Abstract
Organ and tissue replacement therapies are complicated by immune rejection that restricts the long-term effectiveness of implanted devices. With advancements in nanotechnology and tissue engineering, its applications in the biomedical field have gradually increased. To increase the immunologic acceptance of these devices and to mitigate diseased conditions, stem cells have arisen as a suitable choice. The heart is known to recover its function after myocardial infarction with stem cell transplantation. The cardiomyocytes (CMs) to be used can be generated and applied in regenerative medicine by creating tissue-engineered cardiac patches with the evolution of human-induced pluripotent stem cell (hiPSC) technology. Several novel 3D scaffolds have been introduced as stem cell carriers with favorable surface morphologies. Electrospinning-mediated fabrication of tissue engineering scaffolds is considered a method of choice as it can make fibers that best mimic the extracellular matrix of the heart. Stem cells combined with nanofiber carriers to regenerate cardiac tissues show a vast potential to treat cardiac diseases. This chapter gives insights into the production of hiPSC-derived CMs on nanofibrous scaffolds and how these biomaterials can improve stem cell function in the cardiac tissues with potential applications in cardiac regenerative medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Cardiovascular disease (CVD) is a heart and blood vessel disease group and is a significant reason for deaths in the USA. Millions of people have coronary heart disease, and more than 0.7 million new cases are registered of myocardial infarction (MI) every year in the USA (Benjamin et al. 2018). Some of the well-known CVDs are given in Fig. 4.1. When blood flow to the heart is blocked because of damage to the heart muscle, it results in MI and causes necrosis of cardiac tissue (Fig. 4.2). Scar formation and defective responses after MI cause a decrease in function of the left ventricle and, eventually, complete heart failure (Lloyd-Jones et al. 2009; Prabhu and Frangogiannis 2016) (Fig. 4.3). If the heart cannot supply sufficient blood to prepare the needs of the body, it causes problems in heart functions (Kemp and Conte 2012). For the failure of the heart, the fundamental reason is high blood pressure (Nabel and Braunwald 2012). In the past, trials to repair cardiac function using therapeutic delivery did not benefit the pumping function of the heart (Dimmeler et al. 2008). After MI, a quarter of heart cells vanish, and a considerable cell supply is needed for its regeneration (Kajstura et al. 1998; Yoon et al. 2006). To repair cardiac tissue and treat heart failure, cardiac regeneration is seen as the best option (Laflamme and Murry 2011). To treat MI, fabricated cardiac tissue-like constructs have been established by culturing cardiomyocytes (CMs) on nanofibers (Fig. 4.4) (Radisic and Christman 2013; Martins et al. 2014; Li et al. 2017a; Gao et al. 2018). The therapeutic potential of embryonic stem cells (ESCs) is enormous, but their use is limited on account of immunological rejection by the host (Boheler et al. 2002). Yamanaka and colleagues discovered a novel approach to induce stemness in fibroblasts by incorporating genetic factors and named them induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka 2006), which are like ESCs in appearance and differentiation potential. New myocardium and enhanced cardiac function in rats, mice, pigs, and primates have been formed by transplantation of ESCs- or iPSCs-derived cardiac cells and patches (Plowright et al. 2014; Breckwoldt et al. 2016; Foo et al. 2018).
There are mainly parallel-aligned CMs in the cardiac muscle, interspersed with parallel-aligned microvessels (Kaneko et al. 2011). Investigations have been done on the consequences of anisotropic myocardial fabricated tissues on primary (Engelmayr et al. 2008; Bian et al. 2014; Kai et al. 2014; Lin et al. 2014) or stem cell-derived CMs (Parrag et al. 2012). ECM is the essential component of engineered tissues, and it provides signals to cells. Parallel-aligned (anisotropic) scaffolds reflect the native cellular organization and give directions for the cell rearrangements. This leads to the elongated CM morphology (Kai et al. 2011; Orlova et al. 2011; Kharaziha et al. 2014; Khan et al. 2015; Ruan et al. 2016; Li et al. 2017a; Lemoine et al. 2017). Spatially oriented electrospinning is an approach to fabricate anisotropic scaffolds (Zong et al. 2005). When CMs are cultured on these spatially fabricated scaffolds, these arrange the cytoskeleton according to the alignment of fiber (Zong et al. 2005; Parrag et al. 2012; Wanjare et al. 2017; Allen et al. 2019). As the energy consumption of myocardium is high, an uninterrupted blood supply from capillaries is needed for its sustenance (Parker and Ingber 2007). The CM and vascular endothelial cell interaction is necessary in order to establish contact with host vasculature (Sekine et al. 2008; Zamani et al. 2018; Huang et al. 2018). Endothelial cells in engineered heart tissue lead to angiogenesis after myocardial injury because of the presence of endothelial cells within them (Sekine et al. 2008; Gao et al. 2018). Regarding this, stem cell transplantation has drawn enormous attention with the discovery of iPSCs (Takahashi and Yamanaka 2006). Due to the improvement in the direct differentiation of hPSCs, now there is a certainty of producing stem cell-derived CMs and endothelial cells from ESCs or iPSCs with better effectiveness (Burridge et al. 2014). The induced CMs and endothelial cells resemble their appearance and functions to native cells and are tested in animal models for heart tissue regeneration (Rufaihah et al. 2011; Nakayama et al. 2018; Ishida et al. 2019). Many stem cells have the potential to differentiate into cardiac cells, like mesenchymal stem cells (Müller-Ehmsen et al. 2002; Orlic et al. 2003), ESCs (Caplan and Dennis 2006), iPSCs (Lahti et al. 2012), and CPCs (Miyahara et al. 2006), so a lot of attention has been drawn toward PSCs (Braam et al. 2010; Minami et al. 2012; Liang et al. 2013; Navarrete et al. 2013; Mathur et al. 2016). CM constructs that resemble tissues are necessary, not the non-organized clusters of cells (Braam et al. 2010; Matsa et al. 2011; Liang et al. 2013; Navarrete et al. 2013).
The ESCs are taken from the inner mass of cells of the blastocyst (Fig. 4.5). The inner cell mass grows to form ectoderm, endoderm, and mesoderm of the embryo proper, in vivo (Kingham and Oreffo 2013). Because ESCs are mainly produced from preimplantation embryos (Olson 2006; Kattman et al. 2011; Noseda et al. 2011) and iPSCs are generated from somatic cells (Lian et al. 2012; Zhang et al. 2012), these have gained more attention. Recently, there has been a strong surge in the production of hiPSC using fibroblasts under defined factors (Jaffe 2008). Also, there is an increasingly sophisticated capacity of iPSCs to easily differentiate into cell types related to diseases such as CMs (Burridge et al. 2012; Mordwinkin et al. 2013; Matsa et al. 2014).
