Abstract
Achieving successful implantation requires a complex endocrine stimulus of the endometrium. A deep understanding of this highly coordinated process is required for clinicians and investigators attempting to optimize an individual patient’s likelihood of success. Our current level of knowledge is the result of many elegant studies to elucidate these mechanisms. However, our tools for assessing the proper functioning of the endometrium in relation to hormonal response are limited. Modern infertility therapies that rely on gonadotropin stimulation undoubtedly change the chronology and histology of endometrium response. However, many patients achieve success despite these alterations. Patients with recurrent implantation failure, however, may be more sensitive to forces which change the normal physiologic state. Thus, careful attention to these issues in these patients is paramount. This chapter attempts to lay the groundwork for a comprehensive understanding of normal endometrial response, assess current diagnostic options and their limitations, and describe how pathological processes can disrupt normal implantation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Endometrium
- Reproductive endocrinology
- Endometrial histology
- Luteal phase deficiency
- Frozen embryo transfer
- Recurrent implantation failure
- Subclinical hypothyroidism
Introduction
Successful implantation is dependent upon a highly coordinated sequence of hormonal stimuli to prepare the endometrium for pregnancy. In the natural setting, this complex but logical process is beautifully designed to mirror the progress of the preimplantation embryo as it traverses the oviduct and uterotubal junction and approaches the endometrium. However, given the reliance of this delicate system on endocrine signaling, inadequate support or disturbed timing of hormonal stimuli may cause the endometrium to become inhospitable to the preimplantation embryo. Furthermore, alterations in these signals as a result of exogenous gonadotropin administration in modern infertility treatment can have major implications on the receptivity of the endometrium. Additionally, other endocrine processes separate from the hypothalamo-pituitary-ovarian (HPO) axis are involved in optimizing this process, and pathology in these systems can also negatively impact implantation.
While considerable progress has been made in elucidating the mechanisms underlying this complex system, many questions still remain. This chapter seeks to (1) describe the normal coordination of endocrine stimuli required to achieve implantation, (2) review our current understanding of how this system is affected by modern infertility treatment, (3) discuss how pathological endocrine processes disrupt implantation, and (4) discuss treatment options for optimizing the chance for successful implantation.
Endocrine Regulation of Endometrial Growth and Regeneration
For the vast majority of women, the menstrual cycle demonstrates little variability from cycle to cycle, with a normal range of 25–35 days [1]. In the absence of pregnancy, this cycle is repeated approximately 400 times during the adult life of a normally menstruating female. This consistency reflects a predictable cascade of events originating in the hypothalamus and resulting in ovarian sex steroid production. A foundational understanding of the processes coordinating each step is required when attempting to optimize each patient’s chance for successful pregnancy.
Neuroendocrine Control of Menstrual Cycle
Any discussion of the menstrual cycle must begin with an understanding of the neuroendocrine mechanisms underlying its function. This system begins with pulsatile secretion of GnRH from the arcuate nucleus of the hypothalamus under the regulation of kisspeptin. Both pulse frequency and amplitude vary significantly over the course of the menstrual cycle. Early in follicular phase, GnRH pulses slow to every 90–100 min. This period is marked by pituitary FSH production and secretion, which supports follicular recruitment. In the midfollicular phase, GnRH pulse frequency increases to every 60 min. While amplitude is initially low, it increases in parallel with increased estradiol secretion from the follicular unit. The rising estradiol level also induces an increase in pituitary gonadotrope responsiveness to GnRH in the midfollicular phase. At midcycle, this increased responsiveness ultimately facilitates the LH surge, followed by ovulation approximately 36 h later. Following ovulation, progesterone secretion from the corpus luteum slows GnRH pulse frequency to every 4–8 h. Pulse amplitude increases, however, resulting in the persistence of adequate LH levels to support corpus luteum function.
Two-Cell Theory of Ovarian Sex Steroid Production
There is compelling evidence for a cooperative relationship between theca and granulosa cells that is based on specialization of androgen substrate production in the theca for eventual aromatization in the granulosa cells. This process begins in the preceding luteal phase, as FSH levels begin to rise. A slight increase in FSH rescues a cohort of preantral follicles from atresia and initiates growth. As levels continue to rise during the early follicular phase, FSH stimulates granulosa cell proliferation and increases gap junction formation producing a syncytium of granulosa cells in the developing follicle. This mitogenic activity of FSH on granulosa cells works in combination with an FSH-induced increase in aromatase activity to facilitate an increased capacity for estrogen production. Simultaneously, as follicular growth proceeds, theca cells begin to produce an increasing number of LH receptors. In the theca cells, LH binding facilitates cholesterol uptake and conversion into androgens. Preovulatory granulosa cells are unable to complete conversion of the 21-carbon substrates into androgens, and as a result ovarian steroidogenesis is largely dependent on this LH activity in the theca cells. Thus, as follicular development progresses, increasing amounts of androgen substrate are produced as a result of increasing LH activity. These substrates diffuse into adjacent granulosa cells where they are aromatized to estradiol. As the follicular phase advances, increasing LH levels result in additional androgen substrate and ultimately increasing estradiol secretion from the follicular unit. Throughout this process, FSH also increases the number of LH receptors present on the granulosa cells preparing the follicular unit for ovulation.
