Abstract
Machado-Joseph disease (MJD), also known as Spinocerebellar Ataxia type 3 (SCA3), is the most common autosomal dominant ataxia worldwide. MJD integrates a large group of disorders known as polyglutamine diseases (polyQ). To date, no effective treatment exists for MJD and other polyQ diseases. Nevertheless, researchers are making efforts to find treatment possibilities that modify the disease course or alleviate disease symptoms. Since neuroimaging studies in mutation carrying individuals suggest that in nervous system dysfunction begins many years before the onset of any detectable symptoms, the development of therapeutic interventions becomes of great importance, not only to slow progression of manifest disease but also to delay, or ideally prevent, its onset. Potential therapeutic targets for MJD and polyQ diseases can be divided into (i) those that are aimed at the polyQ proteins themselves, namely gene silencing, attempts to enhance mutant protein degradation or inhibition/prevention of aggregation; and (ii) those that intercept the toxic downstream effects of the polyQ proteins, such as mitochondrial dysfunction and oxidative stress, transcriptional abnormalities, UPS impairment, excitotoxicity, or activation of cell death. The existence of relevant animal models and the recent contributions towards the identification of putative molecular mechanisms underlying MJD are impacting on the development of new drugs. To date only a few preclinical trials were conducted, nevertheless some had very promising results and some candidate drugs are close to being tested in humans. Clinical trials for MJD are also very few to date and their results not very promising, mostly due to trial design constraints. Here, we provide an overview of the pharmacological therapeutic strategies for MJD studied in animal models and patients, and of their possible translation into the clinical practice.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Machado-Joseph Disease or Spinocerebellar Ataxia Type 3
Machado-Joseph disease (MJD), also known as spinocerebellar ataxia type 3 (SCA3), is known to exist worldwide [1], representing the most common dominantly inherited ataxia (Reviewed in [1,2,3]) and the second most common polyQ disease [4]. In the last years a large effort has been put forward towards the understanding of the pathologic mechanism(s) underlying polyQ diseases, however, and unfortunately, the therapeutic approaches and drug development did not reach the desirable outcomes yet. Despite the increasing number of therapeutic strategies assessed in mouse models of polyQ diseases (around 250 preclinical therapeutic trials have already been described) [5], there are no effective treatments for these disorders, including MJD, and currently available therapeutic approaches are only able to provide limited symptomatic relief (Reviewed in [6, 7]).
The core clinical feature in MJD is a slowly progressive ataxia starting in adulthood, being the average age at onset of 40 years and the mean survival time of 21 years [8]. Numerous other clinical symptoms , including weight loss, dystonia , dysarthria, spasticity, rigidity , fasciculations, postural instability, proprioceptive loss, dysphagia, amyotrophy, corticospinal and autonomic nervous system dysfunctions and neuropathy, are also frequently observed in MJD patients [9,10,11]. Non-motor symptoms are also present, such as cramps, fatigue, sleep disturbances, mild cognitive affection and mood-related diseases [12,13,14,15,16]. Neuropathologically, MJD is characterized by neuronal loss in the cerebellum , substantia nigra , striatum , thalamus, pontine nuclei, spinal cord and cranial nerves, precerebellar brainstem nuclei, cholinergic and dopaminergic midbrain, as well as visual, auditory, vestibular, somatosensory, and ingestion and urination-related systems (Reviewed in [11]). Retained integrity of the cortical and subcortical regions of the limbic system and mild degeneration of cerebral and cerebellar cortices, white matter of cerebellum , inferior olive and Purkinje cells , are also characteristic of MJD [11]. The ataxin-3 protein (the MJD disease protein) is expressed ubiquitously and when it bears the expanded allele it tends to aggregate forming neuronal nuclear inclusion bodies (NNIs) in the brain [17, 18]. These NNIs are present in functionally affected and non-affected brain regions, indicating that there is no direct correlation between the occurrence of these protein aggregates and neuronal dysfunction [11, 19, 20]. Axonal aggregates have also been found in human patients and, as the intranuclear aggregates , they were immunopositive for ubiquitin and p 62; one can hypothesize that axonal inclusions might be detrimental to axonal transport mechanisms, contributing to degeneration of nerve cells in MJD [21].
The clinical presentation of MJD is highly pleomorphic and led to the definition of four clinical sub-phenotypes: type I, characterised by the predominance of pyramidal and extrapyramidal anomalies, in addition to ataxia and other signs, with an early age-at-onset and fast progression; type II, with typical cerebellar ataxia , progressive external ophthalmoplegia and pyramidal signs appearing at an intermediate age; type III, with late onset and slow progression of peripheral signs, such as loss of proprioception and muscle atrophies; and type IV, the rarest, characterised by the presence of Parkinsonic signs, associated to the core clinical features [10, 22, 23].
Here, we provide an overview of the current situation concerning small molecule therapeutics for MJD, including a brief description of the symptomatic therapies used in the clinics to improve patients’ daily life, followed by a section on the recent drug discovery and development efforts, outlining the disease-modifying therapies tested so far in animal models of this disorder. In the end, we also provide a summary of the clinical trials performed to date in MJD patients.
19.2 Symptomatic Therapies for Machado-Joseph Disease
Despite the lack of efficacious disease-modifying therapies for MJD to date, several treatments, including specific drugs and multi-professional supportive approaches, are used to ameliorate neurological symptoms and increase the quality of life of the patients (Reviewed in [24], updated in 2015).
Non-pharmacological therapies include genetic counselling [25, 26], speech therapy, exercise/physiotherapy [27, 28], and occupational therapy [29]. The occupational therapy combined with antidepressants is thought to be helpful to fight the depression symptoms reported in MJD [30].
The pharmacological therapies prescribed by the physicians are mainly based on the knowledge of other related diseases or based on the patient’s needs. Yet, the efficacy of those therapies has not been proven scientifically in MJD patients. Importantly, none of the clinical trials performed to date in MJD patients were based on data obtained in animal models of the disease. Nowadays, and with available animal models that closely mimic the human condition, the connection between preclinical and clinical studies should be strengthened. Pharmacological therapy includes levodopa or dopamine agonists for the restless leg syndrome as well as for the parkinsonism-like symptoms [31]. Adverse events may occur with levodopa treatment, namely worsening of the motor symptoms as shown for Parkinson’s disease patients [32]. Modafinil, a psychostimulant, can be used to improve daytime fatigue, which is very frequent in MJD, and mexiletine or carbamazepine for cramps [33]. Together, these examples show that symptomatic MJD patients may benefit from available pharmacological approaches, which provide an important combination for the quality of life and the patients’ feeling of independence.
19.3 Disease-Modifying Therapies for Machado-Joseph Disease : Lessons from Preclinical Trials
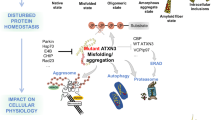
Despite the existence of a variety of MJD rodent models ([34] reviewed in [35]) and their potentialities, only a few preclinical trials have been performed until now using these models (see Table 19.1), and even less have then been translated to clinical trials . Those studies were performed considering different approaches: (i) more directly targeting mutant ataxin-3 synthesis, folding and degradation and (ii) reducing the downstream deleterious effects of mutant ataxin-3 accumulation. The hypothesized pathogenic mechanism(s) involved in MJD and discussed throughout this chapter are represented in Fig. 19.1, as well as the possible therapeutic targets.