Differentiation of human ESC lines: ESCs are derived from the cells of the blastocyst. With their maintenance in culture, they experience self-renewal and proliferation and retain their stem cell state. (They are adapted with permission from (Kingham and Oreffo 2013))
4.2 Gene Expression and Signaling Pathways in CM Differentiation
Many studies have been performed on model organisms that demonstrate that the signaling pathways like Wnt, BMP, and Activin/Nodal/TGF-β play essential roles in establishing the cardiovascular system (Olson 2006; Evans et al. 2010; Noseda et al. 2011). From a mixed population of iPSCs, purification of CMs is attained by non-genetic methods, for example, cell-surface markers (Kattman et al. 2011; Lian et al. 2012; Zhang et al. 2012; Abilez et al. 2014; Sanchez-Freire et al. 2014), mitochondria-specific cells (Uosaki et al. 2011), fluorescent probes (Ban et al. 2013), and glucose deprivation (Tohyama et al. 2013). iPSC differentiation toward CMs at the molecular level is coordinated by the patterned expression of various genes at certain steps, which include genes for the establishment of mesoderm, mesoderm for cardiogenesis, cardiac-specific progenitors, and genes for muscle-related proteins of CMs, respectively (Kattman et al. 2011; Liang et al. 2013; Abilez et al. 2014). The ion channel genes of cardiac tissues are in the left ventricle, such as sodium, potassium, and L-type calcium channels (Liang et al. 2013). iPSC-CMs express genes for Ca2+ cycling machinery, such as inositol triphosphate receptor, sarcoplasmic reticulum Ca2+ ATPase, ryanodine receptor, calsequestrin 2, junctophilin 2, calreticulin, phospholamban, sodium exchanger, and triadin (Itzhaki et al. 2011; Jung et al. 2012a; Rao et al. 2013). Mitochondrial complexes I–V and genes for cholesterol metabolism and genes against apoptotic and oxidative stress processes are expressed in iPSC-CMs (Rana et al. 2012). ROCK signaling pathway, which downregulates cell migration and cell-cell adhesion, is often targeted in cardiac engineering (Riento and Ridley 2003). On screening a diverse compound library using hPSC-CPC, it was found that inhibitor of Wnt pathway signaling (XAV939); bone morphogenetic proteins; a dorsomorphin inhibitor of AMP-activated kinase, RepSox, which acts as an inhibitor of TGF-β type 1 receptor; ALK5; or other inhibitors of ALK5 (Drowley et al. 2016) enhance differentiation of hPSC-CPC.
4.3 Electrospinning Is a Preferable Method for Nanofiber Fabrication
Among many methods employed for tissue engineering, electrospinning has attracted the most attention as it produces nonwoven meshes in the form of scaffolds that structurally resemble the ECM of the heart (Ali et al. 1993; Czyz and Wobus 2001). For the fabrication of fibers, electrospinning is a well-known nanotechnology technique that utilizes electrically charged polymeric solution and is widely used to produce biomaterials for tissue engineering (Ali et al. 1993; Dorfman et al. 1998; Xie et al. 2009) (Fig. 4.6). Electrospun nanofibers produce nanoscale structures that are highly porous and interconnective and have high surface area to volume ratio, imparting the properties of attachment with the cells, better proliferation, and, lastly, their differentiation (Han et al. 2016). It is also a successful technique to fabricate anisotropic scaffolds (Barnes et al. 2007) rather than other conventional methods like soft lithography, microfluidics, photolithography, and two-photon initiated polymerization (Kim et al. 2010; Ma et al. 2014; Xiao et al. 2014).
Electrospinning is a versatile micro-nanofiber production method that uses electric force to form threads of the polymer solutions of a hundred nanometers. On applying a high voltage, the liquid is charged due to the electrostatic repulsion and surface tension; the droplet gets stretched, and then at a point, a stream of liquid flows from the surface. This point is where the Taylor cone formation occurs. The liquid jet, which is now produced, gets elongated by a whipping process due to electrostatic repulsion. Finally, it is deposited on the collector, and uniform fibers of nanometer-scale diameters are perfectly formed. A brief review of the studies done on the differentiation of stem cells into CMs using different nanomaterials is given in Table 4.1.
4.4 Polymers and Bioactive Agents Used in Tissue Engineering
Nanofibrous scaffolds with a diameter range of nanometer to a few microns are utilized for heart muscle generation more than other scaffold types, like sponge scaffolds (Prabhakaran et al. 2011). Whether natural or synthetic, numerous polymers have been successfully used in the production of mammalian culture suitable nanofibers. However, due to the close resemblance of natural polymers to structural proteins of ECM, they are widely used, e.g., collagen, laminin, and gelatin (Jung et al. 2012b; Boccaccini et al. 2015). Synthetic polymers, such as polycaprolactone, poly-lactic-co-glycolic acid, and polyurethane, are mostly used in the production of biocompatible scaffolds (Khil et al. 2005; Vasita and Katti 2006; Song et al. 2008). Other synthetic polymers include chitosan, polyaniline, polyethersulfone, polydimethylglutarimide, peptide amphiphile, and some other peptide nanofibers. Polymers are combined to create co-polymers to be used in the making of scaffolds of desired properties. Other than polymers, e.g., synthetic hydrogels, sometimes based upon polyethylene glycol, are utilized as a scaffold for drug delivery and cell culture (Lin and Anseth 2009). They are biocompatible and have excellent safety records and have established well in the medical field (Van Tomme et al. 2008; Hoffman 2012; Frey et al. 2014). Bioactive agents, like hormones, growth factors, and other small molecules, are introduced within the scaffold matrix to make an ECM-like environment for the proliferation of cells to enhance cell survival, proliferation, integration, and differentiation (Laflamme et al. 2007). Erythropoietin is an example of a bioactive agent successful in clinical studies to reduce cell death and remodel post-MI (Brines and Cerami 2008). A crucial ECM molecule for stem cell differentiation and adhesion is fibronectin (Tate et al. 2002; Wijelath et al. 2004; Van Dijk et al. 2008), which is expressed in the normal heart also. Platelets are the growth factors that are involved in blood clotting, immune response, angiogenesis, and recovery of the damaged tissues in the body. This has attracted the attention of physicians in damaged engineering tissues because the probability of a reaction is near to the ground due to the use of personal blood (Boswell et al. 2012; Lang et al. 2018).
