Roy FD. Post-activation depression in the human soleus muscle using peripheral nerve and transcutaneous spinal stimulation. Neurosci Lett. 2015;589:144–9.
Andrews JC, Stein RB, Roy FD. Reduced postactivation depression of soleus H reflex and root evoked potential after transcranial magnetic stimulation. J Neurophysiol. 2015;114:485–92.
Angeli CA, Edgerton VR, Gerasimenko YP, et al. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–409.
Barnes MP. Management of spasticity. Age Ageing. 1998;27:239–45.
Barolat G, Myklebust JB, Wenninger W. Enhancement of voluntary motor function following spinal cord stimulation–case study. Appl Neurophysiol. 1986;49:307–14.
Barolat G, Myklebust JB, Wenninger W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Appl Neurophysiol. 1988;51:29–44.
Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord. 2006;44:708–22.
Brodal A. Neurological anatomy in relation to clinical medicine. 3rd ed. New York: Oxford University Press; 1981.
Capogrosso M, Wenger N, Raspopovic S, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33:19326–40.
Capogrosso M, Milekovic T, Borton D et al. A brain spinal interface to alleviate lower limb deficits after neuromotor disorders. In: Neurosci Meet Planner, Chicago, IL, Soc Neurosci; 2015. 428.08. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=1cecc2a2-8e4f-4585-9cbe-268cd58172d4&cKey=04840ed1-447c-4af5-a45b-c0a96ed93dc5&mKey=d0ff4555-8574-4fbb-b9d4-04eec8ba0c84.
Cioni B, Dimitrijevic MR, McKay WB, Sherwood AM. Voluntary supraspinal suppression of spinal reflex activity in paralyzed muscles of spinal cord injury patients. Exp Neurol. 1986;93:574–83.
Cook AW. Electrical stimulation in multiple sclerosis. Hosp Pract. 1976;11:51–8.
Cook AW, Weinstein SP. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N Y State J Med. 1973;73:2868–72.
Courtine G, Harkema SJ, Dy CJ, et al. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol. 2007;582:1125–39.
Danner SM, Hofstoetter US, Ladenbauer J, et al. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif Organs. 2011;35:257–62.
Danner SM, Hofstoetter US, Freundl B, et al. Human spinal locomotor control is based on flexibly organized burst generators. Brain. 2015;138:577–88.
Danner SM, Krenn M, Hofstoetter US, et al. Body position influences which neural structures are recruited by lumbar transcutaneous spinal cord stimulation. PLoS ONE. 2016;11:e0147479.
Davidoff RA. The dorsal columns. Neurology. 1989;39:1377–85.
Davis R, Emmonds SE. Spinal cord stimulation for multiple sclerosis: quantifiable benefits. Stereotact Funct Neurosurg. 1992;58:52–8.
Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995;37:574–82.
Dimitrijevic MR, Dimitrijevic MM, Faganel J, et al. Suprasegmentally induced motor unit activity in paralyzed muscles of patients with established spinal cord injury. Ann Neurol. 1984;16:216–21.
Dimitrijevic MM, Dimitrijevic MR, Illis LS, et al. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent Nerv Syst Trauma. 1986;3:129–44.
Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–76.
Dooley DM, Sharkey J. Electrostimulation of the nervous system for patients with demyelinating and degenerative diseases of the nervous system and vascular diseases of the extremities. Appl Neurophysiol. 1977;40:208–17.
Elbasiouny SM, Moroz D, Bakr MM, et al. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair. 2010;24:23–33.
Faganel J, Dimitrijevic MR. Study of propriospinal interneuron system in man. Cutaneous exteroceptive conditioning of stretch reflexes. J Neurol Sci. 1982;56:155–72.
Gildenberg P. Neuromodulation: a historical perspective. In: Krames E, Peckham P, Rezai A, editors. Neuromodulation. London: Elsevier-Academic Press; 2009. p. 9–20.
Guertin PA. Synergistic activation of the central pattern generator for locomotion by l-beta-3,4-dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci Lett. 2004;358:71–4.
Guertin PA. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol. 2012;3:183.
Guertin PA. Preclinical evidence supporting the clinical development of central pattern generator-modulating therapies for chronic spinal cord-injured patients. Front Hum Neurosci. 2014;8:272.
Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–47.
Herman R, He J, D’Luzansky S, et al. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65–8.
Hofstoetter US, Minassian K, Hofer C, et al. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs. 2008;32:644–8.
Hofstoetter US, Hofer C, Kern H, et al. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed Tech (Berl). 2013. https://doi.org/10.1515/bmt-2013-4014.
Hofstoetter US, McKay WB, Tansey KE, et al. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37:202–11.