Schematic representation of the potential pathogenic mechanisms underlying MJD and possible therapeutic targets. Intracellular candidate pathogenesis pathways in MJD are represented in red. These include the formation of cytoplasmic and nuclear aggregates /inclusions , transcriptional deregulation, mitochondrial dysfunction , impairment of degradation mechanisms (autophagy /proteasome) and activation of caspases/calpains. Possible intracellular therapeutic targets are represented in blue
19.3.1 Mutant Ataxin-3 Refolding and Degradation: Autophagy and Proteasome Inducers
Restoration of global protein homeostasis, or proteostasis, is a promising approach to reduce the toxicity of mutant ATXN3 in MJD. Several studies in rodent models demonstrated the efficacy of activating the cellular machinery involved in maintaining adequate conformation and solubility of proteins or, in case this fails, send them for degradation, such as molecular chaperones, the ubiquitin -proteasome system (UPS) and autophagy , which will be discussed hereafter.
For instance, Hsp90 inhibitors are known to possess the unique pharmacological effect of inducing a heat stress response and, in addition to their use as anticancer agents, have also been developed as pharmacological HSP inducers for application in protein folding disorders [36, 37]. Several studies demonstrated the positive effects of 17-AAG and its analogues (including 17-DMAG, which is less toxic) as Hsp90 inhibitors in models of polyQ diseases [38,39,40,41]. The efficacy of 17-DMAG in improving the behavioral deficits was tested in the CMVMJD135 mice [42]. In this study it was shown that the behavioral deficits were transiently improved by 17-DMAG administration and neuropathologic features were ameliorated. Surprisingly, 17-DMAG did not induce the HSR in the brain of CMVMJD135 animals as expected. However, the protein levels of mutant ataxin-3 as well as the aggregate load were diminished after 17-DMAG treatment suggesting that other mechanism(s) would be occurring in the cells. Indeed, it was proposed that 17-DMAG was inducing autophagy and therefore probably the degradation of mutant ataxin-3 through this mechanism (not excluding others, as the UPS). In spite of the promising results in mouse models, establishing proof of concept, 17-DMAG is known to exert several important adverse effects in humans [43], which must be taken in consideration given the expected need for chronic treatment of MJD patients. Chemical modifications should be conducted in 17-DMAG to decrease its toxicity while keeping its beneficial effects; only after that should such an approach be considered for clinical trials in MJD.
Autophagy induction seems to be a promising target to modulate protein aggregation in polyQ diseases and, in addition to the abovementioned results, there is an extensive body of literature demonstrating its beneficial effects in polyQ diseases [43,44,45,46,47,48,49,50,51,52,53,54,55,56]. In order to verify the therapeutic efficacy of autophagy induction in MJD, Menzies and colleagues used the mouse model generated by Bichelmeier et al. [57] which they chronically treated with an autophagy inducer—temsirolimus (codenamed CCI-779), a rapamycin analog. Although the authors were not able to reproduce the phenotype previously described for this model [57], at the end of a two months preclinical trial they report that treated-MJD animals performed better in the accelerating rod when compared to placebo-treated mice, and that this compound had no effect in wild-type (WT) animals in the rotarod. Also, temsirolimus was able to reduce mutant ataxin-3 aggregates in the motor cortex and the soluble cytoplasmic, but not nuclear, mutant ataxin-3 in total brain extracts. Finally, the authors performed a microarray study at basal conditions and after temsirolimus treatment. Overall, the transcriptional alterations found were very small, probably correlating to the absence of a clear phenotype in this cohort of MJD mice. Yet, it was possible to identify genes with decreased expression in MJD-vehicle mice, which was increased after temsirolimus treatment; the opposite effect was not found [48]. The potential beneficial effects of autophagy induction were further reinforced in studies using beclin-1 overexpression in rodent models of MJD [58]. Thus, and also considering the beneficial effects of 17-DMAG, other autophagy inducers were tested in the CMVMJD135 mice: lithium chloride and CCI-779. Unexpectedly, the use of lithium chloride had no overall effect on the behavioral deficits of CMVMJD135 mice, in spite of activating autophagy as expected [59]. Accordingly, a human clinical trial using lithium carbonate was performed in the same year, demonstrating that albeit well tolerated, lithium had no major impact on disease progression in MJD patients [60] (see Sect. 19.4 in the present chapter). In another attempt to increase autophagy , a combination of two autophagy inducers acting independently and dependently of mTOR—lithium and CCI-779, respectively—was tested in the CMVMJD135 mouse model. This combinatory therapy showed no beneficial effects and even proved to be deleterious to both transgenic and wild-type mice, affecting neurological function and general health [61], at doses shown to be safe in mice when administered alone [48, 59]. These results suggest that overactivation of autophagy could also be dangerous, however, other effects of the drug combination cannot be excluded.
Using the mouse model developed by their team [62], Wang and colleagues developed a preclinical trial using H1152, a Rho-kinase (ROCK) inhibitor [63]. ROCK is a kinase and acts as the downstream effector of small GTP-binding proteins of the Rho subfamily, and its abnormal activation has been implicated in several neurodegenerative diseases [64]. Also, ROCK inhibitors were shown to decrease the levels of mutant huntingtin in brain as well as improve motor function in a mouse model of Huntington’s disease (HD) [65]. This study confirmed that H1152 could also decrease the brain level of pathogenic ataxin-3 and exert a therapeutic effect on the MJD mouse model. The authors tested several ROCK inhibitors in vitro and showed that H1152 was the most potent in reducing ataxin-3 protein levels, and that acted by increasing proteasome activity. Daily intraperitoneal injections of H1152 in the MJD mice improved motor coordination and locomotor activity deficits. H1152 administration significantly decreased mutant ataxin-3 levels in the cerebellum , cerebral cortex, pontine nuclei and spinal cord and decreased the cell death (reduction in NeuN positive cells) observed in the pontine nuclei of vehicle-treated transgenic animals [63]. Fasudil, a first-generation ROCK inhibitor, has been studied widely in clinical trials for the treatment of pulmonary arterial hypertension as well as for subarachnoid hemorrhage [66], constituting a safe drug in humans. A phase II clinical trial is ongoing for the study of its safety and efficacy in amyotrophic lateral sclerosis patients (NCT01935518). Indeed, its protective effects were recently shown in a model of HD [67]. In this sense, the inhibition of ROCK can be regarded as a promising avenue for therapeutic intervention in various neurological disorders, including MJD and other polyQ diseases.
19.3.2 Therapies Targeting Downstream Molecular Events
19.3.2.1 Transcriptional Regulation
Transcriptional deregulation is a unifying feature of polyQ disorders [68,69,70,71,72,73]; however, the relationship between polyQ-induced deregulation of gene expression and the ongoing degenerative processes remains unclear.
More than 20 nuclear proteins relevant for transcription are known to interact with polyQ disease associated-proteins [69, 74]. Mutant ataxin-3 has been shown to interact abnormally with several proteins involved in the transcription machinery, namely CREB-binding protein (CBP) and p 300/CREBBP associated factor (PCAF), suppressing their histone acetyltransferase activity [72, 75]. Overexpression of some of these transcription regulators was shown to overcome polyQ toxicity, both in cellular models for MJD, Spinal and Bulbar Muscular Atrophy (SBMA ), and HD [70, 76] as well as in vivo, in a polyQ model in Drosophila [71]. This suggests that expanded polyQ proteins may contribute for the depletion of key transcriptional regulators with toxic effects to the cell and reinforces the idea of an important role for transcription deregulation in polyQ pathogenesis. Acetylation of histones relaxes the DNA structure, promoting transcription, whereas hypoacetylation represses gene activity [77]. The equilibrium of histone acetylation/deacetylation is controlled by histone acetyltransferases (HATs) and deacetylases (HDACs).
