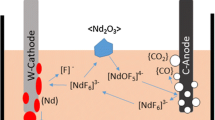
Abstract
During molten salt electrolysis of rare earths greenhouse gas emission occurs causing environmental and climate changes. In this study the electrochemical process window for didymium oxy-fluoride electrolysis is determined by linear sweep voltammetry and staircase chronoamperometry. Simultaneously the composition and the quantity of gas generation are investigated by an in situ FTIR-spectrometry in order to understand the process phenomena happening at anode and the mechanisms behind them. Different electrolyte compositions based on NdF3, PrF3 and LiF and various oxide amounts were employed showing the diversity in off-gas ratios. Effects of using two praseodymium-oxide compounds, namely Pr6O11 and Pr2O3 used as raw materials, on the process and amount of anodic gases is considered as well. Perfluorocarbon (PFC) emission reduction is accomplished by electrolysis automatization by coupling the FTIR-spectrometer with a controller which triggers the oxide dosage preventing full anode effect.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Current increase in price for neodymium (Nd) and praseodymium (Pr) oxide by +50% is direct consequence of their application boost in green technologies, such as wind turbines and electric cars, where didymium alloy (a mixture of Nd and Pr) is one of the main components of permanent magnets. This demand expansion leads to bigger neodymium and praseodymium production and therefore a rise of greenhouse gases (PFCs, CO2) emission. Didymium alloy is usually produced by melting the defined mixture of pure neodymium and praseodymium metals which are obtained by oxy-fluoride molten salt electrolysis similar to aluminium electrolysis. Since separation of neodymium and praseodymium by solvent extraction is difficult and costly process due to their similar physical properties and knowing that praseodymium even improves some magnetic properties of Nd-based magnets [1,2,3] employing direct molten salt electrolysis of their mixed oxides is more advantageous. Another advantage is that the mixed Nd/Pr-oxide supplied as final product from magnet recycling, can be used for direct didymium production [4, 5] in place of primary extraction route during which the radioactive waste is present.
Nevertheless, due to the Chinese monopoly in rare earth metal production, scientists are confronted with the lack of and difficultly accessing scientific articles regarding didymium production by molten salt electrolysis . Some known papers [6, 7] in English report only the viability of the process but are without further explanations and are outdated, whereas accessible Chinese articles do not offer enough data [8,9,10]. Investigation on the influence of electrolyte composition on molten salt electrolysis of didymium was recently done [11] but information on off-gas emission is so far not known, initiating the research in this paper. Off-gas emission during neodymium electrolysis is already known [12,13,14,15] and is used as premise for didymium off-gas analysis.
Experimental
In all experiments a mixture of rare earth fluoride and lithium fluoride is used with 7:1 ratio at working temperature of approximately 1050 °C. The rare earth fluoride composition is varied as shown in Table 1. The chemicals used as electrolyte NdF3 (Treibacher, ≥99.9%), PrF3 (Treibacher, ≥99.9%) and LiF (Less common metals, >99.9%) were firstly dried separately for 24 h at 250 °C, before they were accordingly mixed, pre-melted and homogenized in a high purity graphite crucible placed in a vacuum induction furnace under argon inert atmosphere at ca. 1050 °C. Such prepared electrolyte was stored under high purity argon atmosphere (O2, H2O <1 ppm) within a glovebox (Jacomex). Raw materials used Nd2O3 (Alfa Aesar, ≥99.9%), Pr6O11 (Treibacher, ≥99.0%) and Pr2O3 (Treibacher, ≥99.61%) were dried for 24 h at 120 °C, pressed, crushed in pestle with mortar and sieved to particle size between 0.71 and 1.25 mm. The ratio of the dosed Nd/Pr-oxides was in all experiments 73.4–26.6 wt% and the concentrations used in electrolytes were varied between 0–4 wt%. The experimental setup consisted of pure graphite crucible (SIGRAFINE® R8510) filled with electrolyte (approx. 2 kg) and placed into steel closed cell with Swagelok connections through which the thermocouple type S with molybdenum sheath (Omega Engineering GmbH) and electrodes were inserted (Fig. 1). The lid of the cell is water cooled and had the dosing device for oxides built in. As working electrode (WE) high purity graphite rod (Ø6 mm, SIGRAFINE® R8510) was used, whereas tungsten rod served as quasi-reference (W-QRE) and counter (CE) electrode (Ø6 mm and Ø8 mm, respectively, with purity 99.95%). The prepared steel cell was placed in a resistance heated furnace and flushed with argon during whole experiments. All electrochemical measurements were carried out with an IviumStat potentiostat/galvanostat (Ivium Technologies B.V.). Simultaneously off-gas measurements were done by Gasmet DX4000 Fourier transformation infrared spectrometer (FTIR, Ansyco). For the process control an appropriate software is installed in Yokogawa UT55A Controller for dosing the oxides if a certain voltage value is surpassed and/or CF4 and C2F6 (PFC) gases were detected.
