Abstract
Functional electrical stimulation has been applied for more than half a century to restore and support gait in patients after stroke or after spinal cord injury. Most prevalent are assistive systems for the correction of drop foot in stroke patients using either surface or implanted stimulation technology. For therapeutical use in clinical environments, multi-channel FES systems are often employed in combination with robotic devices or partial body weight support during walking on a treadmill. The restoration of gait in spinal cord injured people is also an ongoing research topic. New implantable stimulation systems and hybrid approaches that combine powered exoskeletons and FES are under investigation. Inertial sensor technology, electromyographic sensing, and advanced feedback control are predicted to be key technologies of future FES systems that allow a more patient and situation-specific gait support.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Stroke is among the leading causes of gait impairments. The prevalence of stroke events is expected to increase as the global population aged over 65 increases. In Europe, the number of stroke events per year is estimated to rise from 1.1 million in 2000 to 1.5 million by 2025 [1]. Worldwide, 15 million people experience a stroke each year; one third die and one third are left permanently disabled [2]. About 50–85% of stroke survivors are able to walk independently within six months after stroke [3, 4]. Although the majority of stroke patients achieve independent walking, chronic gait abnormalities persist, and many stroke survivors do not reach a walking level that enables them to perform all their daily activities. A walking speed of 0.8 m/s or less excludes most individuals from participating in walking-related activities in the community [5]. More than 50% of ambulatory stroke patients fall while walking or because of losing their balance due to muscle weakness or coordination problems [6].
Stroke patients experience a paresis of one side of the body (hemiparesis) and are not able to time and adjust muscle contractions appropriately. These impairments in conjunction with poor endurance and balance adversely affect walking. A few weeks after the stroke event, spasticity and changes in the mechanical properties of the muscles (e.g. stiffness in antagonistic muscles) often develop. Post-stroke hemiplegic gait is a mixture of deviations from normal joint kinematics and compensatory motion dictated by residual functions. This gait is often characterized by inability to flex the hip, knee and ankle joint of the paretic leg due to muscle weakness in the corresponding muscles and increased muscle activity in the antagonistic muscles. The resulting increased leg length causes toe dragging or circumduction of the leg (i.e. stiff-legged gait).
The limited ability to lift the inner (medial) or outer (lateral) edge of the foot, or both, by voluntary muscle activation is known as drop foot syndrome and it is present in about 20% of ambulatory chronic stroke patients [4]. In addition, there is often decreased hip and knee flexion at initial contact and mid-swing, while ankle plantar flexion is reduced at toe-off and increased at initial contact and during the swing phase. The pelvis of the hemiplegic side is mostly elevated (hip hike), and translation of the trunk occurs over the unaffected side to support foot clearance of the affected leg during the swing phase. Abnormal movement patterns can also be observed on the unaffected side, due to compensation movements in addition to muscle weakness.
Spinal cord injury (SCI) is another cause of gait impairment. The prevalence of traumatic SCI is highest in the United States of America (906 per million) [7]. The majority of studies reported by Singh et al. showed a high male-to-female ratio and an age at peak incidence younger than 30 years old. Traffic accidents were typically the most common cause of SCI, followed by falls in the elderly population. Depending on the completeness and level of the lesion, paresis and paralysis of the leg musculature will occur. A complete lesion above the thoracic level will cause a motor and sensory paralysis of both legs (paraplegia), resulting in immobilization of the patient and possible lifelong wheelchair dependence. A complete loss of bladder, bowel and sexual function is also common. These primary effects of SCI lead to a range of secondary medical complications, e.g. atrophy of the paralyzed muscles and decreased cardiovascular fitness.
In stroke patients or individuals with SCI, the signal pathway from the central nervous system (CNS) to the muscles is interrupted. However, the muscles themselves retain their ability to contract and produce force. Functional electrical stimulation (FES) applied to the still intact lower motor neurons can replace the missing signals from the CNS and can be used to generate muscle contractions. In combination with appropriate sensor technology and feedback control, this method can be exploited to elicit or support walking movements.
FES has a direct orthotic or prosthetic effect on walking and can also be used as an assistive technology in daily life. During rehabilitation in a clinical environment, FES is mostly applied to achieve a therapeutic (carry-over) effect. This includes an increase of muscle strength as well as improvements in endurance and cardiovascular fitness. The increased afferent feedback provided by FES is known to modulate motor cortex function and excitability. FES can also be used to give indications about the right timing of muscle activities to sensory-unimpaired patients during walking. Recent findings (Gandolla et al. [8], for example) advocate the use of FES co-incidentally with the voluntary drive to enhance the plasticity of the central nervous system, and thus further improve its therapeutic effects. A recent review of the therapeutic effects of FES on gait in stroke patients [9] provides a detailed discussion of FES-induced brain plasticity and motor learning.
This chapter is organized as follows. In Sect. 1.2, a general overview of technological aspects of FES-assisted gait training is given. Then, the state of the art in drop foot correction for chronic stroke patients is presented in Sect. 1.3. The use of multi-channel FES for gait therapy in clinical environments is investigated in Sect. 1.4. Finally, in Sect. 1.5 we describe the application of FES for the restoration of gait in SCI individuals.
