Abstract
Combustion process of selected woody biomass was investigated. Four types of woody material were used for conducted experiments: poplar, pine, acacia and willow. European Union policy concerning Renewable Energy Resources (RE) is a response to increasing pollution of our globe, and, simultaneously, it introduces additional, independent supplies of energy. With the introduction of legal obligations that regulate these norms, countries that did not have appropriate percentage share of renewable energy had to comply with these regulations by implementing appropriate projects that would allow for supplies of renewable energy shortly. The work presents ecological aspect of selected materials, by showing differences in fuels obtained from woody biomass used in the experiments. It was analyzed the ash and volatile matter content and also calorific value. The experiments also show, which dendromass has the best energetics parameters, and which produces the least pollution. Poplar had the highest net calorific value (18,633 J/g), but during poplar combustion, the highest concentration of carbon oxides and nitrogen oxides was found.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Wood is one of the oldest materials used by man. It has various uses, which, basically, may be divided into two categories, namely, wood may be used for production and for energy-related purposes. The first category involves, i.a. building or furniture industry, and the second category—combustion and all related processes, i.e. gasification, pyrolysis [1], etc. Energy may be obtained according to the rule of sustainable forest management. Conducted experiments confirm huge potential of waste wood biomass, as renewable energy resource [2, 3]. At present, energy derived from biomass must be considered both in the ecological and economic context, which requires the integration of social, environmental and industrial sphere [4]. Supporting the development of renewable energy resources is an important goal for the European Union [1, 5]. It is estimated that in EU countries, biomass may constitute approx. 2/3 of the declared share of renewable energy in 2020 [6].
Woody biomass, also called dendromass, is organic substance derived from woody plants and bushes. It consists of wood, bark, and green mass—branches and conifer needles (coniferous trees) or leaves (deciduous trees). Dry biomass is composed of the following basic elements: C—carbon (48–51%), H—hydrogen (6–7%), N—nitrogen (0.01–3%), O—oxygen (42–45%). Despite the changeability in different parts of the tree, the composition of phytogenic components of individual trees is similar. Inorganic substances such as ash make up 0.4–0.7%, while their share in bark is higher and amounts to 1–1.5%. Building elements in the organic mass of trees are organized as polymer substances such as cellulose, lignin, hemicellulose, and adventive substances. Solid dendromass fuel consists of combustible material which is the most important, as it is responsible for energy supply in the process of oxidization of fuel during combustion, and simultaneous release of heat [7, 8].
During combustion of dendromass, fuel ballast phenomenon occurs. It is undesirable effect during combustion, and it is formed from ash and water present in the fuel. Wood biomass, compared with other solid fuels has a very low fuel ballast percentage coefficient. However, its negative property is bonding with water. Therefore, the fuel must be dried prior to combustion [9,10,11].
In terms of energy efficiency of dendromass combustion, the most important fact, taken into consideration is heat of combustion and heat value. Heat of combustion is the heat released during full combustion of fuel, with simultaneous cooling of the temperature of the combustion gas, where steam condensates, while heat value is the heat released during full combustion of fuel, with simultaneous cooling of the combustion gas to the ambient temperature, where water that formed during evaporation stays in gaseous state. Concluding, heat value is heat of combustion minus heat of water evaporation [12].
Increased use of energy plants as fuel will contribute to solving numerous ecological problems. Plant biomass decreases environmental pollution with harmful substances [13].
The work’s goal was to determine the difference in combustion quality of selected materials using specialist equipment. The scope comprised specifying the calorific value (heat value) of the samples, volatile parts, ash content, and detailed analysis of combustion gas. The conducted experiments allow to determine, which of the tested wood biomass type is the best in energetic and ecological terms.
2 Experimental Section
Four types of material were used for experiments: poplar, pine, acacia and willow. All the materials were dried in a tunnel dryer at the temperature of 60 °C, and reached the moisture content (Mad) of approx. 7%.
Another aspect considered in the research was the ecological aspect, which required verification of samples using fumes analyzer.
