Abstract
Stem cells (SCs) are the main source of biological material used in cell therapy and tissue engineering . Additionally, these cells are being investigated as a potential therapy technique for cardiovascular diseases (CVDs) and heart regeneration . To improve the investigation of SC proliferation and maturation, the Lab-on-a-Chip systems are being developed. There are many reports, which have proven that such microsystems have been successfully used for SC differentiation into different cell lineages. In this chapter, we present Heart-on-a-chip systems based on stem cells —the microsystems utilized for SC differentiation into cardiomyocytes (CMs). Various types of SC differentiation performed in Lab-on-a-chip systems are presented at the beginning of this chapter. Next, biochemical, physical and mechanical stimulations are presented as techniques to perform cardiogenesis. Other promising methods, especially the use of graphene and their other forms, which could be used for cardiac differentiation, are presented at the end of this chapter. Finally, we summarize the research focused on heart regeneration using the Lab-on-a-chip systems , and we outline future perspectives for microsystem usage for SC differentiation into CMs.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Stem Cell Differentiation in Lab-on-a-chip Systems
Regenerative medicine is an alternative method, which can be used to treat cardiovascular diseases (CVDs). Stem cells (SCs) are the main source of biological material used in such therapy. This is because these cells can differentiate into a specific tissue, depending on cell origin and pluripotency (see Chap. 6). Precise control of SC fate is a major challenge to scientists. Stem cell differentiation into cardiomyocytes (CMs) may contribute to the development of an in vitro cardiac model and new methods for the treatment of heart disease. So far, promising results in SC usage for heart regeneration were obtained (Segers et al. 2008). Numerous types of SCs are being investigated nowadays, e.g. bone marrow-derived/circulating progenitor cells (BMP-CSs), mesenchymal stem cells (MSCs), adipose tissue-derived stem cells (ATSCs), endothelial progenitor cells (EPCs), embryonic stem cells (ESCs)-induced pluripotent stem cells (iPSCs) and cardiac stem cells (CSCs) (Emmert et al. 2014; Kawamura et al. 2012). Although many reports based on stem cells have been presented, there are many important issues, which have to be considered (e.g. source of the SCs, low reproducibility and throughput, controllable differentiation) (Discher et al. 2009; Silvestre and Menasché 2015; Ye et al. 2011).
Microscale technologies can provide tools, which allow for improving SC investigation. There are various factors utilized for the determination of SC fate on microscale: biochemical factors (growth factors, vitamins, cytokines, small molecules), physical factors (structure and elasticity of biomaterials, electrical and magnetic fields, thermal gradients ), mechanical factors (fluidic shear stress, mechanical strain), cell–cell interactions (co-culture) and cell-biomaterial interactions (Guilak et al. 2009; Higuchi et al. 2017; Hwang et al. 2008; Tzatzalos et al. 2016). The Lab-on-a-chip systems are powerful tools, which can complete existing laboratory techniques used for SC differentiation studies. The advantages of the Lab-on-a-chip systems were characterized in previous chapters; therefore, here we will focus on benefits which are especially related to SC culture and analysis. First of all, a specific niche with a controllable microenvironment can be created in the microsystems (Duinen et al. 2015; Park et al. 2010). Because of this, SCs can be cultured in conditions close to in vivo and is suitable for cell differentiation. The microsystems made of poly(dimethylsiloxane) (PDMS) are most often fabricated for SC culture and differentiation (Ertl et al. 2014). However, depending on the differentiation method such microsystems are integrated with various components for improving SC operating. For example, other materials used as differentiation factors (nanofibers, microgrooves, hydrogels) are integrated into the microsystems (Higuchi et al. 2017; Tomecka et al. 2017). Hydrogels play an increasingly important role in creating the spatial cell arrangement (three-dimensional, 3D) (Perez et al. 2016). The extracellular matrix (ECM) provided by hydrogels can also influence SC differentiation. These materials determine cell attachment and alignment as well as provide physiologically relevant stiffness, which can influence SC differentiation (Mathur et al. 2016). Various studies with hydrogels based on CMs and SCs have been presented in the literature, e.g. analysis of cell contraction forces (Zimmermann et al. 2006), drug cytotoxicity (Schaafm et al. 2011) and heart disease simulation (Hinson et al. 2015). The combination of both microfluidic systems and hydrogels could be a new approach for SC study; however, it is still a significant challenge for researchers.
The microsystems are also integrated with micropumps, microvalves and electrodes (Zhang et al. 2007). This allows various differential conditions to be created (Zhang and Austin 2012). Thanks to this, defined shear stress conditions, a stable electrical force and precise control of biochemical factor delivery to the SCs are possible to be obtained in microscale (Pavesi et al. 2015; Stoppel et al. 2016). For instance, microarrays have been fabricated for the investigation of protein-protein interaction and to define the properties of the SC microenvironment (Lutolf and Blau 2009). Diffusion of biochemical growth factors of the micropatterned cells and their differentiation have also been investigated (Peerani et al. 2007). The microsystems have also been used for precise control of single cell or high-throughput analysis. It is important to mention the possibility of minimizing the amount of the cells, culture medium and chemical reagent in the experiments performed in the microsystems. It is especially important for the studies performed with SCs.
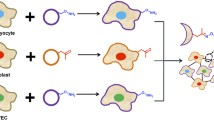
Differentiation methods investigated in Lab-on-a-chip systems and the fate of SCs are shown in Fig. 9.1. SC differentiation into neurons, granular cells (kidney), osteoblasts, hepatocytes, endothelial cells, adipocytes as well as cardiomyocytes have been reported (Figallo et al. 2007; Jeon et al. 2014; Ju et al. 2008; Kim et al. 2014; Ni et al. 2008; Pavesi et al. 2015; Wang et al. 2014; Villa-Diaz et al. 2009; Yang et al. 2015, 2017).
Because SCs can potentially be useful for heart regeneration, SC differentiation into cardiomyocytes have been investigated more and more in the microsystems . In recent years, a few review papers about Heart-on-a-chip based on stem cells have been published (Ghafar-Zadeh et al. 2011; Jastrzebska et al. 2016; Yang and Ma 2012). However, the use of SCs for Heart-on-a-chip is still in the initial phase. SCs are often differentiated in macroscale and afterwards they are seeded in the microsystems and cultured under flow conditions. To differentiate SCs into cardiac cells, three main methods are used: biochemical, mechanical and physical stimulation. The effect of SC differentiation into CMs is investigated by expression studies of specific cardiac cell markers. For this purpose, immunofluorescence staining of the cytoskeletal proteins is performed. Cardiac troponin T (cTnT), α-sarcomeric actin, connexix43, β-myosin heavy chain (β-MHC), Nkx2 are the most common cardiac-specific markers (Qureshi et al. 2012). Specific cardiac gene expressions of, e.g. α-MHC, ANF (atrial natriuretic factor), BNP (B-type natriuretic peptide), MYL2 (myosin light chain 2), MYL7, MYH6 (myosin heavy chain 6), MYH7 are also defined using a polymerase chain reaction (PCR) (Chen et al. 2009; Pavesi et al. 2015). Additionally, the induced stem cell-derived cardiomyocytes (SC-CMs) could contract. Therefore, the amplitude of cell contraction is measured to identify SC differentiation .
A review of the recent literature shows that microscale technologies enable the creation of in vivo-like models of SCs. The Lab-on-a-chip systems have been successfully used for SC culture, SC differentiation, cytotoxicity assays as well as investigation of heart regeneration Regeneration using SCs. Examples and detailed characterization of these microsystems are presented in the next sections of this chapter.
9.2 Biochemical Stimulation
Soluble chemical factors are used for SC differentiation into specific cell types. Such factors are introduced to the cells by their supplementation in a culture medium and incubation with the cell over a period of time. Biochemical factors can influence the fate of the cell, e.g.: cell viability, proliferation, self-renewal and differentiation. Factors such as bone morphogenic protein-2 (BMP-2), vascular endothelial growth factor (VEGF), the Wingless/INT proteins (WNTs) have been used to differentiate SCs into CMs (Dimarakis et al. 2006; Uzel et al. 2014). The fibroblast growth factor (FGF) is also commonly used. The FGF family consists of a large and diverse group of small polypeptide growth factors, which play a crucial role in cardiac differentiation (Kawai et al. 2004; Kofidis et al. 2004; Rosenblatt-Velin et al. 2005). Additionally, the transforming growth factor beta (TGFβ) and 5-azacytidine (5-AZA) have been reported as factors utilized for cardiac SC stimulation (Cheng et al. 2016; Tabar and Studer 2014). The heart is capable of three TGFβ isoform expressions which exhibit a specific function in cardiac differentiation (Jeon et al. 2014; Kumar and Sun 2005). Kumar and Sun investigated the effect of TGFβ isoforms on ESC differentiation . Significant cardiac differentiation and an increase in beating cells were observed for ESC incubation only with TGF-β2 isoform. Most of the presented factors were investigated in the microfluidic systems. The biochemical methods used for SC differentiation into CMs in Lab-on-a-chip systems are summarized in Table 9.1.