2 Technological Aspects of FES-Assisted Gait
2.1 Real-Time Gait Phase Detection
The core element of any FES system for walking is a robust and reliable real-time gait phase detection (GPD). The early systems, which are still the most widely available on the market, use force-resistive switches below the heel to distinguish the stand and swing phases of gait. Recent work extended this approach by integrating knitted resistive strain sensors into socks to derive gait phases indirectly from the measured ankle joint angle [10]. Force/strain sensors generally require patient-specific calibration and have a limited lifespan due to the repeated loading. Therefore, many researchers investigated the use of accelerometers and gyroscopes for GPD (see, for example, Chia Bejarano et al. [11], Rueterbories et al. [12], Seel et al. [13, 14]). Such sensors can be attached to the shoe/foot and/or to the shank and facilitate more detailed GPD. Figure 1 shows a state automaton that describes the gait phases and gait events that can be detected by a full miniature inertial sensor (contains accelerometer and gyroscope, both 3D) placed on the instep of the foot [13, 14]. Current integration of electronics will soon yield wireless inertial sensors with the size of a coin, which can be placed inside the shoe or worn unnoticed under clothes. With implants, gait phases might also be derived from natural sources like nerve activity [15]. Taborri et al. provided a systematic overview of gait partitioning methods [16].
2.2 Real-Time Assessment of Segment Orientations and Joint Angles
Wearable sensor technologies also enable real-time measurements of body segment orientation, e.g. the roll and pitch angle of the foot with respect to the ground, as well as walking velocity, stride lengths and joint angles. Such information can be used to adjust stimulation parameters during walking in order to improve the stimulation outcome, i.e. the motion of the patient. Knitted resistive strain sensors [10] and bio-impedance measurements [17] represent potential technologies for real-time assessment of joint angles. Both technologies can be integrated into textiles. Due to rapidly decreasing form factors, inertial sensors represent a particularly promising technology for ambulatory real-time motion analysis.
For example, by attaching an inertial sensor to each foot, shank and thigh, as well as an additional sensor to the hip, one can derive hip, knee and ankle joint angles with measurement accuracies that are comparable to those of optical motion capture systems. However, a few limitations remain. Precise placement of the inertial sensors or a sequence of predefined precise calibration movements is required, both of which can be challenging for motor-impaired patients. Another limiting factor is that many approaches require the use of magnetometers, which are known to be unreliable inside buildings and near ferromagnetic materials. Just recently, new methods have been proposed that overcome both of these limitations and enable automatic sensor-to-segment calibration of inertial sensor networks from arbitrary motions, including walking itself [18]. In view of these developments, future motion analysis systems are expected to be plug-and-play, in the sense that the sensors are attached in arbitrary orientation and calibrate automatically as the patient starts to walk [19].
2.3 Real-Time Assessment of Muscle Activity
Electromyography (EMG) can be used for multiple purposes in FES gait training. Figure 2 shows an example of a raw surface electromyography (sEMG) recording during active FES. When analyzing EMG signals, one has to distinguish between FES-evoked EMG and patient-induced EMG activity, where the latter includes both intentional (volitional) and unintentional muscle activity [20]. By means of online signal processing, both quantities can be determined from the raw EMG also in between the stimulation pulses, i.e. during active stimulation. The FES-evoked EMG is manifested in the so-called M-wave which is a good measure of the amount of motor units recruited by the last stimulation impulses. Recent studies in the upper extremities show that feedback control of the M-wave magnitude compensates for the effects of muscular fatigue and maintains a desired stimulation effect (e.g. force production) [21, 22].
The EMG activity that is due to patient-induced muscle activity is much smaller than the M-wave. This rather noise-like signal with frequency components in the range of 30–300 Hz [23] can be separated from the M-wave about 20–30 ms after each stimulation pulse by high-pass filtering or by subtraction of an estimated/predicted M-wave (see, for example, Ambrosini et al. [24]). The patient’s EMG can be used to trigger the stimulation onset, to modulate the intensity profile of stimulation or simply to monitor the effect of stimulation on the muscle activity and motor coordination of the patient. It has been demonstrated [25] that patient-induced muscle activity can be detected when using integrated stimulation and measurement electrodes. However, the simultaneous assessment of FES-induced and patient-induced muscle activity still requires the use of separate electrodes for stimulation and EMG monitoring.
2.4 Electrode and Stimulator Technology
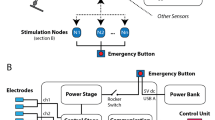
Typically, charge-balanced bi-phasic stimulation impulses are delivered either via surface (skin) electrodes or via implanted electrodes to elicit action potentials in motor nerves of the target muscles. Surface electrodes are self-adhesive with a conductive hydro-gel and can be reused several times. Often, cuffs or body straps are used to improve the electrode-skin contact, whereas integration in trousers is less common. To overcome the poor selectivity of large surface electrodes and the difficulties of electrode placement, the use of electrode arrays (multipad electrodes, see Fig. 3) has been proposed (see, for example, Heller et al. [26], Malesevic et al. [27, 28], Valtin et al. [29]). Such arrays contain many small electrodes and enable the formation of virtual electrodes consisting of a subset of these electrodes. Most arrays still use a common hydrogel layer below all electrodes [30]. This means that stimulation between two virtual electrodes inside one and the same array is not feasible. Instead, two arrays or one array and a conventional counter electrode are required to apply FES.
Electrode arrays are used to overcome the requirement of precise placement and limited selectivity. Shown is a flexible electrode array for the stimulation of the peroneal nerve, as used e.g. in [29]. Each element of the array has a size of 8 mm × 8 mm
Stimulation with surface electrodes also excites pain receptors in the skin, which implies that stimulation intensities and producible forces are limited in patients with intact sensation. Percutaneous electrodes (inserted through the skin) or fully implanted electrodes cause less sensation and allow more selective muscle activation, also of more deeply located muscles. Implanted systems typically use nerve cuffs with a small array of multiple electrodes. To mitigate the problem of fast muscle fatigue in electrically stimulated muscles, alternating activation of these electrodes is performed to simulate the natural asynchronous muscle activation. The same effect is achieved with surface electrode arrays by alternating between virtual electrodes with similar stimulation outcome [31].