All laboratory analysis was conducted in according to the norm. Samples were prepared according to the PN-EN ISO 14780:2017-07 [14] standard requirements. Test sample was milling in IKA A11 basic analytical mill. The milling was repeated until all grains were smaller than 1 mm.
The moisture content (Mad) in as analysed state (air dry) was determined according to the PN-EN ISO 18134-3:2015-11 [15] standard. In this analysis laboratory dryer (SLN 160, POLEKO) was used. The procedure rely on drying of small amount of the material (1 ± 0.05 g) in air at 105 °C. The moisture content was determined according to difference in sample weight before and after drying [15].
The ash content (A) was determined according to the PN-EN ISO 18122:2016-01 [16] standard from the mass of the residue remaining after the sample was combusted in the furnace (SNOL 3/1100) in air under strictly controlled time and temperature. First, the samples were heated for 1 h in 250 °C, then the temperature was increased up to 550 °C and the samples were kept in this temperature for next 2 h. After cooling down the samples to the ambient temperature, the remained ash was weighed and the calculations according to the formula presented in standard [16] were done.
The volatile matter content (V) was determined according to the method based on the PN-EN 15148:2010 standard [17]. A test portion (1 ± 0.05 g) of each sample was heated in combustion crucible with a lid, for 7 min at 910 ± 10 °C. The percentage of volatile matter was calculated based on the loss in mass of the test portion after deducting the loss in mass due to moisture [17].
The gross calorific value (higher heating value, HHV) was determined according to the method based on the PN-EN 14918:2010 standard [18]. It was performed for samples of 1 ± 0.05 g placed in an isoperibol calorimeter (C 6000 Isoperibol, IKA). Each sample was combusted as compressed pellets, in the calorimetric bomb, in pure oxygen and high pressure. The calorific value was calculated following the equations described in the standard [18] which were implemented to the device operating software. The net calorific value (lower heating value, LHV) was calculated from the relation in which gross calorific value is reduced by value equal to heat utilized for vaporization of water, obtained from the fuel in the combustion of hydrogen and moisture content in the sample. This parameter was calculated using the controlling operation software of the calorimeter following the equations presented in the standard [18].
Analyser of fumes, Testo 300-B and an ordinary standalone furnace were used. The analyser was connected to a personal computer, which recorded the results. The probe of the analyser was inserted in the chimney of the furnace. It was decided to make approx. 50 g samples, which were then combusted. The analyser was turned on, and when one sample was combusted, another 50 g sample was put in the furnace. In this way, a set of data was obtained, which were then processed in order to obtain the required data related to gaseous products. Based on the selected data, graphs that show differences in the content of volatile products were generated.
3 Results
The experiments were conducted using the laboratory equipment mentioned above. This allowed to determine such parameters of dendromass as: calorific value, volatile matter, ash (Table 1), and gaseous products during combustion.
The obtained results indicate that poplar has the highest calorific value (provides the most heat), followed by pine (with similar calorific value). It seems that pine should have much higher heat value than poplar as it is a coniferous tree, and contains a lot of resin, extremely flammable material. Additionally, after removal of crucibles from the furnace, it was visible that crucibles used for combustion of pine had most residues. It is caused by the resin, which, during the process of combustion, releases greasy soot, and deposits inside the crucible. Willow and acacia have similar heat values, with the difference only amounting to a dozen or so megajoules.
During the experiments, for volatile matter extremely interesting results were obtained. These results indicate that the values of this parameter of dendromass reach approx. 85%. All materials assume similar values, and the percentage difference is small. Such a high value may be attributed to very low moisture content of the materials.
The results of measurement of ash content were similar to those presented in literature. Poplar and pine had the lowest content of ash among the examined materials, amounting to approx. 0.47%, while willow had the highest content of ash. Such low content of ash results in low emission of dust (approx. 20 times lower than in the case of coal).
During combustion of different substances, various products are released, including gases. During combustion of biomass, the most important side product of this process is carbon oxide (CO) in a gaseous form; other products include hydrocarbons (CH), nitrogen oxides (NOx), and sulphur oxides (SO). Carbon oxides are inorganic compounds, which consist of carbon and oxygen. Carbon monoxide is a side product of combustion process, and is colourless, odorless and lighter than air. It is often a cause of gas poisonings, which may lead to death (in large quantities) [19]. The results of the conducted experiments show that of all compounds, CO amount was the highest.