Ovulation
Ovulation is ultimately facilitated by the increased estradiol produced by the dominant follicle. This increased estradiol causes increased pituitary responsiveness to GnRH and induces the LH surge. The LH surge not only triggers resumption of meiosis in the oocyte and induces ovulation but also induces a significant shift in the activity of the follicular unit. Prior to ovulation, granulosa cell activity is dominated by estradiol production. While progesterone production is initiated in the hours leading up to ovulation, its secretion is significantly increased following ovulation—reaching a peak at approximately 8 h after the LH surge. Progesterone levels remain elevated as long as the corpus luteum is supported by LH stimulus. Meanwhile, estradiol levels decrease to a steady but reduced level following ovulation.
Luteinization and Postovulation Steroid Secretion
The process of luteinization is facilitated by a wide array of mechanisms. The previously avascular follicular unit is transformed into a highly vascular tissue which allows increased exposure to cholesterol substrate for progesterone production. This process is further facilitated by an increased expression of steroidogenic acute regulatory protein (StAR) and side-chain cleavage enzyme which regulate cholesterol transfer into the inner mitochondrial membrane and eventual conversion to pregnenolone. Increased progesterone production is the result of increased 3-beta-hydroxysteroid dehydrogenase activity in the granulosa cells. All of these processes require continued tonic secretion of LH from the anterior pituitary. Thus, the efficiency of the corpus luteum is determined by the extent to which LH receptor accumulation occurred prior to ovulation. An inefficient follicular unit prior to ovulation forebodes a poorly functioning corpus luteum following ovulation. Whether this can be overcome by exogenous support of corpus luteum function or progesterone supplementation is discussed in detail later in this chapter.
Because progesterone secretion is dependent upon the pulsatile release of LH, levels vary throughout the luteal phase. Thus, attempts at correlating progesterone levels with luteal function are limited by the frequent changes in progesterone levels relative to its episodic production from the corpus luteum. This reality has limited the applicability of progesterone measurements in predicting the chance of ongoing gestation. Instead, it appears as though as long as progesterone levels reach a threshold for inducing secretory change in the endometrium, increasing levels due not improve chances at implantation. The more clinically relevant measure of progesterone’s impact on the chance of successful implantation involves determining when progesterone crosses this threshold in relation to when the embryo approaches the endometrium. This concept of endometrial-embryo synchrony is discussed in great detail in Chap. 2 and will also be considered briefly below.
Endometrial Response to Sex Hormone Secretion in the Normal Menstrual Cycle
In the clinical setting, the dynamic changes of ovarian sex steroid production receive the majority of focus when considering the physiology of the menstrual cycle. Providers are prone to focusing on the HPO axis because measuring hormones provides data with which to base treatment decisions during treatment cycles. In contrast, the endometrium provides relatively less information in a given cycle. However, when considering the effect of the endocrine system on implantation, the endometrium must be considered the final recipient of the cascade of messages produced during the menstrual cycle. Without a coordinated endometrial response to the cyclic changes in sex steroid production by the follicular unit, pregnancy will not ensue.
The endometrial cycle is the result of three stages of development in response to ovarian estrogen and progesterone exposure—proliferation, differentiation, and tissue breakdown. At the molecular level, these sex steroids act via cognate receptors to initiate expression of specific cascades of genes and to induce shifts in autocrine, paracrine, and intracrine communication in the endometrium. Estrogen is responsible for the proliferative changes during the follicular phase of the ovarian cycle. Progesterone is required for the establishment and maintenance of pregnancy consequent upon the transformation of the estrogen-primed endometrium into the secretory phase. Menstruation results from withdrawal of both hormones upon demise of the corpus luteum.
Endometrial Structure
A full appreciation of endometrial preparations for implantation requires an understanding of the structure and function of the endometrium. The endometrium is comprised of two major layers: the functionalis and the basalis. The functionalis is a transient and proliferative layer that comprises the upper two-thirds of the endometrium. The basalis is the bottom one-third of the endometrium directly adjacent to the myometrium and is responsible for regenerating the functionalis after menstrual shedding. The basalis contains the basal arteries, which are branches of the radial arteries and are unresponsive to the hormonal changes of follicular unit. The spiral arteries are separate branches of the radial arteries which extend into the functionalis layer and are responsive to cyclic hormonal changes. The other major structural feature of the endometrium is its rich and dynamic endowment of glands. These glands originate in the basalis and extend to the luminal surface of the endometrium. Their presence indicates an important functional component of the endometrium, in that their secretory products serve to communicate with the preimplantation embryo to promote the events leading up to implantation.
A significant body of literature exists for describing the histologic changes that accompany shifts in hormonal stimuli in the endometrium. These morphological changes have been reviewed in detail in classical experiments [2]. The proliferative phase is characterized by a transition from cuboidal, ragged surface epithelium following menstruation to a columnar, pseudostratified luminal epithelium. Gland morphology also develops in the proliferative endometrium from short and narrow in shape to more undulant surfaces with increasing tortuosity. The stromal component demonstrates active mitoses, and its density begins to increase throughout this phase of the cycle.