4.5 Differentiation of CMs from Stem Cells Using Nanofibers
4.5.1 Gelatin Nanofibers
PSCs demand more acclimated 3D cellular microenvironments than conventional 2D surfaces to keep their pluripotency or differentiation homogeneity. Matrigel (gelatinous protein compound of mice tumor cells) (Hughes et al. 2010) recombinant proteins like laminin (Rodin et al. 2014) or vitronectin (Rowland et al. 2010) were introduced for iPSC growth and differentiation. Additional substrates have also been used, like oxygen plasma etched plates (Mahlstedt et al. 2010), porous materials (hydroxyapatite scaffolds) (Kim et al. 2007a), and electrospun nanofibers (Kumar et al. 2015; Li et al. 2017c), which have textured surface morphology. Without improvement to conventional approaches, these will not overcome the risks of genetic instability and tumorigenicity (Okita and Yamanaka 2011; Liyang et al. 2013). These methods do not show the production of rigid and thick cardiac sheets because these have a limitation of cardiac diffusion (Shimizu et al. 2006). For the differentiation of hiPSCs to motor neurons, a patch culture method has been proposed, which has shown an enhanced upregulation of gene expression of the neurons and smooth maturation of motor neurons (Tang et al. 2016a). Tang et al. extended the patch process to culture and differentiated hiPSCs toward functional CMs. A crosslinked monolayer of gelatin nanofibers backed by a poly(ethylene glycol) diacrylate honeycomb frame consisted of the patch. Poly(ethylene glycol) diacrylate, a derivative of polyethylene glycol, could be utilized for multiple drug delivery and tissue engineering-based purposes. UV-based molding method was used for the preparation of the poly(ethylene glycol) diacrylate frame and gelatin nanofibers, electrospinning was used (Fig. 4.7). On the poly(ethylene glycol) diacrylate frame gelatin, nanofibers were electrospun. Due to the wide-sized pores of the patch and natural polymer used, the crosslinked gelatin nanofibers minimized the exogenic substance contact of hiPSCs. Vitronectin or additional extracellular matrix proteins can be used to coat the culture patch if required for more functional cell-nanofiber pairing. As the culture patch was within off-ground conditions, the crosslinked monolayer nanofibers allowed significantly enhancing the exposure field of hiPSCs upon the culture medium. They had also shown in their previous work that preferentially, cells remained confined in the nano-patterned region due to intensified distribution of nutrients and cell uptake (Hu et al. 2010; Tang et al. 2016b). Due to the increased exposure area with the help of monolayer of crosslinked nanofibers supported, cell metabolites could more efficiently be diffused, and in this manner, the culture patch is favorable over other 3D substrate types (Hu and Li 2007). Due to good mechanical stability and weak in-plane resistance to cardiac contraction, the honeycomb structure was chosen (Nishikawa et al. 2003; Arai et al. 2008; Engelmayr et al. 2008), and to aid hiPSC clustering, poly(ethylene glycol) diacrylate was used. Poly(ethylene glycol) diacrylate has been popularly adopted to outline the cell culture surfaces for their weak cell adhesivity and inadequate protein absorption and smooth chemical alteration (Moon et al. 2009). Colonies of ~250μm diameter (~1300 cells) refer to the most suitable size of hiPSC colonies for cardiac differentiation, and a honeycomb structure patch size of 500μm was chosen to generate the hemisphere colonies of this size (Dahlmann et al. 2013). In this study, ROCK inhibitor Y-27632 was utilized to adjust the impact of vitronectin, promoting the integrin-mediated cell adhesivity to the substrate. The cell-substrate interaction decreased due to low vitronectin surface coating and a short interval Y-27632 application given to the cells. It also supports cell to cell adhesion and then augments the cell grouping and development of hemispheric hiPSC colonies. As Y-27632 lasted for 4h or longer, flat hiPSC colonies were generated. However, when the application was restricted to 1–2h, hemisphere colonies with a diameter of around 225μm were obtained. These colonies were selected for cardiac differentiation due to the morphological similarity of hemisphere colonies with embryoid bodies. These are more resembling to in vivo development process of embryos and can assist in CM induction (Mummery et al. 2002, 2003). Poly(ethylene glycol) diacrylate honeycomb compartment prevents the formation of heterogeneous cardiac clusters because of necessary fusion until the later stage of cardiac differentiation.
Cells belonging to the same population exhibit a considerable heterogeneity degree (Janes et al. 2010; Wilson et al. 2015). The determination of the fate of stem cells is also influenced by their inherent heterogeneity (Warren et al. 2006; Musina et al. 2006; Franco et al. 2010). Variations among donor cells result in operative variability and diverse differentiation potentials among other hPSC lines (Adewumi et al. 2007; Kim et al. 2007b; Cahan and Daley 2013). Also, those obtained from different types of tissues, for example, various germ layer tissues, show lineage bias as they go through directed differentiation (Kim et al. 2011), suggesting that hPSCs may hold on to distinct memory about their origin. Furthermore, reprogramming is a complicated and multi-step procedure that adds additional modifications (Liang and Zhang 2013). Building single cell-derived clones could diminish this heterogeneity (Narsinh et al. 2011; Lecault et al. 2011; Smallwood et al. 2014). Leqian et al., in their study (Yu et al. 2019), developed a single hPSC separation and culture platform of gelatin nanofibers. These nanofibers are appropriate for the long-term development of hPSCs supporting the feeder and serum-free culture environments. They established a single cell-derived sub-clone that proved to possess a discrete morphology related to other sub-clones. When this clone was used for differentiation toward CMs, it demonstrated much greater differentiation capability, maturation, and more substantial beating than those obtained from the other sub-clones. These observations present a suitable approach for single-cell separation and culture and illustrate those disparities in differentiation biases among sub-clones belonging to a cell line (Fig. 4.8).
