Abstract
Acute pancreatitis is the most common gastrointestinal disease resulting in hospitalization in the United States, and appropriate critical care is crucial to survival in severe cases. Early adequate fluid resuscitation and nutritional support are cornerstones of initial management. Recognition and treatment of early complications such as abdominal compartment syndrome and later complications such as infected pancreatic necrosis and visceral artery pseudoaneurysm are crucial to survival in the severe cases that confront intensivists. Infected necrosis is the most common complication of severe pancreatitis and should typically be treated using a step-up approach that begins with percutaneous or endoscopic drainage and progresses when necessary to minimally invasive endoscopic or surgical necrosectomy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Pancreatitis
- Acute necrotizing
- Pancreatic fistula
- Pseudocyst
- SIRS
- Abdominal compartment syndrome
- Arterial pseudoaneurysm
Introduction
Acute pancreatitis encompasses a wide range of severity, from mild and self-limited to lethal. This chapter will focus on medical and surgical management of severe pancreatitis requiring intensive care unit admission. We address the most common clinical questions related to care of severe acute pancreatitis, such as which patients should receive antibiotics, the best method of nutrition, which patients require surgery, what is the optimal surgical approach, and others.
Epidemiology and Etiology
Acute pancreatitis is the most common gastrointestinal disorder requiring hospitalization in the United States with an estimated 274,000 hospitalizations in 2009, and its incidence appears to be increasing [1, 2]. The most common causes of acute pancreatitis are ethanol ingestion and gallstones. Less frequent causes include instrumentation of the bile or pancreatic ducts (endoscopic retrograde cholangiopancreatography [ERCP]), medications (especially diuretics, antiepileptics, and protease inhibitors), hypertriglyceridemia, hypercalcemia, congenital anatomic or genetic conditions (e.g., pancreas divisum or CFTR mutation), mumps, pancreatic neoplasm, and trauma or hypoperfusion. In 10–15% of cases, the cause is not identified, though evidence is increasing that a significant percentage of these cases may be due to occult biliary tract disease [3]. The overall mortality is 2–4%, though the mortality rate of patients requiring intensive care is significantly higher [2, 4].
Pathophysiology
The pathophysiology of acute pancreatitis is poorly understood. The most common causes of acute pancreatitis can generally be broken down into mechanical (gallstones, ERCP) or systemic (alcohol, medications, hypercalcemia, hypertriglyceridemia). There are two suggested mechanisms whereby the mechanical causes result in acute pancreatitis: obstruction of the ampulla or bile reflux into the pancreatic ductal system. How systemic agents trigger acute pancreatitis is even less clear.
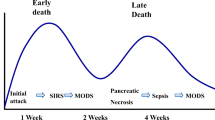
Most investigators agree that, whatever the inciting mechanism, acute pancreatitis results from activation of trypsin within the pancreatic acinar cells. The pancreas has mechanisms for preventing intracellular trypsin activation and counteracting low levels of activation, but when these mechanisms are overwhelmed, pancreatic autodigestion ensues, which can progress beyond the gland itself and into the surrounding peripancreatic tissues. This local injury can in turn activate a variety of local, regional, and systemic inflammatory mediators (complement, interleukins, phospholipase A2) which may be responsible for the systemic effects seen in severe acute pancreatitis [5]. For the intensivist, the relevance is that acute pancreatitis can trigger a profound SIRS response and septic shock-like physiology even in the absence of infection.
Diagnosis , Classification, and Severity
The diagnosis of acute pancreatitis is based on the identification of two of the following three criteria: (1) clinical (central upper abdominal pain, often with associated nausea and vomiting, and sometimes radiating to the back), (2) laboratory (serum amylase or lipase greater than three times the upper limit of normal), and (3) radiographic (imaging (usually CT or MRI) characteristic of acute pancreatitis). Imaging is rarely required to make the diagnosis of acute pancreatitis, which can usually be made on the basis of clinical and biochemical parameters alone. Imaging should only be used acutely when the diagnosis is unclear and is typically more valuable later in the course of disease to better define local complications (discussed below). In critically ill patients in which the diagnosis is unclear, CT with intravenous contrast is highly sensitive and specific and can also assess for many critical alternative diagnoses such as perforated peptic ulcer, aortic pathology, or mesenteric ischemia. The etiology of any episode of pancreatitis should be sought, as it may allow prevention of recurrent episodes. When there is no obvious inciting factor such as heavy alcohol use or recent ERCP, abdominal ultrasound should be performed to evaluate for gallstones as a potential cause.