The Lab-on-a-chip systems can be used for SC stimulation using controlled dosage (concentration, exposition time) of the biochemical factors. Both two-dimensional (2D) and three-dimensional (3D) culture models of SCs have been presented in the literature (Goβmann et al. 2016; Moya et al. 2013; Wan et al. 2011). However, cardiomyocytes derived from SCs have been investigated more often than SC differentiation. An interesting solution was presented by Goβmann et al. (2016). They developed a new device/method, called Cell Drum technology, for mechanical tension analysis of the cells (Fig. 9.2a). Each Cell Drum well contains an ultra-thin (ca. 3.0 µm) circular membrane made of PDMS. Human-induced pluripotent stem cells -derived cardiomyocytes (hiPS-CMs) were obtained (in macroscale) from human skin fibroblasts differentiated by the usage of Yamanaka factors (Oct4, Sox2, cMyc, Klf4) (Takahashi et al. 2007). Both a monolayer and a thin tissue culture were obtained on Cell Drum membrane. Next, the cells regular beating induced membrane deflection, which was monitored using a pressure sensor. The obtained culture models of hiPS-CMs were used for investigation of selective agonists and antagonists of both calcium (S-Bay K8644 and verapamil) and potassium (veratridine and lidocaine) channels. It was found that the beating cell imitated in vivo human heart tissue. Additionally, the tested drugs influenced cell contraction. The developed heart mimicking system can be used for easy and fast pharmacological analysis of cardiac drugs. It should be noted that cardiotoxicity assays are based on cell contraction and the main feature of heart cells. Therefore, the utilization of the Cell Drum technology could have a high impact on heart regeneration research.
a A scheme of Cell Drum tissue analyser: (1) cylindrical Cell Drum culture medium container, (2) ring heater, (3) air pump, (4) laser triangulation sensor, (5) culture medium, (6) membrane and cell monolayer , (7) cell monolayer . Reprinted from Goβmann et al. (2016). Open Access. b A scheme of PDMS microsystem, which contains three microchannels for a 2D cell cultures and two gel microchannels for a 3D cultures. Reprinted with permission from Wan et al. (2011). Copyright 2011 Springer. c A scheme of the perfusion microdevice integrated with a drug reservoir, a connecting channel and a biowire bioreactor. Reprinted with permission from Xiao et al. (2014). Copyright 2013 Royal Society of Chemistry
Microwell/systems technologies are increasingly used to culture and differentiate ESCs (Lee et al. 2011a, b; Mohr et al. 2006; Wan et al. 2011; Xiao et al. 2014). These cells have a specific property, i.e. they are able to create 3D structures called embryoid bodies (EBs). Conventional methods such as dissociated suspension, a methyl cellulose culture, a hanging drop culture, a spinner flask and a bioreactor culture are still often used to obtain EBs as the first step in ESC differentiation (Moya et al. 2013; Thavandiran et al. 2013). EBs can spontaneously differentiate into various cell types. Therefore, the culture medium should be supplemented with factors (e.g. LIF—leukaemia inhibitory factor), which maintain the undifferentiated cell form. These factors should be removed, and differential biochemical agents should be added in the culture medium to differentiate EBs into a specific cell type. It is also important that the size and shape of EBs influence the fate of the cells (Hwang et al. 2008). The utilization of the microtechnology gives the possibility to precisely EB size manipulation. EB dimensions are determined by the geometry and size of the microwells. A microsystem with ca. 200 microwells was proposed as a new and a promising method for creation of EBs (Miyamoto et al. 2016). It was investigated that how different densities of the SCs influence EBs formation. Different EB fates were noticed depending on the initial size of EBs. Large EBs were differentiated into hepatic and cardiac cells, whereas small EBs promoted vascular differentiation. The proposed microplatform could be used as high-throughput technique for EBs formation and SC fate study.
A controllable maintenance of undifferentiated EBs and a precise control of their fate is a big challenge for the scientists. The ESC culture method proposed by Mohr et al. (2006) should be underlined. They presented a microwell system for 3D long-term human ESC culture and EB formation. It was proven that microwell systems allowed undifferentiated ESCs to be obtained for several weeks. Thanks to this, such methods can increase the effectiveness and the reproducibility of undifferentiated cell cultures. Moreover, both controllable ESC differentiation via forming EBs and drug cytotoxicity evaluation could be performed in this microsystem.
So far research indicates that cardiomyogenic differentiation is higher in microscale cultures than in conventional well plates. Moreover, the culture type has influence on SC fate (Wan et al. 2011). An example of a developed PDMS microsystem used for both 2D cultures (coated with 0.1% gelatin) and for 3D cultures (EBs were mixed with collagen I) is shown in (Fig. 9.2b). The usage of a perfusable cardiac biowire, which mimics 3D functional cardiac tissue, provides a new approach for the investigation of heart functions (Xiao et al. 2014). A microplatform made of PDMS and glass plates enabled 3D cardiac cell culture . The microsystem consisted of a drug reservoir, a connecting channel and a biowire bioreactor (Fig. 9.2c). 3D microtissues were generated using human ESC-derived cardiomyocytes (hESC-CMs) and primary neonatal rat cardiomyocytes . hESC-CMs were obtained through EB differentiation with BMP-4, bFGF, activin, VEGF, inhibitor of WNT production-2 (IWP2) using macroscale. The hESC-CMs and neonatal rat CMs (suspended in collagen type I) were seeded in the microfabricated biowire platform and cultured for 14 days. The microplatform was integrated with electrodes (carbon rods), which were used for electrical stimulation (biphasic rectangular signal, 3.5–4 V cm−1, 1 ms duration, 1.2 Hz, 4 days stimulation) of the cultured cells. To characterize cardiac biowires, cardiac markers were immunostained (connexin 43, cTnT, α-actin, F-actin fibres). Such a model was also used for in vitro testing of different compounds. Nitric oxide (NO) and donor sodium nitroprusside (SNP) were investigated in the fabricated platform. The developed microplatform was successfully used for 3D cultures (as biowires) of rat and SC-delivered cardiomyocytes . Such an in vivo-like model can allow a unique opportunity to test drug cytotoxicity as well as to analyse proliferation, interaction and physiology of CMs and SCs. In the future, it can be a good technique not only for study of SC-CMs but also for SC differentiation and analysis of SC influence on heart cell regeneration .
iPSCs are the next kind of stem cells , which are also used to obtain cardiomyocytes . However, iPSCs are the most often differentiated into beating cardiomyocytes (iPSC-CSs) by the usage of biochemial factors and AggreWell or Matrigel-coated plates in macroscale. Next, the beating iPSC-CSs are studied in the microsystems (Bergström et al. 2015; Mathur et al. 2015; Moya et al. 2013). The simulation of a whole cardiovascular system based on iPSC-CMs has been presented by Moya et al. (2013). The geometry of the PDMS/glass microsystem was designed in such a way that a perfusion vascular system was mimicked. It consisted of two fluidic microchannels separated by a central microchannel, in which 12 linear-arranged microchambers were placed (Fig. 9.3a). The beating iPSC-CM organoids were seeded with endothelial colony-forming cells (ECFCs)-derived ECs and normal lung fibroblast in the developed microsystem. A co-culture of the organoids and the cells was performed within the next 28 days. It was observed that during this time a vessel network was created. The obtained 3D model of vascularized cardiac tissue can be used to analyse cardiocytotoxicity of new compounds and heart regeneration using SCs.
a a. Fabricated PDMS-based microfluidic system for 3D cell culture . b. A view of one daisy-chained microchamber. Reprinted from Moya et al. (2013). Open Access. b a. A scheme of introducing cardiac bodies into the microsystem. b. A view of cardiac bodies seeded inside the niches. c. Scheme of the fabricated microfluidic system . Reprinted with permission from Bergström et al. (2015). Copyright 2015 Royal Society of Chemistry. c a. A view of the fabricated microfluidic system with culture microchamber and two-sided microchannels separated by two rows of pillars. b. A confocal fluorescent microscopy of the cardiac microtissue in the microphysiological system (MPS). Inset shows the view of the sarcomeric α-actin (red) and DAPI (blue) staining. Reprinted from Mathur et al. (2015) Open Access
Other microsystems for study of beating iPSC-CMs have been developed in the next years. However, they were mainly used for mimicking of heart functions (not vascularization) and drug cardiotoxicity analysis. Bergström et al. (2015) presented a PDMS-based microfluidic system to analyse cardiotoxicity on cardiac bodies (CBs) derived from iPSCs. The possibility to study the beating of single CB is the main advantage of this research. The microsystem consisted of the main channel connected with two inlets: for CBs seeding and culture medium perfusion (Fig. 9.3b). Ten microchambers used for CB cultures were placed along the main channel. Additionally, each microchamber was connected with three drainage channels. The beating CBs were seeded in the microchambers and incubated with different concentrations of doxorubicin, verapamil and quinidine. In turn, Mathur et al. (2015) presented a cardiac microphysiological system (MPS) with a different geometry. The designed microsystem consisted of a central cell chamber, two- sided media channels and arrays of connecting microchannels (Fig. 9.3c).
3D cardiac cultures of the beating hiPSC-CMs (obtained in macroscale by biochemical stimulation with B27 insulin and WNT inhibitor) were formed in a MPS. Next, the cytotoxicity of four different compounds, i.e. isoproterenol, E-403, verapamil and metoprolol was investigated. It was proven that MPS is an appropriate tool to use for 3D cardiac tissue culture and cytotoxicity analysis. Cell beating frequency was used to investigate cardiotoxicity of drug substances in the microsystems. Because it is the non-invasive method, cardiotoxicity analysis in real-time could be performed.