2.5 Open-Loop and Closed-Loop Control
Most FES systems control the timing of stimulation by detecting gait events, sensing volitional EMG activity or by hand-operated switches. Stimulation profiles are usually pre-programmed (feedforward control/open-loop control) and can only be changed manually. If the muscles fatigue or the muscle tone changes (spasticity), the FES parameters must be adjusted to obtain the same motion as before. Since repeated manual adjustments are undesirable, current research focuses on automatic adjustment of FES parameters to the current situation-dependent needs of the patient. Such closed-loop systems monitor the obtained motion and choose the stimulation intensities in such a way that the desired functional motion is obtained. At the same time, the onset of muscle fatigue can be delayed by avoiding over-stimulation.
Recently, wireless inertial sensors have increasingly been used to realize such closed-loop FES systems. The control algorithm that evaluates the measurements and decides how to adjust the FES parameters is typically implemented on a microcontroller. In conventional feedback control, the current stimulation intensity is adjusted on the basis of the current measurements. Since FES dynamics are slow and gait involves rather quick muscle contractions and motions, this conventional control approach was found to be of limited use. Learning control methods, like run-to-run control (see, for example, Veltink et al. [32]) or iterative learning control (see, for example, Seel et al. [14, 33]), are capable of exploiting the repetitive nature of gait by learning from the measurements obtained in previous strides. While run-to-run control typically aims at improving the amplitude of a stimulation window, iterative learning control can be used to optimize the entire time course of the FES intensities during each stride.
3 Drop Foot Stimulation
Liberson et al. [34] proposed the first clinical application of FES: stimulation of the peroneal nerve for correction of foot drop during the swing phase of gait. The peroneal nerve divides into a superficial and a deep branch, which innervate the m. fibularis longus and m. tibialis anterior, respectively. In a standard drop foot stimulator, both muscles are activated by positioning a pair of surface electrodes on the skin close to the head of fibula and on the insertion of the m. tibialis anterior. Both electrodes must be carefully placed to obtain a sufficient foot lift (dorsiflexion) without exaggerated eversion or inversion. The effect of stimulation is very sensitive to small (~1 cm) changes in the electrode positions and varies “from day to day due to a number of factors: changes in skin resistance due to sweating or dryness of the skin, condition of electrodes and fatigue and changes in resistance to dorsi-flexion caused by spasticity of the calf muscles” [35]. The review articles by Lyons et al. [36], Melo et al. [37] provide an excellent overview of drop foot stimulators in research and industry and classify them in several ways. Until now, all commercially available devices have been based solely on open-loop architectures, i.e. they only use sensors to time the stimulation [37]—typically a simple heel switch.
Several studies have shown the orthotic and therapeutic (carry-over) effects for both transcutaneous and implanted drop foot stimulation systems in terms of improved foot lift and walking speed, reduced Physiological Cost Index (PCI) and improved balance during walking (see, for example, Burridge et al. [35], Hausdorff and Ring [38], Kottink et al. [39, 40], Martin et al. [41], Ring et al. [42], Schiemanck et al. [43], Sheffler et al. [44]; Stein et al. [45], van Swigchem et al. [46], Taylor et al. [47, 48], Wilder et al. [49]). Many patients prefer drop foot stimulators to conventional ankle-foot orthoses [50, 51].
One of the best clinically evaluated systems is the Odstock dropped foot stimulator (ODFS) (Odstock Medical Ltd.). During the last two decades, several new systems have entered the marked, e.g. L300/L300plus/L300 Go (Bioness Inc.), WalkAide (Innovative Neurotronics Inc.), MyGait (Ottobock Healthcare GmbH) and ODFS Leg Cuff (Odstock Medical Ltd.). An important feature of these new systems is the integration of the stimulation device and electrodes in a leg cuff that is worn at the shank below the knee. Furthermore, the systems L300/L300plus, MyGait and ODFS Leg Cuff use a wireless heel switch. WalkAide does not rely on a heel switch at all—instead it uses an inclination sensor in the cuff to determine the gait phase [52]. An additional second stimulation channel for supporting plantar flexion, knee flexion, knee extension or hip extension is available in some of the systems.
In some patients, a desired foot motion cannot be achieved by transcutaneous stimulation, and some do not tolerate the stimulation intensities that are required for sufficient foot lift. Implantable dropped foot stimulators represent potential alternatives for these patients. Two systems have been developed: the two-channel implant Stimustep (Finetech Medical, UK) stimulates the n. peroneus profundus and the n. peroneus superficialis (both branches of the n. peroneus communis) to obtain better control over dorsiflexion and foot eversion/inversion [53]. The ActiGait system (Ottobock Healthcare GmbH) selectively activates the n. peroneus communis by means of a cuff electrode with four stimulation channels to achieve the same goal [54].
Besides these commercial systems, there are a number of promising novel systems being developed in research projects. Some recent contributions suggest the use of electrode arrays in combination with a search algorithm that finds the best position of a virtual electrode (see, for example, Heller et al. [26], Malesevic et al. [27], Prenton et al. [28], Valtin et al. [29]). At the current state of the art, however, this identification takes several minutes, and the virtual electrode is not adjusted when muscle tone or FES dynamics (and thus the induced foot motion) change during walking.
Also for transcutaneous stimulation, Seel et al. [33] proposed and investigated a three-electrode setup to manipulate the recruitment of the m. tibialis anterior and m. fibularis longus via two independent FES channels. Gait phase transitions as well as foot pitch and roll angles were assessed in real time by means of a shoe-mounted wireless inertial sensor. A decentralized iterative learning control scheme was used to adjust the stimulation intensity profiles to the current needs of the individual patient. Starting from conventional stimulation parameters, the controller automatically determined individual stimulation parameters and thereby achieved physiological foot pitch and roll angle trajectories within at most two strides in walking drop foot patients.
