Abstract
Host and symbiont enzymes are involved in lignocellulose processing by termites. A brief description of the structure of the main components of the plant cell wall and the most relevant degrading enzymes is presented. This chapter focuses on the dual cellulolytic system in lower and higher termites and provides an update on the current research strategies through culture-dependent and culture-independent “-omic” approaches. Significance for biofuel production and future perspectives are also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
During the last years, research on insect cellulolytic systems has gained attention because of their potential application in biofuel production. Among cellulose insect feeders, termites constitute one of the most promising sources of novel glycosyl hydrolase enzymes. Cellulose digestion in termites relies on both endogenous and exogenous enzymes. The implication of the former, long time disregarded, begun to emerge less than two decades ago. Even though, the digestive process cannot be accomplished without the action of exogenous enzymes, produced by a variety of endosymbiotic microorganisms hosted in the termite gut. In lower termites, the cellulolytic microbiota is composed mainly of protozoan flagellates. In contrast, higher termites lack such symbiotic protistan communities but have a huge diversity of intestinal bacteria. The increasing, available data provided by metagenomics and metatranscriptomics approaches will help to elucidate the insect’s machinery for lignocellulose degradation. Understanding these mechanisms is a crucial step toward the establishment of efficient enzymatic processes for the bioethanol industry.
5.2 Cellulolytic Enzymes
5.2.1 Lignocellulose Structure
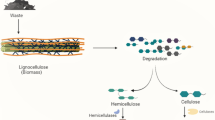
Lignocellulose is a heterogeneous matrix of three macromolecules: cellulose, hemicellulose, and lignin. This highly recalcitrant material makes up the cell wall in plants and therefore represents the most abundant biomass on earth (Isikgor and Becer 2015).
Cellulose, the principal component of lignocellulose, is a polysaccharide consisting of hundreds to thousands glucose monomers linked in linear chains of β-1,4 bonds. This polysaccharide is highly stable and very resistant to chemical attacks. Cellulose occurs in both crystalline and amorphous forms. The crystalline regions are packed very tightly, and consequently even very small active molecules have difficulty in breaking down this structure.
On the other hand, hemicellulose is a branched chain of polymers of structurally heterogeneous sugars: five-carbon sugars (such as D-xylose and L-arabinose), six-carbon sugars (frequently D-galactose, D-glucose, and D-mannose), and uronic acid. Because of its highly branched macromolecular structure, hemicellulose is relatively easy to hydrolyze to simple sugars (Bettiga et al. 2009).
Lignin is an amorphous aromatic polymer of phenolic compounds that are linked to each other and also covalently to hemicellulose by ester bonds. Lignin is usually more difficult to hydrolyze than cellulose and hemicellulose because of its cross-linked structure.
5.2.2 Main Lignocellulose-Degrading Enzymes
5.2.2.1 Cellulases
Cellulases are glycosyl hydrolases (GHs) that can cleave the glycosidic bonds present in cellulose. Indeed, the term “cellulase” encompasses all the cellulolytic enzymes, which include three main types: endoglucanases (1,4-β-D-glucan-4-glucanohydrolases, EC 3.2.1.4), exoglucanases (1,4-β-D-glucan cellobiohydrolases, EC 3.2.1.91 and 1,4-β-D-glucan cellobiohydrolases, EC 3.2.1.74), and β-glucosidases (EC 3.2.1.21).
Endoglucanases cleave amorphous sites in the cellulose chain at random, producing oligosaccharides of different lengths. Exoglucanases attack the ends of cellulose fibers to liberate cello-oligosaccharides (mainly cellobiose) or glucose. Finally, β-glucosidases hydrolyze cellobiose and other cello-oligomers to release glucose monomers from the nonreducing ends. The complete hydrolysis of cellulose usually requires the synergistic action of all these three types of cellulases and other accessory cellulolytic enzymes (Murashima et al. 2002; Tahir et al. 2005; Han and Chen 2010).
5.2.2.2 Hemicellulases
Hemicellulases are a diverse group of enzymes that catalyze the hydrolysis of hemicellulose. These enzymes are important in the digestion process because they expose cellulose to the action of cellulases, making it accessible for depolymerization. Because xylan is one of the main components of hemicellulose (Timell 1967), xylanases (EC 3.2.1.8) play a preponderant role in this respect. Other hemicellulolytic enzymes are also required to hydrolyze the hemicellulose in a synergistic way, including β-xylosidases (EC 3.2.1.37), β-mannanases (EC 3.2.1.78), α-L-arabinases (EC 3.2.1.99), α-L-arabinofuranosidases (EC 3.2.1.55), α-glucoronidases (EC 3.2.1.131), feruloyl esterases (EC 3.2.1.73), etc.
5.2.2.3 Ligninases
The major lignin-degrading enzymes are laccases (E.C. 1.10.3.2) and peroxidases: lignin peroxidase (E.C. 1.11.1.14) and manganese peroxidase (E.C. 1.11.1.13). A number of less significant oxidative ligninolytic enzymes include diaryl propane oxygenases, versatile peroxidases, and dye-decolorizing peroxidases. In addition, several accessory enzymes (oxidases and reductases) act as mediators favoring the ligninolytic activity of the principal enzymes. They participate in H2O2 production, needed by the peroxidases, or catalyze phenolic products reductions. Modification or cleavage of lignin improves the accessibility of cellulases and hemicellulases, thus increasing the efficiency of lignocellulose degradation (Plácido and Capareda 2015).