Results and Discussion
Process Window/off-Gas Composition
Linear sweep voltammograms of NdF3–LiF (87.5–12.5 wt%) and NdF3–PrF3–LiF (64.23–23.27–12.5 wt%) electrolytes with different Nd2O3 and Nd2O3–Pr6O11 (73.4–26.6 wt%) concentrations were recorded on graphite electrode with 5 mV/s scan rate which are shown in Fig. 2. Determined process window and off-gas composition for neodymium electrolysis (Fig. 2a) is used for comparison with phenomena happening during didymium electrolysis (Fig. 2b). Even now any firm conclusion on the mechanism of the anode process in aluminium electrolysis can’t be given, which is used as basis for rare earth electrolysis. It is proposed that at low current densities the discharge of oxygen ions takes place at the most active sites on the anode surface, followed by its penetration into the carbon lattice and by further increase of current density in depletion at the anode surface [16]. Our neodymium experiments are comparable with previous research [12, 15] showing partial passivation with oxygen containing ions attributed to peak O1 at around 1.7 V versus W-QRE where concentration of these electroactive species reaches its maximum resulting in high values of CO and CO2 in off-gas. Anode current density depends on added oxide concentration and reaches a maximal value of 0.9 A/cm2 with oxide concentration >2wt%. Afterwards full anode effect (O2) happens at approx. 2.3 V versus W-QRE followed by current abrupt and PFC, namely CF4 and C2F6 emission (Fig. 2a). In the case of electrolyte that contains PrF3 and with addition of Pr6O11 full anode effect is shifted more positive at around 3.0 V versus W-QRE due to the more complex component system (Fig. 2b). Partial passivation occurs at the same current density values ca. 0.9 A/cm2 but in this case without sharp current drop which is noticed in neodymium electrolysis . This difference can be explained by various simultaneous reactions of neodymium and praseodymium complex species. Proposed anodic reaction for neodymium by Stefanidaki [17] follows Eq. (1):
a Linear sweep voltammograms recorded on graphite electrode at 1050 °C in NdF3–LiF electrolyte with different Nd2O3 concentrations and scan rate = 5 mV/s (above) with off-gases measured with an in situ FTIR-spectrometer generated during electrochemical measurements with 2 wt% oxide (below); b Linear sweep voltammograms recorded on graphite electrode at 1050 °C in NdF3–PrF3–LiF electrolyte with different Nd2O3–Pr6O11 concentrations and scan rate v = 5 mV/s (above) with off-gases measured with in situ FTIR-spectrometer generated during electrochemical measurements with 2 wt% oxide (below)
Based on that we can propose same complex of praseodymium present in melt which oxidation as well generates oxygen on anode according to Eq. ( 2 ):
Since the used Pr6O11 consists of mixture of Pr4+ and Pr3+ some other complexes [PrOFx]y− are probably present. The influence of praseodymium valence in oxide raw material on process and PFC emission is investigated in Sect. “Influence of Praseodymium Fluoride and Oxide on PFC Emission”.
