Abstract
The heart is comprised of three-dimensional components whose size, shape, and spatial interrelationships constantly change in an organized rhythmic fashion. The field of congenital heart disease encompasses the result of a disruption of the normal, but complex, three-dimensional internal anatomy and functioning of the heart. The diagnosis and treatment of congenital heart defects demand a precise and profound understanding of this dynamic anatomy and are therefore heavily reliant on real-time imaging. The open-heart surgeon has the benefit of comprehension and interaction with the real 3D anatomy; however this is usually static during open-heart surgery. The interventionalist wishing to employ minimally invasive transcatheter techniques in the beating heart has to build a mental concept of the anatomy usually derived from 2D echocardiographic and fluoroscopic data displayed on a screen. In general, the surgeon’s understanding of the anatomy is more complete and intuitive than that of the interventionalist even though they may be performing similar procedures, e.g., closure of an ASD secundum. In an attempt to bridge that gap, high-quality 3D volumetric data acquisition systems such as 3D transthoracic and transesophageal echocardiography, 3D rotational angiography, CT, and MRI have been developed. However this volumetric data is typically displayed on 2D screens which restrict the image to a single plane of view, preclude interaction directly with the image, and hamper the perception of depth. The development of a simple, accurate, and accessible 3D display for these data is a challenge, especially when attempting to display true depth.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The heart is comprised of three-dimensional components whose size, shape, and spatial interrelationships constantly change in an organized rhythmic fashion. The field of congenital heart disease encompasses the result of a disruption of the normal, but complex, three-dimensional internal anatomy and functioning of the heart. The diagnosis and treatment of congenital heart defects demand a precise and profound understanding of this dynamic anatomy and are therefore heavily reliant on real-time imaging. The open-heart surgeon has the benefit of comprehension and interaction with the real 3D anatomy; however this is usually static during open-heart surgery. The interventionalist wishing to employ minimally invasive transcatheter techniques in the beating heart has to build a mental concept of the anatomy usually derived from 2D echocardiographic and fluoroscopic data displayed on a screen. In general, the surgeon’s understanding of the anatomy is more complete and intuitive than that of the interventionalist even though they may be performing similar procedures, e.g., closure of an ASD secundum. In an attempt to bridge that gap, high-quality 3D volumetric data acquisition systems such as 3D transthoracic and transesophageal echocardiography, 3D rotational angiography, CT, and MRI have been developed. However this volumetric data is typically displayed on 2D screens which restrict the image to a single plane of view, preclude interaction directly with the image, and hamper the perception of depth. The development of a simple, accurate, and accessible 3D display for these data is a challenge, especially when attempting to display true depth.
Depth perception defines our ability to see an object, comprehend its position in space, and interact precisely with that object. The object’s three dimensionality and distance are perceived owing to the capacity of the visual cortex to simultaneously combine multiple depth cues including binocular stereopsis, convergence, and accommodation. Each eye, since it has a different position in the head, views an object from a slightly different angle, and this disparity, stereopsis, is employed by the brain as one of the cues for depth perception. Stereoscopy is a method which mimics stereopsis by presenting each eye with two slightly different versions of an image which, when combined in the brain, produce an illusion of depth. It is an illusion since all the points of light in the stereoscopic image are focused in a singular focal plane, whereas in a real object, these points arise from multiple different planes, and true depth perception has infinite focal planes (Fig. 44.1).
Even the highest-quality stereoscopic image is confined to one focal plane, and therefore interactions such as placing a device in the image, or manipulating the image with the observer’s hand inside it, are not possible since the device or hand occupies multiple focal planes. In addition, the source of stereoscopic images is at a distance from the observer; however the overlapping of the images to create the 3D illusion occurs up close. When attempting to interact with the up close 3D image, the eyes converge to focus on the hand or tool and lose their focus on the distant source, and the image becomes blurred. This discrepancy between the convergence depth cue and the distant focus creates a conflict in the brain often resulting in nausea. Recent attempts at enhancing the “image-anatomy” interaction and the operator’s experience with stereoscopic displays include virtual reality (VR) and augmented reality (AR). Although these methodologies may provide a better understanding of the patient’s anatomy and spatial relationship to the operator, they lack true depth perception and cannot be used for accurate navigation within the image.
Holography, from the Greek for “whole” and “drawing,” is a methodology which creates an exact visual replication of an object in three physical dimensions including all the depth cues, focal planes, and specific coordinates. The holographic image occupies an actual space and can be likened to an object created by a 3D printer where the material used to print is light. A high-quality hologram should be indistinguishable from the true object it represents (Fig. 44.2).