As it was mentioned before, SC differentiation into CMs is often performed in macroscale and next the SC-delivered CMs are cultured and investigated in Lab-on-a-chip systems. There is one report about SC differentiation into CMs using the biochemical method performed in microscale. Jeon et al. (2014) presented SC differentiation in a PDMS microfluidic system . The geometry of the microsystem is shown in Fig. 9.4. It consisted of a central microchannel for 3D cell culture and two-sided microchannels for media perfusion. Bone marrow-derived human mesenchymal stem cells (BM-hMSC) and vein endothelial cells (HUVECs) mixed with each other and with fibrinogen were cultured in the central channel. To induce vascularisation, the culture medium was supplemented with: 50 ng ml−1 VEGF, 50 ng ml−1 VEGF with 100 ng ml−1 Ang-1 (angiopoietin) or 50 ng ml−1 VEGF with 1 ng ml−1 TGF-β1. Additionally, HUVECs were cultured as a monolayer in the side microchannels. The level of vascularization was monitored by immunostaining α-smooth muscle actin (α-SMA). It was found that, α-SMA in a vascular network significantly increased after the addition of TGF-β1 and Ang-1. The presented microsystem can be successfully used for creation of a 3D functional and a perfusable microvascular network and analysis of SC differentiation. Moreover, this research can have a huge impact on the investigation of heart regeneration in the microsystems .
a A scheme of the microfluidic systems for 3D cell cultures. b A scheme of 3D perfusable microvascular network with endothelial cells (HUVECs) (red) and BM-hMSCs (green) Reprinted with permission from Jeon et al. (2014). Copyright 2014 Royal Society of Chemistry
9.3 Physical Stimulation
Physical cues have been recognized as critical factors during SC differentiation into specific cell types. Structure, topography and elasticity of the biomaterials as well as electrical fields are physical factors which are often used to determine SC fate. These parameters are characterized in the next sections.
9.3.1 Surface and Structural Stimulation
Elasticity/stiffness of the materials may determine, especially in 2D cultures, cell adhesion, morphology and phenotype. It was also proven that the topography of culture surface and extracellular matrix (ECM) influence SC differentiation (Engler et al. 2006; Flaim et al. 2008; Solanki et al. 2010). These factors affect gene and protein expressions which finally improve growth, differentiation and maturation of SCs (Murphy et al. 2014; Pek et al. 2010). Various studies based on surface stiffness and topography (among others on PDMS, polyacrylamide, glass, polystyrene—PS) were performed in macroscale. Surface properties can be changed by micropatterning. In this case, the materials are coated with different proteins (e.g. collagen, poly-L-lysine, fibronectin). So far, studies have shown that micropatterning influences cell morphology, migration, functionality, cytoskeletal structure and nuclear shape.
The combination of surface stimulation (microgrooves) and bioprinting technique is a promising approach to study SC differentiation in CMs. Such a solution has been proposed by Bhuthalingam et al. (2015). Different microgrooves (linear, concentric circles and sinusoidal wave—S-Shaped) were patterned in polystyrene films (Fig. 9.5a). Human MSC suspension was prepared in bioink solution, which contained gelatin dissolved in Dulbecco’s modified Eagle’s medium (DMEM). Then, the cellularized bioink was printed on the polystyrene surfaces containing different grooves. A higher expression of GATA4 (cardiomyocyte marker) was observed on S-shaped patterns than on other geometries. The results showed that the geometry of the microgroove had a high impact on SC fate. Moreover, the bioprinting is a controlled method, which could enhance MSC differentiation . The proposed bioprinting technique can be useful for further investigation of different SC types. Other methods of surface stimulation, presented in the literature, were mainly focused on the study of alignment and maturation of stem cell-derived cardiomyocytes (obtained using biochemical stimulation in macroscale). For example, Salic et al. (2014) tested an increase of sarcomere expression and alignment of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) on 2D micropatterned surfaces with specific geometries (Fig. 9.5b). Surfaces with different rectangular geometries (areas ranging from 2.500 μm2 to 160.000 μm2) were utilized in the experiments. For this purpose, non-adherent poly(ethylene glycol) (PEG) regions were micropatterned and ECM proteins (Matrigel and fibronectin) were put on gold-coated glass slides. Fibronectin-coated microgrooved performed in PDMS were also utilized for investigation of cellular alignment and calcium (Ca2+) cycling of iPSC-CMs (Rao et al. 2013). The scheme of the microgrooved substrate is shown in Fig. 9.5c. It was found that cell alignment and parallel organization of sarcomeres were mainly dependent on the groove width. The above studies also showed that the modified substrates influence Ca2+ cycling in SC-CMs. The proposed highly aligned cell model may be useful in pharmacological studies and understanding how surface geometry influences SC-CMs maturation. In the future, the developed surfaces with microgrooves can be applied to study SC differentiation and heart cell regeneration.
a Polystyrene films with micropatterned grooves: linear (a), sinusoidal wave—S-shaped (b), concentric circles (c) and grooves (g, h). The cells were visualized using fluorescein diacetate (FDA) and DAPI staining (d–f). Reprinted from Bhuthalingam et al. (2015). Open Access. b A 2-day culture (a) and 3-day (b) culture of hESC-CMs seeded onto the micropatterned grooves with different dimensions. Reprinted with permission from Salic et al. (2014). Copyright 2014 Elsevier. c A scheme of a microgrooved flexible scaffold (a). A view of the sarcomeric α-actin (red) and DAPI (blue) immunostaining (b) Reprinted from Rao et al. (2013). Open Access
Dimension and geometry of microstructures also influence the fate of SCs and EBs. An interesting geometry of the microsystem (i.e. tent-like structure) was used to study cardiogenesis (Tanaka and Fujita 2015). A PDMS-based microsystem consisted of a microchannel layer, a microchamber layer with a membrane and a cylindrical block on the membrane (Fig. 9.6). The proposed structure was supposed to perform analysis of cell beating based on periodical oscillation of fluid in the microchannel connected to the membrane. EBs-derived iPSCs were cultured in the microsystem. EBs were attached to a thin PDMS tent-like membrane. Periodical oscillation of fluid in a microchannel and cell beating was observed 14 days after cell seeding. SC differentiation was also proven by immunostaining MHC and cardiac troponin I.
a A scheme of microdevice for iPSC differentiation into cardiomyocytes on the tent-like structure. b Cross-sectional view along line X–Y. c A scheme of method of attaching EBs onto the microchip, which was O2 plasma and gelatin treated. d, f Fluorescent microscopy of anti-cardiac myosin heavy chains antibody (green) and anti-cardiac troponin I antibody (red) immunostaining. Reprinted with permission from Tanaka and Fujita (2015). Copyright 2015 Elsevier
3D polymeric scaffolds, including microspheres, porous forms and nanofibers are also used to culture and differentiate SCs in microscale (Ghafar-Zadeh et al. 2011). Polymer scaffolds next to the hydrogels are physical stimulations, in which no external actuation is used to differentiate SCs (Hoffman 2012; Zuppinger 2016). Nutrients in 3D hydrogel cultures are uniformly distributed to all cells (Annabi et al. 2013; Ghiaasedin et al. 2017). In turn, scaffolds create rigid networks and structures with higher physical stiffness than hydrogels. Functional nanofibers scaffolds produced by electrospinning are meaningful in many SC differentiations. The nanofibers can mimic the native ECM fibres (Alamein et al. 2015; Bianco et al. 2009; Heydarkhan-Hagvall et al. 2008; Liu et al. 2014; Tomecka et al. 2017; Wang et al. 2013). Thanks to this, SC proliferation and interaction with ECM-like nanostructures can be investigated (Luo et al. 2015). For example, collagen (type I)—grafted polyethersulfone (PES) nanofiber matrix was used to culture mouse ESCs. The results showed that ESCs cultured on a PES nanofiber matrix were undifferentiated during the whole culture, whereas cell proliferation was increased. It indicated that a collagen-grafted PES nanofiber matrix can be used to maintain undifferentiated forms of SCs as well as to differentiate SCs under precise control (Hashemi et al. 2011).
Many studies based on regulation of SC fate on nanofiber scaffolds have been discussed in the literature. Nanofibers were utilized to induce SC differentiation into, e.g. neural cells, chondrogenic cells and osteoblasts (Li et al. 2012; Smith et al. 2010). 3D-shaped polymeric scaffolds are also used for SC differentiation into CMs. Ghasemi-Mobarakeh et al. (2014) stimulated EBs delivered from ESCs on Polycaprolactone (PCL)/gelatin nanofibrous scaffolds for 5 days. The presence of CMs was determined by immunostaining cardiac markers (α-sarcomeric actin and conexin43) and cell beating. EB differentiation into CMs was also tested on collagen/Matrigel scaffolds (Zhou et al. 2010). It was found that mouse ESCs seeded onto 3D scaffolds, aggregated and formed EBs. To induce cardiac differentiation, 0.1 mg/ml ascorbic acid was supplemented into the culture medium for 7 days. Beating CMs were observed 7 days after EB seeding; however, synchronous contraction was noticed on the 19th day of culture. Cardiac markers such as cTnT, anti-cytokeratin 18 antibody (CK18), anti-murine antibody (CD31) and nestin were determined to prove SC differentiation into CMs. The obtained results showed that collagen/Matrigel scaffolds can be successfully used to create EBs and ESCs differentiation into CMs. PCL/gelatin and collagen/Matrigel scaffolds can also be useful to investigate the mechanism of SC differentiation and human myocardium regeneration after infarction. It should be noted that the scaffold composition can have an important role in CS differentiation. A new approach was proposed by Yang et al. (2016). They tested how tunnelling nanotubes (TNTs) integrated with a PDMS biochip influences stem cell and cardiomyocyte communication. A novel biological process, unidirectional mitochondrial transfer, mediated by heterotypic TNT connections was discovered. These results could be a base for further research of cardiomyocyte regeneration using SCs.