Previously, based on expression data, Chou and collaborators suggested that a global transcriptional deregulation was occurring in the cerebellum of a MJD transgenic model [62]. More specifically, they have shown a generalized hypoacetylation of H3 and H4. In order to modulate these alterations in the transcriptome, the same authors treated their mouse model with sodium butyrate (SB), an HDAC inhibitor. They observed that daily administration of SB was able to revert histone hypoacetylation as well as the transcription downregulation in the cerebellum . Importantly, SB treatment improved motor performance of transgenic animals in the rotarod, an effect that was less evident in later stages. The gait-related symptoms , quantified through the footprint pattern, were also ameliorated with SB, as well as the spontaneous locomotor activity, body weight loss and survival [78].
In contrast, Esteves S and colleagues, demonstrated that chronic treatment of the CMVMJD135 mice with valproic acid (VPA), also known to act as an HDAC inhibitor led to limited effects concerning the improvement of motor deficits and had no effect on mutant ataxin-3 aggregation in the brain. Nevertheless, VPA treatment increased the levels of GRP78, an endoplasmic reticulum chaperone involved in the folding of newly synthetized proteins and in the translocation of aberrant proteins for degradation by the proteasome, which might explain the small improvement in motor coordination seen after a long treatment duration [79]. These results contrast with the findings of a study in human patients, in which a beneficial effect was observed (see Sect. 19.4 in the present chapter).
19.3.2.2 Calcium Signaling Stabilizers
Calcium signaling is thought to play an important role in polyQ pathogenesis. This hypothesis is based on previous studies demonstrating that mutant huntingtin can bind and activate specifically type 1 inositol 1,4,5-triphosphate receptors (InsP3R1, an intracellular calcium release channel), influencing calcium signaling [80]. Deranged calcium signaling was also observed in neuronal primary cultures from the YAC128 HD mouse model [81, 82]. Later on, mutant ATXN3 was also proven to bind to InsP3R1 and to perturb calcium signaling. Taking advantage of the YAC transgenic model of MJD generated by Cemal et al. in [83], Chen and collaborators performed a chronic treatment to these mice, using food supplemented with dantrolene. This compound is a ryanodine antagonist and a clinically relevant Ca2+ signaling stabilizer, being commonly used as a skeletal muscle relaxant to treat hyperthermia and muscle spasticity [84]. Dantrolene-treated MJD mice showed an improved performance in the balance beam test (taking less time to traverse the different beams, with a number of foot slips identical to WT), reduction of the crawling behavior seen in the MJD-vehicle group, and a significant improvement in the footprinting pattern. To evaluate the neuroprotective effect of dantrolene, the brains of the four groups used were weighed, however there was no improvement in this parameter. Dantrolene food supplementation did, nevertheless, diminish the loss of NeuN positive cells in the pontine nuclei and of TH-positive cells in the substantia nigra of MJD mice [85]. In spite of its beneficial effects, no further studies with this compound were performed in MJD patients. The known side effects of dantrolene originate in the central nervous system, and include drowsiness, lightheadedness, headaches, anorexia, diarrhea, nausea, and vomiting [86]. To our knowledge, no clinical trials with dantrolene have been performed in neurodegenerative diseases, suggesting that this compound might not be a good candidate for MJD treatment.
19.3.2.3 Neuroprotection
Neuronal dysfunction and synaptotoxicity are thought to play a major role in polyQ disease pathogenesis. Indeed, it was previously suggested that neuronal dysfunction may precede neurodegeneration and clinical symptoms in HD [87, 88]. In MJD, loss of synaptic markers was proposed to be an early feature in a lentiviral-based disease model, suggesting a putative role for ataxin-3 in the control of synapse function [89]. Furthermore, Silva-Fernandes and colleagues have shown the presence of a clear motor phenotype in the CMVMJD135 mouse model of MJD, without major early neuronal loss, suggesting once again, that neuronal dysfunction may precede neurodegeneration [42]. These hypotheses were not deeply explored, so far, in MJD; nevertheless, some compounds known to have neuroprotective effects have been tested in MJD models.
Treatment with caffeine (a non-selective adenosine receptor antagonist) as well as with selective blockers of the adenosine A2A receptor (A2AR) have been shown to be neuroprotective in several brain diseases, including HD [90,91,92]. In a study by Gonçalves et al., caffeine was administered to a lentiviral model of MJD (overexpression of human wild-type—atx3-27Q—or mutant ataxin-3 -atx3-72Q) in the drinking water for 3 months (maximum), in a 1 g/L dose, corresponding to a human daily consumption of 5 cups of coffee. Chronic caffeine treatment rescued the striatal shrinkage observed in the mutant ATXN3 transduced animals and slightly reduced the number of pycnotic cells. Also, caffeine was able to avoid the loss of NeuN positive cells observed in the atx3-72Q animals. These data suggest that chronic caffeine treatment is neuroprotective towards ataxin-3 overexpression in the striatum . Furthermore, loss of DARPP-32 staining volume, astrogliosis and putative microgliosis were improved in the treated group. Nevertheless, the beneficial effects of caffeine were shown to be transient. Finally, and intriguingly, caffeine-treated mice showed an increase in the number of nuclear inclusions when compared to water-drinking animals. These observations might indicate that the final stages of aggregation , visible neuronal inclusions , are protective rather than toxic [89], but this was not explored further. Several studies support the use of caffeine for different neurodegenerative diseases (reviewed in [93]). The neuroprotective effects of caffeine observed in the lentiviral-mediated model of MJD, and considering the well-define and side-effect profile, being in general well tolerated comparing to other drugs, support the use of antagonists of adenosine receptors as potential therapeutic tools to treat MJD and other polyQ diseases. Further studies in MJD patients should be performed to prove the clinical utility of this approach.
Recently, Cunha-Santos and colleagues tested the potential of resveratrol, a Sirtuin-1 (SIRT1) activator, as potential therapeutic strategy for MJD [94]. SIRT1 belongs to the group of the histone deacetylase enzymes being a NAD+-dependent histone and protein deacetylase that plays an important role in several cellular and physiological processes, including an important involvement in neurodegeneration [95]. Indeed, induction of SIRT1 was shown to have a protective role in HD and SBMA models [96,97,98]. Resveratrol treatment in the MJD mouse model [99] was shown to improve motor and balance deficits after disease onset. This study pointed SIRT1 activation as a potential therapeutic target for MJD 94]. Resveratrol, being a multitarget compound with several neuroprotective roles, represents an interesting candidate for the treatment of MJD. Nevertheless, it is important to remember resveratrol solubility and bioavailability limitations [100], which can be solved by appropriate chemical modifications. Resveratrol was already tested in a phase 2 clinical trial in Alzheimer’s disease patients and showed to be safe and well tolerated; nevertheless, the small number of participants did not allow researchers to determine whether resveratrol may be beneficial or not.
It was also stated that “More potent and bioavailable SIRT1 activators are also in development” (see Study Results of the NCT01504854 clinical trial), which could be useful for this and other neurodegenerative diseases.
19.3.2.4 Modulators of the Serotonergic and Glutamatergic Systems
Recently, and departing from an unbiased screening of FDA-approved small molecules, Teixeira-Castro and collaborators identified Citalopram (Selective Serotonin Reuptake Inhibitor—SSRI) as a hit compound able to modify the neurotoxic effect of mutant ATXN3 in the nematode C. elegans, but also its aggregation . The effect required early treatment initiation and a minimum duration. The compound was further tested in a mouse model of the disease (CMVMJD135) and shown to delay disease progression, decrease mutant ATXN3 aggregation and neuropathology . This work also demonstrated, using pharmacogenetic approaches, that activation of the serotonergic signaling was beneficial in both animal models of MJD [101]. Intriguingly, improvement in the mouse model happened in spite of normal neurotransmitter levels at the basal state. This intriguing link between serotonin signaling and protein homeostasis has been recognized by the work of Prahlad and colleagues [102], and may imply a new perspective for usage of these established compounds in neurodegenerative diseases, including other polyQ-associated SCAs.