Single-cell isolation system. (a) The process of making culture device for cell isolation. (b) Photograph of the culture device and single-cell isolation. The device is made of a polydimethylsiloxane multiwell array (400 wells) for single-cell isolation, and for the single-cell culture, it consists of gelatin nanofiber substrates. (c) The single-cell distribution rate of the multiwall array. (d) The highest viability was shown by the single cells with a mixture of days 1, 2, and 3 conditioned medium (C. medium) in the beginning 3 days of culture (n≥20). (Adapted from (Yu et al. 2019))
4.5.2 Polycaprolactone Nanofibers
Polycaprolactone is a high-molecular-weight synthetic polymer, which is recognized by the US Food and Drug Administration for therapeutics (Kuppan and Sethuraman 2013). Moreover, the 3D-polycaprolactone nanofibrous scaffolds fabricated by electrospinning are employed for tissue engineering based on stem cells because of their good mechanical and biodegradable characteristics (Hashemi et al. 2009; Lim et al. 2009). These scaffolds provide a possibility to design scaffolds of micro- to nanoscale topography with a significant porosity similar to that of native ECM (Sill and von Recum 2008). Ding et al. (2020) examined the influence of 3D-aligned polycaprolactone nanofiber scaffolds upon cardiac differentiation of hPSC-CPCs. Cells treated with Wnt signaling inhibitors on 3D-aligned nanofiber scaffolds displayed enhanced CM differentiation of hPSC-CPCs. A notable rise in cTnT-positive cells on the 14th day in the 2D culture of differentiation related to cells administered with DMSO vehicle control resulted from treating hPSC-CPCs with Wnt inhibitors (53AH and XAV939). The results show that the Wnt signaling pathway performs an essential role in cardiac differentiation (Patsch et al. 2015; Wang et al. 2011; Willems et al. 2011; Ao et al. 2012; Lian et al. 2013). When the cells were treated with 53AH (i.e., a distinct structural inhibitor of Wnt signaling), the expression of CM marker genes TNNT2 and MYH7 that are predominantly expressed in fetal ventricles was higher, and when the cells were treated with XAV939, the expression of MYH7 was also elevated (Fig. 4.9). These 3D-aligned nanofiber scaffolds mimic the structure of the native ECM.
Real-time RT-PCR analysis of CPC differentiation and proliferation in 3D vs. 2D culture. (a–e) Expression of different cardiac expression markers at day 7 of differentiation in 2D and 3D cultures. (Obtained with permission from (Ding et al. 2020))
Wanjare et al. (2019) fabricated microfibrous polycaprolactone by electrospinning to simulate the established physiological cellular organization of the entire myocardium to control the assemblage of induced-ESCs (iESCs) and induced-CMs (iCMs). By reprogramming peripheral blood mononuclear cells by transduction of cardiac differentiation-related genes (Sox2, Oct3/4, KLF4, and c-myc) mediated by Sendai virus, the hPSC (P356) cell line was developed. hESC (H9) was used along with hPSCs. After the cell seeding, visualization of the in vitro establishment of iCMs and iESCs within scaffolds was achieved according to phenotypic markers of troponin-T (TNNT) for CMs and CD31 for embryonic cells (Fig. 4.10). NOD SCID mice (nonobese diabetic-severe combined immunodeficiency mutant mice) were utilized toward subcutaneous implantation studies. These scaffolds successfully induced and precisely directed the differentiation in the experimental cells.
The vascularization of cardiac tissue after successful implantation in mice model (a). Confocal microscopy images of CD31 staining (green) of engineered tissues obtained from scaffolds containing iCMs, iECs, or iCM+iECs after implantation. Acell means acellular scaffold (b). The orientation of vessels in engineered myocardial tissue relative to the axis of the aligned fibers. (Adapted with permission from (Wanjare et al. 2019))
With the use of an electrospun nanofiber as the substrate instead of tissue culture polystyrene plates (TCPs), CMs display more stable and long-lasting, spontaneous syncytium (Şenel Ayaz et al. 2014). Jingjia et al. (Han et al. 2016) designed the aligned and isotropic polycaprolactone fibers. The fibrous scaffolds were gold or palladium coated and were analyzed for characterization. After the culture of hiPSC-CMs was done on scaffolds coated with Matrigel, the alignment of cells on these substrates was confirmed using a polystyrene substrate as the control. Yan et al. (Chen et al. 2015) studied the impact of 3D polycaprolactone scaffolds on the CM differentiation of murine-iPSCs while performing in vitro examinations. A unique CM-inducing effect exists in the exchanges between the nanofibers of 3D polycaprolactone and the intracellular Wnt/β-catenin signaling of iPSCs. It was found that the gelatin-coated 3D scaffolds were suitable for iPSC cultivation and differentiation. Also, the conventional TCPs are less effective than 3D scaffolds for inducing the CM differentiation of iPSCs using the monolayer culture method.
To increase myogenic differentiation of hESCs, Leung et al. (2013) used chitosan-polycaprolactone fibers resembling the native muscle ECM microenvironment along with the Wnt3a protein. Polycaprolactone nanofibers were fabricated by electrospinning, and hESCs were seeded on this scaffold in media containing Wnt3a. An elongated morphology of hESCs was observed along fiber direction as compared to control substrates. Cells cultured on chitosan-polycaprolactone with Wnt3a expressed a high percentage of myogenic proteins over total hESCs after 2 days of cell culture.
4.5.3 Polydimethylglutarimide Nanofibers
Polydimethylglutarimide is a biocompatible polymer that can be easily electrospun into nanofibers (Orlova et al. 2011). Li et al. (2016) investigated hiPSC-CMs on fibers made of polydimethylglutarimide and pursued their cardiac tissue-like construction. Electrospinning was performed using a rotating drum to prepare aligned polydimethylglutarimide while increasing the rotation speed of the collector. The fibers of concentrations 19% and 16% demonstrated the best alignment than those from lower concentrations. The density of sheets was manipulated by changing the spinning time, and the 90-s electrospun fibers were chosen for CM culture. The as-spun polydimethylglutarimide threads on transferring to the surface of microelectrode array let the extracellular recording of mimicking activities. Recording electrical activity in CMs enables the evaluation of their electrophysiological characteristics (Meiry et al. 2001).