There have been several classification systems for acute pancreatitis severity through the years, each with its inherent strengths and weaknesses. In theory such systems are most practically useful to the intensivist for triage and early identification of patients at high risk of complications who might benefit from initial resuscitation in an ICU setting. The presence of SIRS or organ failure on presentation and at 48 h is considered the best predictor of severity of acute pancreatitis. Complex or pancreatitis-specific severity scoring systems (e.g., Ranson, Glasgow, Balthazar, APACHE II) do not perform better and need not be calculated [6, 7]. Overall, at least 80% of acute pancreatitis is mild, and 20% is severe or moderately severe.
Initial Management
Fluid Resuscitation
Severe or moderately severe pancreatitis patients often manifest systemic signs of inflammation. Fluid resuscitation is required in the acute phase with a balanced electrolyte solution, e.g., Ringer’s lactate [8]. The rate and total amount of fluid used during initial resuscitation can be difficult to predict. The consequences of under-resuscitation include end-organ damage, in particular renal failure. Over-resuscitation can be complicated by pulmonary edema, respiratory failure, and abdominal compartment syndrome. The “sweet spot” between under- and over-resuscitation can be difficult to identify. Studies have shown increased morbidity and mortality when initial resuscitation is undertaken with 10–15 mL/kg/hr. compared to 5–10 mL/kg/hr. We suggest initial resuscitation with Ringer’s lactate solution at 5–10 mL/kg/hr. with continuous reassessment of the endpoints of resuscitation. Relevant endpoints include clinical (e.g., heart rate, blood pressure, urine output), invasive (e.g., stroke volume variation), and biochemical (e.g., base deficit, lactate) parameters [9].
Pancreatitis patients can typically be divided into responders and nonresponders to initial fluid resuscitation. Most responders will manifest signs of improvement clinically and in measured endpoints of resuscitation within the first 4 L of fluid administration. Those who do not respond after 2–4 L may need vasopressor support (i.e., norepinephrine, vasopressin) in addition to ongoing volume resuscitation. Many of the patients who do not respond to this early resuscitation may never respond favorably even to massive resuscitation. One pitfall is to persist with high-volume fluid resuscitation in the hopes of achieving endpoints (low HR, improved urine output, whatever it may be) that will never be achieved with any volume of fluid administration, due to the severity of the underlying inflammation. In these patients, the complications of fluid overload can accumulate without any concomitant improvement in perfusion or organ function. Ask yourself in patients who have not shown significant improvement after high-volume fluid administration (e.g., 6–8 L): what will the ninth or tenth liter of fluid accomplish that the first eight did not? Starting early aggressive fluid resuscitation is the cornerstone of medical therapy of severe acute pancreatitis and is simple but must be judicious in nature . Knowing when to stop is just as important but can be a more difficult and nuanced decision. A balance between fluid resuscitation to meet the needs of the capillary leak and the use of vasopressors to meet the needs of a dilated distributive shock state is important.
Nutrition
In mild pancreatitis oral intake can be resumed as soon as abdominal pain and laboratory parameters are improving, often within the first 24 h after presentation. Neither needs to be completely resolved before resuming oral intake. Oral intake can be rapidly advanced to a full solid diet. Indeed, one randomized controlled trial showed that initial oral intake can be with a full solid diet [10, 11]. In patients with severe pancreatitis requiring nutritional supplementation, enteral feeding should be the primary therapy and initiated early (24–48 h after initiation of resuscitation). No specific formulation or immunonutrition has been shown to improve outcomes. Nasogastric feeding if tolerated is equivalent to nasojejunal feeding, acknowledging that it may be less well tolerated in pancreatitis patients than in other critically ill patients due to mechanical compression of the gastric outlet or relative gastric dysmotility induced by inflammation in the lesser sac, since the pancreas abuts the posterior gastric wall and the duodenum. Parenteral nutrition should only be used in patients who cannot reach nutritional goals with enteral nutrition within 5–7 days [12,13,14,15].