Due to the fact that most investigations of endometrial histology were primarily carried out in infertile patients in an effort to describe alterations in the timing of endometrial structural changes, more attention has been paid to the subtle, daily changes in the secretory endometrium. In a classic paper, Noyes et al. [3] argued that an increased rate of structural change in the secretory endometrium allowed assignment of specific endometrial dating based on histologic assessment. The first, easily identifiable morphologic change due to progesterone exposure occurs on day 3 following ovulation. Prominent subnuclear vacuoles appear and increase in size resulting in loss of pseudostratification and generation of an orderly row of nuclei in the luminal epithelium. On day 4 following ovulation, these vacuoles slip past the nuclei and localize near the luminal surface of the endometrial glands. By day 5 postovulation, few vacuoles are evident indicating intraluminal secretion of their contents. By this time, nuclei are impressively aligned at the basal portion of the glandular epithelium. During this process, gland diameter and tortuosity increases.
The primary morphologic feature at the time of implantation is an appreciable increase in stromal edema, which begins on day 6 postovulation. This feature is responsible for the familiar uniformly echogenic appearance of the endometrium on ultrasound in the secretory phase. Stromal edema reaches its height on days 7 and 8 after ovulation and is accompanied by increased coiling of spiral arterioles. Soon after, polymorphonuclear leukocytes—primarily uterine natural killer cells and macrophages—infiltrate the stroma. A process of pseudodecidualization under the influence of progesterone is also established around the time of implantation. So-called predecidual cells characterized by cytonuclear enlargement, increased mitotic activity, and development of a basement membrane can initially be identified surrounding blood vessels. These cells act in concert with decidual leukocytes to control trophoblastic invasion. At this time, the secretory endometrium is organized into three distinct layers—the unchanged basalis, the lace-like stratum spongiosum (composed of edematous stroma, tightly coiled spiral vessels, and dilated glands), and the superficial stratum compactum (resulting from predecidual transformation).
In the absence of pregnancy and the sustaining actions of human chorionic gonadotropin, the corpus luteum ultimately reaches the end of its predetermined life span. The resulting withdrawal of progesterone and estrogen results in a series of events leading up to menstruation. The primary mechanism responsible for this phenomenon is vasospasm of the spiral arterioles resulting in endometrial ischemia. Furthermore, lytic enzymes including matrix metalloproteinases previously confined to lysosomes under the control of progesterone are released upon progesterone withdrawal. This leads to enzymatic autodigestion of the cellular components, extracellular matrix, and basement membrane of the functionalis. Menstrual sloughing results.
Limitations in Endometrial Dating as a Clinical Tool
Despite significant progress in describing the histologic changes that accompany the sequential hormonal shifts in the menstrual cycle, the utility of endometrial dating as a tool to optimize timing in fertility treatments has been questioned on multiple grounds. First, significant inter- and intra-observer variability on the dating of a given endometrial biopsy sample has been described in multiple papers [4,5,6]. Much of these discrepancies result from difficulties assigning accurate endometrial dating in the case of glandular-stromal dyssynchrony [7]. Furthermore, the utility of endometrial dating as part of the infertility work up was significantly challenged when Coutifaris et al. [8] demonstrated in a large, multicentered prospective trial that out-of-phase biopsy results failed to discriminate between fertile and infertile couples. Thus, while description of endometrial dating has proven a useful framework for studying the effect of modern treatments on endometrial progression, it provides very little clinical value as a screening tool for infertility. As a result, these efforts are not recommended as part of the modern infertility work up.
The Impact of the Supraphysiologic Hormonal Milieu on Endometrial Development in Controlled Ovarian Hyperstimulation
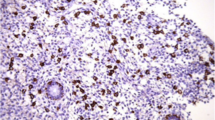
Multifollicular development is the goal of exogenous gonadotropin treatment in an IVF cycle. However, multifollicular development results in supraphysiologic concentrations of estradiol and progesterone. Thus, the endocrine stimuli responsible for the chronologic changes in endometrial histology over the course of the physiologic menstrual cycle are inherently different following administration of exogenous gonadotropins. Not surprisingly, endometrial histology is often also altered. This phenomenon has been extensively described, and the changes in endometrial structure in stimulated cycles are generally divided into two categories: (1) accelerated transition to the secretory changes of the endometrium associated with premature progesterone rise and (2) lack of synchrony of development between the different cellular and structural compartments of the endometrium [9] (Fig. 9.1).
Morphology of the endometrium showing variable stages of glandular and stromal development in the natural cycles and ovarian stimulation cycles of moderate responders and high responders. (a) Natural cycle endometrial biopsy showing in-phase glandular development and lowest amounts of stromal edema (asterisk). (b) Moderate responder: in-phase endometrium showing coordinated development of glands and stroma (asterisk) after ovarian stimulated. (c, d) High responders demonstrating glands stromal dyssynchrony: delayed glandular development and edematous stromal features (asterisk). This arrows show spiral arterial maturation appropriate to the late secretory phase. Bar = 100 μm
The effect of COH on endometrial development has also been demonstrated by identifying aberrations in the endometrial transcriptome following stimulation [10, 11]. Of the identified altered genes, those with known roles in implantation such as leukemia inhibition factor (LIF) and glycodelin have been demonstrated to be downregulated following stimulation. Additional alterations, including microRNA dysregulation have also been described [12]. Most studies attribute these issues to premature progesterone exposure following COH. Indeed, advancement in endometrial histology of >2 days has been reported in 45–100% of cycles with premature progesterone elevations [13, 14]. This advancement in endometrial structure and function has a significant impact on the likelihood of achieving implantation. This conclusion is supported by clinical data that demonstrate a restoration of normal pregnancy rates when embryos created in cycles with prematurely elevated progesterone levels are vitrified and transferred in a subsequent cycle [15, 16]. This issue is explored in much greater detail in Chap. 2.