4.5.4 Poly(lactic-co-glycolic) Acid Nanofibers
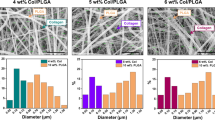
This polymer has ample versatility to some drugs, including hydrophobic, hydrophilic, micromolecular, and macromolecular. It also limits drug degeneration and the chance of easy surface modification to give more considerable interaction with biological surfaces (Pick 2009). Poly(lactic-co-glycolic) acid does not cause any inflammatory response and becomes absorbed well, not concentrating in the tissues or organs (Ali et al. 1993). Torabi et al. (2020), in their study, developed platelet-rich plasma-incorporated poly(lactic-co-glycolic) acid nanofibrous scaffold. The results demonstrated that the fabricated poly(lactic-co-glycolic) acid scaffold, in comparison to standard TCPs, exhibits enhanced biocompatibility. The platelet-rich plasma in poly(lactic-co-glycolic) acid is believed to increase biodegradability and hydrophilicity of scaffold and results in a suitable increase in the CM differentiation potential of hiPSCs. Its enhanced biocompatibility caused a rise in proliferation rate and PSCs survival due to the present growth factors. It may be noted here that there has been a positive impact of platelet-rich plasma on the growth and proliferation rate of other cells (Choi et al. 2005; Drengk et al. 2009). Platelet-rich plasma-incorporated scaffolds showed the maximum expression of cardiac genes, such as MLC2A, ANF, and MLC2V. Prabhakaran et al. (2014) analyzed the ability to differentiate ESCs into CMs on the poly(lactic-co-glycolic) acid and poly(lactic-co-glycolic) acid/collagen electrospun fibrous scaffolds as the cardiac patch. They made uniform bead-free fiber of poly(lactic-co-glycolic) acid and poly(lactic-co-glycolic) acid with collagen using the electrospinning technique. The ESC differentiation was induced by embryoid body formation, which proliferated and differentiated into CMs. Higher proliferation on poly(lactic-co-glycolic) acid with collagen scaffolds was observed by scanning electron microscope; the reason behind this could be the small diameter or high surface area of the fiber.
Most hiPSC-CMs morphologically and functionally look like naive CMs rather than adult ones, limiting their administration. Li et al. (2017a) produced high-quality constructs similar to cardiac tissue by culturing hiPSC-CMs on nanofibers of low girth made of biodegradable poly(lactic-co-glycolic) acid polymer. They described that multi-layered and elongated CMs could be arranged at high density with ordered fibers in a one-step seeding process, developing in upregulated cardiac biomarkers and enhanced cardiac functions. Constructs similar to cardiac tissue were used for assessing drugs, and they were more vigorous than the 2D control. They also highlighted the usability of cardiac constructs for in vitro designing of engraftments and in vivo treatment of MI.
4.5.5 Polyaniline and Polyethersulfone Nanofibers
Polyaniline is receiving much attention due to its excellent electrical conductivity. It has gained wide tissue engineering usage due to its electroactive qualities, high endurance, biocompatibility, easy synthesis, and being economical (Qazi et al. 2014). It scavenges reactive oxygen species and can mitigate oxidative stress in a myocardial injury (Gizdavic-Nikolaidis et al. 2004). It can be doped from the nonconductive form of meraldine base into the conductive state of emeraldine salt by protonic acids (e.g., camphor-10-sulfonic (β)) (Khuspe et al. 2014) and has been employed to assist in the transmission of electrical pulses (Balint et al. 2014). Furthermore, among different doped states of the poly(lactic-co-glycolic) acid, this polymer with camphor-10-sulfonic (β) is considerably aligned in the direction of the fiber. Moreover, the better interchain charge transfer occurs in poly(lactic-co-glycolic) acid with camphor-10-sulfonic (β) due to its dedicated polymeric chain packaging (Pouget et al. 1995). These semiconducting polymers concurrently support the adhesion and reproduction of CMs and induce cardiac differentiation (Bidez et al. 2006). Polyethersulfone nanofibers are other biocompatible materials that induce mesodermal differentiation (Ardeshirylajimi et al. 2013). Polyethersulfone is mechanically stable for differentiation but its electrical conductivity is low. Hence, blending polyethersulfone with polyaniline is practiced to create a scaffold with added mechanical stability for cardiac differentiation and electrical conductivity.
Mohammadi et al. (2017) practiced electrical stimulations onto aligned and random scaffolds to see how it affects the differentiation of PSCs to CMs. The effect of multidirectional electrical stimulation generated by random scaffolds on CM differentiation was negative, but the aligned unidirectional electrical stimulation of the aligned scaffold was positive. Recapitulation of the requirements and events leading to cardiac differentiation and maturation is a significant challenge (Courtney et al. 2006). The electrical impulses were applied to hiPSCs taken from patients with cardiovascular disease (referred to as CVD-iPS cells) seeded on aligned polyaniline/polyethersulfone scaffolds. This setup worked as an electrically effective cell culture system, including features similar to in vivo conditions of the heart that have been shown to generate cardiogenesis (Serena et al. 2009; Chi et al. 2010; Hernández et al. 2016). Through embryogenesis, the primary pacemaker cells originate from fetal CMs in the center of the sinoatrial node to create the first electrical impulses. Studies have been performed about exogenous electrical stimulation and cardiac differentiation of diverse kinds of stem cells (Serena et al. 2009; Hernández et al. 2016) by changing intracellular ion concentrations (Trollinger et al. 2002), yielding reactive oxygen species (Serena et al. 2009), or locating growth factor receptors and lipids in the cell membranes (Zhao et al. 2002). In this work, a bioreactor that applied exogenous electrical impulses was designed to apply electrical stimulation to cells via stainless steel electrodes. Analysis of the properties of this bioreactor showed that it is suitable for cardiac differentiation.
4.5.6 Polydimethylsiloxane Nanofibers
An important factor of the culture substrate that plays a vital role in determining the cell fate is the elasticity (Banerjee et al. 2009; Kshitiz et al. 2012; Sun et al. 2012; Li et al. 2017b). The self-renewal and differentiation of hPSCs are affected by the flexibility of cultures and morphology (Liu et al. 2014, 2017; Macrí-Pellizzeri et al. 2015). Engler et al. showed that the differentiation of mesenchymal stem cells toward a particular lineage is significantly based on substrate elasticity (Engler et al. 2006). The matrix stiffness and cell density assist hiPSCs in intercellular network formation, preserving phenotype and contractile function (Lee et al. 2017). A stencil method was revealed to investigate the outcome of substrate elasticity on the clustering and cardiac differentiation of hiPSCs. Dense elastomer pillars of altering stiffness were designed by adjusting the height of the pillars. To form uniform cell clusters, an elastomer stencil with a honeycomb pattern was created before cell seeding. It was demonstrated that both cell clustering and cardiac differentiation are dependent on the elasticity of substrate. They showed that the pillar of moderate elasticity (9 kPa) was better for both stiffer and softer ones.