Prevention, Diagnosis, and Treatment of Infection
About 20% of pancreatitis is associated with detectable necrosis of pancreatic or peripancreatic tissue. About 20–30% of the time, this necrosis is complicated by infection. This is the primary indication for mechanical intervention (drainage or debridement) in acute pancreatitis, which is discussed in more detail below. Preventing infection could reduce the need for intervention and any associated morbidity, while prompt diagnosis and treatment of infection can limit the morbidity when infection does occur.
A relatively large literature exists on the administration of intravenous antibiotics to patients with either predicted severe acute pancreatitis or radiographic evidence of necrosis for the purpose of preventing infection of the necrosis. A recent meta-analysis and review of 14 randomized controlled trials concluded that there is no evidence to support the routine use of antibiotics in patients with predicted severe pancreatitis. It remains possible that subgroups may be identified and could benefit from antibiotic prophylaxis, but current guidelines recommend against systemic antibiotic administration for prophylactic purposes. Systemic antibiotics should be reserved for the treatment (not the prophylaxis) of infected pancreatic and peripancreatic necrosis [16]. In observational studies, early enteral feeding, as discussed above, is associated with a reduced incidence of infected necrosis. The presumed mechanism is by reducing the permeability of the gut’s mucosal barrier. This benefit has not been supported in randomized trials. Alternative methods of preventing infection include intra-arterial antibiotic administration [17]. There is some evidence that prophylactic selective digestive decontamination (SDD) with enteral antibiotics may be effective in reducing the rate of infected pancreatic necrosis, but this is not strong enough to make SDD a standard recommendation [18]. In one randomized trial, “probiotics” have been found to be harmful [19].
The incidence of infected necrosis increases over the early course of acute pancreatitis and probably peaks in the third and fourth weeks after the onset of the disease. Infected necrosis can be diagnosed definitively by the finding of air in an area of pancreatic necrosis on CT scanning or by gram stain and culture of a fine-needle aspirate of the necrosis. However, infected necrosis remains a clinical diagnosis. It is important to remember that FNA is only approximately 75–90% sensitive for the diagnosis of infection. Thus, patients who are clinically unwell with suspicion for infected necrosis should be treated as if they have infection, since there is no reliable means to exclude it. The common clinical scenario is a patient whose fever curve, leukocytosis, and systemic inflammatory response are improving but begin to return at 3–4 weeks.
When treatment is initiated, carbapenems comprise the best initial regimen based on evidence of effective pancreatic tissue penetration and an appropriate spectrum of antimicrobial activity [20]. Since fungal infection is not uncommon (25%), patients with persistently worsening clinical condition or with microbiologic evidence for fungal infection should be treated with antifungals. If cultures show Candida albicans, fluconazole is appropriate. Although good evidence on the optimal antifungal agents in pancreatitis are lacking, if the indication is severe sepsis, we recommend using broader spectrum antifungal agents until definitive culture and sensitivities are available.
Imaging
CT is the most common imaging modality used for diagnosis of acute pancreatitis and its complications. It is highly sensitive and specific for acute pancreatitis and relevant complications but is overused in general. As noted above, CT is rarely needed to make the diagnosis of acute pancreatitis and should not be used routinely at the time of presentation but should be reserved for cases in which there is diagnostic uncertainty or clinical deterioration in spite of appropriate initial treatment [21,22,23]. Whenever possible, CT should be performed with oral and intravenous contrast. If CT is performed to assess for local complications and the severity of the pancreatitis, the optimal timing is 72–96 h after presentation, as CT scans performed in the first 72 h frequently underestimate the degree of pancreatic and peripancreatic necrosis. Even when early CT shows significant abnormalities, follow-up imaging is not recommended unless there is clinical deterioration or lack of improvement. A patient who continues to improve after an episode of acute pancreatitis, even a severe episode with documented necrosis, does not require serial imaging to monitor resolution. MRI can provide most of the same information as CT. Potential advantages include the lack of ionizing radiation and superiority in delineating liquid and solid components within peripancreatic necrosis. As noted above in the diagnosis section, early ultrasonography should also be used to assess for gallstones as the source of the pancreatitis episode if no other etiology is apparent.