The question of whether or not supraphysiologic estradiol levels alone following exogenous gonadotropin administration affect endometrial receptivity has also been debated. Marchini et al. [17] performed biopsies prior to oocyte retrieval and described accentuated proliferative characteristics and early secretory changes even prior to premature progesterone rise. Furthermore, some have suggested that although estradiol levels at different concentrations can support implantation, the window of uterine receptivity can be narrowed by supraphysiologic estradiol levels. Using a mouse model, Ma et al. [18] demonstrated that LIF, PTGS2, and HEGFL were downregulated sooner when the endometrium was exposed to a higher level of circulating estradiol levels. These authors suggested that this aberrant expression of key genes associated with implantation in the presence of supraphysiologic estradiol levels indicate an accelerated endometrial refractoriness to implantation [18].
This logic has been applied to the clinical setting as well. Paulson et al. initially postulated that higher implantation rates noted in oocyte donation cycles were in part a product of a more physiologic hormonal milieu present in cycles involving recipients of donated oocytes. Supraphysiologic estradiol levels in fresh non-donor IVF cycles were suggested as a potential culprit. This argument was corroborated to some degree by recent data that demonstrated superior live birth rates for frozen transfer compared to fresh embryo transfer in patients with polycystic ovarian syndrome (PCOS) [19]. Patients in the fresh embryo transfer arm in this study had an average maximum estradiol level of 4288 pg/mL [20]. However, it is unclear in this study whether the decrement in pregnancy rates following fresh embryo transfer is solely due to elevated estradiol levels or partially due to the chronic inflammation or aberrant hormonal milieu in PCOS patients. Other studies have failed to demonstrate an association between peak estradiol levels and pregnancy outcomes in fresh IVF cycles. One compelling study comparing implantation rates between patients utilizing autologous oocytes produced in cycles with peak estradiol levels >3000 pg/mL against recipients of donated oocytes produced in cycles with similar peak estradiol levels demonstrated no improvement in implantation rates for recipients of donated oocytes, despite their more physiologic estradiol levels [21]. Multiple additional studies subsequently produced similar findings [19, 20, 22].
Thus, while there exists some evidence of alterations in endometrial histology in the presence of supraphysiologic estradiol levels alone, it is still unclear that this is the primary cause of implantation failure in the most patients. Instead, the primary mechanism by which controlled ovarian hyperstimulation impacts implantation rates in IVF cycles appears to be premature elevation in progesterone and a shift in the window of endometrial receptivity prior to embryo transfer. However, no well-designed studies have been performed by controlling for premature progesterone elevations to isolate the impact of supraphysiologic estradiol levels alone on the incidence of recurrent implantation failure. Thus, more data is needed to definitively answer this question.
Estrogen Administration in Frozen Embryo Transfer Cycles
If supraphysiologic estradiol levels and an increased risk of premature progesterone rise do impact the chance of implantation in fresh cycle following exogenous gonadotropin administration, this can be avoided by proceeding with a frozen embryo transfer. However, a similar debate regarding the optimal endometrial preparation for embryo transfer exists. If the goal is to avoid supraphysiologic estradiol levels, then replacement in a natural cycle would be the ideal choice. Indeed, estradiol levels do tend to be higher in so-called artificial FET cycles than unmedicated cycles [23, 24].
Multiple studies have compared clinical outcomes between natural FET cycles, modified natural FET cycles (with hCG trigger and/or progesterone supplementation), and artificial FET cycles. These studies have been combined into a systematic review [25], and a meta-analysis [26]—neither of which revealed a significant advantage of one specific approach over another. It is important to note that the vast majority of data on this subject comes from retrospective studies, and thus there is still a significant need for more high-quality data. Only two prospective randomized trials on artificial versus natural cycle FET have been performed, and no difference in pregnancy rates was observed in either [27, 28]. One additional prospective trial compared modified natural cycle with an artificial cycle with GnRH agonist downregulation. Similarly, no difference in pregnancy rates was found between the groups [29].
Thus, the available evidence suggests that the typically higher levels of estradiol seen in artificial FET cycles do not result in detrimental outcomes. Furthermore, there is no evidence to support the notion that changing the strategy for endometrial preparation should be expected to improve implantation efficiency. It is also important to note that none of the above studies have focused specifically on patients with recurrent implantation failure. It is possible that RIF represents a unique population of patients that require more specific optimization of the endometrium to achieve pregnancy. This is not clear at the current time, however.
Luteal Phase Deficiency
Given the importance of progesterone production in preparing the endometrium for implantation, it seems logical that suboptimal corpus luteum function may be responsible for some instances of implantation failure. This conclusion is supported by observations that cycles that lead to normal early pregnancy development tend to have higher midluteal progesterone levels than those that result in failed implantation or loss [30]. These lines of thinking have helped generate the concept of luteal phase deficiency (LPD) as cause of infertility in some patients. However, despite being first described in 1949 [31], there is still a lack of high-quality data to support LPD as a plausible and common cause of implantation failure. This section will review the available evidence for LPD and discuss the utility of progesterone supplementation as a therapeutic strategy.