The same object in Fig. 44.1 is projected as a hologram that occupies 3D space, as opposed to one single focal plane. The fixed coordinates and multiple focal planes afford interaction with and within the image at arm’s reach of the observer
Computer-generated holograms (CGH) of patients’ 3D data could be useful in the clinical setting by providing the physician with a 3D image which is a true and spatially accurate representation of the patient’s anatomy, having all the visual depth cues. Since each point of light in the generated hologram is a distinct physical entity in real 3D space, it represents a precise anatomical coordinate in the patient, and interaction with and within the holographic image will simulate such interaction with and within the patients’ particular anatomy. Initial attempts at creating holograms in real time in an actual clinical setting have been recently reported using the RealView 3D dynamic holographic display and interface system [RealView Imaging, Yokneam, Israel]. The system projects high-resolution 3D live holographic images “floating in mid-air” without the need for any human-mounted device, goggles, or a conventional 2D screen. The projected 3D images appear in free space, allowing the user limitless accessibility to the image affording direct and precise interaction with and within the dynamic image. Therefore, a true volumetric display based on current 3D acquisition modalities maintains the interventionalist’s advantage of a minimally invasive approach and provides “real” 3D dynamic anatomy similar or even better than that of the surgeon, since the surgeon sees static 3D surfaces, while the hologram can display the organ at different levels of transparency to the viewer and can be easily cropped and manipulated as required.
The following figures are examples of holographic images created using the RealView system and demonstrate some of the interactions that can be performed and 3D acquisition modalities that can be used to create holograms in the clinical setting. Please note that holograms cannot be fully appreciated on a 2D page, but need to be observed directly. [Videos of holograms shown here in the figures can be viewed at www.realviewimaging.com]
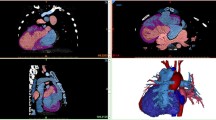
(a) Hologram created from a segmented cardiac CT showing the anterior right ventricle [red] and the posterior left atrium [gray]. The finger is touching the left upper pulmonary vein and rotates the image to the desired viewing angle. (b) The volumetric data of the left atrium is isolated, and the atrium is rotated anteriorly prior to slicing as seen in Fig. 44.3c. (c) The hologram of the left atrial volume has been sliced open and rotated so that the pulmonary venous orifice of the left upper pulmonary vein is viewed en face. (d) Ablation points are simulated directly on the image at the junction of pulmonary vein orifice to the left atrial wall using a tool. These points were intuitively positioned at the exact anatomical position and depth creating a continuous and contiguous line in a similar manner to painting directly on a 3D object
Dynamic color holograms created from volumetric data simulated with the TAVIguide™ technology (FEops, Ghent, Belgium) of a virtually deployed CoreValve® wire frame (Medtronic, Minneapolis, MN, USA). The TAVIguide technology predicts how a certain TAVI device will interact with a specific patient. Upper plane: The aorta and fully deployed CoreValve seen from the ascending aorta. Lower pane: The partially deployed CoreValve is seen by slicing into the ascending aorta, and the interaction with the native aortic leaflets, in blue, can be seen from any angle
Bicaval bidirectional Glenn palliation in a 2-year-old patient: Static hologram created in real time from an intra-procedure 3D rotational angiogram using a Philips Allura Cath Lab System [Philips Medical, Eindhoven, Netherlands]. Contrast was injected into the right SVC and left arm [hand injection] simultaneously
ASD secundum in a 16-year-old girl. Dynamic color holograms created in real time from intra-procedure 3D transesophageal electrocardiography using a Philips ie33 echocardiography system [Philips Medical, Eindhoven, Netherlands]. Upper plane: The ASD secundum is demonstrated from the left atrial aspect in the center of the septum. Lower pane: The ASD is closed by a septal occlude, and the mitral valve is seen en face, while the observer holds the heart in his hand
Acknowledgement
Conflict of interest: EB and CR are stockholders and employees of RealView Imaging.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Bruckheimer, E., Rotschild, C. (2019). Holography in Congenital Heart Disease: Diagnosis and Transcatheter Treatment. In: Butera, G., Chessa, M., Eicken, A., Thomson, J.D. (eds) Atlas of Cardiac Catheterization for Congenital Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-72443-0_44
Download citation
DOI: https://doi.org/10.1007/978-3-319-72443-0_44
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72442-3
Online ISBN: 978-3-319-72443-0
eBook Packages: MedicineMedicine (R0)