ERCP
ERCP with sphincterotomy and common bile duct stone extraction is most commonly used to relieve biliary obstruction in cases of gallstone pancreatitis associated with cholangitis. Since the vast majority of gallstones responsible for gallstone pancreatitis pass spontaneously out of the common bile duct, ERCP is usually not necessary. Routine ERCP for all cases of biliary pancreatitis increases the rate of complications, so ERCP should only be employed when there is ongoing biliary obstruction. Sphincterotomy has the added benefit of reducing the risk of recurrent biliary complications. Occasionally biliary obstruction may result later in the course of disease when inflammation or necrosis in the region of the pancreatic head compresses the common bile duct; this usually requires biliary stenting to relieve the obstruction.
Abdominal Compartment Syndrome
As noted above, abdominal compartment syndrome in acute pancreatitis is usually related to high-volume fluid resuscitation, through retroperitoneal mass effect from peripancreatic edema and bowel edema. Diagnosis in acute pancreatitis is as for ACS of other etiologies with intra-abdominal pressures estimated by transduction of bladder pressures. The difficulty in diagnosing ACS in acute pancreatitis is that even in the absence of intra-abdominal hypertension, acute pancreatitis can result in all the clinical hallmarks of ACS such as acute kidney injury, respiratory failure with high peak inspiratory pressures, hypotension, and a tense distended abdomen. When these signs and symptoms occur in a patient with severe pancreatitis, it can be difficult to know if they are a manifestation of the systemic inflammation induced by pancreatitis or if they are a direct result of the intra-abdominal hypertension and thus whether they will improve with treatment of the intra-abdominal hypertension. Treatment is as for ACS of any etiology usually beginning with nasogastric and rectal decompression, volume removal with diuresis or ultrafiltration , and sedation or neuromuscular blockade to increase abdominal wall compliance. Ascites is often a major contributor to intra-abdominal hypertension in acute pancreatitis patients with ACS, and if significant ascites is present, it should be percutaneously drained [24]. When all of these measures fail, surgical decompression of the abdomen is the last resort. If abdominal decompression is performed, pancreatic necrosectomy should not be undertaken. ACS typically occurs early in the course of pancreatitis when surgical necrosectomy increases mortality. Although tempting, do not perform necrosectomy “just because you are there.” If the indication for surgery is ACS, then treat the ACS. After decompression, diuresis and staged closure should achieve a high rate (>90%) of primary fascial closure [25].
Intervention
Initial medical management for pancreatitis can be easily provided at most hospitals, but intervention requires a facility with a multidisciplinary team including at least surgeons, interventional radiologists, and gastroenterologists experienced in managing the disease [26]. The clearest consensus indication for intervention is infected pancreatic necrosis.