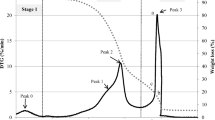
Figure 1 shows a graph presenting stages of biomass combustion. In the measured period kindling of biomass takes place within 45 s, characterized by high concentration of CO. Kindling biomass chokes itself, which causes release of large amounts of CO. The next stage is proper combustion, where appropriate flame forms, burning the sample, and supplying energy in the form of heat. This period lasts from 45th to 420th second. This period is characterized by sharp decrease in CO concentration, and increase in oxygen concentration. The last stage is full combustion. In this period, the sample burns to ashes, and the level of CO increases slightly.
Among the tested samples, the highest CO concentration was measured for poplar, exceeding the concentration of CO for other samples by up to 5 times, and reaching the value of 4730 ppm.
Nitrogen oxides (NOx) are inorganic compounds, and consist of oxygen and nitrogen. They are considered one of the most harmful substances that pollute the atmosphere. They are approx. 10 times more harmful than carbon oxides, and a few times more harmful than sulphur oxides. Their presence can be attributed to extremely high temperatures during combustion process with the presence of air.
NOx concentration during biomass combustion behaves in a similar way to CO concentration. Despite the fact that NOx concentration is more than a dozen times lower than CO concentration, it is just as toxic and harmful to our health. Figure 2 shows that the highest concentration is reached at the moment of kindling biomass, when the temperature increases sharply. In the next stages of combustion, NOx concentration changes even with the slightest changes in the way the sample burns, e.g. when the sample starts to glow more violently, NOx concentration also increases (in case of CO the concentration changes are not so significant). The highest concentration of NOx during kindling was recorded for poplar, though during proper combustion, both poplar and acacia samples behave in a similar way, with NOx concentration being higher.
Figure 3 shows a graph presenting H2 concentration during combustion in the measured period.
In the process of combustion, hydrogen is one of products of the process; it does not have a significant negative impact on the environment. During biomass combustion, mail side-products included carbon oxides and sulphur oxides. Additionally, probes were inserted in the analyser to measure concentrations of hydrogen, sulphur oxides and oxygen.
Oxygen concentration behaved in the similar way as CO concentration (the higher CO concentration the lower oxygen concentration and vice versa) (Fig. 4). Interestingly, sulphur oxides concentration was 0 for all the samples (with the exception of one experiment, where the probe detected SO concentration near 0). It is connected with the changeability and versatility of the composition.
4 Conclusion
Among all the examined samples of biomass, poplar had the highest net calorific value, i.e. generated the most energy (18,633 J/g), while acacia had the lowest net calorific value, with the average heat value being equal 17,978 J/g. Volatile matter responsible for the flame, had a similar value in each sample, amounting to 85%. It is a very high value, indicative of low moisture of the material. Willow had the highest share of ash among the examined dendromasses, with the percentage share of ash amounting to approx. 1.2%, i.e. more than two times higher than for pine and poplar, for which ash share is equal approx. 0.47%. The best woody biomass material is poplar, due to the highest calorific value and the lowest share of ash, followed by pine, the only representative of coniferous trees. The worst materials of are acacia and willow, as high share of ash (willow) means more frequent cleaning of fire grate, which, considering mass use, would mean the necessity to implement additional technical solutions, or undesirable, increased outlay of labour.
During qualitative analysis of the material, it was determined which dendromass was the most ecological one. Primary pollution can be attributed to carbon oxides and nitrogen oxides. Among the examined materials, the highest concentration of these compounds was found during poplar combustion. However, it is worth noting that mass combustion (e.g. in power plants) does not require repeated kindling since once the material lights up, the fire is fed, and burns continuously. To sum up, the least ecological samples were those of pine and willow. However, the differences between them are small, compared to conventional fuels, such as hard coal or oil; conventional fuels assume values equal to zero.