5.3 The Cellulolytic Systems of Termites
Lignocellulose degradation in termites depends on a dual system that includes activities of both the host and its intestinal symbionts. Mechanical digestion works together with the enzymatic action to maximize lignocellulose degradation (Fig. 5.1). For almost a century, the theory was accepted that cellulose digestion in termites was mediated only by the hindgut microbiota (Ohkuma 2003; Hongoh 2011; Ni and Tokuda 2013). The key role of a variety of enzyme-producing symbiotic microorganisms (including protistans, archaea, bacteria, and fungi) is well documented. Nevertheless, since the first description of an endogenous cellulase (an endoglucanase) in the lower termite Reticulitermes speratus (Watanabe et al. 1998), accumulated evidence proved that host hydrolytic enzymes contribute to lignocellulose processing in a non-negligible manner.
5.3.1 Lower Termites
In lower termites, which are generally xylophages, the cellulolytic process starts in the foregut; the wood fragments cut by the mandibles are further triturated by the muscular gizzard into smaller particles (10–20 μm in diameter). The endogenous enzymes secreted by the salivary glands into the foregut initiate cellulose hydrolysis. A number of papers and reviews have signaled the salivary glands as a source of endoglucanases, primarily (Watanabe et al. 1997; Zhou et al. 2007; Brune 2014). The production of β-glucosidases in this organ has also been established for several termite species (Tokuda et al. 2002; Zhang et al. 2012a; Shimada and Maekawa 2014). In the midgut, the high concentration of endoglucanases breaks down the amorphous regions of the cellulose fibers, and the synergistic action of β-glucosidases prevents product inhibition by reducing cellobiose accumulation (Watanabe and Tokuda 2010; Ni and Tokuda 2013; Brune 2014). Finally, the protistan flagellates housed in the hindgut produce the three principal types of cellulases (endoglucanases, exoglucanases, and β-glucosidases), as well as hemicellulases. Inside the protozoan digestive vacuoles, these enzymes cooperate in hydrolyzing hemicellulose, crystalline cellulose, and other remnants of ingestion.
The expression of cellulolytic enzymes in the termite gut varies according to the termite caste and developmental stages (Fujita et al. 2010; Shimada and Maekawa 2010). Regarding the classification, Henrissat and Bairoch (1993) demonstrated that, despite having different substrate specificities, members of the same GH family often have a common evolutionary origin, as revealed by their structural similarities. All the endogenous endoglucanases identified so far belong to the GH 9 family (Teather and Wood 1982; Watanabe and Tokuda 2010; Leonardo et al. 2011; Zhang et al. 2012b; Scharf 2015a). On the other hand, the protistan endoglucanases correspond to GHs 5, 7, and 45, which seem to belong to a core enzyme set conserved during symbiotic evolution (Ni and Tokuda 2013; Scharf 2015a). To date, host β-glucosidases were found to belong to GH 1 family (Slaytor 2000; Tokuda et al. 2002). Concerning hemicellulases, some works have reported xylanases from the host (GH 11) and protist symbionts (GH 45), which were recombinant expressed (Sasagawa et al. 2011; Sethi et al. 2013). Several ligninolytic enzymes, such as laccases, peroxidases, aldo-keto reductases, phenol oxidases, and esterases, have been also identified in termites (Coy et al. 2010; Chandrasekharaiah et al. 2011; Sethi et al. 2013).
In lower termites, the bacterial microbiota seems not to have a lead role in lignocellulose hydrolysis. It has been stated that when wood particles enter the hindgut, they are immediately arrested in the food vacuoles of the flagellate protists (Brune 2014). However, the recent discovery of a bacterial feruloyl esterase from Coptotermes formosanus (Rashamuse et al. 2014) opens new insights in this matter.
5.3.2 Higher Termites
During the Eocene period (around 60 Myrs ago), the higher termites lost the endosymbiont flagellates and, in consequence, diverged by evolving new strategies for cellulose hydrolysis (Lo and Eggleton 2011; Brune 2014). The higher termites have different feeding habits, including organic matter of soil, wood, herbivore dung, litter, lichen, and dry grass (Konig et al. 2013). Except for the fungi-associated termites (referred later), their hindguts evolved to increased length, compartmentalization, and alkalinity in order to partially palliate the absence of protozoans. Lignocellulose digestion in higher termites is poorly studied and needs further clarification. Endoglucanase secretion by the salivary glands seems to be the exception rather than the rule (Tokuda et al. 1997; Tokuda et al. 2004). Instead, there is a consensus about the enzymatic production in the midgut epithelium that is much more relevant to cellulose processing than in lower termites (Lo et al. 2011; Brune 2014). Thus, the cellulose hydrolysis is performed mainly by host and bacterial endosymbiont endoglucanases secreted by the midgut and the hindgut, respectively (Watanabe et al. 1998; Tokuda et al. 2004). Both host and bacterial GH 9 endoglucanases have been reported, while GH 5 and 45 are considered of bacterial origin only. Concerning the β-glucosidases (GH 1), the organs involved vary among termite species. In majority of Nasutitermes sp., β-glucosidases have been detected mainly in the salivary glands and midgut, whereas in other species, this activity occurs mostly in the hindgut (Slaytor 2000; Uchima and Arioka 2012; Wang et al. 2012; Ni and Tokuda 2013; Rashamuse et al. 2014). Also, two xylanases (GH 10 and 11) have been isolated and recombinant expressed from Nasutitermes sp. and Globitermes brachycerastes bacterial symbionts, respectively (Brennan et al. 2004; Han et al. 2013). A less frequent, but conspicuous association, can be found in the subfamily Macrotermitinae, in which a basidiomycete fungus, cultivated inside the nest, contributes to lignocellulose degradation (Johjima et al. 2006; Liu et al. 2013).