Once the diagnosis of infected necrosis is made, treatment is with the supportive care described above. When possible intervention should be delayed to 28 days or more from the onset of the pancreatitis episode. This may be impossible if patients are clinically unstable. Whenever the first intervention is undertaken, a minimally invasive percutaneous or endoscopic drainage procedure should be the initial procedure as the first step in a so-called “step-up” approach [27, 28]. Between 20% and 45% of patients with infected necrosis can be successfully treated with percutaneous drainage alone, though this may require several repeat drainage procedures. Drains should be placed taking into account the planned strategies for subsequent stages of the step-up approach. When possible, this may involve placing at least one drain into the area of infected necrosis via a retroperitoneal route to allow for video-assisted retroperitoneal debridement (VARD) along the drain tract. This involves a small subcostal flank incision, dissection along the drain tract into the necrosis cavity, and blunt debridement of the necrotic and infected fluid and tissue. Long retractors are used to expose the tract and cavity, and a standard laparoscope is used to improve visualization of the cavity, though there is typically no insufflation [29]. For patients with necrosis anatomically amenable to such a “step-up” approach, short-term benefits include the ability to avoid any surgery in a significant subset of the population and less new-onset organ failure. Long-term benefits include reduced incidence of diabetes mellitus and incisional hernia. For patients debrided entirely via an endoscopic route, there may also be a mortality advantage compared to open surgery without any preoperative drainage procedure [30]. When no retroperitoneal drainage route is available, sinus tract endoscopy can utilize even transperitoneal drain tracts for debridement of necrosis that does not resolve with percutaneous drainage. This technique involves dilation of the drain tract to allow introduction of a debriding instrument (rigid nephroscopes, flexible endoscopes, and laparoscopic instruments have all been used) into the necrosis cavity [31]. Rarely, some patients with infected pancreatic necrosis will not be amenable to endoscopic or retroperitoneal debridement, in which case laparotomy or laparoscopy may be used for transperitoneal debridement. Additionally, it must be noted that while minimally invasive drainage – either percutaneous or endoscopic – as a first step likely confers significant advantages, when surgery is subsequently required, the evidence is less compelling that any one surgical approach is superior to others. The general principles of delay beyond 4 weeks and preoperative drainage of some form should still be applied whatever the approach is. Tansabdominal debridement should involve removal of all or nearly all infected or necrotic pancreatic and peripancreatic tissue with closed suction drainage of the necrotic cavity. The use of different incisions (midline versus subcostal), approaches to the pancreas (transmesocolic, through the gastrocolic omentum, or retroperitoneal), and drainage (closed packing, closed suction alone, continuous lavage) is at the discretion of the surgeon [32].
Intervention is less often needed for sterile pancreatic necrosis. The most common indication is gastric outlet obstruction. Intervention can often be delayed longer, as this complication will often resolve with time. If patients with presumed sterile necrosis remain persistently unwell, the possibility of occult infection must be considered. Intervention can include surgical debridement or bypass.
Other complications that may prompt intervention in the acute setting include abdominal compartment syndrome (ACS) , hemorrhage of a visceral artery (usually splenic artery) pseudoaneurysm, and bowel perforation or fistula. When these complications arise in the acute setting, they should, as a rule, be treated by the least invasive methods possible. The treatment of ACS is discussed above. Bleeding from a visceral artery pseudoaneurysm should be controlled endovascularly by angioembolization whenever possible as direct surgical control in a region of active or recent pancreatitis is extremely difficult [33]. Intestinal perforation is similarly difficult to manage. Contained perforations or controlled fistulas may be manageable with drainage or diversion . When there is bowel ischemia or uncontrolled enteric spillage, resection will likely be necessary.
Late Complications
Pancreatic fistulas and pseudocysts result from disruption of the pancreatic duct due to destruction of the surrounding parenchyma. Fistulas may result from severe pancreatitis or as a complication of pancreatic debridement, after which they are common. One advantage of endoscopic transluminal debridement may be that such leaks from the pancreatic duct drain internally, rather than forming external fistulas. Whatever the cause, when the fistula is controlled with percutaneous drains, it will usually close, though it may require many weeks. Pancreatic duct stenting, octreotide administration, and restriction of enteral nutrition have all been advocated to aid in fistula closure but are not routinely helpful and should be used very selectively. In the special situation of a disconnected distal pancreatic remnant in which a segment of the gland has been completely separated from any route of drainage into the gastrointestinal tract, spontaneous closure is impossible. Such patients may either be treated endoscopically by transluminal stenting to attempt to convert the external fistula into a controlled internal fistula or may be treated surgically by either Roux-en-Y jejunostomy to the distal pancreatic remnant or resection of the disconnected distal segment.