Pathophysiology of LPD
Given that ovarian progesterone secretion follows a cascade of signaling originating in the central nervous system, many authors have proposed that some instances of inadequate luteal progesterone secretion originates with disorders in the neuroendocrine support of the corpus luteum. These mechanisms include disorders associated with altered GnRH pulsatility (including hypothalamic amenorrhea, thyroid disease, and hyperprolactinemia) and dysregulated LH secretion (obesity). Others have suggested that ovarian aging alone may result in suboptimal luteal function. However, while the mechanistic changes associated with these pathologies have biologic plausibility, the pulsatile secretion of progesterone and associated challenges of obtaining accurate measurements makes direct evidence of luteal deficiency as the primary mechanism of poor outcomes in these patients difficult to establish.
Furthermore, there is not a widely accepted profile of ideal progesterone secretion required for implantation. While it is well established that progesterone levels peak 6–8 days after ovulation, these levels demonstrate rapid and significant variability according to LH pulsatility in the luteal phase [32]. Levels as low as 2.3 and as high as 40.1 ng/mL have been observed in the same patient in a 90-min interval [33]. This presents challenges for determining peak progesterone concentrations in the luteal phase in any given cycle. In addition, low progesterone levels early in pregnancy may be more reflective of poor hCG secretion from an abnormal early pregnancy than poor corpus luteum function.
The other logical strategy for evaluating luteal phase function is an assessment of luteal dating via endometrial biopsy. However, the same shortcomings for endometrial histology described above apply for diagnosing LPD. These include (1) high intercycle variability in dating for individual patients [34], (2) high interobserver diagnostic variability among pathologists [4], and (3) no difference in the incidence of delayed endometrial maturation between infertile patients and fertile controls [8]. Furthermore, once progesterone levels cross a given threshold for inducing secretory changes, it is unclear whether low normal and high normal levels have different impact on endometrial histology [35].
Therapeutic Strategies for Optimizing the Luteal Phase
In the absence of evidence for the above noted pathologies’ association with poor central support of corpus luteal function, all strategies for enhancing luteal function are empiric in nature. Multiple different therapeutic approaches have been examined in many different clinical contexts. One approach is to increase progesterone and/or estrogen exposure of the endometrium by directly supplying these hormones exogenously during the luteal phase. One study demonstrated that increasing serum progesterone levels in the luteal phase after controlled ovarian hyperstimulation were associated with an increased clinical pregnancy rate. In this study [36], higher serum levels were achieved with vaginal progesterone. While the overall pregnancy rates between the routes of administration were no different, higher levels were associated with an increase in pregnancy rates. Another strategy is to artificially augment corpus luteum function by administering hCG. Some practitioners argue that tailoring the route of administration of progesterone or enhancing endogenous progesterone may be a consideration in patients with recurrent implantation failure to achieve high levels of progesterone due to the limited downside. However, there is limited data to suggest improvement in outcomes.
Direct progesterone supplementation is often utilized in COH cycles. While supplementation can be administered in multiple ways, vaginal progesterone is the most commonly utilized strategy. However, while there is little downside to administering supplemental progesterone, there is also no evidence to suggest that luteal phase progesterone supplementation is beneficial in increasing COH cycle implantation rates, though this is common practice due to the presumption that increased sex steroid production associated with multifollicular growth may suppress LH support of luteal function [37]. The only clinical scenario with high-quality evidence for progesterone supplementation is for use in ART cycles utilizing GnRH agonists [38] and antagonists [39] due to their strong association with premature luteolysis. Use in these cycles significantly improves clinical pregnancy rates.
Administration of hCG to support endogenous production of progesterone from the corpus luteum is another strategy for improving luteal function. Many programs measure progesterone levels following ovulation in COH cycles and administer an additional dose of hCG if progesterone remains below a predefined threshold. This is physiologically sound but again has not been demonstrated outside of ART to improve the chances of pregnancy. Like exogenous progesterone supplementation, hCG administration has been demonstrated to improve success rates in IVF cycles utilizing GnRH analogues. However, the increased risk of OHSS makes this a less desirable strategy than direct progesterone supplementation in ART cycles [40].
Thyroid
Physiology of Thyroid Signaling and Implantation
There is substantial experimental and clinical evidence that implicate thyroid hormones (TH) in the implantation process. While it is unclear whether these actions are mediated through classical endocrine regulation of the hypothalamic-pituitary-thyroid axis, or through paracrine and intracrine signaling at the implantation site, there is little doubt that thyroid hormones help regulate the cascade of events culminating in implantation. Interestingly, there is convincing evidence that TH influences both embryonic and endometrial activity. The following will review the physiology of thyroid hormone actions at the implantation site and discuss recommendations for clinical thyroid management in the infertile patient.
The best evidence for thyroid regulation of implantation is the variation in endometrial expression of nuclear thyroid hormone receptors (TR) and G protein-coupled thyroid stimulating hormone receptors (TSHR) across the menstrual cycle [41]. TRα1, TRβ1, and TSHR are present in the glandular and luminal epithelium of the endometrium, and all demonstrate an increase in expression during the secretory phase followed by a subsequent decrease. Each receptor reaches peak expression at the same time that endometrial pinopodes appear and receptivity is established. Whether these receptors respond primarily to hormones secreted by the thyroid gland or to locally produced TH is still up for debate as evidence exists for TH production in the endometrium. Transcripts encoding deiodinases, thyroglobulin, and thyroid peroxidase are all expressed in the endometrium [42]. Additionally, there is strong evidence suggesting that the endometrium serves as a target tissue of pituitary TSH. During the window of implantation, TSH increases leukemia inhibitory factor (LIF) and LIF receptor expression—both of which are essential components in the implantation cascade. TSH also regulates glucose transport by increasing expression of glucose transporter-1 (GLUT-1) [43]. This biosynthetic activity in the endometrium appears to be partially regulated by the presence of progesterone, as mifepristone administration reduces expression of TR and thyroglobulin. This theory may also explain, in part, menstrual irregularity experienced by patients with thyroid dysfunction [44].