5.4 Omics Approaches Applied to the Discovery of Novel Cellulolytic Enzymes
Since the first genomic studies, the suffix “-omics” has been used to denote large-scale research in different fields, including metagenomics, transcriptomics, proteomics, and metabolomics. The term digestome was used to describe the ensemble of endogenous and symbiont genes that contribute to lignocellulose digestion in the digestive tract of animals, including termites (Scharf and Tartar 2008; Tartar et al. 2009).
A current scheme of the “-omics” approach to the cellulolytic systems in termites is illustrated in Fig. 5.2. There are two main ways to study the termite digestome. The first one explores genomic strategies through culture-dependent approaches, i.e., the isolation of microorganisms containing cellulolytic genes or the characterization of enzymatic extracts from cellulolytic enrichment cultures (Ben Guerrero et al. 2015; Butera et al. 2016). This method focuses on insect-associated microorganisms and therefore does not take into account the host genome. Its potential is further limited by the fact that it is widely accepted that only about 1% of the gut microbial diversity can be cultured through conventional techniques.
The second approach seeks to overcome these constraints applying strategies (which include metagenomics, metatranscriptomics, metaproteomics, and metabolomics) that do not require microorganism cultivation in artificial media and also considers the insect host. These studies can be performed independently or in combination. The main strategies dealing with metagenomics are (1) biodiversity analyses (symbionts); (2) functional metagenomics (through the construction of metagenomic libraries in fosmids or plasmids, functional screening, and selection of individual clones with cellulolytic activity); and (3) sequence-based analysis through next-generation sequencing (NGS) by Illumina, 454 pyrosequencing technologies, etc. To date, successful results in functional metagenomics and NGS allowed the identification of genes encoding lignocellulolytic enzymes or novel protein families in both host and symbionts, as well as their heterologous expression, purification, and biochemical characterization (Fig. 5.2).
In termites, near 50 articles referring to “-omics” have been referenced in PubMed by June 2016, at least 25 concerned metagenomics (Warnecke et al. 2007; He et al. 2013; Liu et al. 2013; Do et al. 2014), around 18 metatranscriptomics (Huang et al. 2012; Sethi et al. 2013; Rajarapu et al. 2015), and 5 metaproteomics (Burnum et al. 2011; Sillam-Dusses et al. 2012; Bauwens et al. 2013). Several of them combined different approaches. Table 5.1 provides a summary of some relevant “-omics” studies concerning termites.
Most metagenomic studies have been based on the identification of lignocellulases from enriched lignocellulosic cultures of microorganisms (Liu et al. 2011; Matteotti et al. 2011; Matteotti et al. 2012; Nimchua et al. 2012; Wang et al. 2012; Rashamuse et al. 2014). Other studies have targeted total gut microbiota in termites with different feeding habits (Warnecke et al. 2007; He et al. 2013; Liu et al. 2013). Many transcriptomic studies have focused on host transcriptome by employing different techniques, such as subtractive hybridization or cDNA macroarrays, random or de novo cDNA library sequencing, and cDNA oligonucleotide microarrays, to reveal differentially expressed genes (Warnecke et al. 2007; Weil et al. 2009; Ishikawa et al. 2010; Hojo et al. 2012; Huang et al. 2012; Husseneder et al. 2012; Terrapon et al. 2014). Other studies have considered symbiont metatranscriptomic analyses by using traditional and NGS technologies (Scharf et al. 2005; Todaka et al. 2010; Rosenthal et al. 2011; Xie et al. 2012; Zhang et al. 2012b; He et al. 2013).
In higher termites, very few proteomic studies have been published. The results obtained were of limited resolution or just sufficient to corroborate the presence of some bacterial cellulases predicted by transcriptomic or metagenomic approaches (Warnecke et al. 2007; Burnum et al. 2011; Ben Guerrero et al. 2015). Proteomic studies in lower termites were more consistent and have allowed researchers to identify protist cellulases previously determined by metagenomic approaches (Todaka et al. 2007; Sethi et al. 2013) and describe symbiont diversity (Bauwens et al. 2013).
Several metabolomic studies have focused on lignocellulose digestion, paying special attention to lignin modifications. As mentioned earlier, termites are very efficient degraders of lignocellulose. However, lignin degradation constitutes one of the less-known aspects of the process and still needs to be clarified (Geib et al. 2008; Ke et al. 2011, 2013; Tokuda et al. 2014). The extent of lignin decomposition is actually a subject of controversy. Previous works identified gut bacteria able to degrade aromatic compounds, and peroxidase producers as well, suggesting that these bacteria could be involved in lignin modifications or degradation (Bugg et al. 2011; Ke et al. 2012). It is possible that termites are capable of degrading only some functional groups, monomers, or dimers but not large lignin molecules (Ke et al. 2011). However, no genes encoding ligninolytic enzymes have been identified in termite guts so far. Other metabolomic studies helped to understand the contribution of endogenous and symbiont microorganisms enzymes in the overall digestion process (Scharf et al. 2011; Tokuda et al. 2014).