Although evidence for excitotoxicity is not as strong as for HD, perturbed glutamate transmission has also been proposed to play a role in MJD [62, 103, 104], namely through very intriguing links to mutant protein cleavage and aggregation . Interestingly, clinical trials using the antiglutamatergic drug riluzole demonstrated a beneficial effect in patients with different ataxias [105, 106]. Unfortunately, MJD patients were not included in these clinical trials . Considering this, and also the fact that riluzole was shown to have protective effects in cellular models of HD [107, 108], Schmidt and colleagues have studied the potential beneficial effects of riluzole in a conditional MJD mouse model [109]. Post-symptomatic chronic treatment with riluzole had no effect on motor deficits of the mouse despite the observed reduction of soluble mutant ataxin-3 protein levels. Furthermore, riluzole increased the levels of ataxin-3 aggregation . Also, and very importantly, the authors showed that treatment with riluzole decreased the Calbindin expression in Purkinje cells of the cerebellum , suggestive of possible toxicity, which might indicate that this compound might not be commendable to test in humans with MJD, or, at least, that it should be tested with caution [110].
19.4 Clinical Trials in MJD Patients
Currently, no disease modifying treatment exists for MJD. Yet, some symptomatic treatment is available, including genetic counseling, physical therapy programs, and speech and swallowing training as discussed above. The translation of findings from model systems to human patients is an important and urgent issue. Considering the lack of information on the key aspects of the pathogenic mechanism(s), the clinical and molecular heterogeneity of MJD patients and the scarcity of human biological tissues available for research, the development of translational approaches is very difficult. Still, some clinical trials have been performed for MJD (see Table 19.2). The detection of undesired side effects is also of major importance in clinical trials and must be taken in consideration. Most of the MJD clinical trials to date were performed using very few patients (less than 10) and only short-term effects were investigated, thus their outcome assessment might be compromised.
The combination of sulphamethoxazole and trimethoprim (Bactrim, a broad-spectrum antibiotic used in ear and urinary infections) was suggested to reduce disease symptoms in a small double-blind clinical trial using 8 MJD patients. The authors observed mild improvements in some of the parameters evaluated, such as hyperreflexia of knee jerks and rigospasticity of the legs in the patients treated with Bactrim. It was also shown that the levels of biopterins and homovanillic acid were reduced in the cerebrospinal fluid (CSF) of MJD patients when compared with controls with other neurodegenerative diseases, and that the short-term treatment with Bactrim increased these levels [111]. In the same year, another double-blind clinical trial was performed using Bactrim in 8 additional patients. In this study, three parameters were evaluated: subjective performance, neurological examination and timed tests. The treatment with Bactrim again demonstrated an improvement on gait and coordination. The authors suggested that further clinical trials using Bactrim should be performed due to the promising results obtained with this small number of patients [112]. Indeed, in 2001, a third double-blind clinical trial using Bactrim was performed in 22 MJD patients. In this trial, and in contrast to previous observations, chronic treatment with Bactrim had no effect in the parameters evaluated, such as ataxia ranking scale, self-assessment score, posturography and computer assisted motor performance test of Schoppe. The visual system function and mental health were also evaluated, but no effect was observed with Bactrim treatment [113].
The progression of MJD usually confines the patients to a wheelchair and ultimately the patients will be bedridden. In this condition, and in contrast to cognitive preservation, the patients might suffer depressive symptoms . Furthermore, the serotoninergic system in the cerebellum seems to play a role in motor output, such as locomotion. Serotonergic system impairment in the cerebellum was demonstrated to induce cerebellar ataxia [114]. The selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, are commonly used in the treatment of depression and present few side-effects [115]. In fact, as discussed above, citalopram (a commonly used antidepressant) proved to ameliorate the phenotype and neuropathology of the CMVMJD135 mouse model of MJD, suggesting that serotonergic system modulation might have an important role in MJD counteracting pathogenesis. Indeed, and long before this preclinical evidence emerged, some clinical trials using antidepressants have been performed in MJD patients, however the trial design was often less than optimal for detection of an effect. Monte et al. performed an open-label trial in 13 molecularly confirmed MJD patients, and saw that after 6 weeks of treatment, fluoxetine had no overall effect on motor abilities measured by functional scales and had no beneficial effect on the other neuropsychological tests [116]. Again, the outcome of the study may have been compromised by the small number of patients and particularly by the short duration of the study.
The use of 5-HT1A agonists has been controversial for the treatment of cerebellar ataxia , but several reports have suggested the efficacy of these agonists for the treatment of MJD [117,118,119,120]. Indeed, Friedman and collaborators have shown mild effects of buspirone in one MJD patient [121]. Later, Takei et al., reported the positive effects of tandospirone, another 5-HT1A agonist, in one MJD patient, that showed improvement in ataxia, depression, insomnia, anorexia and leg pain [122]. These positive effects led the authors to pursue a larger clinical trial using 10 MJD patients. In this trial, the patients started tandospirone treatment at an initial dose of 15 mg/kg (as the previous case study) that was further increased to 30 mg/kg for 7 weeks. The patients were examined using the international cooperative ataxia ranking scale (ICARS), the total length traveled (TLT) by stabolimetry test and the self-rating depression scale (SDS). All these parameters were alleviated with tandospirone treatment. Interestingly, all the symptoms were aggravated after a transient stop of tandospirone, and improved when the therapy was restarted [123]. These results suggested that 5-HT1A agonists could be effective in MJD, although more studies need to be performed to confirm these assumptions. Interestingly, it was suggested that the effects of 5-HT1A agonists might be potentiated by the concomitant use of SSRI’s (e.g. citalopram) and vice versa [124, 125], which could be an interesting approach considering the results of these human trials and the promising data resultant of the study showing the beneficial effects of citalopram (but also of 5-HT receptor agonists) in MJD animal models [101]
The involvement of excessive N-methyl-d-aspartate (NMDA)-mediated signaling in the mechanism of neuronal inclusion formation has been proposed [126]. It was recently shown that L-glutamate-induced excitation of iPSC cells of MJD patients leads to Ca2+-dependent proteolysis of ATXN3 followed by the formation of insoluble aggregates . The formation of those aggregates was also dependent on Na+ and K+ channels as well as on voltage-gated Ca2+ channels [103]. These very intriguing observations could provide a link between excitotoxicity and ATXN3 aggregation . A pilot study was performed in 6 MJD patients using Lamotrigine (25 mg twice a day during 9 weeks), a commonly used antiepileptic drug acting as a sodium channel-blocking agent that might be related to the reduction of NMDA-induced toxicity. In this trial, the patients were evaluated in the one leg standing test (OLST) and tandem gait index (TGI). Both OLST and TGI were improved during Lamotrigine treatment, comparing the values obtained with the normal values for Chinese population. Furthermore, and given these positive results, the authors cultured lymphoblastoid cells of one MJD patient and treated those cells with Lamotrigine. Mutant, but not normal ataxin-3 , was reduced with Lamotrigine at concentrations within the therapeutic range in humans. The mechanism underlying the reduction in mutant ataxin-3 levels was not investigated in this work and this effect was not confirmed in the trial subjects [127].