Experimental evidence also suggests that the embryo responds to thyroid hormone both prior to and during implantation. Oocytes, cleavage-stage embryos, and blastocysts all possess TRα mRNA. The preimplantation blastocyst expresses deiodinases and thyroid hormone transporters, such as monocarboxylate transporter 8 (MCT8). Multiple studies have suggested that TH exposure augments early embryo development. Experiments in bovine embryo culture have reported improved embryo cleavage, blastulation rates, and hatching rates when culture media is supplemented with TH [45, 46] Others have theorized that thyroid hormone utilization may result in blastocyst secretion of human chorionic gonadotropin (hCG), thus facilitating embryo-endometrial communication during the time of implantation [43] (Fig. 9.2). After implantation, TH promotes normal placental growth and invasion by inhibiting expression of pro-apoptotic factors Fas, Fas ligand, and Bcl-2 and preventing cleavage of caspase-3 in the trophoblast [47].
During the window of implantation, TSH increases LIF and LIFR expression and regulates glucose transport by increasing GLUT-1 expression. Low oxygen tension at the implantation site increases HIFα expression which increases deiodinases. The trophectoderm cells express TRs, thyroid hormone transporters, such as MCT8 and deiodinases. T3 helps regulate the production of hCG and later, hPL
Clinical Management of Thyroid Dysfunction
A substantial body of literature has developed to address the optimal clinical management of thyroid dysfunction in the context of reproduction. This literature has been challenging to interpret due to disagreements in definitions of thyroid pathologies and discrepancies in recommendations for clinical management among national and international professional organizations. However, there is little doubt that gross abnormalities in thyroid function negatively impacts implantation and that treatment improves outcomes. The controversy lies in the more subtle interruptions in thyroid homeostasis.
Overt hypothyroidism is associated with a number of reproductive pathologies. Abnormal thyroid homeostasis can interfere with normal LH pulsatility and can cause hyperprolactinemia [48]. Furthermore, hypothyroidism is associated with an increased risk of miscarriage, preterm birth, gestational hypertension, placental abruption, fetal growth restriction, and impaired neuropsychological development of the offspring [49]. Thus, there is no debate regarding the utility of levothyroxine replacement in patients with overt hypothyroidism.
There is less clarity, however, regarding the proper management subclinical hypothyroidism (SCH) in the context of reproduction. Much of the confusion has stemmed from a disagreement regarding the proper definition of subclinical hypothyroidism. The upper limit of the reference range for TSH levels was established by the National Health and Nutritional Examination Survey (NHANES III) to be 4.5–5 mIU/L [50]. Thus, the classical definition of subclinical hypothyroidism is a TSH level >4.5 mIU/L, with normal free thyroxine (T4) levels. However, the National Academy of Clinical Biochemistry suggested in 2002 that the normal reference range for TSH be reduced to 2.5 mIU/L after reporting that 95% of rigorously screened euthyroid individuals had serum TSH values between 0.4 and 2.5 mIU/L [51]. In addition, the Endocrine Society and the American Society for Reproductive Medicine recommend that 2.5 mIU/L be used as the upper limit of normal for the first trimester of pregnancy [52, 53]. As a result, most IVF programs treat patients with levothyroxine if they demonstrate a TSH value above this range prior to initiating treatment.
The best data in support improved implantation rates in levothyroxine treated SCH patients comes from two randomized controlled trials. Using a cutoff of >4.5 mIU/L, Kim et al. [54] randomized patients with SCH to either levothyroxine (50 micrograms daily) versus no treatment. The implantation rate was significantly higher in the treatment arm than in the control group (26.9 vs. 14.9%, p = 0.044). A similar study by Abdel Rahman et al. [55] used a TSH cutoff of 4.2 mIU/L to diagnose SCH and randomized 70 patients to levothyroxine or placebo. In this study, the clinical pregnancy rate was also significantly higher in the treatment group (35 vs. 10%, p = 0.02). Thus, there is high-quality data demonstrating that untreated SCH negatively impacts implantation rate after IVF. However, no studies have evaluated whether this effect persists at TSH levels >2.5 mIU/L but <4.2 mIU/L. Furthermore, tighter control of TSH levels below 2.5 do not appear to impact implantation or live birth rates.
One study did evaluate IVF outcomes according to TSH levels in the first 11 weeks of pregnancy. In this study, levels between 2.5 and 5 mIU/L were associated with a significant increase in pregnancy loss (6.1 vs. 3.6%, p = 0.006) [56]. However, this study did not control for the chromosomal status of the embryo. Thus, it is possible that the lower hCG levels associated with aneuploid gestations may have contributed to the failure of TSH to fall below 2.5 mIU/L in this cohort. Thus, the ideal TSH level within the normal range for optimizing implantation success is unclear, but levels about 2.5 mIU/L during early pregnancy may increase miscarriage risk.