Sequence surveys targeting 16S rRNA have been used in diversity analyses of bacteria and archaea (Wang and Qian 2009), whereas 18S rRNA screenings identified protist symbionts (Tai and Keeling 2013). Cloning-dependent and cloning-independent approaches were performed through low- and high-throughput analyses, focusing on both functional and taxonomic topics (Warnecke et al. 2007; He et al. 2013). In general, six major bacterial phyla are represented across higher and lower termites: Bacteroidetes, Firmicutes, Spirochaetes, Proteobacteria, Fibrobacteres, and Elusimicrobia (Brune 2014). Many hundreds to more than thousand species have been recorded in the different termite species investigated so far (Boucias et al. 2013). Interestingly, the 16S surveys revealed that lignocellulosic diet shifts have no short-term impacts on the microbiota composition of termites (Sanyika et al. 2012; Boucias et al. 2013). Otani et al. (2014) suggested a core microbiota of 42 genera shared among 9 termite species tested. Although less diverse than bacteria, the number of protist taxa revealed by 18S rRNA high throughput sequencing was found to be higher than estimated by morphology.
The advanced “-omics” research on termites has contributed to elucidate molecular and physiological issues of the host/symbiont relationships and the cellulolytic digestion processes mediated by the different actors involved. Further work is needed to better understand the functional significance of the data obtained by genome (and metagenome), transcriptome, and proteome sequencing.
5.5 Potential Industrial Applications and Future Perspectives
Termites are an interesting biotechnological model for various industrial applications, including fuels, food, breweries, pulp, and paper. The termite symbiotic system is a rich resource, promising the discovery of new genes and enzymes.
As lignocellulose is the main component of the plant cell wall, it is the most abundant, widespread, and renewable biofuel resource available on Earth. It is composed mainly of cellulose, hemicellulose, and lignin in different proportions, depending on the plant taxa. The conversion of the plant biomass into bioethanol can be divided into three main processes: pretreatment, hydrolysis, and fermentation (Merino and Cherry 2007). The major limitation in this process is the cost of the hydrolysis step and the inefficiency of industrial lignocellulose pretreatments (Scharf 2015b; Yang and Wyman 2008). Termites, and their associated microorganisms, contain enzymes that could be useful in order to remove or modify lignin and hemicellulose in the biomass pretreatment, then to hydrolyze cellulose into sugar monomers, and finally to ferment them into acetate. Thus, the study of the termite cellulolytic system could be a valuable tool for reducing the high costs experienced in the biofuel industry. Future research should increasingly focus on the characterization of these enzymes and on the genetic engineering work needed to make them more suitable for an industrial use.
5.6 Conclusion
The maintenance of a dual cellulolytic system across evolutionary time reveals that the combined action of both strategies is essential for termites’ survival. More “-omics” efforts are needed to better understand the contribution of endogenous (host) and exogenous (symbionts) enzymes in the digestion of lignocellulose. The ability of termites to degrade recalcitrant plant biomasses and their ubiquitous distribution make them an ideal biological model for the industrial processing of cellulosic material. The discovery of novel enzymes, of either host or symbiont origin, is regarded as a major concern for biorefinery improvement.
References
Bastien, G., Arnal, G., Bozonnet, S., Laquerre, S., Ferreira, F., Fauré, R., Henrissat, B., Lefèvre, F., Robo, P., Bouchez, O., Noirot, C., Dumond, C., & O’Donohue, M. (2013). Mining for hemicellulases in the fungus-growing termite Pseudacanthotermes militaris using functional metagenomics. Biotechnology for Biofuels, 6, 78.
Bauwens, J., Millet, C., Tarayre, C., Brasseur, C., Destain, J., Vandenbol, M., Thonart, P., Portetelle, D., De Pauw, E., Haubruge, E., & Francis, F. (2013). Symbiont diversity in Reticulitermes santonensis: Investigation strategy through proteomics. Environmental Entomology, 42, 882–887.
Ben Guerrero, E., Arneodo, J., Campanha, R. B., Oliveira, P. A., Labate, M. T. V., Regiani, T., Campos, E., Cataldi, A., Labate, C. A., Rodrigues, C. M., & Talia, P. (2015). Prospection and evaluation of (hemi) cellulolytic enzymes using untreated and pretreated biomass in two Argentinean native termites. PLoS One, 10, e0136573.
Benjamino, J., & Graf, J. (2016). Characterization of the core and caste-specific microbiota in the termite, Reticulitermes flavipes. Frontiers in Microbiology, 7, 171.
Bettiga, M., Bengtsson, O., Hahn-Hagerdal, B., & Gorwa-Grauslund, M. F. (2009). Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Microbial Cell Factories, 8, 40. https://doi.org/10.1186/1475-2859-8-40.
Boucias, D. G., Cai, Y., Sun, Y., Lietze, V. U., Sen, R., Raychoudhury, R., & Scharf, M. E. (2013). The hindgut lumen prokaryotic microbiota of the termite Reticulitermes flavipes and its responses to dietary lignocellulose composition. Molecular Ecology, 22, 1836–1853.
Brennan, Y., Callen, W. N., Christoffersen, L., Dupree, P., Goubet, F., Healey, S., Hernandez, M., Keller, M., Li, K., Palackal, N., Sittenfeld, A., Tamayo, G., Wells, S., Hazlewood, G. P., Mathur, E. J., Short, J. M., Robertson, D. E., & Steer, B. A. (2004). Unusual microbial xylanases from insect guts. Applied and Environmental Microbiology, 70, 3609–3617.