The next technique, which enables 3D culture of the cells, is the microsphere method. Microspheres can be used to precisely deliver SCs into the damaged tissue. Moreover, growth factors and therapeutic molecules can be placed inside the microspheres. The usage of encapsulated and differentiated SCs can be an effective method for CVD treatment. Trimethyl ammonium-coated polystyrene microspheres were used for 3D culture and differentiation of human ESCs (Phillips et al. 2008). ESCs have the capacity to differentiate towards pancreatic, neuronal and cardiomyocyte cells. The utility of microspheres in CVD treatment is also studied in vivo. Microspheres made of alginate were used for encapsulation of human MSCs and transplantation into rats with ischaemia-reperfusion myocardial infarction (Yu et al. 2010). Alginate was used as a non-toxic and semi-permeable material. The alginate microspheres with the encapsulated MSCs are shown in Fig. 9.7. Properties of the fabricated microspheres improve cell attachment and growth on the injured heart. The effects of treatment after microsphere introduction into the rats were investigated by echocardiography and immunostaining of the histopathological preparation. The results showed that the encapsulated MSCs into microspheres induced angiogenesis and heart regeneration Regeneration .
Human mesenchymal stem cells two days after encapsulation into alginate microbeads. Magnification 4× (a) and 10× (b). Reprinted with permission from Yu et al. (2010). Copyright 2010 Elsevier
9.3.2 Electrical Stimulation
Electrical field can play an important role in many biological and medical applications. The tests to prove the usage of electrical field for the treatment of various diseases are also performed. The mechanisms of electrical stimulation have been investigated for many years and so far they are not understood in many cases (Ghafar-Zadeh et al. 2011). A knowledge of electrical field functions for heart stimulation could have an important meaning for the study of heart properties under normal and pathological conditions. Many reports based on electrical stimulations in macroscale have been presented in the literature (Barash et al. 2010; Kujala et al. 2012; Maidhof et al. 2012). Microtechnology is investigated as an effective method for electrical manipulation of SCs and differentiation of SCs into cardiac cells. Electrodes used for stimulation of SCs in the microsystems are fabricated from the same materials such electrodes for CM stimulation (see Chap. 8).
The type of electrical signal, cell culture (single cell, monolayer and 3D cultures) and a culture system have an influence on SC differentiation into cardiac cells (Tandon et al. 2009). A chamber with shielded electrodes, flasks and dishes integrated with electrodes and customized microchamber can be utilized for electrical stimulations. SC differentiation after electrical field action can be determined by analysing contractile cell activity, cell elongation, cell morphology, the force of cell contraction, electrical cell activity and gene expression. The parameters which can influence electrical stimulation for cardiac tissue engineering are shown in Fig. 9.8.
Overview of the parameters which can influence electrical stimulation during stem cell differentiation into cardiomyocytes including: a scale (single cell (i), monolayer (ii), 3D culture(iii)), b the electrical signal monophasic pulses (i), charge-balanced biphasic pulses (ii), charge-balanced biphasic pulses with interphase delay (iii) charge-balanced biphasic pulses with slow reversal (iv), direct current (v), c the culture system (a chamber with shielded electrodes (i), a T-flask (ii), a Petri-dish (iii), a customized chamber (iv) and d the analytics performed to evaluate contractile activity (i), elongation (ii), force of concretion (iii), electrical activity (iv), morphology (v) and gene expression (vi) Reprinted with permission from Tandon et al. (2009). Copyright 2009 Nature Publishing Group
Biphasic square pulses have most often been reported as electrical signal used for SC differentiation into CMs. For example, Pavesi et al. (2015) developed a PDMS-based microdevice useful for mechanical and electrical stimulation of human BM-MSCs. The microdevice consisted of three layers: a pneumatic layer for mechanical stimulation, a fluidic layer for the SC culture and a conductive layer for electrical stimulation (Fig. 9.9a). An interesting technique was used to fabricate the conductive layer. A mixture of carbon nanotubes (CNTs) and PDMS was deposited on the silicon wafer. Biphasic square pulses (5 V cm−1, 1 Hz, 1 ms) were generated 24 h after SC seeding in the microdevice. An efficiency of human BM-MSC differentiation into CMs was evaluated on the 14th day of the culture. For this purpose, specific cardiac markers (MYH7, Nkx2.5, cTnT, MEF2C—myocyte enhancer factor 2C and TUBB—tubulin beta) were defined using quantitative real-time polymerase chain reactions (qRT-PCR). High expression of the markers mentioned above was noticed. Not only biphasic but also monophasic square pulses were utilized for SC differentiation in microscale. An example of a PDMS-based microdevice used for electrical stimulation of SCs using monophasic square pulses is shown in Fig. 9.9b (Tandon et al. 2010). It was found that such stimulation enhanced SC proliferation, elongation and perpendicular orientation to the electrodes.
a A scheme of the microfluidic platform containing central channel (red, to provide culture medium), the pneumatic channels (light blue, to perform mechanical stimulation) and the electrical layer with two conductive regions composed of carbon nanotubes and polydimethylosiloxane (light grey). Red arrows represent the electric field. Reprinted from Pavesi et al. (2015). Open Access. b Views of the slide with electrode arrays (a) and polydimethylosiloxane layer with two culture wells (b) Reprinted with permission from Tandon et al. (2009). Copyright 2009 Royal Society of Chemistry. c A view of the polydimethylosiloxane-based bioreactor for electrical stimulation with 16 culture wells. Reprinted with permission from Serena et al. (2009). Copyright 2009 Elsevier. d A scheme of the microfluidic system . Stem cells (pink spheres) were laser-patterned to form a bridge connecting two separated muscle fibres (green). Reprinted with permission from Ma et al. (2012). Copyright 2011 Royal Society of Chemistry
They can be fabricated using some wires/rods (Serena et al. 2009; Thavandiran et al. 2013) or planar electrodes patterned on the substrate (Tandon et al. 2010; Zhou et al. 2016). A PDMS micro-bioreactor with 4 × 4 wells was integrated with electrodes made of different rods is shown in Fig. 9.9c (Serena et al. 2009). 10 cm × 1.3 mm diameter 304 stainless steel, titanium and titanium nitride-coated titanium rods were integrated with three independent microdevices. Such microsystems were utilized for EB differentiation studies. As it was mentioned before, the mechanisms responsible for electrical field-induced therapy are still not fully known. Serena et al. (2009) investigated that the intracellular reactive oxygen species (ROS) could take part in SC differentiation into CMs. Therefore, the effect of H2O2 (hydrogen peroxide) on the SCs was additionally investigated to prove this hypothesis. It was found that stainless steel electrodes induced the highest cardiac differentiation . These electrodes and 1 nM of H2O2 generated a comparable ROS level. Based on these results, the authors proved that electrical stimulation can differentiate SCs into CMs based on mechanisms associated with intracellular ROS generation.
A planar microelectrode array technology (MEA) with integrated recording and stimulation electrodes is also used to stimulate cells (Chen et al. 2009). MEA platforms enable precisely localized current to be injected into the cell culture and differentiated cell properties to be detected in the same culture chamber. A potential impact in regenerative medicine could have a MEA-based microsystem presented by Ma et al. (2012). MEA-based biochip was utilized to mimic the cardiac model. First, rat CMs were seeded on a biochip to form muscle fibres. After 4 days, small gaps (120 μm length) were created to mimic a myocardial infarction. Next, rat mesenchymal stem cells from bone marrow (rMSCs-BM) were laser-patterned into the gaps to create a bridge between cardiomyocytes and rMSCs-MB (Fig. 9.9d). Fibroblasts laser-patterned in the gaps were used as control samples. The electrical conductivity of SCs and fibroblasts was determined using MEA. Additionally, cardiac markers were immunostained (sarcomeric α-actin and connexin 43). It was found that SCs-CMs bridges showed higher and more stable conduction through the gap junction than CMs-fibroblasts interactions. The above results proved that SCs can be a promising method for heart regeneration, and the proposed microchip can be successfully used for cell monitoring using MEA.