Multiple studies have addressed whether the evidence of thyroid autoimmunity (anti-thyroperoxidase or antithyroglobulin antibodies) impacts IVF success. A meta-analysis of seven studies including 330 thyroid antibody-positive patients and 1430 controls demonstrated no difference in implantation rate after IVF (odds ratio 0.67, 95% confidence interval 0.36–1.4, p = 0.67) [57]. One prospective, randomized controlled trial evaluated empiric treatment with levothyroxine in euthyroid patients with evidence of thyroid autoimmunity. In that study, there was a trend to improvement in clinical pregnancy rates between the treated and untreated patients (56 vs. 49%); however, transfer order was not reported in each group, limiting the conclusion [58]. Furthermore, this data has not been replicated in other trials, and thus further evaluation is merited. However, both the meta-analysis and prospective trials listed above suggested that thyroid autoimmunity increased the risk of miscarriage and preterm delivery, which could be prevented by replacement therapy. Thus, if a decision is made to not treat women with TSH levels between 2.5 and 5.0 mIU/L, it may be prudent to measure thyroid peroxidase antibodies and treat if positive [59].
Summary
A proper endocrine stimulus is required to allow the endometrium to accept and support pregnancy. A significant body of literature has contributed to our understanding of how this complex system coordinates endometrial development and prepares the uterus for implantation. However, many of the current tools for improving the efficiency of a given cycle, such as exogenous gonadotropins, may negatively impact endometrial receptivity. Thus, special attention is required in patients with recurrent implantation failure to ensure that modern therapies are not inadvertently decreasing their chances of achieving sustained implantation. More data is needed in this regard.
Furthermore, our tools for assessing the health of the endocrine system in relation to implantation remain limited. Many new and exciting diagnostic methods are currently in development that may help elucidate the effect of endocrine stimuli on the endometrial receptivity. As with many aspects of modern infertility care, it is likely that a strong understanding of the physiologic basis of normal implantation, combined with state of the art molecular technologies, will help develop treatments strategies that mimic the natural setting while harnessing the power of modern assisted reproductive techniques. These advanced diagnostics are needed as we push to optimize outcomes.
References
Treloar AE, Boynton RE, Begn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126.
Rock J, Bartlett MK. Biopsy studies of human endometrium. JAMA. 1937;108(24):2022–8.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25.
Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–43.
Scott RT, Snyder RR, Strickland DM, Tyburski CC, Bagnall JA, Reed KR, Adair CA, Hensley SB. The effect of interobserver variation in dating endometrial histology of the diagnosis of luteal phase defects. Fertil Steril. 1988;50:888–92.
Smith S, Hosid S, Scott L. Endometrial biopsy dating. Interobserver variation and its impact on clinical practice. J Reprod Med. 1995;76:782–91.
Deglidisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285–94.
Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP, Silva S, Vogel DL, Leppert PC. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–72.
Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, Salamonsen LA, Rombauts LJF, Hincks C, Rombauts LJ, Salamonsen LA. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. 2014;0:1–14.
Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, Simon C. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005;11:195–205.
Van Vaerenbergh I, Van Lommel L, Ghislain V, In’t Veld P, Schuit F, Fatemi HM, Devroey P, Bourgain C. In GnRH antagonist/rec-FSH stimulated cycles, advanced endometrial maturation on the day of oocyte retrieval correlates with altered gene expression. Hum Reprod. 2009;24:1085–91.
Li R, Qiao J, Wang L, Li L, Zhen X, Liu P, Zheng X. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29.
Kolibianakis EM, Bourgain C, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Prolongation of follicular phase by delaying hCG administration results in higher incidence of endometrial advancement on the day of oocyte retrieval in GnRH antagonist cycles. Hum Reprod. 2005;20:2453–6.
Ubaldi F, Bourgain C, Tournaye H, Smitz J, Van Steirteghem A, Devroey P. Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil Steril. 1997;67:521–6.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–8.
Yang S, Pang T, Li R, Yang R, Zhen X, Chen X, Wang H, Ma C, Liu P, Qiao J. The individualized choice of embryo transfer timing for patients with elevated serum progesterone level on the HCG day in IVF/ICSI cycles: a prospective randomized clinical study. Gynecol Endocrinol. 2015;31(5):355–8.
Marchini M, Fedele L, Bianchi S, Losa GA, Ghisletta M, Gandiani GB. Secretory changes in preovulatory during controlled ovarian hyperstimulation with buserelin acetate and human gonadotropins. Fertil Steril. 1991;55:717–21.
Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–8.
Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, Tian L, Hao C, Yang D, Zhou F, Shi J, Xu Y, Li J, Yan J, Qin Y, Zhao H, Zhang H, Legro R. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375:523–33.
Chen HC, Zhang X, Barnes R, Confino E, Milad M, Puscheck E, Kazer R. Relationship between peak serum estradiol levels and treatment outcome in in vitro fertilization cycles after embryo transfer on day 3 or day 5. Fertil Steril. 2003;80:75–9.
Levi AJ, Drews MR, Bergh PA, Miller BT, Scott RT Jr. Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. 2001;76(4):670.
Papageorgiou T, Guibert J, Goffinet F, Patrat C, Fulla Y, Janssens Y, Zorn AR. Percentile curves of serum estradiol levels during controlled ovarian stimulation in 905 cycles stimulated with recombinant FSH show that high estradiol is not detrimental to IVF outcome. Hum Reprod. 2001;17:2846–50.