The application of EMG-derived stimulation intensity profiles has been investigated by Byrne et al. [55], Chen et al. [56], O’Keeffe and Lyons [57]. A stimulation proportional to the residual volitional activity of the m. tibialis anterior has been advocated by different authors [58–60]. Kesar et al. [61] suggested the use of variable-frequency pulse trains to enhance correction of foot drop compared with traditional FES systems that deliver constant-frequency pulse trains.
4 FES-Assisted Gait Therapy in Stroke Patients
Intensive FES training programs are often applied in clinics to restore and enhance gait patterns after stroke, both in sub-acute and chronic patients [9]. The meta-analysis by Teasell et al. [62] suggests FES as an adjunctive therapy in gait training. Another meta-analysis by Robbins et al. [63] showed a significant positive effect of FES on walking speed. A randomized controlled study even demonstrated recovery of coordinated gait in chronic stroke patients [64].
In clinical studies, the number of stimulation channels has been very diverse. In the study by Salisbury et al. [65], for example, a single-channel drop foot stimulator was employed for gait therapy of sub-acute stroke patients. By contrast, one of the first randomized studies with multi-channel FES considered heel-switch-triggered stimulation of the peroneal nerve as well as of the muscles triceps surae, hamstring, quadriceps femoris, gluteus maximus and triceps brachii [66]. Most clinical systems use transcutaneous stimulation, but the use of intramuscular (percutaneous) electrodes is also feasible, as demonstrated by Daly et al. [64].
The stimulation can be applied during level-ground walking, during treadmill walking (in possible combination with partial body weight support) (see, for example, Cho et al. [67], Daly et al. [64], Hesse et al. [68], Kesar et al. [69], Lindquist et al. [70], or in combination with a gravity-balanced orthosis [71], electromechanical gait trainers (e.g. [72]), or robotic locomotion devices (e.g. Dohring and Daly [73], McCabe et al. [74]). In the latter two cases, the timing of stimulation is usually derived from the mechanical support system and not by means of inertial sensors or heel switches. In order to better prepare non-ambulatory patients for gait training, FES cycling ergometers [75] or bed-side stimulation settings (for acute stroke patients) [76] can also be used.
Due to the necessary cabling effort, currently available clinical stimulation systems are cumbersome to apply—especially if many stimulation channels are used. In future, distributed wireless stimulation and sensor systems will enhance the usability of multichannel FES systems. The first prototypes have already been presented [77, 78].
5 FES-Assisted Ambulation After Spinal Cord Injury
The realization of standing and stepping in paraplegic individuals requires several stimulation channels. Bilateral stimulation of the m. quadriceps (for knee extension), peroneal nerve and, optionally, the m. gluteus maximus using surface electrodes represents a common approach [79]. To stand up and during standing, both quadriceps muscles are activated. To initiate a step, activation of the quadriceps stimulation is paused on one side, and the peroneal nerve is excited to elicit the withdrawal reflex (causing a flexion of the leg). The user will transfer his flexed leg forward by means of the upper body while holding on to a roller walker for balance. The initiation of steps is typically controlled by hand switches that are mounted on the handles of the walker. A commercially available system that uses this principle is the ParaStep system (Sigmedics, Inc.) [80, 81]. It is recommended for paraplegics with a lesion level between T4 and T12. The resulting stepping movement is not very physiological and not comparable to normal walking. Before using the system, a long-lasting and intensive program is required to build up fatigue-resistant muscles by electrical stimulation. A review of technical aspects of FES control of standing and stepping after SCI is provided by Braz et al. [82].
Implanted FES systems with up to 16 channels provide mobility in paraplegics [19, 83, 84, 85] and in individuals with incomplete spinal cord injury (iSCI) [86]. The use of implants reduces donning time and improves day-to-day repeatability compared to transcutaneous FES systems. Individuals with iSCI retain some control of the partially paralyzed muscles, which requires careful integration of FES. Dutta et al. [87] successfully exploited volitionally induced surface EMG activity to trigger stimulation via an implanted stimulation system in an iSCI individual.
The combination of FES with reciprocating gait orthoses (RGO) or powered exoskeletons offers hybrid solutions for the ambulation of SCI individuals. The mechanical structures stabilize the leg joints and trunk and provide good postural stability, while ambulation is supported by the artificially activated paralyzed muscles. Powered exoskeletons ensure precise movement execution by the motors even in the presence of time-varying muscle dynamics, providing consistent and repeatable gait. Hence, hybrid systems can combine the advantages of FES and orthoses/exoskeletons and thereby offer more advantages than the individual components alone. Examples of hybrid FES orthoses are described in papers by Durfee and Rivard [88], Kobetic et al. [89]. A good overview of hybrid FES-powered exoskeletons is given by del Ama et al. [90]. First results for the use of a hybrid FES-powered exoskeleton on three paraplegic subjects with motor-complete SCI are presented by Ha et al. [91]. For case studies with iSCI individuals using the hybrid Kinesis system (see Fig. 4) see del-Ama et al. [92], Del-Ama et al. [93].
Patient with incomplete spinal cord injury walking with the hybrid FES-powered exoskeleton Kinesis [92]
6 Conclusions
Functional electrical stimulation is a highly useful technology for the restoration and support of gait in stroke patients and spinal cord-injured individuals. Several studies have shown the orthotic and therapeutic (carry-over) effects for both transcutaneous and implanted drop foot stimulation systems.
Besides assistive systems for gait support of chronic drop foot patients, multichannel FES systems are available for therapeutic use in clinical environments.