Brune, A. (2014). Symbiotic digestion of lignocellulose in termite guts. Nature Reviews Microbiology, 12, 681–180.
Bugg, T. D. H., Ahmad, M., Hardiman, E. M., & Singh, R. (2011). The emerging role for bacteria in lignin degradation and bioproduct formation. Current Opinion in Biotechnology, 22, 394–400.
Burnum, K. E., Callister, S. J., Nicora, C. D., Purvine, S. O., Hugenholtz, P., Warnecke, F., Scheffrahn, R. H., Smith, R. D., & Lipton, M. S. (2011). Proteome insights into the symbiotic relationship between a captive colony of Nasutitermes corniger and its hindgut microbiome. The ISME Journal, 5, 161–164.
Butera, G., Ferraro, C., Alonzo, G., Colazza, S., & Quatrini, P. (2016). The gut microbiota of the wood-feeding termite Reticulitermes lucifugus (Isoptera; Rhinotermitidae). Annals of Microbiology, 66, 253–260.
Chandrasekharaiah, M., Thulasi, A., Bagath, M., Kumar, D. P., Santosh, S. S., Palanivel, C., Jose, V. L., & Sampath, K. T. (2011). Molecular cloning, expression and characterization of a novel feruloyl esterase enzyme from the symbionts of termite (Coptotermes formosanus) gut. BMB Reports, 44, 52–57.
Coy, M. R., Salem, T. Z., Denton, J. S., Kovaleva, E. S., Liu, Z., Barber, D. S., Campbell, J. H., Davis, D. C., Buchman, G. W., Boucias, D. G., & Scharf, M. E. (2010). Phenol-oxidizing laccases from the termite gut. Insect Biochemistry and Molecular Biology, 40, 723–732.
Do, T. H., Nguyen, T. T., Nguyen, T. N., Le, Q. G., Nguyen, C., Kimura, K., & Truong, N. H. (2014). Mining biomass-degrading genes through Illumina-based de novo sequencing and metagenomic analysis of free-living bacteria in the gut of the lower termite Coptotermes gestroi harvested in Vietnam. Journal of Bioscience and Bioengineering, 6, 665–671.
Fujita, A., Hojo, M., Aoyagi, T., Hayashi, Y., Arakawa, G., Tokuda, G., & Watanabe, H. (2010). Details of the digestive system in the midgut of Coptotermes formosanus Shiraki. Journal of Wood Science, 56, 222–226.
Geib, S. M., Filley, T. R., Hatcher, P. G., Hoover, K., Carlson, J. E., Jimenez-Gasco, M. M., Nakagawa-Izumi, A., Sleighter, R. L., & Tien, M. (2008). Lignin degradation in wood-feeding insects. Proceedings of the National Academy of Sciences, 105, 12932–12937.
Han, Y. J., & Chen, H. Z. (2010). Synergism between hydrophobic proteins of corn stover and cellulase in lignocellulose hydrolysis. Biochemical Engineering Journal, 48, 218–224.
Han, Q., Liu, N., Robinson, H., Cao, L., Qian, C., Wang, Q., Xie, L., Ding, H., Wang, Q., Huang, Y., Li, J., & Zhou, Z. (2013). Biochemical characterization and crystal structure of a GH10 xylanase from termite gut bacteria reveal a novel structural feature and significance of its bacterial Ig-like domain. Biotechnology and Bioengineering, 110, 3093–3103.
He, S., Ivanova, N., Kirton, E., Allgaier, M., Bergin, C., Scheffrahn, R. H., Kyrpides, N. C., Warnecke, F., Tringe, S. G., & Hugenholtz, P. (2013). Comparative metagenomic and metatranscriptomic analysis of hindgut paunch microbiota in wood- and dung-feeding higher termites. PLoS One, 8, e61126.
Henrissat, B., & Bairoch, A. (1993). New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. The Biochemical Journal, 293, 781–788.
Hojo, M., Maekawa, K., Saitoh, S., Shigenobu, S., Miura, T., Hayashi, Y., Tokuda, G., & Maekawa, H. (2012). Exploration and characterization of genes involved in the synthesis of diterpene defence secretion in nasute termite soldiers. Insect Molecular Biology, 21, 545–557.
Hongoh, Y. (2011). Toward the functional analysis of uncultivable, symbiotic microorganisms in the termite gut. Cellular and Molecular Life Sciences, 68, 1311–1325.
Huang, Q., Sun, P., Zhou, X., Lei, C., Huang, Q., Sun, P., Zhou, X., & Lei, C. (2012). Characterization of head transcriptome and analysis of gene expression involved in caste differentiation and aggression in Odontotermes formosanus (Shiraki). PLoS One, 7, e50383.
Husseneder, C., Simms, D. M., Aluko, G. K., & Delatte, J. (2010a). Colony breeding system influences cuticular bacterial load of Formosan subterranean termite workers. Environmental Entomology, 39, 1715–1723.
Husseneder, C., Ho, H. Y., & Blackwell, M. (2010b). Comparison of the Bacterial symbiont composition of the Formosan subterranean termite from its native and introduced range. Open Microbiology Journal, 4, 53–66.