Pseudocysts form when the ductal disruption is walled off by the body into an organized collection of pancreatic juice encased by reactive inflammatory tissue – a process that occurs 4 weeks or more after damage to the pancreatic duct. Asymptomatic pseudocysts do not require intervention. The most common symptoms are early satiety and abdominal pain. Pseudocysts may also cause true gastric outlet obstruction, become infected, or lead to pseudoaneurysm formation. Internal drainage is the treatment of choice for pseudocysts requiring intervention. For pseudocysts closely opposed to the stomach or duodenum, endoscopic pseudocyst-gastrostomy or duodenostomy is the treatment of choice. For very large or endoscopically inaccessible pseudocysts, surgical cyst gastrostomy or Roux-en-Y cyst jejunostomy is necessary [34].
Vascular complications of pancreatitis include arterial pseudoaneurysm and venous thrombosis. These most commonly involve the splenic vessels, but in pancreatitis primarily affecting the head, pseudoaneurysms of the pancreaticoduodenal or gastroduodenal arteries may occur along with thrombosis of the superior mesenteric or portal veins. Pseuodaneurysms result from the action of pancreatic enzymes on the arterial wall. They may be identified incidentally on contrast-enhanced CT or can present with catastrophic hemorrhage. We recommend intervening even on asymptomatic pseudoaneurysms in most cases, since there is no reliable way to predict hemorrhage. As above, whether elective or emergent, they are best treated with angioembolization [33]. Splenic vein thrombosis due to pancreatitis can usually be observed. We occasionally anticoagulate if clot extends into the main portal vein. Even without anticoagulation the thrombus can resolve. If it does not, the most common late complication is gastric varices. If these result in gastrointestinal bleeding, they can be eliminated by splenectomy [35].
Efforts should be made to prevent recurrence after an episode of pancreatitis by cessation of ethanol abuse for alcoholic pancreatitis, treatment of the underlying condition in hypercalcemia and hypertriglyceridemia, and cessation of any offending medications in cases of medication-induced pancreatitis. In patients with gallstone pancreatitis, cholecystectomy during the same hospitalization is recommended for mild cases. In patients undergoing transperitoneal necrosectomy, cholecystectomy at the time of necrosectomy is safe and reduces recurrent biliary complications [36]. In patients with peripancreatic fluid collections, cholecystectomy should be delayed for 6 weeks. In especially poor operative candidates, ERCP with sphincterotomy reduces the risk of recurrent gallstone pancreatitis and can be considered as an alternative to cholecystectomy.
References
Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, Dibonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–87.
Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA Jr. Increasing United States hospital admissions for acute pancreatitis, 1988–2003. Ann Epidemiol. 2007;17(7):491–7.
Räty S, Pulkkinen J, Nordback I, Sand J, Victorzon M, Grönroos J, Helminen H, Kuusanmäki P, Nordström P, Paajanen H. Can laparoscopic cholecystectomy prevent recurrent idiopathic acute pancreatitis?: a prospective randomized multicenter trial. Ann Surg. 2015;262(5):736–41.
Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371(9607):143–52.
Sarr MG, Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Tsiotos GG, Vege SS. The new revised classification of acute pancreatitis 2012. Surg Clin North Am. 2013;93(3):549–62.
Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:1247e51.
Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435e41.
BU W, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–7.
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15.
Eckerwall GE, Tingstedt BB, Bergenzaun PE, Andersson RG. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery-a randomized clinical study. Clin Nutr. 2007;26:758–63.
Moraes JM, Felga GE, Chebli LA, Franco MB, Gomes CA, Gaburri PD, Zanini A, Chebli JM. A full solid diet as the initial meal in mild acute pancreatitis is safe and result in a shorter length of hospitalization: results from a prospective, randomized, controlled, double-blind clinical trial. J Clin Gastroenterol. 2010;44:517–22.
Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1):CD002837.
Petrov MS, Loveday BP, Pylypchuk RD, McIlroy K, Phillips AR, Windsor JA. Systematic review and meta-analysis of enteral nutrition formulations in acute pancreatitis. Br J Surg. 2009;96:1243–52.
Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, Imrie CW. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol. 2005;100:432–9.
Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. J Clin Gastroenterol. 2006;40:431–4.
Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP, Isenmann R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol. 2010;46(3):261–70.