The latter might be used in combination with robotic devices or partial body weight support during walking on a treadmill. The restoration of gait in spinal cord-injured people, however, remains an ongoing research topic. New implantable stimulation systems and hybrid approaches that combine powered exoskeletons and FES are under investigation.
Remaining drawbacks and limitations of dropped foot stimulators, multi-channel FES systems and hybrid gait support systems might be overcome by present and future technological progress in the fields of inertial sensor motion analysis, electromyographic sensing and advanced feedback control. Future systems may be expected to adjust automatically to the individual user and to provide a more patient- and situation-specific gait support.
References
Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13(6):581–98.
Mackay J, Mensah GA, Mendis S, Greenlund K (2004) The atlas of heart disease and stroke. World Health Organization.
Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke study. Arch Phys Med Rehabil. 1995;76(1):27–32.
Wade DT, Wood VA, Heller A, Maggs J, Hewer RL. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987;19(1):25–30.
Robinson CA, Shumway-Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil. 2011;33(12):1033–42.
Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83(2):165–70.
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Gandolla M, Ferrante S, Molteni F, Guanziroli E, Frattini T, Martegani A, Ferrigno G, Friston K, Pedrocchi A, Ward NS. Re-thinking the role of motor cortex: context-sensitive motor outputs? NeuroImage. 2014;91:366–74.
Kafri M, Laufer Y. Therapeutic effects of functional electrical stimulation on gait in individuals post-stroke. Ann Biomed Eng. 2015;43(2):451–66. https://doi.org/10.1007/s10439-014-1148-8.
Preece SJ, Kenney LP, Major MJ, Dias T, Lay E, Fernandes BT. Automatic identification of gait events using an instrumented sock. J Neuroengineering Rehabil. 2011;8(1):1.
Chia Bejarano N, Ambrosini E, Pedrocchi A, Ferrigno G, Monticone M, Ferrante S. A novel adaptive, real-time algorithm to detect gait events from wearable sensors. IEEE Trans Neural Syst Rehabil Eng. 2015;23(3):413–22. https://doi.org/10.1109/TNSRE.2014.2337914.
Rueterbories J, Spaich EG, Andersen OK. Gait event detection for use in FES rehabilitation by radial and tangential foot accelerations. Med Eng Phys. 2014;36(4):502–8.
Seel T, Escobar VC, Raisch J, Schauer T. Online gait phase detection with automatic adaption to gait velocity changes using accelerometers and gyroscopes. Biomed Eng/Biomed Techik. 2014;59(s1):S795–8. https://doi.org/10.1515/bmt-2014-5011.
Seel T, Werner C, Raisch J, Schauer T. Iterative learning control of a drop foot neuroprosthesis—generating physiological foot motion in paretic gait by automatic feedback control. Control Eng Pract. 2016;48:87–97.
Sinkjaer T, Haugland M, Inmann A, Hansen M, Nielsen KD. Biopotentials as command and feedback signals in functional electrical stimulation systems. Med Eng Phys. 2003;25(1):29–40.
Taborri J, Palermo E, Rossi S, Cappa P. Gait partitioning methods: a systematic review. Sensors 16(1):66. http://doi.org/10.3390/s16010066.
Nahrstaedt H, Schauer T, Shalaby R, Hesse S, Raisch J. Automatic control of a drop foot stimulator based on angle measurement using bioimpedance. Artif Organs. 2008;32(8):649–54.
Seel T, Raisch J, Schauer T. IMU-based joint angle measurement for gait analysis. Sensors. 2014;14(4):6891–909. https://doi.org/10.3390/s140406891.
Graurock D, Schauer T, Seel T. Automatic pairing of inertial sensors to lower limb segments—a plugand-play approach. Current Direct Biomed Eng. 2016;2:715–8. https://doi.org/10.1515/cdbme-2016-01552364-5504.
Merletti R, Knaflitz M, De Luca CJ. Electrically evoked myoelectric signals. Crit Rev Biomed Eng. 1992;19(4):293–340.
Klauer C, Raisch J, Schauer T. Linearisation of electrically stimulated muscles by feedback control of the muscular recruitment measured by evoked EMG. In: 2012 17th international conference on IEEE, Methods and Models in Automation and Robotics (MMAR); 2012. p. 108–13.
Klauer C, Ferrante S, Ambrosini E, Shiri U, Dahne F, Schmehl I, Pedrocchi A, Schauer T. A patient-controlled functional electrical stimulation system for arm weight relief. Med Eng Phys. 2016;38(11):1232–43.
De Luca CF, Knaflitz M. Surface electromyography, what’s new?. Turin, Italy: CLUT Publishers; 1992.
Ambrosini E, Ferrante S, Schauer T, Klauer C, Gaffuri M, Ferrigno G, Pedrocchi A. A myocontrolled neuroprosthesis integrated with a passive exoskeleton to support upper limb activities. J Electromyogr Kinesiol. 2014;24(2):307–17. https://doi.org/10.1016/j.jelekin.2014.01.006.
Shalaby R, Schauer T, Liedecke W, Raisch J. Amplifier design for EMG recording from stimulation electrodes during functional electrical stimulation leg cycling ergometry. Biomed Technik Biomed Eng. 2011;56(1):23–33.
Heller BW, Clarke AJ, Good TR, Healey TJ, Nair S, Pratt EJ, Reeves ML, van der Meulen JM, Barker AT. Automated setup of functional electrical stimulation for drop foot using a novel 64 channel prototype stimulator and electrode array: results from a gait-lab based study. Med Eng Phys. 2013;35(1):74–81. https://doi.org/10.1016/j.medengphy.2012.03.012.