The composition of the off-gases during didymium electrochemical measurements is the same as in case of neodymium consisting of CO, CO2, CF4 and C2F6 as can be seen in Fig. 2a, b (showing one exemplar measurement with 2 wt% oxide for simplicity). Nevertheless, the ratio and amount differs and more gas is produced, especially CO/CO2 ratio increases. C2F6 emission starts after CF4 evolution showing the same tendency but with significantly smaller amount then during neodymium electrolysis . This increase in gas production is related to praseodymium molarity in its oxide and fluoride, i.e. higher oxygen and fluoride content and its availability. Additionally, Pr4+-oxide present in Pr6O11 reacts spontaneously with carbon (ΔG < 0) following the Eq. ( 3 ) and evolution of CO and CO2.
Influence of Praseodymium Fluoride and Oxide on PFC Emission
In aluminium industrial electrolysis anode effect can occur up to several times per cell per day producing around 37.4 million metric tons of greenhouse gases worldwide [18]. This frequency of anode effect can be assumed for rare earth electrolysis requiring the analysis in which system and under which conditions the PFC has the highest emission. In Fig. 3 can be seen the tendency of anode effect and accordingly CF4 evolution with increasing praseodymium content in NdF3–PrF3–LiF electrolyte and different Nd2O3–Pr6O11 oxide concentration. In this figure the maximal CF4 concentrations obtained during the measurements are taken into consideration after which electrolysis is interfered. It can be noticed that CF4 amount decreases with increase of oxides in system and above 2 wt% remains relatively constant with increasing praseodymium content in electrolyte. This behavior is similar to aluminium electrolysis where wetting of the electrodes is worsened by alumina decrease leading to spread out of the gas bubbles and increase of their concentration on an anode [19] which in our case due to vertical position of used anode manages to escape the bath. There is so far no known research on the solubility of mixed didymium oxide in its fluoride salts but those results can implicate this chemical property as well. Nevertheless, the off-gas concentration values should be more considered for qualitative description of the systems and processes rather than for quantification of the off-gases due to many diverse influencing factors.
Influence of Praseodymium Valence on Process Window and off-Gas Composition
The systems containing Pr2O3 with Pr3+ and Pr6O11 with mixture of Pr3+ and Pr4+ are investigated and compared. Linear sweep voltammogram of NdF3–PrF3–LiF system with different concentrations of Nd2O3–Pr2O3 is shown in Fig. 4a expressing quite similar trend as the voltammograms done in the neodymium system without praseodymium (Fig. 2a). This behavior was expected, since in this case praseodymium and neodymium have the same valence 3+ and close decomposition potentials of −1.276 V and −1.297 V, respectively calculated by FactSage® thermodynamical software at 1050 °C. The partial passivation with oxygen containing ions (peak O1) is clear to distinguish from anode effect (peak O2) and these reactions are happening at almost the same potentials, ca. 1.7 V versus W-QRE and ca. 2.3 V versus W-QRE, respectively. Higher current densities are notable owing it to simultaneous oxidation reactions of neodymium and praseodymium oxy-fluoride complexes shown in Eqs. (1) and (2) reaching the values of around 1.4 A/cm2. The process window difference between systems using Pr3+ and mixture of Pr3+ and Pr4+ in same electrolyte presented in Fig. 4b clearly shows that additional oxidation reactions are taking part in systems containing Pr4+ ions. As assumed Pr4+ present in Pr6O11 forms as well some other oxy-fluoride complexes which are shifting the critical potential values more positive and disable visibility of partial passivation by oxygen containing species.