Husseneder, C., McGregor, C., Lang, R. P., Collier, R., & Delatte, J. (2012). Transcriptome profiling of female alates and egg-laying queens of the Formosan subterranean termite. Comparative Biochemistry and Physiology, 7, 14–27.
Ishikawa, Y., Okada, Y., Ishikawa, A., Miyakawa, H., Koshikawa, S., & Miura, T. (2010). Gene expression changes during caste-specific neuronal development in the damp-wood termite Hodotermopsis sjostedti. BMC Genomics, 11, 314.
Isikgor, F. H., & Becer, C. R. (2015). Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polymer Chemistry, 6, 4497–4559.
Johjima, T., Taprab, Y., Noparatnaraporn, N., Kudo, T., & Ohkuma, M. (2006). Large-scale identification of transcripts expressed in a symbiotic fungus (Termitomyces) during plant biomass degradation. Applied Microbiology and Biotechnology, 73, 195–203.
Ke, J., Laskar, D. D., Singh, D., & Chen, S. (2011). In situ lignocellulosic unlocking mechanism for carbohydrate hydrolysis in termites: Crucial lignin modification. Biotechnology for Biofuels, 4, 17.
Ke, J., Singh, D., & Chen, S. (2012). Metabolism of polycyclic aromatic hydrocarbons by the wood-feeding termite Coptotermes formosanus (Shiraki). Journal of Agricultural and Food Chemistry, 60, 1788–1797.
Ke, J., Laskar, D. D., & Chen, S. (2013). Tetramethylammonium hydroxide (TMAH) thermochemolysis for probing in situ softwood lignin modification in each gut segment of the termite. Journal of Agricultural and Food Chemistry, 61, 1299–1308.
Kohler, T., Dietrich, C., Scheffrahn, R. H., & Brune, A. (2012). High-resolution analysis of gut environment and bacterial microbiota reveals functional compartmentation of the gut in wood-feeding higher termites (Nasutitermes sp.) Applied and Environmental Microbiology, 78, 4691–4701.
Konig, H., Li, L., & Frohlich, J. (2013). The cellulolytic system of the termite gut. Applied Microbiology and Biotechnology, 97, 7943–7962.
Leonardo, F. C., da Cunha, A. F., da Silva, M. J., Carazzolle, M. F., Costa-Leonardo, A. M., Costa, F. F., & Pereira, G. A. (2011). Analysis of the workers head transcriptome of the Asian subterranean termite, Coptotermes gestroi. Bulletin of Entomological Research, 101, 383–391.
Liu, N., Xing, Y., Zhang, M., Xie, L., Wang, Q., Huang, Y., Zhou, X., Wang, S., & Zhou, Z. (2011). Microbiome of fungus-growing termites: A new reservoir for lignocellulase genes. Applied and Environmental Microbiology, 77, 48–56.
Liu, N., Zhang, L., Zhou, H., Zhang, M., Yan, X., Wang, Q., Long, Y., Xie, L., Wang, S., Huang, Y., & Zhou, Z. (2013). Metagenomic insights into metabolic capacities of the gut microbiota in a fungus-cultivating termite (Odontotermes yunnanensis). PLoS One, 8, e69184.
Lo, N., & Eggleton, P. (2011). Termite phylogenetics and co-cladogenesis with symbionts. In D. E. Bignell, Y. Roisin, & N. Lo (Eds.), Biology of termites: A modern synthesis (pp. 27–50). Dordrecht: Springer.
Lo, N., Tokuda, G., & Watanabe, H. (2011). Evolution and function of endogenous termite cellulases. In D. E. Bignell, Y. Roisin, & N. Lo (Eds.), Biology of termites: A modern synthesis (pp. 51–67). Dordrecht: Springer.
Matteotti, C., Haubruge, E., Thonart, P., Francis, F., De Pauw, E., Portetelle, D., & Vandenbol, M. (2011). Characterization of a new β-glucosidase/β-xylosidase from the gut microbiota of the termite (Reticulitermes santonensis). FEMS Microbiology Letters, 314, 147–157.
Matteotti, C., Bauwens, J., Brasseur, C., Tarayre, C., Thonart, P., Destain, J., Francis, F., Haubruge, E., De Pauw, E., Portetelle, D., & Vandenbol, M. (2012). Identification and characterization of a new xylanase from Gram-positive bacteria isolated from termite gut (Reticulitermes santonensis). Protein Expression and Purification, 83, 117–127.
Merino, S. T., & Cherry, J. (2007). Progress and challenges in enzyme development for biomass utilization. Advances in Biochemical Engineering/Biotechnology, 108, 95–120.
Mikaelyan, A., Dietrich, C., Kohler, T., Poulsen, M., Sillam-Dusses, D., & Brune, A. (2015). Diet is the primary determinant of bacterial community structure in the guts of higher termites. Molecular Ecology, 24, 5284–5295.
Murashima, K., Kosugi, A., & Doi, R. H. (2002). Thermostabilization of cellulosomal endoglucanase EngB from Clostridium cellulovorans by in vitro DNA recombination with non-cellulosomal endoglucanase EngD. Molecular Microbiology, 45, 617–626.
Ni, J., & Tokuda, G. (2013). Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnology Advances, 31, 838–850.
Nimchua, T., Thongaram, T., Uengwetwanit, T., Pongpattanakitshote, S., & Eurwilaichitr, L. (2012). Metagenomic analysis of novel lignocellulose-degrading enzymes from higher termite guts inhabiting microbes. Journal of Microbiology and Biotechnology, 22, 462–469.