The electrodes integrated with the microsystems are used not only for stimulation but also for electrical conduction monitoring. MEA and electrical impedance spectroscopy (EIS) are used to measure SCs after their differentiations (Ma et al. 2012; Zhou et al. 2016). Zhou et al. (2016) presented a microsystem for quantitative analysis of the changes in electrical parameters of mouse ESCs at different stages of differentiation . A 0 h—undifferentiated state, 24 h—transition state and 48 h fully-differentiated state of SCs were investigated in the microsystem. EIS was used as non-invasive, label-free and real-time analysis of cell conditions based on their dielectric properties and biological structure. The microfluidic device composed of PDMS, and glass with patterned titanium and gold electrodes was used for hydrodynamic trapping of single mouse ESC (Fig. 9.10a). The differences between SC stage and magnitude of the cell impedance were noticed. Cell impedance increased with an increase in cell size and SC differentiation state. A label-free cell cytometry can also be used to distinguish differentiated and undifferentiated SCs (Fig. 9.10b). For this purpose, a microsystem consisting of a PDMS layer and a glass slide integrated with two platinum electrodes and a Si3N4 passivation layer was tested (Myers et al. 2013). Undifferentiated human iPSC- and human iPSC-derived CMs were successfully distinguished based on the recording of extracellular field potential (FP) signals from suspended cells in flow. Such a technique can be useful for investigating differentiation stages of SCs and for optimizing SC stimulation parameters (Table 9.2).
a A view of the fabricated microsystem (a). Three-dimensional scheme of the trapping channels (grey) and electrodes (yellow) (b). Reprinted from Zhou et al. (2016). Open Access. b A scheme of the electrophysiology-activated cell cytometry system composed of microfluidic flow chamber integrated with microelectrode and the measurement simulation, Reprinted with permission from Myers et al. (2013). Copyright 2012 Royal Society of Chemistry
9.4 Mechanical Stimulation
9.4.1 Mechanical Strain
Cardiac cells are continuously exposed to a variety of mechanical stimulations under native conditions, i.e. action of muscle forces, gravity or blood flow. Moreover, the interactions between the cells are crucial for their stretching (Gupta et al. 2010). The above-mentioned forces regulate cellular physiology and functions. Therefore, mechanical forces are mimicked in the experiments performed in vitro. Thanks to this, cardiac cells can be cultured under conditions similar to in vivo. It was proven that mechanical stimulations can enhance SC differentiation into CMs. Cell stretching has been simulated both in macroscale and the microsystems (Gwak et al. 2008; Pavesi et al. 2015; Ruan et al. 2015; Simmons et al. 2012). 2D and 3D mechanical strains have been reported in the literature (Marsano et al. 2016; Mummery et al. 2012; Pavesi et al. 2015; Shimko and Claycomb 2008). A deformable and elastic substrate (membrane) is exposed to 2D strains with controllable magnitude and frequency. 2D strain can be uniaxial (exerted along one axis), biaxial (exerted in two directions) and equiaxial (exerted in all directions). Air pressure microchannels are most often used to deform a thin membrane, on which cells are cultured. SCs 2D or 3D cultured on elastic membranes can be exposed on cyclic stretch. Range value during the stimulation equalled 5–20% strain, 1–3 Hz. The cells were often stimulated for 1 or 2 days (Clause et al. 2009; Kurpinski et al. 2006; Park et al. 2004). However, long-strain stimulation (7 days or 2 weeks) was also investigated (Gwak et al. 2008; Shimko and Calycomb 2008; Zimmerman et al. 2006).
Marsano et al. (2016) designed a Heart-on-a-chip platform to generate mature and highly functional micro-engineered cardiac tissues (μECTs). The microsystem was composed of three PDMS-based layers. The top and bottom layers had two rows of micropillars, which created a central microchannel and two-sided microchannels. The top and bottom PDMS layers were separated by a thin PDMS membrane. Cyclic uniaxial strains (10–15%) were generated in the microsystem because of their integration with an electronically controlled pressure regulator system. 3D culture of neonatal rat CMs and human iPSC-CMs suspended in a fibrin gel matrix were performed in a culture microchannel. Stretching of 3D cultured cells was monitored using a microscope and analysed based on ImageJ software. Immunofluorescence staining was used to detect cardiac markers: troponin I, connexin43 and sarcomeric α-actin. It was found that 5 days after cell injection, expression of cardiac markers increased. The fabricated microsystem enabled not only generation of 3D cardiac microtissue but also controllable mechanical stimulation and quantitative analysis of cell culture .
The mechanical strain with the usage of additional factors can enhance cardiac differentiation of SCs. Different factors (e.g. biochemical factors, electrical fields and mechanical stimulations) are often simultaneously used to precisely control the fate of SCs in the microsystems. Surprising results were obtained in a hybrid PDMS-hydrogel microfluidic platform fabricated by (Wan et al. 2011). They used both biochemical stimulation and controllable uniaxial cyclic stretching for induction of cardiogenesis. A 4-day cultured EBs were stretched at 10% strain and 1 Hz frequency for 24 h. Expression of α-MHC was analysed to evaluate the effectiveness of SC differentiation. Surprisingly, the expression of α-MHC was lower after uniaxial cyclic stretching. This indicated that mechanical stimulation can be stopped and reduced the SC differentiation into CSs. The usage of an additional factor most often enhances cardiogenesis. However, cell type, kind of stimulation and value of the used parameters have impact on a degree of SC differentiation. Therefore, such a type of research should still be performed.
It should be noted that a microsystem for SC culture under fast changes in gas partial pressure and cyclic stretching was also developed (Campillo et al. 2016). The PDMS-based microsystem consists of a thin membrane for rat bone marrow-derived mesenchymal stem cell (BM-MSC) culture. Such culture was exposured to hypoxia conditions and cyclic stretch. The proposed microsystem can be a useful tool for investigation of SC response to hypoxia and stretch. Moreover, it has promising application in regenerative and personalized medicine in the future.
9.4.2 Shear Stress
The effect of shear stress on endothelial cells (ECs) and CMs has been investigated by many research groups. Although SC fate under exposure to shear stress has not been very well investigated, there is research being performed with conventional methods and the microfluidic systems. Shear stress in macroscale was successfully used to differentiate SCs into the cells of the cardiovascular system: CMs (Huang et al. 2010), vascular smooth muscle cells (Huang et al. 2005) and endothelial cells (Metallo et al. 2008; Wu et al. 2008). Microscale allows for a creation of controllable flow of culture medium and other biochemical factors. Thanks to this, SC fate can be investigated under controllable conditions. Due to the fact that the microstructures with various dimensions can be developed, different values of shear stress can be exposed to SCs. The effect of shear stress on various SCs was investigated in the microfluidic systems (Jeon et al. 2014; Kang et al. 2010; Toh and Voldman 2010; Xiao et al. 2014). A shear stress value in the range of 1–20 dyn cm−1 was utilized to stimulate cells. SC differentiation into CMs was investigated in a simple geometry of a microsystem (Villa-Diaz et al. 2009). For this purpose, human ESCs were cultured under dynamic and static conditions in the microfluidic system for 48 h. The PDMS-based microsystem consisted of three inlet microchannels, which merge in a main cell culture microchannel (Fig. 9.11a).
a A scheme of the designed microfluidic system with three inlets microchannels converting in the cell culture microchannel (a) A view of the fabricated microsystem (b) Reprinted with permission from Villa-Diaz et al. (2009). Copyright 2009 Royal Society of Chemistry. b Scheme of the micro-bioreactor array, containing 12 independent cell culture microwells (a). A scheme of the two configuration used in the experiments: BIO (a bottom inlet/outlet) and MIO (a middle inlet/outlet). While BIO and MIO configuration allowed for two-dimensional cell culture , the usage of hydrogel in MIO configuration allowed three-dimensional cell culture (b). Reprinted with permission from Figallo et al. (2007). Copyright 2007 Royal Society of Chemistry
In this case, non-significant differences were observed between static and perfusion conditions. It should be noted that the type of cell culture and value of shear stress have been crucial in SC differentiation into cardiac cells. Figallo et al. (2007) presented a PDMS micro-bioreactor array (MBA) for study SC differentiation based on culture and conditions types. The microsystem consisted of twelve independent culture wells (Fig. 9.11b). The MBA platform enabled both 2D culture (the cells attached to the substrate) and 3D culture (the cells were encapsulated in a hydrogel) of human ESCs. Additionally, two types of experiments were performed in this microsystem (a) BIO configuration—culture medium flowed directly over the attached cells (b) MIO configuration—a culture medium flows above the main chamber with the cells cultured in a monolayer (2D) or encapsulated in hydrogel (3D). hESC differentiation was investigated in this microsystem under both static and dynamic conditions for 4 days. To induce differentiation , hESCs were additionally cultured with a medium containing human VEGF. It was noticed that MIO configuration more closely imitated the native environment and reduced hydrodynamic stress. Cardiac differentiation was evaluated by immunostaining α-SMA. It was noticed that higher shear stress influences higher vascular differentiation .