Recently, Zesiewicz and collaborators carried out a short-term clinical trial in 20 MJD patients using Varenicline (Chantix, 1 mg twice a day for 9 weeks) [128]. Chantix (partial agonist of the α4β2 neuronal nicotinic acetylcholine receptors) is used for smoking cessation. The rationale for this study was the fact that, although the major components of the cholinergic system seem to be spared in MJD, which may be reflected by the absence of dementia in MJD patients, the midbrain cholinergic pars compacta of the pedunculopontine nucleus suffers cell loss during disease progression [11], contributing for example to REM sleep disturbances, hence targeting the cholinergic neurotransmission could be a good approach. Chantix was also shown to be beneficial in SCA patients in previous case studies [129,122,, 130]. In this trial, patients were evaluated at baseline and at the end of the treatment (after 8 weeks) primarily using the Scale for the Assessment and Rating of Ataxia (SARA scale). Secondary measurements consisted of a timed 25-foot walk, a 9-hole peg test, Beck depression inventory (BDI), Beck anxiety inventory (BAI), clinical global impression (CGI), patient global impression (PGI) and the Short-Form 36 (SF36) to evaluate daily living. Chantix was able to significantly improve some subscores of the SARA scale, such as gait and rapid alternating movements. Also, the timed 25-foot walk was ameliorated by Chantix treatment, as well as the BDI score. The BDI score improved in both groups (Chantix and placebo) probably because the patients that were enrolled in the trial became hopeful regarding new treatment possibilities. A problem concerning this study was a high rate of dropout in the placebo group (4 out of 10 patients), interpreted as probably reflecting the difficulty of patients to reach the academic center. Regarding adverse events, it is possible to observe that Chantix caused, to a higher extent, gastrointestinal effects when compared to placebo. The mechanism by which Chantix improves ataxic symptoms was not evaluated in this study or elsewhere [131]. No follow up studies with larger groups of patients have been undertaken after this first promising result.
More recently, Saute and colleagues conducted a phase II clinical trial in 62 MJD patients using Lithium Carbonate. Lithium is commonly used to treat bipolar disorder, and is also used adjunctively with mood stabilizers and antidepressants to enhance, prolong and facilitate treatment response and remission of mood disorders [132, 133]. Lithium treatment was shown to have beneficial effects in several models of different neurodegenerative diseases [134,135,136,137,138], by the inhibition of glycogen synthase kinase-3β (GSK-3β) and autophagy activation. Importantly, however, irreversible cerebellar toxicity, leading to ataxia, nystagmus and dysarthria has also been observed due to lithium intoxication (reviewed in [139]). In this long-term clinical trial, Lithium (at therapeutic dosages of 0.5–0.8 mEq/L) was well tolerated by patients. After 48 weeks of follow-up, patients treated with Lithium did not show significant differences in disease progression, given by the results by Neurological Examination Score for the Assessment of Spinocerebellar Ataxia (NESSCA) and SARA scale. Nevertheless, the authors were able to observe that Lithium-treated MJD patients had a slower progression concerning the PATA test (word speed) and the Click test (finger-pointing coordination) as well as in the SCAFI (spinocerebellar ataxia functional index) and CCFS (composite cerebellar functional score), when compared to patients receiving placebo [133]. They suggested that larger clinical trials should be performed in order to understand the value of Lithium in the treatment of MJD.
The vast literature regarding transcription deregulation involvement in polyQ pathogenesis, led some researchers to conduct a clinical trial in MJD patients using Valproic Acid (VPA). VPA is commonly used as an anticonvulsant drug in the treatment of bipolar disorder. It has several known functions, including the increase in GABA neurotransmission, inhibition of voltage-gated sodium channels, T-type sodium channels and HDAC. In the preclinical trial field, the literature is controversial, since it was shown to be neuroprotective in a Drosophila MJD model [141], but showing limited therapeutic effects in a transgenic mouse model of the disease [79], as discussed above. Nevertheless, a clinical trial was recently performed in MJD patients using VPA. In this study, Lei and collaborators used two different study designs. In the first, a randomized, open-label, dose-escalation study was performed to evaluate safety of VPA administration. In this first part of the study, it was possible to observe that VPA was safe in all the dosages tested (400, 600 and 800 mg/twice a day). In the second approach, 36 MJD patients were enrolled and randomly allocated to placebo, 800 and 1200 mg/day VPA dosing. After 12 weeks of treatment, the patients were evaluated using the SARA scale, and it was possible to observe a decrease in the total SARA score in both VPA dosages, indicating a significant improvement of the patients’ motor coordination [142].
There are many concerns regarding the clinical trials performed to date in MJD: (i) the small cohorts of patients, which might be difficult to overcome due to the fact that this is a rare disorder and also the collaboration of patients might represent a problem; (ii) the clinical heterogeneity of the patients; (iii) the short-term observation of the patients, that contrasts with the slow progression of the disease (except for the Lithium Carbonate trial, which had a duration of 48 weeks); (iv) the outcomes used for ataxia measurement, which might be difficult to analyze due to the multisystem involvement in this disease; (v) the design of the studies, as randomized double-blinded trials with quantifiable ataxia scales and non-ataxia measurements should be used, which was not often the case, and (vi) the lack of useful biomarkers . Despite the existence of several scales to measure ataxia (reviewed in [143]), other non-ataxia scores should be applied to MJD patients since these patients also present non-ataxia symptoms , such as pyramidal and extrapyramidal signs, as well as peripheral findings [144].
19.5 Concluding Remarks
The search for disease-modifying therapeutic approaches for most neurodegenerative diseases has not been very productive to date; in the specific case of MJD, an important link between preclinical and clinical studies is still lacking. It is important to pursue well-designed clinical trials based on robust preclinical studies. Certainly, efforts are being made to perform good preclinical trials, and the scientific community is nowadays conducting better clinical studies with promising results for MJD. Other, non-pharmacological, disease-modifying therapeutic strategies may also be very promising.
Despite being rare diseases, MJD and other SCAs affect a large number of people worldwide. Given our current efficacy measures, large clinical trials , involving multiple centers and of long duration, are necessary which, in turn, implies high costs. Pharmaceutical companies are increasingly aware of the relevance of studying diseases of well-defined etiology, such as MJD, and their contribution could help to speed this process in a significant manner.