Hofstoetter US, Krenn M, Danner SM et al. Short- and long-term effects of intermittent transcutaneous spinal cord stimulation on spinal spasticity and residual motor control. In: Neurosci Meet Planner, Washingotn, DC, Soc Neurosci; 2014b. 630.04. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=d4a1ebd1-790a-474a-865c-6def8b901ee8&cKey=04026d5f-dacb-410d-b5f9-12ff12fd7243&mKey=54c85d94-6d69-4b09-afaa-502c0e680ca7.
Hofstoetter US, Danner SM, Freundl B, et al. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J Neurophysiol. 2015;114:400–10.
Hofstoetter US, Krenn M, Danner SM, et al. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39:E176–86.
Holsheimer J. Concepts and methods in neuromodulation and functional electrical stimulation: an introduction. Neuromodulation. 1998;1:57–61.
Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation. 2002;5:25–31.
Huang H, He J, Herman R, et al. Modulation effects of epidural spinal cord stimulation on muscle activities during walking. IEEE Trans Neural Syst Rehabil Eng. 2006;14:14–23.
Hunter JP, Ashby P. Segmental effects of epidural spinal cord stimulation in humans. J Physiol. 1994;474:407–19.
Illis LS, Oygar AE, Sedgwick EM, et al. Dorsal-column stimulation in the rehabilitation of patients with multiple sclerosis. Lancet. 1976;1:1383–6.
Illis LS, Sedgwick EM, Tallis RC. Spinal cord stimulation in multiple sclerosis: clinical results. J Neurol Neurosurg Psychiatry. 1980;43:1–14.
Jackson A, Zimmermann JB. Neural interfaces for the brain and spinal cord—restoring motor function. Nat Rev Neurol. 2012;8:690–9.
Jilge B, Minassian K, Rattay F, et al. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res. 2004;154:308–26.
Kakulas BA. Pathology of spinal injuries. Cent Nerv Syst Trauma. 1984;1:117–29.
Kakulas A. The applied neurobiology of human spinal cord injury: a review. Paraplegia. 1988;26:371–9.
Kakulas BA, Kaelan C. The neuropathological foundations for the restorative neurology of spinal cord injury. Clin Neurol Neurosurg. 2015;129:S1–7.
Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–12.
Krames E, Rezai A, Peckham P, et al. What is neuromodulation? In: Krames E, Peckham P, Rezai A, editors. neuromodulation. London: Elsevier-Academic Press; 2009. p. 3–8.
Krenn M, Hofstoetter US, Danner SM, et al. Multi-electrode array for transcutaneous lumbar posterior root stimulation. Artif Organs. 2015;39:834–40.
Kumar K, Lind G, Winter J, et al. Spinal cord stimulation: placement of surgical leads via laminotomy—techniques and benefits. In: Krames E, Peckham P, Rezai A, editors. Neuromodulation. London: Elsevier-Academic Press; 2009. p. 1005–11.
Kumru H, Murillo N, Samso JV, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24:435–41.
Ladenbauer J, Minassian K, Hofstoetter US, et al. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng. 2010;18:637–45.
Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg lecture. Neurology. 1980;30:1303–13.
Lloyd DPC. Reflex action in relation to pattern and peripheral source of afferent stimulation. J Neurophysiol. 1943;6:111–9.
Maegele M, Müller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma. 2002;19:1217–29.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9.
Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210–6.
Minassian K, Jilge B, Rattay F, et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401–16.
Minassian K, Persy I, Rattay F, et al. Effect of peripheral afferent and central afferent input to the human lumbar spinal cord isolated from brain control. Biocybern Biomed Eng. 2005;25:11–29.
Minassian K, Persy I, Rattay F, et al. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci. 2007;26:275–95.
Minassian K, Persy I, Rattay F. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve. 2007;35:327–36.
Minassian K, Hofstoetter US, Rattay F. Transcutaneous lumbar posterior root stimulation for motor control studies and modification of motor activity after spinal cord injury. In: Dimitrijevic M, Kakulas B, McKay W, Vrbova G, editors. Restorative neurology of spinal cord injury. New York: Oxford University Press; 2011. p. 226–55.
Minassian K, Hofstoetter U, Tansey K et al. Neuromodulation of lower limb motor control in restorative neurology. Clin Neurol Neurosurg. 2012;114:489–497.
Minassian K, McKay WB, Binder H et al. Targeting lumbar spinal neural circuitry by Epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics. 2016a. https://doi.org/10.1007/s13311-016-0421-y.
Minassian K, Hofstoetter US. Spinal cord stimulation and augmentative control strategies for leg movement after spinal paralysis in humans. CNS Neurosci Ther CNS Neurosci Ther. 2016. https://doi.org/10.1111/cns.12530.
