Abstract
Purpose
To evaluate the outcome of frozen-thawed embryo transfer (FET) when freezing takes place at the pronuclear stage, a retrospective analysis was performed comparing spontaneous and artificial cycles.
Methods
148 women received FET in a spontaneous cycle (Group A) and 55 women received FET in an artificial cycle (Group B) induced by administering estrogen (E2) and progesterone (P). Pregnancy rates, endometrial thickness and serum levels of E2, P and luteinizing hormone (LH) were measured. Statistical analysis included the mean, the standard deviation, the Chi-squared test and the T-test.
Results
The clinical pregnancy rate was 34.5% for Group A and 21.8% for Group B (p = 0.084), with a live birth rate of 20.9% and 12.7% respectively (p = 0.15). There was no difference in endometrial thickness or the P levels, while LH and E2 levels were significantly higher in group B (p < 0.0001).
Conclusion
Our retrospective study shows a trend towards higher pregnancy rates and live birth rates with the administration of FET during a spontaneous cycle compared to FET during an artificial cycle. Large randomized controlled trials are needed to confirm this trend.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryopreservation of embryos is widely used after assisted reproduction technology (ART), such as in-vitro-fertilisation (IVF) or intracytoplasmic sperm injection (ICSI), and has become a fundamental part of ART. However, the cryopreservation of embryos is not allowed in Germany. Instead, the pronuclear stages are frozen with a standard slow-freezing protocol.
The advantages of frozen-thawed embryo transfer (FET) are a less invasive procedure for patients, lower costs than ART treatment, and ease of application within a short space of time [10]. Nevertheless, compared to fresh IVF cycles, FET tends to show lower pregnancy rates [7]. Performing FET in a spontaneous cycle is favourable because medication is not used and no adverse events are expected. A further advantage is a significant cost reduction compared to hormone therapy.
Spontaneous FET does have problems however, such as the timing of ovulation in women with an irregular cycle. The date of embryo transfer is difficult to plan and has a higher rate of cancellation than with an artificial cycle [9]. Additionally, endometrial development in the follicular phase is strongly affected by age with a lower pregnancy rate in older women [10]. Administering exogenous estrogen (E) and progesterone (P) in an artificial cycle can be favorable as it allows for easy management, flexibility in timing the FET [1, 2, 18, 24] and has a lower cancellation rate [10]. However, the reported pregnancy rate of a spontaneous FET cycle was often lower, about 22% [16], than with an artificial FET cycle, up to 36% [4, 23], though a study by Sathanandan et al. comparing both protocols that was semi-randomized showed no difference [27].
We retrospectively examined the outcome of spontaneous versus artificial FET cycles to shed light on the controversy as to which protocol is superior.
Material and methods
Subjects
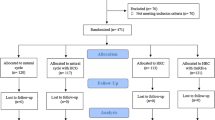
This retrospective study evaluated the data of all women who attended the Department of Endocrinology and Reproduction at the University of Ulm between 2000 and 2010 to undergo frozen-thawed embryo transfer (FET) after unsuccessful fresh ART, in which the protocol was GnRH agonist and FSH. Indications for the previous ART were either three unsuccessful intrauterine inseminations, tubal factor (IVF) or a male factor (ICSI). The GnRH agonist protocol using FSH was mainly performed.
Inclusion criteria were women undergoing FET in the spontaneous cycle, administration of human choriogonadotropin (hCG) for final oocyte maturation only and women undergoing FET in the artificial cycle with estradiol (E) in the follicular phase. Exclusion criteria were stimulation protocols with either clomiphene citrate (CC), gonadotrophins, gonadotropin-releasing hormone agonist (GnRHa), -antagonist (GnRHant), or women receiving hCG in the luteal phase.
Out of 1243 FET cycles, 1040 were excluded primarily because different stimulation protocols were used. 203 women met the inclusion criteria, of which 148 received FET in the spontaneous cycle with hCG for the induction of ovulation only (Group A) and 55 women underwent the artificial cycle with E/P replacement (Group B). Each woman underwent only one FET.
FET—protocol
The decision to proceed with a spontaneous or artificial cycle was reached through a combination of patient preference and physician guidance. In both protocols, women attended the Endocrinology and Reproduction Unit on day two of their regular menstrual cycle where a transvaginal sonography was performed to exclude any ovarian cysts.