References
Paulson H (2012) Machado-Joseph disease/spinocerebellar ataxia type 3. Handb Clin Neurol 103:437–449
Bettencourt C, Lima M (2011) Machado-Joseph disease: from first descriptions to new perspectives. Orphanet J Rare Dis 6:35
Matos CA, de Macedo-Ribeiro S, Carvalho AL (2011) Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog Neurobiol 95(1):26–48
Schols L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 3(5):291–304
Switonski PM, Szlachcic WJ, Gabka A, Krzyzosiak WJ, Figiel M (2012) Mouse models of polyglutamine diseases in therapeutic approaches: review and data table. Part II. Mol Neurobiol 46(2):430–466
Bauer PO, Nukina N (2009) The pathogenic mechanisms of polyglutamine diseases and current therapeutic strategies. J Neurochem 110(6):1737–1765
Saute JAM, Jardim LB (2015) Machado Joseph disease: clinical and genetic aspects, and current treatment. Expert Opin Orphan Drugs 3(5):517–535
Coutinho P, Sequeiros J (1981) Clinical, genetic and pathological aspects of Machado-Joseph disease. J Genet Hum 29(3):203–209
Riess O, Rub U, Pastore A, Bauer P, Schols L (2008) SCA3: neurological features, pathogenesis and animal models. Cerebellum 7(2):125–137
Rosenberg RN (1992) Machado-Joseph disease: an autosomal dominant motor system degeneration. Mov Disord 7(3):193–203
Rub U, Brunt ER, Deller T (2008) New insights into the pathoanatomy of spinocerebellar ataxia type 3 (Machado-Joseph disease). Curr Opin Neurol 21(2):111–116
Franca MC Jr, D’Abreu A, Nucci A, Lopes-Cendes I (2008) Muscle excitability abnormalities in Machado-Joseph disease. Arch Neurol 65(4):525–529
Friedman JH, Amick MM (2008) Fatigue and daytime somnolence in Machado Joseph disease (spinocerebellar ataxia type 3). Mov Disord 23(9):1323–1324
Schols L, Haan J, Riess O, Amoiridis G, Przuntek H (1998) Sleep disturbance in spinocerebellar ataxias: is the SCA3 mutation a cause of restless legs syndrome? Neurology 51(6):1603–1607
Kawai Y, Takeda A, Abe Y, Washimi Y, Tanaka F, Sobue G (2004) Cognitive impairments in Machado-Joseph disease. Arch Neurol 61(11):1757–1760
Silva UC, Marques W Jr, Lourenco CM, Hallak JE, Osorio FL (2015) Psychiatric disorders, spinocerebellar ataxia type 3 and CAG expansion. J Neurol 262(7):1777–1779
Paulson HL, Perez MK, Trottier Y, et al (1997) Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 19(2):333–344
Schmidt T, Landwehrmeyer GB, Schmitt I, et al (1998) An isoform of ataxin-3 accumulates in the nucleus of neuronal cells in affected brain regions of SCA3 patients. Brain Pathol 8(4):669–679
Rub U, Seidel K, Ozerden I, et al (2007) Consistent affection of the central somatosensory system in spinocerebellar ataxia type 2 and type 3 and its significance for clinical symptoms and rehabilitative therapy. Brain Res Rev 53(2):235–249
Yamada M, Tan CF, Inenaga C, Tsuji S, Takahashi H (2004) Sharing of polyglutamine localization by the neuronal nucleus and cytoplasm in CAG-repeat diseases. Neuropathol Appl Neurobiol 30(6):665–675
Seidel K, den Dunnen WF, Schultz C, et al (2010) Axonal inclusions in spinocerebellar ataxia type 3. Acta Neuropathol 120(4):449–460
Coutinho P, Andrade C (1978) Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology 28(7):703–709
Lima L, Coutinho P (1980) Clinical criteria for diagnosis of Machado-Joseph disease: report of a non-Azoren Portuguese family. Neurology 30(3):319–322
Paulson H (1993). Spinocerebellar ataxia type 3. In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews(R). Seattle, WA
Maciel P, Costa MC, Ferro A, et al (2001) Improvement in the molecular diagnosis of Machado-Joseph disease. Arch Neurol 58(11):1821–1827
Rodrigues CS, de Oliveira VZ, Camargo G, et al (2012) Presymptomatic testing for neurogenetic diseases in Brazil: assessing who seeks and who follows through with testing. J genet couns 21(1):101–112
Miyai I, Ito M, Hattori N, et al (2012) Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair 26(5):515–522
Svensson M, Lexell J, Deierborg T (2015) Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior: what we can learn from animal models in clinical settings. Neurorehabil Neural Repair 29(6):577–589
Silva RC, Saute JA, Silva AC, Coutinho AC, Saraiva-Pereira ML, Jardim LB (2010) Occupational therapy in spinocerebellar ataxia type 3: an open-label trial. Braz J Med Biol Res = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 43(6):537–542
Cecchin CR, Pires AP, Rieder CR, et al (2007) Depressive symptoms in Machado-Joseph disease (SCA3) patients and their relatives. Commun Genet 10(1):19–26
Nandagopal R, Moorthy SG (2004) Dramatic levodopa responsiveness of dystonia in a sporadic case of spinocerebellar ataxia type 3. Postgrad Med J 80(944):363–365
Thanvi B, Lo N, Robinson T (2007) Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J 83(980):384–388
D’Abreu A, Franca MC Jr, Paulson HL, Lopes-Cendes I (2010) Caring for Machado-Joseph disease: current understanding and how to help patients. Parkinsonism Relat Disord 16(1):2–7
Szlachcic WJ, Switonski PM, Kurkowiak M, Wiatr K, Figiel M (2015) Mouse polyQ database: a new online resource for research using mouse models of neurodegenerative diseases. Mol Brain 8(1):69
Colomer Gould VF (2012) Mouse models of spinocerebellar ataxia type 3 (Machado-Joseph disease). Neurotherapeutics 9(2):285–296
Whitesell L, Bagatell R, Falsey R (2003) The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets 3(5):349–358
Bagatell R, Paine-Murrieta GD, Taylor CW, et al (2000) Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin Cancer Res 6(8):3312–3318
Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T (2008) Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem 283(38):26188–26197
Tokui K, Adachi H, Waza M, et al (2009) 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse. Hum Mol Genet 18(5):898–910
Waza M, Adachi H, Katsuno M, et al (2005) 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 11(10):1088–1095
Thomas M, Harrell JM, Morishima Y, Peng HM, Pratt WB, Lieberman AP (2006) Pharmacologic and genetic inhibition of hsp90-dependent trafficking reduces aggregation and promotes degradation of the expanded glutamine androgen receptor without stress protein induction. Hum Mol Genet 15(11):1876–1883
Silva-Fernandes A, Duarte-Silva S, Neves-Carvalho A, et al (2014) Chronic treatment with 17-DMAG improves balance and coordination in a new mouse model of Machado-Joseph disease. Neurother: j Am Soc Exp NeuroTher 11(2):433–449
Jhaveri K, Taldone T, Modi S, Chiosis G (2012) Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta 1823(3):742–755
del Cano-Espinel M, Acebes JR, Sanchez D, Ganfornina MD (2015) Lazarillo-related Lipocalins confer long-term protection against type I Spinocerebellar ataxia degeneration contributing to optimize selective autophagy. Mol Neurodegeneration 10:11
Menzies FM, Garcia-Arencibia M, Imarisio S, et al (2015) Calpain inhibition mediates autophagy-dependent protection against polyglutamine toxicity. Cell Death Differ 22(3):433–444
Tsunemi T, Ashe TD, Morrison BE, et al (2012) PGC-1alpha rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med 4(142):142–197
Roscic A, Baldo B, Crochemore C, Marcellin D, Paganetti P (2011) Induction of autophagy with catalytic mTOR inhibitors reduces huntingtin aggregates in a neuronal cell model. J Neurochem 119(2):398–407
Menzies FM, Huebener J, Renna M, Bonin M, Riess O, Rubinsztein DC (2010) Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain : J neurol 133(Pt 1):93–104
Sarkar S, Krishna G, Imarisio S, Saiki S, O’Kane CJ, Rubinsztein DC (2008) A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet 17(2):170–178
Williams A, Jahreiss L, Sarkar S, et al (2006) Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol 76:89–101
Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11(9):1107–1117