Piascik M, Rydzewska G, Milewski J, Olszewski S, Furmanek M, Walecki J, et al. The results of severe acute pancreatitis treatment with continuous regional arterial infusion of protease inhibitor and antibiotic: a randomized controlled study. Pancreas. 2010;39:863e7.
Luiten EJ, Hop WC, Lange JF, Bruining HA. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995;222:57e65.
Besselink MG, Van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651e9.
Dellinger EP, Tellado JM, Soto NE, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674–83.
Fleszler F, Friedenberg F, Krevsky B, Friedel D, Braitman LE. Abdominal computed tomography prolongs length of stay and is frequently unnecessary in the evaluation of acute pancreatitis. Am J Med Sci. 2003;325:251–5.
Spanier BW, Nio Y, van der Hulst RW, Tuynman HA, Dijkgraaf MG, Bruno MJ. Practice and yield of early CT scan in acute pancreatitis: a Dutch Observational Multicenter Study. Pancreatology. 2010;10:222–8.
Mortele KJ, Ip IK, BU W, Conwell DL, Banks PA, Khorasani R. Acute pancreatitis: imaging utilization practices in an urban teaching hospital--analysis of trends with assessment of independent predictors in correlation with patient outcomes. Radiology. 2011;258:174–81.
Radenkovic DV, Bajec D, Ivancevic N, Bumbasirevic V, Milic N, Jeremic V, Gregoric P, Karamarkovic A, Karadzic B, Mirkovic D, Bilanovic D, Scepanovic R, Cijan V. Decompressive laparotomy with temporary abdominal closure versus percutaneous puncture with placement of abdominal catheter in patients with abdominal compartment syndrome during acute pancreatitis: background and design of multicenter, randomised, controlled study. BMC Surg. 2010;10:22.
Boone B, Zureikat A, Hughes SJ, Moser AJ, Yadav D, Zeh HJ, Lee KK. Abdominal compartment syndrome is an early, lethal complication of acute pancreatitis. Am Surg. 2013;79(6):601–7.
Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, Horvath KD, vanSonnenberg E, Bollen TL, Vege SS, International Multidisciplinary Panel of Speakers and Moderators. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–94.
van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG, Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–502.
van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG, Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141(4):1254–63.
van Santvoort HC, Besselink MG, Horvath KD, Sinanan MN, Bollen TL, van Ramshorst B, Gooszen HG, Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford). 2007;9(2):156–9.
Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, Poley JW, van Ramshorst B, Vleggaar FP, Boermeester MA, Gooszen HG, Weusten BL, Timmer R, Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307(10):1053–61.
Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232(2):175–80.
Mantke R, Lippert H, Buchler MW, Sarr MG. International practices in pancreatic surgery. Part IV, Surgery of acute pancreatitis. New York: Springer; 2013.
Kalva SP, Yeddula K, Wicky S, Fernandez del Castillo C, Warshaw AL. Angiographic intervention in patients with a suspected visceral artery pseudoaneurysm complicating pancreatitis and pancreatic surgery. Arch Surg. 2011;146(6):647–52.
Samuelson AL, Shah RJ. Endoscopic management of pancreatic pseudocysts. Gastroenterol Clin N Am. 2012;41(1):47–62.
Besselink MG. Splanchnic vein thrombosis complicating severe acute pancreatitis. HPB (Oxford). 2011;13(12):831–2.
Fong ZV, Peev M, Warshaw AL, Lillemoe KD, Fernández-del Castillo C, Velmahos GC, Fagenholz PJ. Single-stage cholecystectomy at the time of pancreatic necrosectomy is safe and prevents future biliary complications: a 20-year single institutional experience with 217 consecutive patients. J Gastrointest Surg. 2015;19(1):32–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Fagenholz, P., de Moya, M. (2018). Acute Pancreatitis. In: Salim, A., Brown, C., Inaba, K., Martin, M. (eds) Surgical Critical Care Therapy . Springer, Cham. https://doi.org/10.1007/978-3-319-71712-8_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-71712-8_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71711-1
Online ISBN: 978-3-319-71712-8
eBook Packages: MedicineMedicine (R0)