-
Spontaneous cycle (Group A): Women with a spontaneous cycle did not have any medication during their follicular phase. After exclusion of ovarian cysts on day two of the ovarian cycle a transvaginal sonography was performed on day ten. Subsequently, ultrasounds were performed until the endometrium thickness was at least 8 mm and the main follicle reached 18–22 mm. At this point the ovulation was induced with 5,000 I.E. hCG s.c. self-administered by the patient. FET was performed four days later. The luteal phase was supported with progesterone applied vaginally two days before FET, 600 mg/d (3 × 200 mg).
-
Artificial cycle (Group B): Women undergoing an artificial cycle started on the first day of their natural menstrual cycle and received transdermal estrogen patches that released 100 μg estradiol per 24 h. Each person used the same protocol during their menstrual cycle: one patch on days 1, 3 and 5; two patches on days 7 and 9; four patches on days 11 and 13; three patches on days 15 and 17; and two patches on days 19, 21, 23, 25, 27, 29 and 31. The transfer was performed on day 17 and required an endometrial thickness of at least 8 mm. If the endometrial thickness was less than 8 mm the transfer was cancelled and shifted to the next cycle. On day 15 of the menstrual cycle, progesterone was started for the luteal phase support (200 mg vaginally three times a day). Estrogen (2 mg estradiolvalerat) was applied orally when patches were not tolerated, with an analogous protocol: one pill on days 1, 2, 3 and 4; two pills on days 5, 6 and 7; three pills on days 8, 9, 10 and 11; four pills on days 12, 13, 14, 15 and 16; and two pills on each day 17 to 31.
All women were screened for endometrial responsiveness using a transvaginal sonography and blood samples were taken to measure levels of LH, E2 and P prior to administration of hCG and before FET in both groups. The embryos were transferred two days after thawing for all women. The transfer was guided under transabdominal ultrasound; the women were advised to rest for 15 min following transfer. The number of embryos transferred was chosen individually by each patient.
The main goal of our study was to demonstrate a difference in clinical pregnancy rates between spontaneous and artificial cycles. Pregnancy was defined as the presence of a fetal heart beat detected by ultrasound. Additionally, subgroup analyses were performed in both groups receiving exactly two embryos. Furthermore, endometrial receptivity, E2, LH and P measurements were compared between the groups.
The quality of each embryo was classified after Hill et al. [14]. A quality score of A or B was classified as good quality in single embryo transfers. If more than one embryo was transferred the combination of AA, AB or BB score was classified as good quality.
Statistical analysis
Data were analyzed using Excel and SAS (Statistical Analyzing System). The mean and standard deviation (SD) were calculated. The Chi-squared test and T-test were used to determine differences between the groups. A p-value <0.05 was considered statistically significant.
Results
Study group (Table 1)
The mean age was 34.8 and 34.1 in Groups A and B respectively (p = 0.17). The mean number of embryos per FET in Group A was 2.07 (SD 0.61) and 2.09 (SD 0.59) in Group B. The quality of embryos was best in 70.4% of Group A and 65.2% of Group B (p = 0.088).
Clinical pregnancy rate and live birth rate (Fig. 1)
Out of the 148 women in Group A attending our institution for FET, 51 pregnancies were achieved (a 34.5% pregnancy rate). In Group B a pregnancy rate of 21.8% was achieved (12 pregnancies out of 55 FETs). This apparent lower pregnancy rate in women undergoing an artificial cycle was not statistically significant (p = 0.07).
In total, Group A (n = 51/148) and B (n = 12/55) combined, resulted in 31 (60.7% of clinical pregnancies) and 7 (58.3% of clinical pregnancies) live births, 8 (15.7% of clinical pregnancies) and 2 (16.6% of clinical pregnancies) abortions, and 12 (23.5% of clinical pregnancies) and 3 (25% of clinical pregnancies) women that could not be followed up, respectively.
Mean endometrial thickness, LH, E2 and P (Table 2)
Mean endometrial thickness was 9.17 mm in Group A and 9.79 mm in Group B (p = 0.091, Table 2). Mean serum levels of LH were statistically significantly lower in Group A, 10.41 IU/l and 18.31 IU/l respectively (p < 0.0001). There was also a significant difference between Groups A and B in the mean E2 level: 231.69 ng/l and 368.53 ng/l (p < 0.0001) respectively. No significant difference was shown in the mean P levels: 0.55 μg/l and 0.57 μg/l (p = 0.786) for Groups A and B respectively.
