Abstract
In the current study, the fluid flow and desulfurization in a 160 t KR ladle were simulated using FLUENT combined with user-developed subroutines. The velocity distribution of the hot metal, the motion and distribution of CaO particles, and the desulfurization rate of the hot metal were calculated. With the increase of rotation speed of the impeller, the velocity of CaO particles increased as well, which resulted in the increase of desulfurization efficiency. The desulfurization rate varied at different positions of the ladle, with a higher rate in the higher speed zone and slower in the dead zone. The desulfurization rate increased slightly with growing rotation speed of the impeller. When the particle diameter increased from 1.3 to 2 mm, the desulfurization rate decreased approximately 3 or 4 times.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
As demand for high-quality steel increases, high efficiency of hot metal desulfurization has become one of main objectives in the steelmaking process. Sulfur which is harmful in most steel always leads to the crack on the surface of steel when the temperature is very high [1]. Almost all of the sulfur in hot metal can be removed by hot metal pretreatment in the process of steelmaking. Kambara Reactor (KR) desulfurization is widely used as a high efficiency and low cost desulfurization method [2, 3]. During KR stirring process, the CaO particles are involved into hot metal by the cross impeller which is rotating at a high speed under the liquid level [4]. The desulfurization reaction between sulfur and CaO particles that occurred in hot metal was investigated by CFD software, because the fluid flow and particles distribution in invisible hot metal internal were invisible only by the experiment. In the current study, the multi-phase flow and the distribution of added CaO particle during KR stirring process were firstly discussed. Then, the characteristics of desulfurization reaction within the metal steel was investigated with the in-house user-developed subroutines. Finally, the effects of rotation speed of the impeller, immersion depth of impeller, and size of CaO particles on the desulfurization reaction were compared.
Models
Calculation Domain and Mesh System
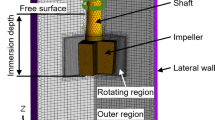
A schematic of calculation domain and meshes of the current model are shown in Fig. 1. In order to simplify the calculation and avoid the convergence problem caused by complex mesh, the bottom of hot metal ladle was regarded as flat which was round in fact. The impeller was cross shape and the diameter of connecting rod was 150 mm. The mesh type was unstructured hexahedral mesh with a total mesh number of about 400,000. The sizes of the 160 t hot metal ladle and the impeller are showed in Table 1 and Table 2, respectively [5].
Models Used and Boundary Conditions
The turbulence model used in the calculation was standard k–\( \varepsilon \) model. In order to obtain the vortex surface distribution, multiphase flow VOF model was used [6]. The desulfurizer particles were added via a discrete phase model (DPM) [7]. The diameter of all the particles was 1 mm. The diffusion of S in hot metal and the desulfurization of CaO particles were performed by solving UDS equations [8].
In the stirring process of KR desulfurization , CaO particles were added into the hot metal to react with sulfur. The main reaction was shown as Eq. 1.
According to chemical reaction kinetics, the desulfurization rate can be expressed as Eq. 2 by assuming that the rate-controlling step is the mass transfer of [S] in hot metal.
where, k is mass transfer rate, A (m2) is the interfacial area that can be expressed as the sum of surface area of all particles, V m is hot metal volume (m3), and [S] (ppm) is the sulfur concentration in hot metal. The mass transfer rate, k, was obtained by Eq. 3, which was proposed by Nakanishi et al. [9].
where D s is the diffusion coefficient of S in hot metal (= 2.58 × 10−9 m2/s), v t is the relative velocity of particle compared with hot metal (m/s), and D t is the diameter of particles (m). Desulfurization behaviors during KR stirring process were estimated by Eqs. (1)–(3).
The boundary conditions were set as follows:
-
a.
The bottom and side wall of the ladle were non-slip walls;
-
b.
The upper outlet of the ladle was a plane without shear stress;
-
c.
The impeller wall was a rotation wall;
-
d.
The rotation of hot metal and the stirrer were calculated by the multiple reference frame model (MRF) [10].
The properties of materials used in the calculation are shown in Table 3 [11, 12].
Results and Discussion
Flow Field
After the flow was stable, the streamline and the distribution of flow velocity under the condition of the rotation speed was 120 rpm (revolution per minute) are presented in Fig. 2. As the figure shows, the flow of hot metal was divided into two parts of the upper and lower circulation. The flow velocity of hot metal at different positions is very different. It can be seen that the flow velocity of hot metal is inversely proportional to the distance from the stirring impeller. The flow velocity besides the impeller reaches 7.0 m/s while near the wall it is below 2.5 m/s. The movement of hot metal under the impeller is much worse than other positions. In this paper, the zone in which the flow velocity of hot metal is below 0.5 m/s is defined as dead zone. During the hot metal pretreatment , the dead zone always leads to an increase of sulfur content after the desulfurization is finished.
Distribution of CaO Particles
Figure 3 shows the distribution of 20000 particles at different calculation times after adding into the hot metal, when the rotation speed of the impeller was 100 rpm. It can be seen that under the stirring effect from the impeller, the added particles moved with the hot metal, and at 60 s, the distribution of CaO particles was dispersed well within the hot metal. As the main desulfurizer , the movement and distribution of the CaO particles in the hot metal have significant effect on the desulfurization rate. It is obvious that the CaO particles were moving with hot metal, so the movement situation of CaO particles can be optimized by the optimization of velocity field.