Malesevic J, Malesevic N, Bijelic G, Keller T, Konstantinovic L. Multi-pad stimulation device for treating foot drop: Case study. In: Annual conference of the Functional Electrical Stimulation Society (IFESS), IEEE, IEEE; 2014. p. 1–4.
Prenton S, Kenney LP, Stapleton C, Cooper G, Reeves ML, Heller BW, Sobuh M, Barker AT, Healey J, Good TR, et al. Feasibility study of a take-home array-based functional electrical stimulation system with automated setup for current functional electrical stimulation users with foot-drop. Arch Phys Med Rehabil. 2014;95(10):1870–7.
Valtin M, Seel T, Raisch J, Schauer T. Iterative learning control of drop foot stimulation with array electrodes for selective muscle activation. In: Proceedings of the 19th IFAC World Congress; 2014. p. 6587–92.
Cooper G, Barker AT, Heller BW, Good T, Kenney LP, Howard D. The use of hydrogel as an electrode-skin interface for electrode array FES applications. Med Eng Phys. 2011;33(8):967–72.
Sayenko DG, Nguyen R, Popovic MR, Masani K. Reducing muscle fatigue during transcutaneous neuromuscular electrical stimulation by spatially and sequentially distributing electrical stimulation sources. Eur J Appl Physiol. 2014;114(4):793–804.
Veltink PH, Slycke P, Hemssems J, Buschman R, Bultstra G, Hermens H. Three dimensional inertial sensing of foot movements for automatic tuning of a two-channel implantable drop-foot stimulator. Med Eng Phys. 2003;25(1):21–8.
Seel T, Werner C, Schauer T. The adaptive drop foot stimulator—multivariable learning control of foot pitch and roll motion in paretic gait. Med Eng Phys. 2016;38(11):1205–13.
Liberson W, Holmquest H, Scott M. Functional electrotherapy: stimulation of the common peroneal nerve synchronised with the swing phase of gait of hemiplegic subjects. Arch Phys Med Rehabil. 1961;42:202–5.
Burridge J, Taylor P, Hagan S, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11(3):201–10.
Lyons GM, Sinkjaer T, Burridge JH, Wilcox DJ. A review of portable FES-based neural orthoses for the correction of drop foot. IEEE Trans Neural Syst Rehabil Eng. 2002;10(4):260–79.
Melo PL, Silva MT, Martins JM, Newman DJ. Technical developments of functional electrical stimulation to correct drop foot: sensing, actuation and control strategies. Clin Biomech. 2015;30(2):101–13. https://doi.org/10.1016/j.clinbiomech.2014.11.007.
Hausdorff JM, Ring H. Effects of a new radio frequency-controlled neuroprosthesis on gait symmetry and rhythmicity in patients with chronic hemiparesis. Am J Phys Med Rehabil. 2008;87(1):4–13. https://doi.org/10.1097/PHM.0b013e31815e6680.
Kottink AIR, Oostendorp LJM, Buurke JH, Nene AV, Hermens HJ. IJzerman MJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004;28(6):577–86. https://doi.org/10.1111/j.1525-1594.2004.07310.x.
Kottink AIR, Hermens HJ, Nene AV, Tenniglo MJ, Groothuis-Oudshoorn CG. IJzerman MJ. Therapeutic effect of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized controlled trial. Phys Ther. 2008;88(4):437–48. https://doi.org/10.2522/ptj.20070035.
Martin KD, Polanski W, Schackert G, Sobottka SB. New therapeutic option for drop foot with the ActiGait peroneal nerve stimulator—a technical note. World Neurosurg. 2015;84(6):2037–42. https://doi.org/10.1016/j.wneu.2015.06.074.
Ring H, Treger I, Gruendlinger L, Hausdorff JM. Neuroprosthesis for foot-drop compared with an ankle-foot orthosis: effects on postural control during walking. J Stroke Cerebrovasc Dis. 2009;18(1):41–7.
Schiemanck S, Berenpas F, van Swigchem R, van den Munckhof P, de Vries J, Beelen A, Nollet F, Geurts AC. Effects of implantable peroneal nerve stimulation on gait quality, energy expenditure, participation and user satisfaction in patients with post-stroke drop foot using an ankle-foot orthosis. Restorative Neurol Neurosci. 2015;33(6):795–807. https://doi.org/10.3233/rnn-150501.
Sheffler LR, Hennessey MT, Naples GG, Chae J. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabilitation Neural Repair. 2006;20(3):355–60. https://doi.org/10.1177/1545968306287925.
Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J, Kuether G. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24(2):152–67. https://doi.org/10.1177/1545968309347681.
van Swigchem R, Weerdesteyn V, van Duijnhoven HJ, den Boer J, Beems T, Geurts AC. Near-normal gait pattern with peroneal electrical stimulation as a neuroprosthesis in the chronic phase of stroke: a case report. Arch Phys Med Rehabil. 2011;92(2):320–4.
Taylor P, Humphreys L, Swain I. The long-term cost-effectiveness of the use of functional electrical stimulation for the correction of dropped foot due to upper motor neuron lesion. J Rehabil Med. 2013;45(2):154–60. https://doi.org/10.2340/16501977-1090.
Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, Swain ID. Clinical use of the odstock dropped foot stimulator: Its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80(12):1577–83. https://doi.org/10.1016/S0003-9993(99)90333-7.
Wilder RP, Wind TC, Jones EV, Crider BE, Edlich RF. Functional electrical stimulation for a dropped foot. J Long Term Eff Med Implants. 2002;12(3):149–59.