Discussion
The results of our retrospective study demonstrate that the live birth rates of the spontaneous and artificial FET cycles are not statistically different, although the data does suggest a trend towards higher pregnancy and live birth rates in spontaneous FET cycles.
There are many non-randomized studies comparing artificial and spontaneous FET cycles that show results comparable to ours with a similar number of included cycles [3, 6, 20, 22], ([3], n = 100), ([22], n = 164), ([6], n = 386) and ([20], n = 212).
Catolli et al. [3], for example, could not demonstrate different pregnancy rates when comparing artificial (21.4%) and spontaneous cycles (20.4%). Gelbaya et al. [9] evaluated 417 FET cycles, using GnRH in the artificial cycle, and achieved similar pregnancy rates (10.2%) compared to the natural cycle (11.6%). These overall pregnancy rates were far lower than in our study, and in all other studies, making it difficult to take these data into consideration. A Cochrane analysis (2008) concluded that no protocol is superior.
Nevertheless, the 8.2% difference in live birth rates we discovered between our groups is significant for our patients. This would justify a switch from an artificial to a spontaneous cycle, which is also much more patient friendly. In keeping with this, a randomized controlled trial recently found significantly higher pregnancy rates (31.1% vs. 14.3%) in natural FET cycles without any medication, compared to spontaneous FET cycles administering only 5000 IE HCG [8]. In our study HCG was administered for induction of ovulation, but the pregnancy rate was much higher than the 14.3% reported by Fatemi et al. [8]. They argue that administration of HCG may have an unfavorable impact on endometrial receptivity, although LH and HCG act on the same receptor [28]. We cannot confirm this in our results.
Despite the fact that our results illustrated no statistically significant difference between the natural and the artificial cycle, a recent analysis by Hill et al. [15] showed higher live birth rates in 1,391 FET cycles after GnRH following E2 and P (32.2%) compared to the natural cycle (20.4%), with a relative risk (RR) of 1.58. Another study including 1205 women with 1677 FET cycles showed higher pregnancy rates in a programmed FET (artificial cycle) than in a natural FET, though the rate of delivered pregnancies was similar (29.4% vs. 28.4% programmed and natural FET respectively) [11].
However, the data available at present more often demonstrate similar pregnancy rates than the superiority of either the artificial or the spontaneous cycle.
Application of exogenous estradiol lead to higher E2 levels, measured two days before FET, in our cohort. The question remains whether or not these higher E2 levels influence the pregnancy rate. In our study the pregnancy rate was lower in the artificial cycle with higher estradiol levels. The high concentration of estrogen has been made mostly accountable for the lower pregnancy rate. It has been suggested that high levels of estrogen have an inhibitory effect on embryo implantation [5]. Our measured E2 levels are similar to the those in other publications [1, 12, 26, 30]. Niu et al. [25] suggested that the measured endometrial thickness and the timing of FET is more important than the serum levels of E2. Navot et al. [24] reported that a shortened artificial cycle (5–10 days) and accompanying lower dosage result in lower pregnancy rates. Ma et al. [21] on the other hand stated that E2 levels are critical determinants in transforming uterine receptivity to a refractory state, suggesting the hypothesis that the implantation window closes much faster at higher E2 levels. Furthermore, high levels of estradiol after controlled ovarian hyperstimulation might impair endometrial receptivity [17]. Others found that an altered gene expression profile in the endometrium of women with high estradiol levels might also impair implantation [13].
Another important consideration is that high pre-ovulatory E2 levels may trigger the mid-cycle LH peak, which is dependent on the estradiol threshold. However, if preparation of the artificial cycle is started very early in the follicular phase (day 1) spontaneous ovulation is usually inhibited [25]. In an artificial FET this mid-cycle LH peak does not have an endocrine or sonographic correlation and therefore cannot be proved [1, 19]. Another question posed is how LH itself affects pregnancy rates. High LH levels may negatively affect endometrial receptivity and implantation since LH receptors are present in the endometrium [29]. In our study the significantly higher LH levels in the artificial cycle group, with lower pregnancy rates, are in accordance with this assumption. Griesinger et al. [12], however, showed that there is no difference in the pregnancy rate whether LH levels on day 14 are high or low. Therefore we may only state that LH levels are significantly higher in the artificial cycle, though this might not affect pregnancy rates.