Ohkuma, M. (2003). Termite symbiotic systems: Efficient biorecycling of lignocellulose. Applied Microbiology and Biotechnology, 61, 1–9.
Otani, S., Mikaelyan, A., Nobre, T., Hansen, L. H., Kone, N. A., Sorensen, S. J., AD, K., Boomsma, J. J., Brune, A., & Poulsen, M. (2014). Identifying the core microbial community in the gut of fungus-growing termites. Molecular Ecology, 23, 4631–4644.
Placido, J., & Capareda, S. (2015). Ligninolytic enzymes: A biotechnological alternative for bioethanol production. Bioresource Bioprocess, 2, 23. https://doi.org/10.1186/s40643-015-0049-5.
Rajarapu, S. P., Shreve, J. T., Bhide, K. P., Thimmapuram, J., & Scharf, M. E. (2015). Metatranscriptomic profiles of Eastern subterranean termites, Reticulitermes flavipes (Kollar) fed on second generation feedstocks. BMC Genomics, 16, 332. https://doi.org/10.1186/s12864-015-1502-8.
Rashamuse, K., Ronneburg, T., Sanyika, W., Mathiba, K., Mmutlane, E., & Brady, D. (2014). Metagenomic mining of feruloyl esterases from termite enteric flora. Applied Microbiology and Biotechnology, 98, 727–737.
Rosenthal, A. Z., Matson, E. G., Eldar, A., & Leadbetter, J. R. (2011). RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture. The ISME Journal, 5, 1133–1142.
Sanyika, T. W., Rashamuse, K. J., Hennesy, F., & Brady, D. (2012). Luminal hindgut bacterial diversities of the grass and sugarcane feeding termite Trinervitermes trinervoides. African Journal of Microbiology Research, 6, 2639–2648.
Sasagawa, T., Matsui, M., Kobayashi, Y., Otagiri, M., Moriya, S., Sakamoto, Y., Ito, Y., Lee, C. C., Kitamoto, K., & Arioka, M. (2011). High-throughput recombinant gene expression systems in Pichia pastoris using newly developed plasmid vectors. Plasmid, 65, 65–69.
Scharf, M. E. (2015a). Omic research in termites: An overview and a roadmap. Frontiers in Genetics, 6, 1–19.
Scharf, M. E. (2015b). Termites as targets and models for biotechnology. Annual Review of Entomology, 60, 77–102.
Scharf, M. E., & Tartar, A. (2008). Termite digestomes as sources for novel lignocellulases. Biofuels, Bioproducts and Biorefining, 2, 540–552.
Scharf, M. E., Wu-Scharf, D., Zhou, X., Pittendrigh, B. R., & Bennett, G. W. (2005). Gene expression profiles among immature and adult reproductive castes of the termite Reticulitermes flavipes. Insect Molecular Biology, 14, 31–44.
Scharf, M. E., Karl, Z. J., Sethi, A., & Boucias, D. G. (2011). Multiple levels of synergistic collaboration in termite lignocellulose digestion. PLoS One, 6, e21709.
Sethi, A., Slack, J. M., Kovaleva, E. S., Buchman, G. W., & Scharf, M. E. (2013). Lignin-associated metagene expression in a lignocellulose-digesting termite. Insect Biochemistry and Molecular Biology, 43, 91–101.
Shimada, K., & Maekawa, K. (2010). Changes in endogenous cellulase gene expression levels and reproductive characteristics of primary and secondary reproductives with colony development of the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Journal of Insect Physiology, 56, 1118–1124.
Shimada, K., & Maekawa, K. (2014). Gene expression and molecular phylogenetic analyses of beta-glucosidase in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Journal of Insect Physiology, 65, 63–69.
Sillam-Dusses, D., Krasulova, J., Vrkoslav, V., Pytelkova, J., Cvacka, J., Kutalova, K., Bourguignon, T., Miura, T., & Sobotnik, J. (2012). Comparative study of the labial gland secretion in termites (Isoptera). PLoS One, 7, e46431.
Slaytor, M. (2000). Energy metabolism in the termite and its gut microbiota. In T. Abe, D. E. Bignell, & M. Higashi (Eds.), Termites: Evolution, sociality, symbioses, ecology (pp. 307–332). Dordrecht: Kluwer Academics Publishers.
Tahir, M., Saleh, F., Ohtsuka, A., & Hayashi, K. (2005). Synergistic effect of cellulase and hemicellulase on nutrients utilization and performance in broilers fed corn-soybean meal diet. Animal Science Journal, 76, 559–565.
Tai, V., & Keeling, P. J. (2013). Termite hindguts and the ecology of microbial communities in the sequencing age. The Journal of Eukaryotic Microbiology, 60, 421–428.
Tartar, A., Wheeler, M. M., Zhou, X., Coy, M. R., Boucias, D. G., & Scharf, M. E. (2009). Parallel meta-transcriptome analyses of host and symbiont gene expression in the gut of the termite Reticulitermes flavipes. Biotechnology for Biofuels, 2, 25.
Teather, R. M., & Wood, P. J. (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied and Environmental Microbiology, 43, 777–780.
Terrapon, N., Li, C., Robertson, H. M., Ji, L., Meng, X., Booth, W., Chen, Z., et al. (2014). Molecular traces of alternative social organization in a termite genome. Nature Communications, 5, 3636. https://doi.org/10.1038/ncomms4636.