a Linear sweep voltammograms recorded on graphite electrode at 1050 °C in NdF3 –PrF3 –LiF (64.23–23.27–12.5 wt%) electrolyte with different Nd2O3 –Pr2O3 (73.4–26.6 wt%) concentrations and scan rate = 5 mV/s (above) with off-gases measured with an in situ FTIR-spectrometer generated during these measurements in case with 2 wt% oxide (below) b) Comparison of process windows in NdF3 –PrF3 –LiF electrolyte with 2.5 wt% Nd2O3 –Pr6O11 and Nd2O3 –Pr2O3
Principle and Implementation of Process Control
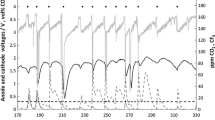
Electrolysis process control is usually focused on cell potential and oxide concentration values. Since oxide concentration is quite difficult to measure in situ and online, critical cell potential data are used to build a controller with help of company Yokogawa, which would trigger the dosing of the oxides by reaching this pre-set potential value [20]. Chronoamperometry measurements are done on chosen potentials near oxidation reactions (O1 and O2) (Fig. 5a), allowing longer reaction time, during which off-gases are measured (Fig. 5b) in order to confirm our results and assumptions that CF4 emission is detectable before full anode effect occurs. As it can be seen CF4 evolution happens prior the full anode effect and after partial passivation, whereas C2F6 is detectable afterwards when an anode effect starts. From it can be concluded and confirmed that in partial passivation only oxygen containing species are taking part and CF4 emission starts later at higher potentials. These findings led to possibility for installment of an advanced process control by connecting the FTIR-spectrometer (Ansyco) with new model controller UT55A. As soon as previously-set PFC concentrations are detected by in situ FTIR-spectrometer, the signal will be send to controller, triggering again the dosing of the oxides and preventing full anode effect.
Conclusion and Outlook
Process windows for neodymium electrolysis (as reference) and didymium molten salt electrolysis in different electrolytes, using diverse mixed oxide concentrations and with two different praseodymium oxides, namely Pr2O3 and Pr6O11, were determined. Critical potential for full anode effect differs only in case when Pr6O11 is used as the raw material leading to a more positive shift of anode potential values and impedes visibility of partial passivation by oxygen containing species. Presumably, next to present [NdOF5]4− and assumed [PrOF5]4− at least one more praseodymium oxy-fluoride complex exists taking part in oxidation reactions on the anode. Simultaneously, the off-gases were measured in situ by FTIR-spectrometer and analyzed. In regard to PFC emission the use of higher praseodymium fluoride content in electrolyte showed higher CF4 and C2F6 concentrations in case of anode effect, when the oxide amount was below 2 wt%. Investigations show that CF4 can be detected before full anode effect, whereas C2F6 is emitted at and after this phenomenon. This fact was used for installment of a controller triggering the oxide dosing after first CF4 detection preventing the system entering full anode effect. Next step will be implementation of those fundamental findings into scaled-up long-term electrolysis with similar setup to an industrial one in order to estimate the feasibility of the process control, i.e. PFC emission reduction.
References
Sun H et al. (2011) Coercivity enhancement in Nd–Fe–B sintered permanent magnet doped with Pr nanoparticles. Journal of Applied Physics 109 (7):07A749.
McGuiness PJ, Podmiljsak B, Kobe S (2003) The effect of Pr and Zr substitutions on the disproportionation reaction in Nd-Fe-B-based materials. IEEE Transactions on Magnetics 39(5):2956–2958.
Kablov EN et al. (2005) Effect of Praseodymium on Magnetic Properties and Phase Composition of a Material of the Nd–Pr–Dy–Fe–Co–B System. Metal Science and Heat Treatment 47(5–6):227–231.
Nakamoto M et al. (2012) Extraction of Rare Earth Elements as Oxides from a Neodymium Magnetic Sludge. Metallurgical and Materials Transactions B 43(3):468–476.
Kruse S et al. (2017) Influencing Factors on the Melting Characteristics of NdFeB-Based Production Wastes for the Recovery of Rare Earth Compounds. Journal of Sustainable Metallurgy 3(1):168–178.