Minassian K, Hofstoetter US, Danner SM, et al. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil Neural Repair. 2016;30:233–43.
Murg M, Binder H, Dimitrijevic MR. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches—a functional method to define the site of stimulation. Spinal Cord. 2000;38:394–402.
Nathan PW, Smith MC. Fasciculi proprii of the spinal cord in man. Brain. 1959;82:610–68.
Penn RD. Intrathecal drugs for spasticity. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. Berlin: Springer; 2009. p. 1973–81.
Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord. Cambridge: Cambridge University Press; 2012.
Pinter MM, Gerstenbrand F, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord. 2000;38:524–31.
Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord. 2000;38:473–89.
Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS ONE. 2015;10:e0133998.
Rekand T. Clinical assessment and management of spasticity: a review. Acta Neurol Scand Suppl. 2010;190:62–6.
Richardson RR, McLone DG. Percutaneous epidural neurostimulation for paraplegic spasticity. Surg Neurol. 1978;9:153–5.
Richardson RR, Cerullo LJ, McLone DG, et al. Percutaneous epidural neurostimulation in modulation of paraplegic spasticity. Six case reports. Acta Neurochir (Wien). 1979;49:235–43.
Roy FD, Gibson G, Stein RB. Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp Brain Res. 2012;223:281–9.
Roy FD, Bosgra D, Stein RB. Interaction of transcutaneous spinal stimulation and transcranial magnetic stimulation in human leg muscles. Exp Brain Res. 2014;232:1717–28.
Saadé NE, Tabet MS, Atweh SF, et al. Modulation of segmental mechanisms by activation of a dorsal column brainstem spinal loop. Brain Res. 1984;310:180–4.
Sayenko DG, Atkinson DA, Dy CJ, et al. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J Appl Physiol. 2015;118:1364–74.
Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–76.
Sedgwick EM, Illis LS, Tallis RC, et al. Evoked potentials and contingent negative variation during treatment of multiple sclerosis with spinal cord stimulation. J Neurol Neurosurg Psychiatry. 1980;43:15–24.
Shealy CN, Taslitz N, Mortimer JT. Electrical inhibition of pain: experimental evaluation. Anesth Analg. 1967;46:299–305.
Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–91.
Sheean G (2002) The pathophysiology of spasticity. Eur J Neurol. 2002; S1:3–9.
Siegfried J, Krainick JU, Haas H, et al. Electrical spinal cord stimulation for spastic movement disorders. Appl Neurophysiol. 1978;41:134–41.
Siegfried J, Lazorthes Y, Broggi G. Electrical spinal cord stimulation for spastic movement disorders. Appl Neurophysiol. 1981;44:77–92.
Singh A, Tetreault L, Kalsi-Ryan S. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Sköld C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–57.
Steuer I, Rouleau P, Guertin PA. Pharmacological approaches to chronic spinal cord injury. Curr Pharm Des. 2013;19:4423–36.
Struijk JJ, Holsheimer J, Boom HB. Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans Biomed Eng. 1993;40:632–9.
Szava Z, Danner SM, Minassian K. Transcutaneous electrical spinal cord stimulation: Biophysics of a new rehabilitation method after spinal cord injury. Müller, Saarbrücken: VDM Verlag Dr; 2011.
Veltink PH, Van Alsté JA, Boom HB. Influences of stimulation conditions on recruitment of myelinated nerve fibers: a model study. IEEE Trans Biomed Eng. 1988;35:917–24.
Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108–9.
Waltz JM. Spinal cord stimulation: a quarter century of development and investigation. A review of its development and effectiveness in 1336 cases. Stereotact Funct Neurosurg. 1997;69:288–99.
Waltz JM. Chronic stimulation for motor disorders. In: Gindelberg PL, Tasker RR, editors. Textbook for stereotactic and functional neurosurgery. New York: McGraw-Hill; 1998. p. 1087–99.
Wenger N, Moraud EM, Raspopovic S et al. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci Transl Med. 2014;6:255ra133.
Wernig A, Müller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992;30:229–38.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Hofstoetter, U.S., Freundl, B., Binder, H., Minassian, K. (2018). Spinal Cord Stimulation as a Neuromodulatory Intervention for Altered Motor Control Following Spinal Cord Injury. In: Sandrini, G., Homberg, V., Saltuari, L., Smania, N., Pedrocchi, A. (eds) Advanced Technologies for the Rehabilitation of Gait and Balance Disorders. Biosystems & Biorobotics, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-72736-3_33
Download citation
DOI: https://doi.org/10.1007/978-3-319-72736-3_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72735-6
Online ISBN: 978-3-319-72736-3
eBook Packages: EngineeringEngineering (R0)