4.5.7 Collagen Nanofibers
Joanne et al., in one of their works, have shown the feasibility of deriving collagen scaffolds mixed with biological solvents and crosslinking agents (Kitsara et al. 2015). In their other study (Joanne et al. 2016), they generated collagen scaffolds and cultured hiPSC-CMs and injected these scaffolds epicardially in a dilated cardiomyopathy (DCM) mouse model. Atelocollagen extracted from animal dermal tissue was electrospun by applying a proper voltage between the collector and the syringe needle. The suturing of collagen scaffolds on the ventricles of mice was done. The results showed the feasibility of the hiPSC-CM-seeded scaffold for the treatment of DCM. Hansen et al. (2018) developed and characterized hiPSC-CM-seeded fibrin suture that could be used for the delivery platform in repairing cardiac issues. hiPSC-CMs were seeded onto micro fibrin threads, and their contractile properties with time were characterized. These researchers fabricated a fibrin microthread suture for direct cell delivery to the myocardium (Guyette et al. 2013). In their study, various ECM and surface coatings were applied for improving cell attachment. Collagen IV and fibronectin were selected because of their occurrence in the cardiac basement membrane (Moyes et al. 2013; Rodriguez et al. 2014). Other hiPSC-CMs seeded on microthreads showed contraction within 7 days after seeding. The contraction of cells was in the direction of fiber for over 21 days. Also, this ordering was proved by immunohistochemical stains as over 21 days, the cells ordered further close to the thread, having the final alignment within 8° to the thread. Their findings suggest that hiPSC-CMs are able to attach to fibrin microthreads while the collagen IV protein-coating improves the potential of hiPSC-CMs attachment to fibrin microthreads. By the 14th day, the fibers contracted at a frequency that resembled the human heart and generated strains like those developed by myocardium. Like the previous studies (Guyette et al. 2013; Hansen et al. 2016; Tao et al. 2017), this microthread scaffold could impact cardiac delivery approaches.
A blood vessel is composed of fibrillar ECMs and the other cell composition at the micro-/nanoscale (Stehbens and Martin 1993). Nakayama et al. (2015) designed a bi-layered vascular graft extracted from hiPSCs that reiterates the cell construction, alignment, and anti-inflammatory operation of blood vessels. In this graft, longitudinal-aligned nanofibrillar collagen-containing endothelial stem cells consisted of the luminal layer. Collagen with iPSC-derived smooth muscle cells consisted of the outer layer. Cells aligned on aligned fibers showed the association of F-actin within 8° from the direction of scaffolds. Endothelial stem cells seeded on ordered scaffolds had significantly lowered immune response due to attachment to monocytes. There is a significant influence of anisotropic scaffolds in directing cell construction and function.
4.5.8 Peptide Nanofiber
RADA-16-I (Ac-(RADA)4-CONH2) is a peptide nanofiber that causes the attachment, development, and differentiation of stem cells and mature somatic cells (Davis et al. 2005). RADA16-I can be modified with the addition of functional peptides to its C-terminal end. One useful peptide, e.g., PRG (Ac-(RADA)4GPRGDSGYRGDS-CONH2), contains RGD (a natural cell adhesion motif to regulate the localization and proliferation of cells by ligation with integrin). Another operative peptide, i.e., KLT (Ac-(RADA)4G4KLTWQELYQLKYKGI-CONH2), augments angiogenesis by copying vascular endothelial growth factor (Liu et al. 2012). Functionalized self-assembling peptide nanofibers are of good biological histocompatibility. They have the capability to sustain the reproduction and differentiation of cells for repairing nervous tissues in vertebrate studies and are having applications for cardiovascular diseases as well. Peptide amphiphile is self-assembling peptides that join a hydrophobic and a hydrophilic peptide sequence exhibiting an excellent approach to gain this purpose (Hartgerink et al. 2001). Self-adhesive ligand Arg-Gly-Asp-Ser (RGDS), when incorporated with peptide amphiphile (PA-RGDS) and matrix metalloprotease-2 (MMP-2) degradable sequence, Gly-Thr-Ala-Gly-Leu-Ile-Gly-Gln (GTAGLIGQ), forms an ECM mimicking injectable nano-matrix. When interjected into PA, RGDS and fibronectin-derived ligand enhance cell adhesion and endurance and are verified by several cell types, including mesenchymal, umbilical, aortic, and pancreatic beta cells (Benton et al. 2009; Yu et al. 2009, 2010; Sapir et al. 2011; Gandaglia et al. 2012; Mihardja et al. 2013). MMP-2 degradable sequences on incorporation into peptide amphiphile cause a gradual degeneration of the fiber and are replaced by ECM of cells because the damaged tissue exhibits enhanced MMP production under ischemic conditions (Bendeck et al. 1994; Spinale et al. 1998; Cheung et al. 2000). This increases the migration of CMs into the myocardium (Jun et al. 2005). PA-RGDS nearly simulates the physical and biochemical complexity of ECM. Peptide amphiphile is amphiphilic and provides assembly into 3D networks of nanofibers similar to ECM proteins under biological conditions. To know the underlying performance and machinery of transplanted stem cells in vivo, molecular imaging by dual-modal or multi-modal is more accepted to comprehend and review stem cell therapy with comprehensive information (Nguyen et al. 2014). To trace the transplanted stem cells and estimate their clinical effects, steadfast dual-modal imaging that utilizes bioluminescence and magnetic resonance imaging is set up (Cao et al. 2015). Li et al. (2017c) co-transplanted the bone marrow-derived mesenchymal stem cells using RAD/PRG or RAD/KLT, which promoted their localization and endurance in the infarcted myocardium, and their therapeutic effect was increased by co-transplantation with either RAD/PRG or RAD/KLT.
Ban et al. (2014) derived the CMS from mouse ESCs and encapsulated them in peptide amphiphile-RGDS to check their usage in MI therapy (Fig. 4.11). The incorporation of RGDS and GTAGLIGQ did the generation of peptide amphiphile-RGDS into peptide amphiphile. The CMs were taken from the rat model. They elucidated that most CMs survived in PA-RGDS for a week. The CMs were injected into the myocardium of mice model with PA-RGDS, and a threefold increase was seen in the incorporation in the models with CM+PA-RGDS compared to those with only CMs. A well-established cardiac function was seen in the group of mice with CM+PA-RGDS from 3 weeks and was sustained for 12 weeks.
PA-RGDS intensifies the endurance of mouse ESC-CMs and boosts heart function post-MI. (Adapted with permission from (Ban et al. 2014))
4.5.9 Polyurethane Nanofibers
Tissue constructs with similar properties of structure and mechanics as that of myocardium were generated by Guan et al. (2011). In this regard, the electrospun polyurethane nanofibers were produced. The tissue construct mesenchymal differentiation was recorded by analyzing the expression of cardiac markers and the development of ion channels. The differentiation of cardiac cells was seen to be initiated by recording the mRNA expression. Tissue constructs were stretched statically to achieve cell alignment. The strain was increased from 25% to 75%, and it increased the degree of 3D alignment of cells. The RT-PCR determined that with a strain of 75%, the expression of GATA4, Nkx2.5, and MEF2C, which are the markers of differentiation of CMs, increased. Their work suggests that the pre-differentiation of mesenchymal stem cells into CMs before injection results in a higher cardiac regeneration rather than only injecting undifferentiated mesenchymal stem cells into the heart.