9.5 Challenges
There are other methods, which can be applied in the microfluidic systems to differentiate SCs into CMs. It was proven that optical, magnetic, ultrasonic and thermal stimulations can influence the fate of SCs and maturation (Guess et al. 2014; Jenkins et al. 2010; Lucchetta et al. 2005; Oberti et al. 2010). However, these methods were initially tested in macroscale. Therefore, there are many issues, which have to be deeply investigated before using these methods for cardiogenesis in microscale. The usage of graphene and their forms for differentiation is present in the literature as a promising technique in regeneration therapy. Biological studies based on graphene (G) and their forms: graphene oxide (GO) and its reduced form (rGO) have been rapidly growing in the last few years (Charlier et al. 2008; Sun et al. 2008). Graphene and rGO are hydrophobic, not very soluble in water and also require surfactants or surface modifications for biological applications. GO is hydrophilic and can be dispersed in water to form stable colloids (Zhang et al. 2010). Graphene, GO and rGO can be used in gene therapy and photodynamic therapy, especially as drug carriers (Matteini et al. 2014). Moreover, graphene materials have mainly been explored for a construction of matrices and biosensor components in tissue engineering . Study of the cytotoxicity and the influence of graphene and its form to differentiate SCs is a relatively new area of research. There are several reports describing the process of SC differentiation using graphene and GO. These materials were often used to differentiate MSCs into cell lineages such as adipocytes, osteoblasts and chondrocytes (Bitounis et al. 2013; Yoon et al. 2014). However, in these studies, graphene or GO was used to modify the surface of cell growth to improve cell attachment to the culture surface, proliferation and differentiation. Additional factors, most often biochemical, were used for differentiation . Lee et al. investigated the effects of G and GO substrates on the adipogenic and osteogenic differentiation of MSCs (Lee et al. 2011a, b). In order to determine the role of G and GO in differentiation and proliferation, MSCs were seeded on PDMS coated with graphene and GO and PDMS layers with the culture medium. They noticed that MSCs seeded on PDMS were round and poorly attached as compared to G and GO. Although GO is hydrophilic like PDMS, due to the presence of oxygenated groups, it can bind to serum proteins via electrostatic interactions. Moreover, it was proved that MSCs is differentiated into osteoblasts at low cell density (3000 cells per cm2) in a medium with ascorbate (0.2 mM), dexamethasone (10−8 M) and β—glycerolphosphate (10 mM). After twelve days of osteogenic induction, a 7-fold increase in the extent of mineralization in the MSCs cultured on G compared to those cultured on PDMS has been proven (Lee et al. 2011a, b). There are also reports based on graphene differentiation of SCs into CMs. Lee et al. reported that graphene at least partially enhances cardiomyogenic differentiation of hESC. (Lee et al. 2014) hESC cultures were performed on: (1) Matrigel-coated glass, (2) Vitronectin (VN)-coated glass and (3) VN-coated graphene for 21 days. In this study, cardiac mesodermal gene expressions were determined by a qRT-PCR assay. The total RNA was extracted from the differentiated hESCs on days 4, 7, 14 and 21 and reverse-transcribed into cDNA. On the 14th day, it was noted that the graphene group showed an increase in the cardiac mesodermal gene (MESP1) and mesodermal gene (T and M-CAD) expression compared to the glass and Matrigel groups. Moreover, higher gene expression of cardiomyogenic proteins (α-MHC, β-MHC, MLC2a, cTnT), transcriptional factors (NKX2-5, GATA4, and MEF2C) and gap junction proteins (Connexin43) in the graphene group than the Matrigel and the glass groups at 14th and 21st days was observed.
Apart from graphene, GO was studied in adhesion, proliferation and differentiation of mouse myoblasts —C2C12 cells (Ku and Park 2013). The authors have proven that myogenic differentiation was enhanced by GO, as evidenced by the analysis of myogenic protein expression, multinucleate myotube formation and the expression of differentiation genes (MyoD, myogenin, Troponin T and MHC). According to the results, myoblasts grew well on the graphene oxide surface. The morphology of the cells was analysed by cytoskeleton staining after a 1-day culture. Real-time PCR confirmed that gene expression levels were the highest on GO sheets. Although there are reports describing the usage of graphene and GO to SC differentiation into SMs, the experiments in microscale are not performed. So far, the investigation has shown that graphene and its forms can be combined with PDMS—the most commonly used material for Lab-on-a-chip system fabrication. Thus, previous research has indicated that the studies based on graphene and its forms in microscale will be a promising method for investigation of SC differentiation into cardiac cells.
9.6 Summary and Perspectives
Regenerative medicine is a promising method, which can be used to treat CVDs. Although advanced research based on heart regeneration is being performed, it needs to be investigated further. The Lab-on-a-chip systems are fabricated to obtain conditions similar to the native environment. The microsystems are used to differentiate SCs using biochemical, physical (electrical, structural, surface) and mechanical (strain, shear stress) factors. However, the SC differentiation is most often started in macroscale (for example using biochemical factors) and next the cells are additionally stimulated in microscale. It should be noted that stem cells -derived cardiomyocytes (SC-CMs) are most often differentiated in the microsystems. There are few studies performed in the microchips, in which SC types, e.g. ESCs, MSCs and iPSCs were stimulated. This seems to be important to investigate how full microscale conditions influence cardiogenesis of SCs.
There are key signalling parameters, which enhance cell growth and enable the mimicking of native myocardium in the microsystems . They are: dynamic conditions, stretching, spatial and parallel cell arrangement (by the usage of hydrogels, scaffolds or nanofibers) and electrical field. To obtain a highly effective cardiogenesis, the above-mentioned features should be provided in the microsystems dedicated for SC culture. It should be noted that SC research is most often performed on 2D than 3D cultures. On the other hand, it is known that spatial and parallel cell arrangement enhance the expression of cardiac markers. Therefore, to obtain a high efficient of cardiogenesis, three-dimensional SC culture should be maintained in the microsystems . Thanks to this, a native myocardium could be mimicked in SC culture model and enhance SC differentiation into CMs more than in research performed so far. A fully functionalized microsystem mimicking heart features is a perspective of cardiac culture microtechnologies based on stem cells . Moreover, there are only a few studies based on CVD mimicking and CM regeneration . Therefore, a diseased heart model maintained with a defined vascular system seems to be important to test cell regeneration by the usage of SCs. The optimized CM/SC model could be used to investigate SC differentiation using techniques potentially useful for cardiogenesis (e.g. graphene, magnetic and thermal stimulations). The combination of advanced microfluidic technologies with a diseased heart model and SC differentiation methods would become highly crucial Heart-on-a-chip systems for personalized medicine and therapeutic.
References
Alamein AM, Wolvetag EJ, Ovchinnikov DA, Stephens S, Sanders K, Warnke PH (2015) Polymeric nanofibrous substrates stimulate pluripotent stem cells to form three-dimensional multilayered patty-like spheroids in feeder-free culture and maintain their pluripotency. J Tissue Eng Regen Med 9:1078–1083
Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, Weiss AS (2013) Highly elastic micropatterned hydrogel for engineering functional cardiac tissue. Adv Funct Mater 23:4950–4959
Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S (2010) Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods 16:1417–1426
Bergström G, Christoffersson J, Schwanke K, Zweigerdt R, Mandenius CF (2015) Stem cell derived in vivo-like human cardiac bodies in a microfluidic device for toxicity testing by beating frequency imaging. Lab Chip 15:3242–3249
Bhuthalingam R, Lim PQ, Irvine SA, Agrawal A, Mhaisalkar PS, An J, Chua CK, Venkatraman S (2015) A novel 3D printing method for cell alignment and differentiation. Int J Bioprinting 1:57–65
Bianco A, Di Federico E, Moscatelli I, Camaioni A, Armentano I, Campagnolo L, Dottori M, Kenny JM, Siracusa G, Gusmano G (2009) Electrospun poly(e-prolactone)/Ca-deficient hydroxyapatite nanohybrids: microstructure, mechanical properties and cell response by murine embryonic stem cells. Mat Sci Eng C 29:2063–2071
Bitounis D, Ali-Boucetta H, Hong BH, Min D-H, Kostarelos K (2013) Prospects and challenges of graphene in biomedical applications. Adv Funct Mater 25:2258–2268
Campillo N, Jorba I, Schaedel L, Casals B, Gozal D, Farré R, Almendros I, Navajas D (2016) A novel chip for cyclic stretch and intermittent hypoxia cell exposures mimicking obstructive sleep apnea. Front Physiol 7: 319_1–319_12
Charlier JC, Eklund PC, Zhu J, Ferrari AC (2008) Electron and phonon properties of graphene: their relationship with carbon nanotubes. In: Jorio A, Dresselhaus G, Dresselhaus MS (eds) Carbon nanotubes. Topics in applied physics, vol 111. Springer: Berlin, Heidelberg, p 673–709