Ravikumar B, Vacher C, Berger Z, et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36(6):585–595
Xilouri M, Stefanis L (2015) Chaperone mediated autophagy to the rescue: a new-fangled target for the treatment of neurodegenerative diseases. Mol Cell Neurosci 66(Pt A):29–36
Frake RA, Ricketts T, Menzies FM, Rubinsztein DC (2015) Autophagy and neurodegeneration. J Clin Invest 125(1):65–74
Harris H, Rubinsztein DC (2012) Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 8(2):108–117
Cortes CJ, La Spada AR (2015) Autophagy in polyglutamine disease: imposing order on disorder or contributing to the chaos? Mol Cell Neurosci 66(Pt A): 53–61
Bichelmeier U, Schmidt T, Hubener J, et al (2007) Nuclear localization of ataxin-3 is required for the manifestation of symptoms in SCA3: in vivo evidence. J Neurosci: Official J Soc Neurosci 27(28):7418–7428
Nascimento-Ferreira I, Santos-Ferreira T, Sousa-Ferreira L, et al (2011) Overexpression of the autophagic beclin-1 protein clears mutant ataxin-3 and alleviates Machado-Joseph disease. Brain: J neurol 134(Pt 5):1400–1415
Duarte-Silva S, Neves-Carvalho A, Soares-Cunha C, et al (2014) Lithium chloride therapy fails to improve motor function in a transgenic mouse model of Machado-Joseph disease. Cerebellum 13(6):713–727
Saute JA, de Castilhos RM, Monte TL, et al (2014) A randomized, phase 2 clinical trial of lithium carbonate in Machado-Joseph disease. Mov Disord 29(4):568–573
Duarte-Silva S, Silva-Fernandes A, Neves-Carvalho A, Soares-Cunha C, Teixeira-Castro A, Maciel P (2016) Combined therapy with m-TOR-dependent and -independent autophagy inducers causes neurotoxicity in a mouse model of Machado-Joseph disease. Neuroscience 313:162–173
Chou AH, Yeh TH, Ouyang P, Chen YL, Chen SY, Wang HL (2008) Polyglutamine-expanded ataxin-3 causes cerebellar dysfunction of SCA3 transgenic mice by inducing transcriptional dysregulation. Neurobiol Dis 31(1):89–101
Wang HL, Hu SH, Chou AH, Wang SS, Weng YH, Yeh TH (2013) H1152 promotes the degradation of polyglutamine-expanded ataxin-3 or ataxin-7 independently of its ROCK-inhibiting effect and ameliorates mutant ataxin-3-induced neurodegeneration in the SCA3 transgenic mouse. Neuropharmacology 70:1–11
Mueller BK, Mack H, Teusch N (2005) Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 4(5):387–398
Li M, Huang Y, Ma AA, Lin E, Diamond MI (2009) Y-27632 improves rotarod performance and reduces huntingtin levels in R6/2 mice. Neurobiol Dis 36(3):413–420
Zhao J, Zhou D, Guo J, et al (2006) Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol Med Chir 46(9):421–428
Ahmed LA, Darwish HA, Abdelsalam RM, Amin HA (2015) Role of Rho kinase inhibition in the protective effect of Fasudil and Simvastatin against 3-Nitropropionic acid-induced striatal neurodegeneration and mitochondrial dysfunction in Rats. Mol Neurobiol
Lin Y, Hubert L Jr, Wilson JH (2009) Transcription destabilizes triplet repeats. Mol Carcinog 48(4):350–361
Okazawa H (2003) Polyglutamine diseases: a transcription disorder? Cell Mol Life Sci 60(7):1427–1439
McCampbell A, Taylor JP, Taye AA, et al (2000) CREB-binding protein sequestration by expanded polyglutamine. Hum Mol Genet 9(14):2197–2202
Taylor JP, Taye AA, Campbell C, Kazemi-Esfarjani P, Fischbeck KH, Min KT (2003) Aberrant histone acetylation, altered transcription, and retinal degeneration in a Drosophila model of polyglutamine disease are rescued by CREB-binding protein. Genes Dev 17(12):1463–1468
Li F, Macfarlan T, Pittman RN, Chakravarti D (2002) Ataxin-3 is a histone-binding protein with two independent transcriptional corepressor activities. J Biol Chem 277(47):45004–45012
Li SH, Cheng AL, Zhou H, et al (2002) Interaction of Huntington disease protein with transcriptional activator Sp1. Mol Cell Biol 22(5):1277–1287
Cohen-Carmon D, Meshorer E (2012) Polyglutamine (polyQ) disorders: the chromatin connection. Nucleus 3(5):433–441
Chai Y, Wu L, Griffin JD, Paulson HL (2001) The role of protein composition in specifying nuclear inclusion formation in polyglutamine disease. J Biol Chem 276(48):44889–44897
Dunah AW, Jeong H, Griffin A, et al (2002) Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science 296(5576):2238–2243
Grewal SI, Moazed D (2003) Heterochromatin and epigenetic control of gene expression. Science 301(5634):798–802
Chou AH, Chen SY, Yeh TH, Weng YH, Wang HL (2011) HDAC inhibitor sodium butyrate reverses transcriptional downregulation and ameliorates ataxic symptoms in a transgenic mouse model of SCA3. Neurobiol Dis 41(2):481–488
Esteves S, Duarte-Silva S, Naia L, et al (2015) Limited effect of chronic valproic acid treatment in a mouse model of Machado-Joseph disease. PloS one 10(10):e0141610
Tang TS, Tu H, Chan EY, et al (2003) Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 39(2):227–239
Tang TS, Slow E, Lupu V, et al (2005) Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci USA 102(7):2602–2607
Wu J, Tang T, Bezprozvanny I (2006) Evaluation of clinically relevant glutamate pathway inhibitors in in vitro model of Huntington’s disease. Neurosci Lett 407(3):219–223
Cemal CK, Carroll CJ, Lawrence L, et al (2002) YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum mol genet 11(9):1075–1094
Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F (2004) Dantrolene–a review of its pharmacology, therapeutic use and new developments. Anaesthesia 59(4):364–373
Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I (2008) Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci 48:12713–12724
Stutzmann GE (2005) Calcium dysregulation, IP3 signaling, and Alzheimer’s disease. Neuroscientist: Rev J Bringing Neurobiol, Neurol Psychiatry 11(2):110–115
Andrews TC, Weeks RA, Turjanski N, et al (1999) Huntington’s disease progression. PET and clinical observations. Brain: J Neurol 122(Pt 12):2353-2363
Li H, Li SH, Yu ZX, Shelbourne P, Li XJ (2001) Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci: Official J Soc Neurosci 21(21):8473–8481
Goncalves N, Simoes AT, Cunha RA, de Almeida LP (2013) Caffeine and adenosine A(2A) receptor inactivation decrease striatal neuropathology in a lentiviral-based model of Machado-Joseph disease. Ann Neurol 73(5):655–666
Chen JF, Sonsalla PK, Pedata F, et al (2007) Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol 83(5):310–331
Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA (2011) Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808(5):1380–1399
Popoli P, Blum D, Martire A, Ledent C, Ceruti S, Abbracchio MP (2007) Functions, dysfunctions and possible therapeutic relevance of adenosine A2A receptors in Huntington’s disease. Prog Neurobiol 81(5–6):331–348
Rivera-Oliver M, Diaz-Rios M (2014) Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: a review. Life Sci 101(1–2):1–9
Cunha-Santos J, Duarte-Neves J, Carmona V, Guarente L, Pereira de Almeida L, Cavadas C (2016) Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway. Nat Commun 7:11445
Martin A, Tegla CA, Cudrici CD, et al (2015) Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol Res 61(3):187–197
Jeong H, Cohen DE, Cui L, et al (2012) Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med 18(1):159–165
Jiang M, Wang J, Fu J, et al (2012) Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med 18(1):153–158
Montie HL, Pestell RG, Merry DE (2011) SIRT1 modulates aggregation and toxicity through deacetylation of the androgen receptor in cell models of SBMA. J Neurosci 31(48):17425–17436
Torashima T, Koyama C, Iizuka A, et al (2008) Lentivector-mediated rescue from cerebellar ataxia in a mouse model of spinocerebellar ataxia. EMBO reports 9(4):393–399
Walle T (2011) Bioavailability of resveratrol. Ann NY Acad Sci 1215:9–15
Teixeira-Castro A, Jalles A, Esteves S, et al (2015) Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain: J Neurol 138(Pt 11):3221–3237