Desulfurization Process
The variation of sulfur content in the hot metal after the injection of CaO particles is shown in Fig. 4, in which the rotation speed of impeller was 100 rpm and the initial sulfur content was 600 ppm. As the figure shows, with the addition of CaO particles, the desulfurization reaction occurred in the hot metal. Figure 5 shows the change of sulfur content monitored in three positions. Obviously, the desulfurization rate at P-3 was much higher than P-1. The reason for this phenomenon is that P-1 is in the dead zone where the flow is the worst in the ladle. On the contrary, P-3 is besides the impeller where the stirring is much stronger than other positions in the ladle. In combination with Eq. (3), it can be seen that the rate of desulfurization was increased due to the high stirring strength near the impeller and the faster rate of hot metal regeneration.
Effect of Parameters on Desulfurization
In this paper, the desulfurization rate under different conditions were simulated, respectively. Figure 6 shows the desulfurization results with different rotation speeds (under the immersion depth of 1.85 m). From the result, it indicated that the desulfurization rate was slightly increased by the increase of rotation speed. That is because the desulfurization rate is not directly effected by the rotation speed, but by the relative velocity of particles compared with hot metal. The increasing rotation speed has a little effect on the relative velocity.
The results of desulfurization rate with different immersion depths and particle sizes are shown in Fig. 7 and Fig. 8, respectively. As Fig. 7 shows, the increasing of immersion depth has no effect on the desulfurization rate. This is because with the increasing of immersion depth, the desulfurization rate of the lower part of the ladle was accelerated, but at the same time the desulfurization rate of the upper part of the ladle was decreased, resulting in no change in the overall desulfurization rate. Figure 8 shows the relationship between the desulfurization rate and particle size. Here, three different diameters of CaO particles were calculated as a contrast. As the result shows, the desulfurization rate was greatly effected by the particle size. When the particle diameter increased from 1.3 to 2.0 mm, the overall desulfurization efficiency reduced by 3 times mainly due to the surface area reduction of particles.
Conclusions
The KR desulfurization process in hot metal under different conditions were investigated by CFD. The conclusions are summarized as follows.
-
(1)
During KR stirring desulfurization process in the ladle, the flow of hot metal was greatly effected by the rotation speed.
-
(2)
The movement and dispersion of CaO particles were simulated by DPM model. After the desulfurizer was added into the ladle, it can be well dispersed in the hot metal. The desulfurization reaction between the CaO particles and the S in hot metal was also evident.
-
(3)
Desulfurization behavior was simulated using the reaction model. The results obtained show that the increasing of rotation speed and immersion depth have a little effect on the desulfurization rate. It is a significant method to improve the desulfurization rate by controlling the CaO particle size.
-
(4)
The simulation of the KR desulfurization process seems to be feasible and parameter studies give plausible results. Nevertheless, some important phenomena like aggregation of the desulfurizer have not be considered so far. Therefore, it should be careful to analyze simulation results. Further investigations should incorporate these effects in order to achieve more realistic predictions.
References
Yao N, Xing C, Li SX (2011) KR desulfurization effect of hot metal pretreatment. J Mater Metall 10(2):93–95
Yang SS, Jia M, Wang H (2009) Brief introduction of KR hot stirring pretreatment process. Bao-Steel Technol 35(1):85–87
Huang CY (2013) KR stirring desulfurization process. Met World 1(6):56–59
Koichi T, Keita U, Hiroyuki S (2012) Influence of solid CaO and liquid slag on hot metal desulfurization. ISIJ Int 52(1):10–17
Gao YL (2012) Numerical simulation of molten iron flow in molten iron desulphurization by KR. Sci Technol Get Rich Guide 1(9):377–378
Long P (2013) Numerical investigation on kinetics of desulfurization by mechanical mixing of hot metal. PhD thesis, Huazhong University of Science and Technology
Zhang LF, Wang YF, Zuo XJ (2008) Flow transport and inclusion motion in steel continuous-casting mold under submerged entry nozzle clogging condition. Metall Mater Trans B 39(4):534–550
Lou WT, Zhu MY (2014) Numerical simulation of desulfurization behavior in gas-stirred systems based on computation fluid dynamics–simultaneous reaction model (CFD–SRM) coupled model. Metall Mater Trans B 45(5):1706–1722
Yoshie N, Ikuhiro S, Naoki K (2013) Aggregation behavior of desulfurization flux in hot metal desulfurization with mechanical stirring. ISIJ Int 53(8):1411–1419
Cao MJ, Yang FL, Wang HZ (2011) Numerical simulation of turbulent flow in a stirred tank. Drying Technol Equip 9(4):191–199
Liu P (2010) KR mechanical desulfurization stirrer inside the fluid flow characteristics. PhD thesis, Northeastern University
Stefan P, Philipp G (2002) CFD, a design tool for a new hot metal desulfurization technology. Appl Math Model 26(2):337–350
Acknowledgements
The authors are grateful for support from the National Science Foundation China (Grant No. 51504020 and No. 51404019), Beijing Key Laboratory of Green Recycling and Extraction of Metals (GREM), the Laboratory of Green Process Metallurgy and Modeling (GPM2) and High Quality steel Consortium (HQSC) at the School of Metallurgical and Ecological Engineering at University of Science and Technology Beijing (USTB); The authors are also grateful for support from Chongqing Key Technology Innovation of Industries (cstc2016zdcy-ztzx20006), Chongqing Social Technology Innovation Foundation (cstc2016shmszx20015), China.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Fan, C., GoSa, A., Zhang, L., Liu, Q., Chen, D. (2018). Mathematical Modeling on the Fluid Flow and Desulfurization During KR Hot Metal Treatment. In: & Materials Society, T. (eds) TMS 2018 147th Annual Meeting & Exhibition Supplemental Proceedings. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72526-0_41
Download citation
DOI: https://doi.org/10.1007/978-3-319-72526-0_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72525-3
Online ISBN: 978-3-319-72526-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)