Hancke K, More S, Kreienberg R, Weiss JM. Patients undergoing frozen-thawed embryo transfer have similar live birth rates in spontaneous and artificial cycles. J Assist Reprod Genet. 2012;29:403–7.
Tomax C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy loss after frozen-embryo transfer – a comparison of three protocols. Fertil Steril. 2012;98:1165–9.
Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2008;7:CD003414.
Groenewoud ER, Cantineau AEP, Kollen BJ, Mackon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19:458–70.
Cattoli M. A randomized prospective study on cryopreserved-thawed embryo transfer: natural versus hormone replacement cycles. Abstracts of the 10th Annual Meeting of the ESHRE Brussels 1994;356:139.
Mounce G, McVeigh E, Turner K, Child TJ. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015;104:915–20.
Greco E, Litwicka K, Arrivi C, Varrichio MT, Caragia A, Greco A, Minasi MG, Fiorentino G. The endometrial preparation for frozen thawed euploid blastocyst transfer: a prospective randomized trial comparing clinical results from natural modified cycle with exogenous hormone stimulation with GnRH agonist. J Assist Reprod Genet. 2016;33:873–84.
Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PL, Collins DC. Hormonal profiles of natural conception cycles in ending in early, unrecognized pregnancy loss. New Engl J Med. 1999;340:1796–9. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88(9):4186–92
Jones GES. Some newer aspects of management of infertility. JAMA. 1949;141:1123–9.
Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
Filicori M, Butler JP, Crowley WF Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638–47.
Davis OK, Berkeley AS, Naus GJ, Cholst IN, Freedman KS. The incidence of luteal phase defect in normal, fertile women, determine by serial endometrial biopsies. Fertil Steril. 1989;51:582–56.
Usadi RS, Groll JM, Lessey BA, Lininger RA, Zaino RJ, Fritz MA. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93:4058–64.
Mitwally MF, Diamond MP, Abuzeid M. Vaginal micronized progesterone versus intramuscular progesterone for luteal support in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2010;93(20):554–69.
American Society for Reproductive Medicine Practice Committee. Current clinical irrelevance of luteal phase deficiency: a committee opinion. Fertil Steril. 2015;103:327–e32.
Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod. 2002;17:2287–99.
Beckers NG, Latteau P, Eijkemans MJ, Macklon NS, de Jong FH, Devroey P, Fauser BC. The early luteal phase administration of estrogen and progesterone does not induce premature luteolysis in normo-ovulatory women. Eur J Endocrinol. 2006;1559(2):355–63.
Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev. 2004;10:CD004830.
Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Skjoldebrand Sparre L, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone-receptors are involved in human endometrial physiology. Fertil Steril. 2011;95:230–7.
Catalano RD, Critchley HO, Heikinheimo O, Baird DT, Hapangama D, Sherwin JRA, Charnock-Jones DS, Smith SK, Sharkey AM. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in the human endometrium. Mol Hum Reprod. 2007;13:641–54.
Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update. 2014;20(6):884–904.
Scoccia B, Demir H, Kang Y, Fierro MA, Winston NJ. In vitro fertilization pregnancy rates in levothyroxine-treated women with hypothyroidism compared to women without thyroid dysfunction disorders. Thyroid. 2012;22:631–6.
Ashkar FA, Semple E, Schmidt CH, St John E, Bartlewski PM, King WA. Thyroid hormone supplementation improves bovine embryo development in vitro. Hum Reprod. 2010;25:334–44.
Costa NN, Cordeiro MS, Silva TV, Sastre D, Santana PP, Sa AL, Sampaio RV, Santos SS, Adona PR, Miranda MS. Effect of triiodothyronine on developmental competence of bovine oocytes. Theriogenology. 2013;80:295–301.
Laoag-Fernandez JB, Matsuo H, Murakoshi H, Hamada AL, Tsang BK, Maruo T. 3,5,3′-Triiodothyronine down-regulates Fas and Fas ligand expression and suppresses caspase-3 and poly (adenosine 5′-diphosphate-ribose) polymerase cleavage and apoptosis in early placental extravillous trophoblasts in vitro. J Clin Endocrinol Metab. 2004;89:4069–77.
Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–55.
Vissenberg R, van den Boogaard E, van Wely M, van der Post JA, Filers E, Bisschop PH, Goddijn M. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2012;18:360–73.
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–99.
National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis of thyroid disease, vol. 13. Washington, DC: The National Academy of Clinical Biochemistry; 2002.
American Society for Reproductive Medicine Practice Committee. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545–53.
De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–65.
Kim CH, Ahn JW, Kang SP, Kim SH, Chae HD, Kang BM. Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2011;95(5):1650–4.
Abdel Rahman AH, Aly Abbassy H, Abbassy AA. Improved in vitro fertilization outcomes after treatment of subclinical hypothyroidism in infertile women. Endocr Pract. 2010;16:792–7.
Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, Locorotondo G, Caroli P, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod. 2005;20:1529–33.
van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17:605–19.
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44–8.
Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105:873–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Morin, S., Ata, B., Seli, E. (2018). Endocrine Causes of Implantation Failure. In: Franasiak, J., Scott Jr., R. (eds) Recurrent Implantation Failure. Springer, Cham. https://doi.org/10.1007/978-3-319-71967-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-71967-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71966-5
Online ISBN: 978-3-319-71967-2
eBook Packages: MedicineMedicine (R0)