References
Nussbaumer, T.: Combustion and co-combustion of biomass: fundamentals, technologies, and primary measures for emission reduction. Energy Fuels 17(6), 1510–1521 (2003)
Yang, Y.B., Sharifi, V.N., Swithenbank, J., Ma, L., Darvell, L.I., Jones, J.M., Pourkashanian, M., Williams, A.: Combustion of a single particle of biomass. Energy Fuels 22(1), 306–316 (2008)
Brodziński, Z., Kryszk, H., Kurowska, K.: Market of producers and processors of agricultural biomass for energy purposes. Pol. J. Environ. Stud. 23(2), 619–627 (2014)
Dobrowolska, E., Dzurenda, L., Jabłoński, M., Kłosińska, T.: Wykorzystanie energetyczne dendromasy. Wydawnictwo SGGW, Warszawa (2010)
Erol, M., Haykiri-Acma, H., Küçükbayrak, S.: Calorific value estimation of biomass from their proximate analyses data. Renew. Energy 35(1), 170–173 (2010). https://doi.org/10.1016/j.renene.2009.05.008
Frączek, J.: Produkcja biomasy na cele energetyczne. PTIR, Kraków (2009). ISBN 978-83-917053-8-4
Gołos, P., Kaliszewski, A.: Wybrane aspekty wykorzystania biomasy drzewnej do celów energetycznych. Biomasa Leśna Na Cele Energetyczne. IBL, Sękocin Stary 76(1), 78–87 (2015). https://doi.org/10.1515/frp-2015-0009
Skodras, G., Grammelis, P., Basinas, P., Kakarass, E., Sakellaropoulos, G.: Pyrolysis and combustion characteristics of biomass and waste-derived feedstock Ind. Eng. Chem. Res. 45(11), 3791–3799 (2006). https://doi.org/10.1021/ie060107g
Gendek, A., Głowacki, S.: Convectional drying of chips for energy purposes. Ann. Warsaw Univ. Life Sci.—SGGW Agric. 53, 67–72 (2009)
Głowacki, S., Gendek, A.: Application of forced drying methods in preparation of forest chips for energy purposes. Ann. Warsaw Univ. Life Sci.—SGGW Agric. 58, 29–34 (2011)
Jasinskas, A., Ulozevićiute, I., Rutkauskas, G.: Plant biomass production and use as an environmentally-friendly local fuel. Pol. J. Environ. Stud. 21(1), 89–94 (2012)
Jasiulewicz, M.: Possibility of liquid bio-fuels, electric and heat energy production from biomass in polish agriculture. Pol. J. Environ. Stud. 19(3), 479–483 (2010)
Najjar, Y.S.H.: Gaseous pollutants formation and their harmful effects on health and environment. Innovative Energy Policies 1, 1–9 (2011)
PN-EN ISO 14780:2017-07 Solid biofuels. Sample preparation
PN-EN ISO 18134-3:2015-11 Solid biofuels—Determination of moisture content—Oven dry method—Part 3: Moisture in general analysis sample
PN-EN ISO 18122:2016-01 Solid biofuels—Determination of ash
PN-EN 15148:2010 Solid fuels—Determination of volatile content by gravimetric method
PN-EN 14918:2010 Solid Biofuels—Determination of calorific value
Woch, F., Hernik, J., Wyrozumska, P., Czesak, B.: Residual woody waste biomass as an energy source—case study. Pol. J. Environ. Stud. 24(1), 355–358 (2015). https://doi.org/10.15244/pjoes/29689
Acknowledgements
This research was financed by the Ministry of Science and Higher Education of the Republic of Poland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Głowacki, S. et al. (2018). Analysis of the Combustion Process of Selected Wood Biomass. In: Mudryk, K., Werle, S. (eds) Renewable Energy Sources: Engineering, Technology, Innovation. Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-319-72371-6_71
Download citation
DOI: https://doi.org/10.1007/978-3-319-72371-6_71
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72370-9
Online ISBN: 978-3-319-72371-6
eBook Packages: EnergyEnergy (R0)