Chen MQ, Xie X, Wilson KD, Sun N, Wu JC, Giovangrandi L, Kovacs GT (2009) Current-controlled electrical point-source stimulation of embryonic stem cells. Cell Mol Bioeng 2:625–635
Cheng J, Ding Q, Wang J, Deng L, Yang L, Tao L, Lei H, Lu S (2016) 5-azacytidine delivered by mesoporous silica nanoparticles regulates the differentiation of P19 cell into cardiomyocytes. Nanoscale 8:2011–2021
Clause KC, Tinney JP, Liu LJ, Keller BB, Tobita K (2009) Engineered early embryonic cardiac tissue increases cardiomyocyte proliferation by cyclic mechanical stretch via p38-MAP kinase phosphorylation. Tissue Eng Part A 15:1373–1380
Dimarakis I, Levicar N, Nihoyannopoulos P, Hbib NA, Gordon MY (2006) In vitro stem cell differentiation into cardiomyocytes: Part 1. Culture medium and growth factors. JCRR 1:107–114
Discher DE, Mooney DJ, Zandstra PW (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–1677
Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T (2015) Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotech 35:118–126
Emmert MY, Hitchcock RW, Hoerstrup SP (2014) Cell therapy, 3D culture systems and tissue engineering for cardiac regeneration. Adv Drug Deliver Rev 69:254–269
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689
Ertl P, Sticker D, Charwat V, Kasper C, Lepperdinger G (2014) Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol 32:245–253
Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, Elvassore N, Vunjak-Novakovic G (2007) Micro-bioreactor array for controlling cellular microenvironments. Lab Chip 7:710–719
Flaim CJ, Teng D, Chien S, Bhatia SN (2008) Combinatorial signalling microenvironments for studying stem cell fate. Stem Cell Dev 17:29–39
Geuss LR, Wu DC, Ramamoorthy D, Alford CD, Suggs LJ (2014) Paramagnetic beads and magnetically mediated strain enhance cardiomyogenesis in mouse embryoid bodies. PLoS ONE 9:1–20
Ghafar-Zadeh E, Waldeisen JR, Le LP (2011) Engineered approaches to the stem cell microenvironment for cardiac tissue regeneration. Lab Chip 11:3031–3048
Ghasemi-Mobarakeh L, Prabhakaran MP, Nematollahi M, Karbalaie K, Ramakrishna S, Nasr-Esfahani MH (2014) Embryonic stem cells differentiation to cardiomyocytes on nanostructured scaffolds for myocardial tissue regeneration. Int J Polym Mater 63:240–245
Ghiaseddin A, Pouri H, Soleimani M, Vasheghani-Farahani E, Ahmadi Tafti H, Hashemi-Najafabadi S (2017) Cell laden hydrogel construct on-a-chip for mimicry of cardiac tissue in-vitro study. Biochem Biophys Res Commun 484:225–230
Goβmann M, Frotscher R, Linder P, Neumann S, Bayer R, Epple M, Staat M, (Temiz) Artmann A, Artmann GM (2016) Mechano-pharmacological characterisation of cardiomyocytes derived from human induced pluripotent stem cells. Cell Physiol Biochem 38:1182–1198
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtje W, Chen CS (2009) Control stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5:17–26
Gupta K, Kim D-H, Ellison D, Smith C, Kundu A, Tuan J, Suh KY, Levchenko A (2010) Lab-on-a-chip devices as an emerging platform for stem cell biology. Lab Chip 10:2019–2031
Gwak S-J, Bhang SH, Kim I-K, Kim S-S, Cho S-W, Jeon O, Yoo KJ, Putnam AJ, Kim B-S (2008) The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials 29:844–856
Hashemi SM, Soudi S, Shabani I, Naderi M, Soleimani M (2011) The promotion of stemness and pluripotency following feeder-free culture of embryonic stem cells on collagen-grafted 3-dimensional nanofibrous scaffold. Biomaterials 32:7363–7374
Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygiu RE, MacLellan WR (2008) Three-dimentional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 29:2907–2914
Higuchi A, Kumar SS, Ling QD, Alarfaj AA, Munusamy MA, Murugan K, Hsu S-T, Benelli G, Umezawa A (2017) Polymeric design of cell culture materials that guide the differentiation of human pluripotent stem cells. Prog Polym Sci 65:83–126
Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE (2015) HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349:644–654
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Deliv Rev 64:18–23
Huang A, Sun D, Jcobson A, Carroll MA, Falck JR, Kaley G (2005) Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarisation of arteriolar smooth muscle. Circ Res 96:376–383
Huang Y, Jia X, Bai X, Gong X, Fan Y (2010) Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch Med Res 41:497–505
Hwang NS, Varghese S, Elisseeff J (2008) Controlled differentiation of stem cells. Adv Drug Deliv Rev 60:199–214
Jastrzebska E, Tomecka E, Jesion I (2016) Heart-on-a-chip based on stem cell biology. Biosens Bioelectron 75:67–81
Jenkins MW, Duke AR, Gu S, Chiel HJ, Fujioka H, Watanabe M, Jansen ED, Rollins AM (2010) Optical pacing of the embryonic heart. Nat Photonics 4:623–626
Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD (2014) Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic system. Integr Biol 6:555–563
Ju X, Li D, Gao N, Shi Q, Hou H (2008) Hepatogenic differentiation of mesenchymal stem cells using microfluidic chips. Biotechnology 3:383–391
Kang E, Choi YY, Jun Y, Chung BG, Lee SH (2010) Development of a multi-layer microfluidic array chip to culture and replate uniform-sized embryoid bodies without manual cell retrieval. Lab Chip 10:2651–2654
Kawai T, Takahashi T, Esaki M, Ushikoshi H, Nagano S, Fujiwara H, Kosai K (2004) Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenic protein 2. Circ J 68:691–702
Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y (2012) Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126:29–37
Kim KM, Choi YJ, Hwang J-H, Kim AR, Cho HJ, Hwang ES, Park JY, Lee S-H, Hong J-H (2014) Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS ONE 9:e92427-1–e92427-9
Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC (2004) Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells 22:1239–1245
Ku SH, Park CB (2013) Myoblast differentiation on graphene oxide. Biomaterials 34:2017–2023
Kujala K, Ahola A, Hyttinen J, Kerkela E, Aalto-Setala K (2012) Electrical field stimulation with a novel platform: effect on cardiomyocyte gene expression but not on orientation. Int J Biomed Sci 8:109–120
Kumar D, Sun B (2005) Transforming growth factor-beta2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem Biophys Res Commun 332:135–141
Kurpinski K, Chu J, Hashi C, Li S (2006) Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA 103:16095–16100
Lee JM, Kim J, Kang E, Lee SH, Chung BG (2011a) An integrated microfluidic culture device to regulate endothelial cell differentiation from embryonic stem cells. Electrophoresis 32:3133–3137
Lee WC, Lim CHYX, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP (2011b) Origin enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 5:7334
Lee T-J, Park S, Bhang SH, Yoon J-K, Jo I, Jeong G-J, Hong BH, Kim B-S (2014) Graphene enhances the cardiomyogenic differentiation of human embryonic stem cells. Biochem Bioph Res Co 452:174–180
Li Q, Cheung WH, Chow KL, Ellis-Behnke RG, Chau Y (2012) Factorial analysis of adaptable properties of self-assembling peptide matrix on cellular proliferation and neuronal differentiation of pluripotent embryonic carcinoma. Nanomedicine 8:748–756
Liu L, Yoshioka M, Nakajima M, Ogasawara A, Liu J, Hasegawa K, Li S, Zou J, Nakatsuji N, Kamei K, Chen Y (2014) Nanofibrous gelatin substrates for long-term expansion of human pluripotent stem cells. Biomaterials 35:6259–6267
Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF (2005) Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature 434:1134–1138
Luo Y, Shen H, Fang Y, Cao Y, Huang J, Zhang M, Dai J, Shi X, Zhang Z (2015) Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on grapheme oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl Mater Interfaces 7:6331–6339
Lutolf MP, Blau HM (2009) Artificial stem cell niches. Adv Mater 21:3255–3268
Ma Z, Liu Q, Liu H, Yang H, Yun JX, Eisenberg C, Borg TK, Xu M, Gao BZ (2012) Laser-patterned stem-cell bridges in a cardiac muscle model for on-chip electrical conductivity analyses. Lab Chip 12:566–573
Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G (2012) Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med 6:e12–e23
Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M (2016) Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissue. Lab Chip 16:599–610
Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE (2015) Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 5:1–7
Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE (2016) In vitro cardiac tissue models: current status and future prospects. Adv Drug Deliv Rev 15:203–213
Matteini P, Tatini F, Cavigli L, Ottaviano S, Ghini G, Pini R (2014) Graphene as a photothermal switch for controlled drug release. Nanoscale 6:7947–7953
Metallo CM, Vodyanik MA, de Pablo JJ, Slukvin II, Palecek SP (2008) The response of human embryonic stem cell-derived endothelial cells to shear stress. Biotechnol Bioeng 100:830–837
Miyamoto D, Ohno K, Hara T, Koga H, Nakazawa K (2016) Effect of separation distance on the growth and differentiation of mouse embryoid bodies in micropatterned cultures. J Biosci Bioeng 121:105–110
Mohr JC, de Pablo JJ, Palecek SP (2006) 3-D microwell culture of human embryonic stem cells. Biomaterials 27:6032–6042
Moya M, Tran D, George SC (2013) An integrated in vitro model of perfused tumor and cardiac tissue. Stem Cell Res Ther 4:S15-1–S15-6
Mummery CI, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ (2012) Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 111:344–358