One limitation of our study is the different quality of embryos in our groups. The spontaneous group had a higher rate of best quality embryos, which may explain the higher percentage of resulting pregnancies. This difference is not significant and we assume that other unknown factors assist the implantation. Another limitation in our study was its nature as a retrospective analysis rather than a prospective randomized design as well as the small number of cycles included. Nevertheless, retrospective studies are able to show a trend that may initiate prospective randomized trials. Limitations aside, a strength of our study was its monocentric nature with standardized protocols and the same management applied to every cycle.
Conclusion
Our study confirms previous reports that live birth rates are not significantly higher in women undergoing FET in a spontaneous, opposed to artificial, cycle. Further randomized controlled studies are needed to show a significant effect on live birth rates in FET.
References
Bals-Pratsch M, Al-Hasani S, Schöpper B, Diedrich C, Hoepfner AS, Weiss J, Küpker W, et al. A simple, inexpensive and effective artificial cycle with exogenous transdermal oestradiol and vaginal progesterone for the transfer of cryopreserved pronucleated human oocytes in women with normal cycles. Hum Reprod (Oxford, England). 1999;14 Suppl 1:222–30.
Ben-Nun I, Shulman A. Induction of artificial endometrial cycles with s.c. oestrogen implants and injectable progesterone in in-vitro fertilization treatment with donated oocytes: a preliminary report. Hum Reprod (Oxford, England). 1997;12(10):2267–70.
Catolli M, Ciotti P, Seraccholi R. A randomized prospective study on cryopreserved-thawed embryo transfer: natural versus hormone replacement cycles. Abstract of the 10th Annual Meeting of the ESHRE 356 1994;(Brussels):139.
Davies DW, Jenkins JM, Anthony FW, Gadd SC, Watson RH, Sakhrani LR, Masson GM. Biochemical monitoring during hormone replacement therapy cycles for transfer of cryopreserved embryos in patients with functional ovaries. Hum Reprod (Oxford, England). 1991;6(7):934–8.
Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database of Systematic Reviews (Online). 2004;(3):CD004830. doi:10.1002/14651858.CD004830.
Dor J, Rudak E, Davidson A, Levran D, Ben-Rafael Z, Mashiach S. Endocrine and biological factors influencing implantation of human embryos following cryopreservation. Gynecol Endocrinol: The Official Journal of the International Society of Gynecological Endocrinology. 1991;5(3):203–11.
Edgar DH, Archer J, Bourne H. The application and impact of cryopreservation of early cleavage stage embryos in assisted reproduction. Hum Fertil (Cambridge, England). 2005;8(4):225–30. doi:10.1080/14647270500054779.
Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94(6):2054–8. doi:10.1016/j.fertnstert.2009.11.036.
Gelbaya TA, Nardo LG, Hunter HR, Fitzgerald CT, Horne G, Pease EEH, Brison DR, Lieberman BA. Cryopreserved-thawed embryo transfer in natural or down-regulated hormonally controlled cycles: a retrospective study. Fertil Steril. 2006;85(3):603–9. doi:10.1016/j.fertnstert.2005.09.015.
Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database of Systematic Reviews (Online). 2008; (1):CD003414. doi:10.1002/14651858.CD003414.pub2.
Givens CR, Markun LC, Ryan IP, Chenette PE, Herbert CM, Schriock ED. Outcomes of natural cycles versus programmed cycles for 1677 frozen-thawed embryo transfers. Reprod Biomed Online. 2009;19(3):380–4.
Griesinger G, Weig M, Schroer A, Diedrich K, Kolibianakis EM. Mid-cycle serum levels of endogenous LH are not associated with the likelihood of pregnancy in artificial frozen-thawed embryo transfer cycles without pituitary suppression. Hum Reprod (Oxford, England). 2007;22(10):2589–93. doi:10.1093/humrep/dem207.
Haouzi D, Assou S, Dechanet C, Anahory T, Dechaud H, De Vos J, Hamamah S. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects. Biol Reprod. 2010;82(4):679–86. doi:10.1095/biolreprod.109.081299.
Hill GA, Freeman M, Bastias MC, Rogers BJ, Herbert 3rd CM, Osteen KG, Wentz AC. The influence of oocyte maturity and embryo quality on pregnancy rate in a program for in vitro fertilization-embryo transfer. Fertil Steril. 1989;52(5):801–6.
Hill MJ, Miller KA, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: an analysis of 1391 cycles. Fertil Steril. 2010;93(2):416–22. doi:10.1016/j.fertnstert.2008.11.027.