Tatum MC, Ooi FK, Chikka MR, et al (2015) Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr Biol 25(2):163–174
Koch P, Breuer P, Peitz M, et al (2011) Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature 480(7378):543–546
Konno A, Shuvaev AN, Miyake N, Miyake K, et al (2014) Mutant ataxin-3 with an abnormally expanded polyglutamine chain disrupts dendritic development and metabotropic glutamate receptor signaling in mouse cerebellar Purkinje cells. Cerebellum 13(1):29–41
Ristori G, Romano S, Visconti A, et al (2010) Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology 74(10):839–845
Romano S, Coarelli G, Marcotulli C, et al (2015) Riluzole in patients with hereditary cerebellar ataxia: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 14(10):985–991
Hockly E, Tse J, Barker AL, et al (2006) Evaluation of the benzothiazole aggregation inhibitors riluzole and PGL-135 as therapeutics for Huntington’s disease. Neurobiol Dis 21(1):228–236
Ortega Z, Diaz-Hernandez M, Maynard CJ, Hernandez F, Dantuma NP, Lucas JJ (2010) Acute polyglutamine expression in inducible mouse model unravels ubiquitin/proteasome system impairment and permanent recovery attributable to aggregate formation. J Neurosci 30(10):3675–3688
Boy J, Schmidt T, Wolburg H, et al (2009) Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Hum mol genet 18(22):4282–4295
Schmidt J, Schmidt T, Golla M, et al (2016) In vivo assessment of riluzole as a potential therapeutic drug for spinocerebellar ataxia type 3. J Neurochem 138(1):150–162
Sakai T, Matsuishi T, Yamada S, Komori H, Iwashita H (1995) Sulfamethoxazole-trimethoprim double-blind, placebo-controlled, crossover trial in Machado-Joseph disease: sulfamethoxazole-trimethoprim increases cerebrospinal fluid level of biopterin. J Neural Transm Gen Sect 102(2):159–172
Correia M, Coutinho P, Silva MC, Guimaraes J, Amado J, Matos E (1995) Evaluation of the effect of sulphametoxazole and trimethoprim in patients with Machado-Joseph disease. Rev Neurol 23(121):632–634
Schulte T, Mattern R, Berger K, et al (2001) Double-blind crossover trial of trimethoprim-sulfamethoxazole in spinocerebellar ataxia type 3/Machado-Joseph disease. Arch Neurol 58(9):1451–1457
Chan-Palay V (1977) Indoleamine neurons and their processes in the normal rat brain and in chronic diet-induced thiamine deficiency demonstrated by uptake of 3H-serotonin. J Comp Neurol 176(4):467–493
Wenthur CJ, Bennett MR, Lindsley CW (2014) Classics in chemical neuroscience: fluoxetine (Prozac). ACS Chem Neurosci 5(1):14–23
Monte TL, Rieder CR, Tort AB, et al (2003) Use of fluoxetine for treatment of Machado-Joseph disease: an open-label study. Acta Neurol Scand 107(3):207–210
Lou JS, Goldfarb L, McShane L, Gatev P, Hallett M (1995) Use of buspirone for treatment of cerebellar ataxia. An open-label study. Arch Neurol 52(10):982–988
Trouillas P, Brudon F, Adeleine P (1988) Improvement of cerebellar ataxia with levorotatory form of 5-hydroxytryptophan. A double-blind study with quantified data processing. Arch Neurol 45(11):1217–1222
Trouillas P, Xie J, Adeleine P (1997) Buspirone, a serotonergic 5-HT1A agonist, is active in cerebellar ataxia. A new fact in favor of the serotonergic theory of ataxia. Prog Brain Res 114:589–599
Trouillas P, Xie J, Adeleine P, et al (1997) Buspirone, a 5-hydroxytryptamine1A agonist, is active in cerebellar ataxia. Results of a double-blind drug placebo study in patients with cerebellar cortical atrophy. Arch Neurol 54(6):749–752
Friedman JH (1997) Machado-Joseph disease/spinocerebellar ataxia 3 responsive to buspirone. Mov Disord: Official J Mov Disord Soc 12(4):613–614
Takei A, Honma S, Kawashima A, et al (2002) Beneficial effects of tandospirone on ataxia of a patient with Machado-Joseph disease. Psychiatry Clin Neurosci 56(2):181–185
Takei A, Fukazawa T, Hamada T, et al (2004) Effects of tandospirone on “5-HT1A receptor-associated symptoms” in patients with Machado-Josephe disease: an open-label study. Clin Neuropharmacol 27(1):9–13
Lanfumey L, Hamon M (2004) 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord 3(1):1–10
Blier P, Ward NM (2003) Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry 53(3):193–203
Ientile R, Caccamo D, Macaione V, Torre V, Macaione S (2002) NMDA-evoked excitotoxicity increases tissue transglutaminase in cerebellar granule cells. Neuroscience 115(3):723–729
Liu CS, Hsu HM, Cheng WL, Hsieh M (2005) Clinical and molecular events in patients with Machado-Joseph disease under lamotrigine therapy. Acta Neurol Scand 111(6):385–390
Zesiewicz TA, Sullivan KL (2008) Treatment of ataxia and imbalance with varenicline (Chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14). Clin Neuropharmacol 31(6):363–365
Zesiewicz TA, Sullivan KL, Freeman A, Juncos JL (2009) Treatment of imbalance with varenicline Chantix®: report of a patient with fragile X tremor/ataxia syndrome. Acta Neurol Scand 119(2):135–138
Zesiewicz TA, Sullivan KL, Gooch CL, Lynch DR (2009) Subjective improvement in proprioception in 2 patients with atypical Friedreich ataxia treated with varenicline (Chantix). J Clin Neuromuscul Dis 10(4):191–193
Zesiewicz TA, Greenstein PE, Sullivan KL, Wecker L, Miller A, Jahan I, Chen R, Perlman SL (2012) A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology 78(8):545–550
Goodwin FK (2003) Rationale for using lithium in combination with other mood stabilizers in the management of bipolar disorder. J Clin Psychiatry 64(Suppl 5):18–24
Lin D, Mok H, Yatham LN (2006) Polytherapy in bipolar disorder. CNS Drugs 20(1):29–42
Wood NI, Morton AJ (2003) Chronic lithium chloride treatment has variable effects on motor behaviour and survival of mice transgenic for the Huntington’s disease mutation. Brain res bull 61(4):375–383
Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM (2008) Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 155(3):567–572
Jia DD, Zhang L, Chen Z, et al (2013) Lithium chloride alleviates neurodegeneration partly by inhibiting activity of GSK3beta in a SCA3 Drosophila model. Cerebellum 12(6):892–901
Fornai F, Longone P, Cafaro L, et al (2008) Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 105(6):2052–2057
Watase K, Gatchel JR, Sun Y, et al (2007) Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med 4(5):e182
van Gaalen J, Kerstens FG, Maas RP, Harmark L, van de Warrenburg BP (2014) Drug-induced cerebellar ataxia: a systematic review. CNS Drugs 28(12):1139–1153
Saute JA, Castilhos RM, Monte TL, et al (2014) A randomized, phase 2 clinical trial of lithium carbonate in Machado-Joseph disease. Mov Disord: Official J Mov Disord Soc
Yi J, Zhang L, Tang B, et al (2013) Sodium valproate alleviates neurodegeneration in SCA3/MJD via suppressing apoptosis and rescuing the hypoacetylation levels of histone H3 and H4. PLoS ONE 8(1):e54792
Lei LF, Yang GP, Wang JL, et al (2016) Safety and efficacy of valproic acid treatment in SCA3/MJD patients. Parkinsonism Relat Disord 26:55–61
Saute JA, Donis KC, Serrano-Munuera C, et al (2012) Ataxia rating scales—psychometric profiles, natural history and their application in clinical trials. Cerebellum 11(2):488–504
Kieling C, Morales Saute JA, Jardim LB (2007) When ataxia is not just ataxia. Nat Clin Pract Neurol 3(5):E2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Duarte-Silva, S., Maciel, P. (2018). Pharmacological Therapies for Machado-Joseph Disease. In: Nóbrega, C., Pereira de Almeida, L. (eds) Polyglutamine Disorders. Advances in Experimental Medicine and Biology, vol 1049. Springer, Cham. https://doi.org/10.1007/978-3-319-71779-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-71779-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71778-4
Online ISBN: 978-3-319-71779-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)