Murphy WL, McDevitt TC, Engler AJ (2014) Materials as stem cell regulators. Nat Mater 13:547–557
Myers FB, Zarins CK, Abilez OJ, Lee LP (2013) Label-free electrophysiological cytometry for stem cell-derived cardiomyocyte cluster. Lab Chip 13:220–228
Ni XF, Crozatier C, Sensebe L, Langonne A, Wang L, Fan Y, He PG, Chen Y (2008) On-chip differentiation of human mesenchymal stem cells into adipocytes. Microelectron Eng 85:1330–1333
Oberti S, Möller D, Neild A, Dual J, Beyeler F, Nelson BJ, Gutmann S (2010) Strategies for single particle manipulation using acoustic and flow fields. Ultrasonics 50:247–257
Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S (2004) Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng 88:356–368
Park JY, Takayama S, Lee S-H (2010) Regulating microenvironmental stimuli for stem cells and cancer cells using microsystems. Integr Biol 2:229–240
Pavesi A, Adriani G, Rasponi M, Zervantonakis IK, Fiore GB, Kamm RD (2015) Controlled electromechanical cell stimulation on-a-chip. Sci Rep 5:1–12
Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, Kumacheva E, Zandstra PW (2007) Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J 26:4744–4755
Pek YS, Wan AC, Ying JY (2010) The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 31:385–391
Perez RA, Choi S-J, Han C-M, Kim J, Shim H, Leong KW, Kim H-W (2016) Biomaterials control of pluripotent stem cell fate for regenerative medicine. Prog Mater Sci 82:234–293
Phillips BW, Horne R, Lay TS, Rust WL, Teck TT, Crook JM (2008) Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol 138:24–32
Qureshi A, Gurbuz Y, Niazi JH (2012) Biosensors for cardiac biomarkers detection: a review. Sens Actuat B Chem 171–172:62–76
Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM (2013) The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 34:2399–2411
Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T (2005) FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest 115:1724–1733
Ruan JL, Tulloch NL, Saiget M, Paige SL, Razumova MV, Regnier M, Tung KC, Keller G, Pabon L, Reinecke H, Murry CE (2015) Mechanical stress promotes maturation of human myocardium from pluripotent stem cell-derived progenitors. Stem Cells 33:2148–2157
Salic MR, Napiwocki BN, Sha J, Knight GT, Chindhy SA, Kamp TJ, Ashton RS, Crone WC (2014) Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials 35:4454–4464
Schaaf S, Schibamiya A, Mewe M, Eder A, Stöhr A, Hirt MN, Rau T, ZimmermannW-H, Conradi L, Eschenhagen T, Hansen A (2011) Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One 6:e26397-1–e26397-11
Segers VF, Lee RT (2008) Stem-cell therapy for cardiac disease. Nature 451:937–942
Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, Vunjak-Novakovic G (2009) Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp Cell Res 315:3611–3619
Shimko VF, Claycomb WC (2008) Effect of mechanical loading on three-dimentional cultures of embryonic stem cell-derived cardiomyocytes. J Mol Med 75:901–920
Silvestre J-S, Menasché P (2015) The evolution of the stem theory for heart failure. EBioMedicine 2:1871–1879
Simmons CS, Petzold BC, Pruitt BL (2012) Microsystems for biomimetic stimulation of cardiac cells. Lab Chip 12:3235–3248
Smith LA, Liu X, Hu J, Ma PX (2010) The enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials 31:5526–5535
Solanki A, Shah S, Memoli KA, Park SY, Hong S, Lee KB (2010) Controlling differentiation of neural stem cells using extracellular matrix protein patterns. Small 6:2509–2513
Stoppel WL, Kaplan DL, Black LD III (2016) Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliver Rev 96:135–155
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H (2008) Nano-graphene oxide for cellular imaging and drug delivery. Nano Res 1:203–212
Tabar V, Studer L (2014) Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15:82–92
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Tanaka Y, Fujita H (2015) Fluid driving system for a micropump by differentiating iPS cells into cardiomyocytes on a tent-like structure. Sens Actuat B Chem 210:267–272
Tandon N, Cannizzaro C, Chao P-HG, Maidhof R, Marsano A, Au HTH, Radisic M, Vunjak-Navakovic G (2009) Electrical stimulation systems for cardiac tissue engineering. Nat Protoc 4:155–173
Tandon N, Marsano A, Maidhof R, Numata K, Montouri-Sorrentino C et al (2010) Surface-patterned electrode bioreactor for electrical stimulation. Lab Chip 10:692–700
Thavandiran N, Dubois N, Mikryukov A, Massé S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K, Keller GM, Radisic M, Zandstra PW (2013) Design and formulation of functional pluripotent stem cell-derived cardiac microtissue. Proc Natl Acad Sci 110:E4698–E4707
Toh YC, Voldman J (2010) Multiplex microfluidic perfusion identifies shear stress mechanosensing mediators in mouse embryonic stem cells. In: 14th international conference on miniaturized systems for chemistry and life sciences, Groningen, The Netherlands, 3–7 October 2010
Tomecka E, Wojasinski M, Jastrzebska E, Chudy M, Ciach T, Brzozka Z (2017) Poly(l-lactic acid) and polyurethane nanofibers fabricated by solution blow spinning as potential substrates for cardiac cell culture. Mater Sci Eng C 75:305–316
Tzatzalos E, Abilez OJ, Shukla P, Wu JC (2016) Engineered heart tissue and induced pluripotent stem cells: macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliver Rev 96:234–244
Uzel SGM, Pavesi A, Kamm RD (2014) Microfabrication and microfluidics for muscle tissue models. Prog Biophys Mol Biol 115:279–293
Villa-Diaz LG, Torisawa Y, Uchida T, Ding J, Nogueira-de-Souza NC, O’Shea KS, Takayama S, Smith GD (2009) Microfluidic culture of single human embryonic stem cell colonies. Lab Chip 9:1749–1755
Wan CR, Chung S, Kamm RD (2011) Differentiation of embryonic stem cells into cardiomyocytes in a compliant microfluidic system. Ann Biomed Eng 39:1840–1847
Wang X, Ding B, Li B (2013) Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today 16:229–241
Wang B, Jedlicka S, Cheng X (2014) Maintenance and neuronal cell differentiation of neural stem cells c17.2 correlated to medium availability sets design criteria in microfluidic systems. PLoS ONE 9:e109815-1–e109815-15
Wu CC, Chao YC, Chen CN, Chien S, Chen YC, Chien CC, Chiu JJ, Yen BL (2008) Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech 41:813–821
Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M (2014) Microfabricated perfusable cardiac biowire: a platform that mimic native cardiac bundle. Lab Chip 14:869–882
Yang H, Ma Z (2012) Microsystem for stem cell-based cardiovascular research. BioNano Sci 2:305–315
Yang K, Park H-J, Han S, Lee J, Ko E, Kim J, Lee JS, Yu JH, Song KY, Cheong E, Cho SR, Chung S, Cho SW (2015) Recapitulation of in vivo-like paracrine signals of human mesenchymal stem cells for functional neuronal differentiation of human neural stem cells in a 3D microfluidic system. Biomaterials 63:177–188
Yang H, Borg TK, Ma Z, Xu M, Wetzel G, Saraf LV, Markwald R, Runyan RB, Gao BZ (2016) Biochip-based study of unidirectional mitochondrial transfer from stem cells to myocytes via tunneling nanotubes. Biofabrication 4:015012
Yang SH, Choi JW, Huh D, Jo HA, Kim S, Lim CS, Lee JC, Kim HC, Kwon HM, Jeong CW, Kwak C, Joo KW, Kim YS, Kim DK (2017) Roles of fluid shear stress and retinoic acid in the differentiation of primary cultured human podocytes. Exp Cell Res 354:48–56
Ye Z, Zhou Y, Cai H, Tan W (2011) Myocardial regeneration: roles of stem cells and hydrogels. Adv Drug Deliver Rev 63:688–697
Yoon HH, Bhang SH, Kim T, Yu T, Hyeon T, Kim B-S (2014) Dual roles of graphene oxide in chondrogenic differentiation of adult stem cells: cell-adhesion substrate and growth factor-delivery carrier. Adv Funct Mater 24:6455–6464
Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ (2010) The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31:7012–7020
Zhang Q, Austin RH (2012) Applications of microfluidics in stem cell biology. Bionanoscience 2:277–286
Zhang C, Xing D, Li Y (2007) Micropumps, microvalves, and micromixers within PCR microfluidic chip: advances and trends. Biotechnol Adv 25:483–514
Zhang J, Yang H, Shen G, Cheng P, Zhang J, Guo S (2010) Reduction of graphene oxide via L-ascorbic acid. Chem Commun 46:1112–1114
Zhou J, Zhang Y, Lin Q, Liu Z, Wang H, Duan C, Wang Y, Hao T, Wu K, Wang C (2010) Embryoid bodies formation and differentiation from mouse embryonic stem cells in collagen/Matrigel scaffolds. J Genet Genomics 37:451–460
Zhou Y, Basu S, Laue E, Seshia AA (2016) Single cell studies of mouse embryonic stem cell (mESC) differentiation by electrical impedance measurements in a microfluidic device. Biosens Bioelectron 81:249–258
Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T (2006) Engineered heart tissue grafts improve systolic and diastolic function in infracted rat heart. Nat Med 12:452–458
Zuppinger C (2016) 3D culture for cardiac cells. Biochim Biophys Acta 1863:1873–1881
Acknowledgements
This work was realized with the frame of project LIDER No. LIDER/026/573/L-4/12/NCBR/2013.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Kobuszewska, A., Sokolowska, P., Jastrzebska, E. (2018). Cardiac Cell Culture Microtechnologies Based on Stem Cells. In: Brzozka, Z., Jastrzebska, E. (eds) Cardiac Cell Culture Technologies. Springer, Cham. https://doi.org/10.1007/978-3-319-70685-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-70685-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70684-9
Online ISBN: 978-3-319-70685-6
eBook Packages: EngineeringEngineering (R0)