Bulley C, Shiels J, Wilkie K, Salisbury L. User experiences, preferences and choices relating to functional electrical stimulation and ankle foot orthoses for foot-drop after stroke. Physiotherapy. 2011;97(3):226–33. https://doi.org/10.1016/j.physio.2010.11.001.
van Swigchem R, Vloothuis J, den Boer J, Weerdesteyn V, Geurts AC. Is transcutaneous peroneal stimulation beneficial to patients with chronic stroke using an ankle-foot orthosis? A within-subjects study of patients’ satisfaction, walking speed and physical activity level. J Rehabil Med. 2010;42(2):117–21.
Weber DJ, Stein RB, Chan KM, Loeb G, Richmond F, Rolf R, James K, Chong SL. BIONic WalkAide for correcting foot drop. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2005;13(2):242–6. https://doi.org/10.1109/TNSRE.2005.847385.
Kenney L, Bultstra G, Buschman R, Taylor P, Mann G, Hermens H, Holsheimer J, Nene A, Tenniglo M, van der Aa H, Hobby J. An implantable two channel drop foot stimulator: initial clinical results. Artif Organs. 2002;26(3):267–70.
Burridge JH, Haugland M, Larsen B, Svaneborg N, Iversen HK, Christensen PB, Pickering RM, Sinkjaer T. Patients’ perceptions of the benefits and problems of using the ActiGait implanted drop-foot stimulator. J Rehabil Med. 2008;40(10):873–5. https://doi.org/10.2340/16501977-0268.
Byrne CA, O’Keeffe DT, Donnelly AE, Lyons GM. Effect of walking speed changes on tibialis anterior EMG during healthy gait for FES envelope design in drop foot correction. J Electromyogr Kinesiol. 2007;17(5):605–16. https://doi.org/10.1016/j.jelekin.2006.07.008.
Chen M, Wu B, Lou X, Zhao T, Li J, Xu Z, Hu X, Zheng X. A self- adaptive foot drop corrector using functional electrical stimulation (FES) modulated by tibialis anterior electromyography (EMG) dataset. Med Eng Phys. 2013;35(2):195–204. https://doi.org/10.1016/j.medengphy.2012.04.016.
O’Keeffe DT, Lyons GM. A versatile drop foot stimulator for research applications. Med Eng Phys. 2002;24(3):237–42.
Chen WL, Chen SC, Chen CC, Chou CH, Shih YY, Chen YL, Kuo TS. Patient-driven loop control for ambulation function restoration in a non-invasive functional electrical stimulation system. Dis Rehabil. 2010;32(1):65–71. https://doi.org/10.3109/09638280903026564.
Thorsen R, Ferrarin M, Veltink P. Enhancement of isometric ankle dorsiflex- ion by automyoelectrically controlled functional electrical stimulation on subjects with upper motor neuron lesions. Neuromodulation. 2002;5(4):256–63.
Yeom H, Chang YH. Autogenic EMG-controlled functional electrical stimulation for ankle dorsiflexion control. J Neurosci Methods. 2010;193(1):118–25. https://doi.org/10.1016/j.jneumeth.2010.08.011.
Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS, Binder-Macleod SA. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther. 2010;90(1):55–66. https://doi.org/10.2522/ptj.20090140.
Teasell RW, Bhogal SK, Foley NC, Speechley MR. Gait retraining post stroke. Topics Stroke Rehabil. 2003;10(2):34–65. https://doi.org/10.1310/UDXE-MJFF-53V2-EAP0.
Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. 2006;87(6):853–9.
Daly JJ, Zimbelman J, Roenigk KL, McCabe JP, Rogers JM, Butler K, Burdsall R, Holcomb JP, Marsolais EB, Ruff RL. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabilitation and Neural Repair. 2011;25(7):588–96. https://doi.org/10.1177/1545968311400092.
Salisbury L, Shiels J, Todd I, Dennis M. A feasibility study to investigate the clinical application of functional electrical stimulation (FES), for dropped foot, during the sub-acute phase of stroke—a randomized controlled trial. Physiotherapy Theor Pract. 2013;29(1):31–40. https://doi.org/10.3109/09593985.2012.674087.
Bogataj U, Gros N, Kljajic M, Acimovic R, Malezic M. The rehabilitation of gait in patients with hemiplegia: a comparison between conventional therapy and multichannel functional electrical stimulation therapy. Phys Ther. 1995;75(6):490–502.
Cho MK, Kim JH, Chung Y, Hwang S. Treadmill gait training combined with functional electrical stimulation on hip abductor and ankle dorsiflexor muscles for chronic hemiparesis. Gait Posture. 2015;42(1):73–8.
Hesse S, Malezic M, Schaffrin A, Mauritz K. Restoration of gait by combined treadmill training and multichannel electrical stimulation in non-ambulatory hemiparetic patients. Scand J Rehabil Med. 1995;27(4):199–204.
Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, Binder-Macleod SA. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011;33(2):309–13. https://doi.org/10.1016/j.gaitpost.2010.11.019.
Lindquist AR, Prado CL, Barros RM, Mattioli R, Da Costa PHL, Salvini TF. Gait training combining partial body-weight support, a treadmill, and functional electrical stimulation: effects on poststroke gait. Phys Ther. 2007;87(9):1144–54.
Krishnamoorthy V, Hsu WL, Kesar TM, Benoit DL, Banala SK, Perumal R, Sangwan V, Binder-Macleod SA, Agrawal SK, Scholz JP. Gait training after stroke: a pilot study combining a gravity-balanced orthosis, functional electrical stimulation, and visual feedback. J Neurol Phys Ther JNPT. 2008;32(4):192–202. https://doi.org/10.1097/NPT.0b013e31818e8fc2.
Tong RK, Ng MF, Li LS. Effectiveness of gait training using an electromechanical gait trainer, with and without functional electric stimulation, in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87(10):1298–304. https://doi.org/10.1016/j.apmr.2006.06.016.