Morrice E, Henrie TA (1967) Electrowinning high-purity neodymium, praseodymium, and didymium metals from their oxide. Report of investigations, United States Department of the Interior, Bureau of Mines, Washington D.C.
Tamamura H (1990) Process for preparing praseodynium metal or praseodymium-containing alloy US. Patent US4966662 A. 30 October 1990.
Liu Y et al. (2016) Effects of Physical Properties of Pr-Nd Oxide on its Electrolysis Preparation and Improvement Measures (in Chinese). Rare Metals and Cemented Carbides 44 (2):50–53.
Liu M, Wang L (2014) Study on the Effects of Electrolysis Temperature on Technical Indexes of Rare Earth Electrolytic Cell (in Chinese). Rare Metals and Cemented Carbides 42 (6):16–19.
Li J et al (2008) Carbon Content Control for Pr-Nd Alloy Production Process Using 6000 A Electrolysis Cell (in Chinese). Rare Metals Letters 27 (11):41–43.
Milicevic K, Meyer T, Friedrich B (2017) Influence of electrolyte composition on molten salt electrolysis of didymium. Presentation at The 2nd conference on European Rare Earth Resources, Santorini, Greece, 28–31 May 2017. https://doi.org/10.13140/RG.2.2.13137.33122.
Liu S et al. (2014) Anode processes for Nd electrowinning from LiF–NdF. Electrochimica Acta 147:82–86.
Vogel H et al. (2016) Reducing Greenhouse Gas Emission from the Neodymium Oxide Electrolysis. Part I: Analysis of the Anodic Gas Formation. Journal of Sustainable Metallurgy 3(1):99–107. https://doi.org/10.1007/s40831-016-0086-0.
Keller R, Larimer KT (1997) Anode effect in neodymium oxide electrolysis. In: Bautista, RG et al. (ed) Rare earths—science, technology and applications III. The Minerals, Metals and Materials Society, Warrendale, p 175–180.
Li B et al. (2014) Electrochemistry for Nd electrowinning from fluoride-oxide molten salts. In: Neale R et al. (ed) Rare Metal Technology. The Minerals, Metals & Materials Society, San Diego, p 95–98.
Grjotheim K et al. (1977) Aluminium electrolysis: The chemistry of the Hall-Héroult process. Aluminium-Verlag, Düsseldorf.
Stefanidaki E, Hasiotis C, Kontoyannis C (2001) Electrodeposition of neodymium from LiF–NdF3–Nd2O3 melts. Electrochimica Acta 46 (17):2665–2670.
Chase R, Gibson R, Marks J (2005) PFC Emissions performance for the global primary aluminium industry. In: Kvande H (ed) Light metals 2005. The Minerals Metals & Materials Society, Warrendale, p 279–282.
Thonstad J, Utigard TA, Vogt H (2000) On the Anode Effect in Aluminum Electrolysis. In: Peterson RD (ed) Light Metals 2000. The Minerals Metals & Materials Society, Warrendale, p 249–256.
Vogel H, Friedrich B (2017) Reducing Greenhouse Gas Emission from the Neodymium Oxide Electrolysis. Part II: Basics of a Process Control Avoiding PFC Emission. International Journal of Nonferrous Metallurgy, 6 (3):27–46.
Acknowledgements
This work was supported by the FP-7 EU Program Grant No. 309373 ‘‘Development of a Sustainable Exploitation Scheme for Europe’s rare earth Deposits’’ (EURARE).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Milicevic, K., Feldhaus, D., Friedrich, B. (2018). Conditions and Mechanisms of Gas Emissions from Didymium Electrolysis and Its Process Control. In: Martin, O. (eds) Light Metals 2018. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72284-9_187
Download citation
DOI: https://doi.org/10.1007/978-3-319-72284-9_187
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72283-2
Online ISBN: 978-3-319-72284-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)