Timell, T. E. (1967). Recent progress in the chemistry of wood hemicelluloses. Wood Science and Technology, 1, 45–70.
Todaka, N., Moriya, S., Saita, K., Hondo, T., Kiuchi, I., Takasu, H., Ohkuma, M., Piero, C., Hayashizaki, Y., & Kudo, T. (2007). Environmental cDNA analysis of the genes involved in lignocellulose digestion in the symbiotic protist community of Reticulitermes speratus. FEMS Microbiology Ecology, 59, 592–599.
Todaka, N., Inoue, T., Saita, K., Ohkuma, M., Nalepa, C. A., Lenz, M., Kudo, T., & Moriya, S. (2010). Phylogenetic analysis of cellulolytic enzyme genes from representative lineages of termites and a related cockroach. PLoS One, 5, e8636.
Tokuda, G., Watanabe, H., Matsumoto, T., & Noda, H. (1997). Cellulose digestion in the wood-eating higher termite, Nasutitermes takasagoensis (Shiraki): Distribution of cellulases and properties of endo-beta-1,4-glucanase. Zoological Science, 14, 83–93.
Tokuda, G., Saito, H., & Watanabe, H. (2002). A digestive β-glucosidase from the salivary glands of the termite, Neotermes koshunensis (Shiraki): Distribution, characterization and isolation of its precursor cDNA by 5′- and 3′-RACE amplifications with degenerate primers. Insect Biochemistry and Molecular Biology, 32, 1681–1689.
Tokuda, G., Lo, N., Watanabe, H., Arakawa, G., Matsumoto, T., & Noda, H. (2004). Major alteration of the expression site of endogenous cellulases in members of an apical termite lineage. Molecular Ecology, 13, 3219–3228.
Tokuda, G., Tsuboi, Y., Kihara, K., Saitou, S., Moriya, S., Lo, N., & Kikuchi, J. (2014). Metabolomic profiling of 13C-labelled cellulose digestion in a lower termite: Insights into gut symbiont function. Proceedings of the Biological Sciences, 281, 1789.
Uchima, C. A., & Arioka, M. (2012). Expression and one-step purification of recombinant proteins using an alternative episomal vector for the expression of N-tagged heterologous proteins in Pichia pastoris. Bioscience, Biotechnology, and Biochemistry, 76, 368–371.
Wang, Y., & Qian, P. Y. (2009). Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One, 4, e7401.
Wang, Q., Qian, C., Zhang, X. Z., Liu, N., Yan, X., & Zhou, Z. (2012). Characterization of a novel thermostable β-glucosidase from a metagenomic library of termite gut. Enzyme and Microbial Technology, 51, 319–324.
Warnecke, F., Luginbuhl, P., Ivanova, N., Ghassemian, M., Richardson, T. H., Stege, J. T., Cayouette, M., McHardy, A. C., Djordjevic, G., Aboushadi, N., Sorek, R., Tringe, S. G., et al. (2007). Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature, 450, 560–565.
Watanabe, H., & Tokuda, G. (2010). Cellulolytic Systems in Insects. Annual Review of Entomology, 55, 609–632.
Watanabe, H., Nakamura, M., Tokuda, G., Yamaoka, I., Scrivener, A. M., & Noda, H. (1997). Site of secretion and properties of endogenous endo-β-1,4-glucanase components from Reticulitermes speratus (Kolbe), a Japanese subterranean termite. Insect Biochemistry and Molecular Biology, 27, 305–313.
Watanabe, H., Noda, H., Tokuda, G., & Lo, N. (1998). A cellulase gene of termite origin. Nature, 394, 330–331.
Weil, T., Korb, J., & Rehli, M. (2009). Comparison of queen-specific gene expression in related lower termite species. Molecular Biology and Evolution, 26, 1841–1850.
Xie, L., Zhang, L., Zhong, Y., Liu, N., Long, Y., Wang, S., Zhou, X., Zhou, Z., Huang, Y., & Wang, Q. (2012). Profiling the metatranscriptome of the protistan community in Coptotermes formosanus with emphasis on the lignocellulolytic system. Genomics, 99, 246–255.
Yang, B., & Wyman, C. E. (2008). Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2, 26–40.
Zhang, D., Allen, A. B., & Lax, A. R. (2012a). Functional analyses of the digestive β-glucosidase of Formosan subterranean termites (Coptotermes formosanus). Journal of Insect Physiology, 58, 205–210.
Zhang, D., Lax, A. R., Henrissat, B., Coutinho, P., Katiya, N., Nierman, W. C., & Fedorova, N. (2012b). Carbohydrate-active enzymes revealed in Coptotermes formosanus (Isoptera: Rhinotermitidae) transcriptome. Insect Molecular Biology, 21, 235–245.
Zhou, X., Smith, J. A., Oi, F. M., Koehler, P. G., Bennett, G. W., & Scharf, M. E. (2007). Correlation of cellulase gene expression and cellulolytic activity throughout the gut of the termite Reticulitermes flavipes. Gene, 395, 29–39.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Talia, P., Arneodo, J. (2018). Lignocellulose Degradation by Termites. In: Khan, M., Ahmad, W. (eds) Termites and Sustainable Management. Sustainability in Plant and Crop Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-72110-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-72110-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72109-5
Online ISBN: 978-3-319-72110-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)