Dohring ME, Daly JJ. Automatic synchronization of functional electrical stimulation and robotic assisted treadmill training. IEEE Trans Neural Syst Rehabil Eng. 2008;16(3):310–3. https://doi.org/10.1109/TNSRE.2008.920081.
McCabe JP, Dohring ME, Marsolais EB, Rogers J, Burdsall R, Roenigk K, Pundik S, Daly JJ. Feasibility of combining gait robot and multichannel functional electrical stimulation with intramuscular electrodes. J Rehabil Res Dev. 2008;45(7):997–1006.
Ambrosini E, Ferrante S, Pedrocchi A, Ferrigno G, Molteni F. Cycling induced by electrical stimulation improves motor recovery in postacute hemiparetic patients a randomized controlled trial. Stroke. 2011;42(4):1068–73.
Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: A randomized placebo-controlled trial. Stroke. 2005;36(1):80–5.
Jovicic NS, Saranovac LV, Popovic DB. Wireless distributed functional electrical stimulation system. J Neuroengineering Rehabil. 2012;9:54.
Mecheraoui CA, Swain I, Cobb J. A distributed three-channel wireless Functional electrical stimulation system for automated triggering of stimulation to enable coordinated task execution by patients with neurological disease. Biomed Signal Proc Control. 2013;8(2):176–83. https://doi.org/10.1016/j.bspc.2012.08.006.
Kralj A, Bajd T, Turk R. Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin Orthop Relat Res. 1988;233:34–43.
Graupe D, Kohn KH. Functional neuromuscular stimulator for short-distance ambulation by certain thoracic-level spinal-cord-injured paraplegics. Surg Neurol. 1998;50(3):202–7.
Graupe D, Davis R, Kordylewski H, Kohn KH. Ambulation by traumatic t4–12 paraplegics using functional neuromuscular stimulation. Crit Rev Neurosurg. 1998;8(4):221–31.
Braz GP, Russold M, Davis GM. Functional electrical stimulation control of standing and stepping after spinal cord injury: a review of technical characteristics. Neuromodulation J Int Neuromodulation Soc. 2009;12(3):180–90. https://doi.org/10.1111/j.15251403.2009.00213.x.
Guiraud D, Stieglitz T, Koch KP, Divoux JL, Rabischong P. An implantable neuroprosthesis for standing and walking in paraplegia: 5-year patient follow-up. J Neural Eng. 2006;3(4):268–75. https://doi.org/10.1088/1741-2560/3/4/003.
Guiraud D, Coste CA, Benoussaad M, Fattal C. Implanted functional electrical stimulation: case report of a paraplegic patient with complete sci after 9 years. J. Neuroengineering Rehabil. 2014;11:15.
Kobetic R, Triolo RJ, Uhlir JP, Bieri C, Wibowo M, Polando G, Marsolais EB, Davis JA Jr, Ferguson KA. Implanted functional electrical stimulation system for mobility in paraplegia: a follow-up case report. IEEE Trans Rehabil Eng. 1999;7(4):390–8.
von Wild K, Rabischong P, Brunelli G, Benichou M, Krishnan K. Computer added locomotion by implanted electrical stimulation in paraplegic patients (SUAW). Acta Neurochir Suppl. 2002;79:99–104.
Hardin E, Kobetic R, Murray L, Corado-Ahmed M, Pinault G, Sakai J, Bailey SN, Ho C, Triolo RJ. Walking after incomplete spinal cord injury using an implanted FES system: a case report. J Rehabil Res Dev. 2007;44(3):333–46.
Dutta A, Kobetic R, Triolo RJ. Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Trans Bio-Med Eng. 2008;55(2 Pt 1):791–4. https://doi.org/10.1109/TBME.2007.902225.
Durfee WK, Rivard A. Design and simulation of a pneumatic, stored- energy, hybrid orthosis for gait restoration. J Biomech Eng. 2005;127(6):1014–9.
Kobetic R, To CS, Schnellenberger JR, Audu ML, Bulea TC, Gaudio R, Pinault G, Tashman S, Triolo RJ. Development of hybrid orthosis for standing, walking, and stair climbing after spinal cord injury. J Rehabil Res Dev. 2009;46(3):447–62.
del-Ama AJ, Koutsou AD, Moreno JC, de-los Reyes A, Gil-Agudo A, Pons JL. Review of hybrid exoskeletons to restore gait following spinal cord injury. J Rehabil Res Dev. 2012;49(4):497–514.
Ha KH, Murray SA, Goldfarb M. An approach for the cooperative control of FES with a powered exoskeleton during level walking for persons with paraplegia. IEEE Trans Neural Syst Rehabil Eng. 2016;24(4):455–66. https://doi.org/10.1109/TNSRE.2015.2421052.
del-Ama AJ, Gil-Agudo A, Bravo-Esteban E, Perez-Nombela S, Pons JL, Moreno JC. Hybrid therapy of walking with kinesis overground robot for persons with incomplete spinal cord injury: a feasibility study. Robot Auton Syst. 2015;73:44–58.
Del-Ama AJ, Gil-Agudo A, Pons JL, Moreno JC. Hybrid gait training with an overground robot for people with incomplete spinal cord injury: a pilot study. Frontiers Hum Neurosci. 2014;8:298.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Schauer, T., Seel, T. (2018). Gait Training by FES. In: Sandrini, G., Homberg, V., Saltuari, L., Smania, N., Pedrocchi, A. (eds) Advanced Technologies for the Rehabilitation of Gait and Balance Disorders. Biosystems & Biorobotics, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-72736-3_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-72736-3_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72735-6
Online ISBN: 978-3-319-72736-3
eBook Packages: EngineeringEngineering (R0)