Irianni FM, Veeck LL, Toner JP, Muasher SJ. Influence of number of pre-embryos transferred, progesterone level and oestradiol/progesterone ratio at thaw on pregnancy results during replacement of cryopreserved pre-embryos in natural cycles. Hum Reprod (Oxford, England). 1992;7(6):797–800.
Krikun G, Schatz F, Taylor R, Critchley HOD, Rogers PAW, Huang J, Lockwood CJ. Endometrial endothelial cell steroid receptor expression and steroid effects on gene expression. J Clin Endocrinol Metab. 2005;90(3):1812–8. doi:10.1210/jc.2004-1814.
Leeton J, Rogers P, King C, Healy D. A comparison of pregnancy rates for 131 donor oocyte transfers using either a sequential or fixed regime of steroid replacement therapy. Hum Reprod (Oxford, England). 1991;6(2):299–301.
Lelaidier C, de Ziegler D, Gaetano J, Hazout A, Fernandez H, Frydman R. Controlled preparation of the endometrium with exogenous oestradiol and progesterone: a novel regimen not using a gonadotrophin-releasing hormone agonist. Hum Reprod (Oxford, England). 1992;7(10):1353–6.
Loh SK, Leong NK. Factors affecting success in an embryo cryopreservation programme. Ann Acad Med, Singapore. 1999;28(2):260–5.
Ma W-G, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100(5):2963–8. doi:10.1073/pnas.0530162100.
Morozov V, Ruman J, Kenigsberg D, Moodie G, Brenner S. Natural cycle cryo-thaw transfer may improve pregnancy outcome. J Assist Reprod Genet. 2007;24(4):119–23. doi:10.1007/s10815-006-9100-y.
Muasher SJ, Kruithoff C, Simonetti S, Oehninger S, Acosta AA, Jones GS. Controlled preparation of the endometrium with exogenous steroids for the transfer of frozen-thawed pre-embryos in patients with anovulatory or irregular cycles. Hum Reprod (Oxford, England). 1991;6(3):443–5.
Navot D, Anderson TL, Droesch K, Scott RT, Kreiner D, Rosenwaks Z. Hormonal manipulation of endometrial maturation. J Clin Endocrinol Metab. 1989;68(4):801–7.
Niu Z, Feng Y, Sun Y, Zhang A, Zhang H. Estrogen level monitoring in artificial frozen-thawed embryo transfer cycles using step-up regime without pituitary suppression: is it necessary? J Exp Clin Assist Reprod. 2008;5:4. doi:10.1186/1743-1050-5-4.
Queenan JT, Veeck LL, Toner JP, Oehninger S, Muasher SJ. Cryopreservation of all prezygotes in patients at risk of severe hyperstimulation does not eliminate the syndrome, but the chances of pregnancy are excellent with subsequent frozen-thaw transfers. Hum Reprod (Oxford, England). 1997;12(7):1573–6.
Sathanandan M, Macnamee MC, Rainsbury P, Wick K, Brinsden P, Edwards RG. Replacement of frozen-thawed embryos in artificial and natural cycles: a prospective semi-randomized study. Hum Reprod (Oxford, England). 1991;6(5):685–7.
Segaloff DL, Ascoli M. The lutropin/choriogonadotropin receptor … 4 years later. Endocr Rev. 1993;14(3):324–47.
Ziecik AJ, Derecka-Reszka K, Rzucidło SJ. Extragonadal gonadotropin receptors, their distribution and function. J Physiol Pharmacol: An Official Journal of the Polish Physiological Society. 1992;43(4 Suppl 1):33–49.
de Ziegler D, Cornel C, Bergeron C, Hazout A, Bouchard P, Frydman R. Controlled preparation of the endometrium with exogenous estradiol and progesterone in women having functioning ovaries. Fertil Steril. 1991;56(5):851–5.
Acknowledgments
None.
Conflict of interest
The authors confirm no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Outcome of frozen-thawed embryo transfer (FET) - a retrospective analysis.
Rights and permissions
About this article
Cite this article
Hancke, K., More, S., Kreienberg, R. et al. Patients undergoing frozen-thawed embryo transfer have similar live birth rates in spontaneous and artificial cycles. J Assist Reprod Genet 29, 403–407 (2012). https://doi.org/10.1007/s10815-012-9724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9724-z