4.6 Conclusion and What Is Next
Cardiac tissue engineering is a field that repairs, reconstructs, and replaces cardiovascular structures, especially the heart, with engineered tissues. With the technology of deriving CMs from iPSCs, CVD phenotypes can be modeled, screening of the drugs can be done, and new ways of producing regenerative medicines can be achieved. It also offers ways to isolate iPSC-CMs from patients with genetic mutations or understand their pathophysiology. Direct reprogramming of the somatic cells into CMs without a transitionally pluripotent state is possible now. Genes are introduced at particular loci, and gene mutations are introduced to reverse the mutations that lead to diseases in iPSC-based CVD models in vitro. It is a novel and fast budding technology having thrilling applications. In the future, with more refinements, it will create means for the progress of personalized medicine for CVDs. Biomaterials can enhance stem cell function, and more knowledge of biomaterial engineering will help in molecularly designing biomaterials that resemble naturally occurring ECM of cardiac tissues. This way, biomaterials can guide the differentiation and function of progenitor cells. Biomaterials can be designed molecularly to control many of the factors that drive the differentiation of progenitor cells and function. Injectable nano-matrices could be designed to maintain biophysical and biochemical microenvironments of transplanted cells for better engineered cardiac tissues.
References
Abilez OJ, Plews JR, Diecke S, Mordwinkin NM, Huber B, Churko JM, Matsa E, Lin ZC, Cui B, Gold JD, Lan F, Burridge PW, Ebert AD, Wu JC, Shukla P (2014) Chemically defined generation of human cardiomyocytes. Nat Methods 11:855–860
Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo ABH, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RDG, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O’Brien CM, Oh SKW, Olsson C, Otonkoski T, Park KY, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RAR, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, Van Den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W (2007) Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol 25:803–816
Ali SAM, Doherty PJ, Williams DF (1993) Mechanisms of polymer degradation in implantable devices. 2. Poly(DL-lactic acid). J Biomed Mater Res 27:1409–1418
Allen ACB, Barone E, Momtahan N, Crosby CO, Tu C, Deng W, Polansky K, Zoldan J (2019) Temporal impact of substrate anisotropy on differentiating cardiomyocyte alignment and functionality. Tissue Eng Part A 25:1426–1437
Ao A, Hao J, Hopkins CR, Hong CC (2012) DMH1, a novel BMP small molecule inhibitor, increases cardiomyocyte progenitors and promotes cardiac differentiation in mouse embryonic stem cells. PLoS One 7:e41627
Arai K, Tanaka M, Yamamoto S, Shimomura M (2008) Effect of pore size of honeycomb films on the morphology, adhesion and cytoskeletal organization of cardiac myocytes. Colloids Surfaces A Physicochem Eng Asp 313–314:530–535
Ardeshirylajimi A, Hosseinkhani S, Parivar K, Yaghmaie P, Soleimani M (2013) Nanofiber-based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Mol Biol Rep 40:4287–4294
Balint R, Cassidy NJ, Cartmell SH (2014) Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 10:2341–2353
Ban K, Wile B, Kim S, Park HJ, Byun J, Cho KW, Saafir T, Song MK, Yu SP, Wagner M, Bao G, Yoon YS (2013) Purification of cardiomyocytes from differentiating pluripotent stem cells using molecular beacons that target cardiomyocyte-specific mRNA. Circulation 128:1897–1909
Ban K, Park HJ, Kim S, Andukuri A, Cho KW, Hwang JW, Cha HJ, Kim SY, Kim WS, Jun HW, Yoon YS (2014) Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair. ACS Nano 8:10815–10825
Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS (2009) The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30:4695–4699
Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL (2007) Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev 59:1413–1433
Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA (1994) Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res 75:539–545
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, De Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, MacKey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P (2018) Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137:E67–E492
Benton JA, Fairbanks BD, Anseth KS (2009) Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials 30:6593–6603
Bian W, Jackman CP, Bursac N (2014) Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 6:024109
Bidez PR, Li S, Macdiarmid AG, Venancio EC, Wei Y, Lelkes PI (2006) Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J Biomater Sci Polym Ed 17:199–212
Boccaccini AR, Tallawi M, Rosellini E, Barbani N, Cascone MG, Rai R, Saint-Pierre G (2015) Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review. J R Soc Interface 12:20150254
Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM (2002) Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 91:189–201
Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA (2012) Platelet-rich plasma: a milieu of bioactive factors. Arthrosc J Arthrosc Relat Surg 28:429–439
Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL (2010) Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res 4:107–116
Breckwoldt K, Weinberger F, Eschenhagen T (2016) Heart regeneration. Biochim Biophys Acta Mol Cell Res 1863:1749–1759
Brines M, Cerami A (2008) Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med 264:405–432
Burridge PW, Keller G, Gold JD, Wu JC (2012) Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10:16–28
Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC (2014) Chemically defined generation of human cardiomyocytes. Nat Methods 11:855–860
Cahan P, Daley GQ (2013) Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol 14:357–368
Cao J, Li X, Chang N, Wang Y, Lei J, Zhao D, Gao K, Jin Z (2015) Dual-modular molecular imaging to trace transplanted bone mesenchymal stromal cells in an acute myocardial infarction model. Cytotherapy 17:1365–1373
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084
Chen Y, Zeng D, Ding L, Li XL, Liu XT, Li WJ, Wei T, Yan S, Xie JH, Wei L, Zheng QS (2015) Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biol 16:22
Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R (2000) Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101:1833–1839
Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin JE, Shaw RM, Martin GR, Stainier DYR (2010) Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci U S A 107:14662–14667
Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH (2005) Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg 34:420–424
Chow A, Stuckey DJ, Kidher E, Rocco M, Jabbour RJ, Mansfield CA, Darzi A, Harding SE, Stevens MM, Athanasiou T (2017) Human induced pluripotent stem cell-derived cardiomyocyte encapsulating bioactive hydrogels improve rat heart function post myocardial infarction. Stem Cell Rep 9:1415–1422
Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR (2006) Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 27:3631–3638
Czyz J, Wobus AM (2001) Embryonic stem cell differentiation: the role of extracellular factors. Differentiation 68:167–174
Dahlmann J, Kensah G, Kempf H, Skvorc D, Gawol A, Elliott DA, Dräger G, Zweigerdt R, Martin U, Gruh I (2013) The use of agarose microwells for scalable embryoid body formation and cardiac differentiation of human and murine pluripotent stem cells. Biomaterials 34:2463–2471
Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT (2005) Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 111:442–450
Dimmeler S, Burchfield J, Zeiher AM (2008) Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol 28:208–216
Ding M, Andersson H, Martinsson S, Sabirsh A, Jonebring A, Wang QD, Plowright AT, Drowley L (2020) Aligned nanofiber scaffolds improve functionality of cardiomyocytes differentiated from human induced pluripotent stem cell-derived cardiac progenitor cells. Sci Rep 10:13575
Dorfman J, Duong M, Zibaitis A, Pelletier MP, Shum-Tim D, Li C, Chiu RCJ, Chachques JC, Yacoub M, Rose EA, Carpentier AF (1998) Myocardial tissue engineering with autologous myoblast implantation. J Thorac Cardiovasc Surg 116:744–751
Drengk A, Zapf A, Stürmer EK, Stürmer KM, Frosch KH (2009) Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs 189:317–326
Drowley L, Koonce C, Peel S, Jonebring A, Plowright AT, Kattman SJ, Andersson H, Anson B, Swanson BJ, Wang Q-D, Brolen G (2016) Human induced pluripotent stem cell-derived cardiac progenitor cells in phenotypic screening: a transforming growth factor-β type 1 receptor kinase inhibitor induces efficient cardiac differentiation. Stem Cells Transl Med 5:164–174
Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE (2008) Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater 7:1003–1010
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689
Evans SM, Yelon D, Conlon FL, Kirby ML (2010) Myocardial lineage development. Circ Res 107:1428–1444
Foo KS, Lehtinen ML, Leung CY, Lian X, Xu J, Keung W, Geng L, Kolstad TRS, Thams S, Tik WA, Wong N, Bylund K, Zhou C, He X, Jin SB, Clarke J, Lendahl U, Li RA, Louch WE, Chien KR (2018) Human ISL1 + ventricular progenitors self-assemble into an in vivo functional heart patch and preserve cardiac function post infarction. Mol Ther 26:1644–1659
Franco CB, Chen CC, Drukker M, Weissman IL, Galli SJ (2010) Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell 6:361–368
Frey N, Linke A, Süselbeck T, Müller-Ehmsen J, Vermeersch P, Schoors D, Rosenberg M, Bea F, Tuvia S, Leor J (2014) Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv 7:806–812
Gandaglia A, Huerta-Cantillo R, Comisso M, Danesin R, Ghezzo F, Naso F, Gastaldello A, Schittullo E, Buratto E, Spina M, Gerosa G, Dettin M (2012) Cardiomyocytes in vitro adhesion is actively influenced by biomimetic synthetic peptides for cardiac tissue engineering. Tissue Eng Pt A 18:725–736
Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J (2018) Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 137:1712–1730
Gizdavic-Nikolaidis M, Travas-Sejdic J, Bowmaker GA, Cooney RP, Kilmartin PA (2004) Conducting polymers as free radical scavengers. Synth Met 140:225–232
Guan J, Wang F, Li Z, Chen J, Guo X, Liao J, Moldovan NI (2011) The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials 32:5568–5580
Guyette JP, Fakharzadeh M, Burford EJ, Tao ZW, Pins GD, Rolle MW, Gaudette GR (2013) A novel suture-based method for efficient transplantation of stem cells. J Biomed Mater Res Pt A 101(A):809–818
Han J, Wu Q, Xia Y, Wagner MB, Xu C (2016) Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation. Stem Cell Res 16:740–750
Hansen KJ, Favreau JT, Guyette JP, Tao ZW, Coffin ST, Cunha-Gavidia A, D’Amore B, Perreault LR, Fitzpatrick JP, Demartino A, Gaudette GR (2016) Functional effects of delivering human mesenchymal stem cell-seeded biological sutures to an infarcted heart. Biores Open Access 5:249–260
Hansen KJ, Laflamme MA, Gaudette GR (2018) Development of a contractile cardiac fiber from pluripotent stem cell derived cardiomyocytes. Front Cardiovasc Med 5:52
Hartgerink JD, Beniash E, Stupp SI (2001) Self-assembly and mineralization of peptide-amphiphile nanofibers. Science (80- ) 294:1684–1688
Hashemi S, Soleimani M, Zargarian SS et al (2009) In vitro differentiation of human cord blood-derived unrestricted somatic stem cells into hepatocyte-like cells on poly (ε-caprolactone) nanofiber scaffolds. Cells Tissues Organs 190(3):135–149
Hernández D, Millard R, Sivakumaran P, Wong RCB, Crombie DE, Hewitt AW, Liang H, Hung SSC, Pébay A, Shepherd RK, Dusting GJ, Lim SY (2016) Electrical stimulation promotes cardiac differentiation of human induced pluripotent stem cells. Stem Cells Int 2016:1–12
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Deliv Rev 64:18–23
Hu G, Li D (2007) Three-dimensional modeling of transport of nutrients for multicellular tumor spheroid culture in a microchannel. Biomed Microdevices 9:315–323
Hu J, Shi J, Zhang F, Lei L, Li X, Wang L, Liu L, Chen Y (2010) High resolution and hybrid patterning for single cell attachment. Microelectron Eng 87:726–729
Huang NF, Serpooshan V, Morris VB, Sayed N, Pardon G, Abilez OJ, Nakayama KH, Pruitt BL, Wu SM, Yoon Y, Zhang J, Wu JC (2018) Big bottlenecks in cardiovascular tissue engineering. Commun Biol 1:199
Hughes CS, Postovit LM, Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10:1886–1890
Ishida M, Miyagawa S, Saito A, Fukushima S, Harada A, Ito E, Ohashi F, Watabe T, Hatazawa J, Matsuura K, Sawa Y (2019) Transplantation of human-induced pluripotent stem cell-derived cardiomyocytes is superior to somatic stem cell therapy for restoring cardiac function and oxygen consumption in a porcine model of myocardial infarction. Transplantation 103:291–298
Itzhaki I, Rapoport S, Huber I, Mizrahi I, Zwi-Dantsis L, Arbel G, Schiller J, Gepstein L (2011) Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS One 6:e18037
Jaffe RB (2008) Induction of pluripotent stem cells from adult human fibroblasts by defined factors: Commentary. Obstet Gynecol Surv 63:153
Janes KA, Wang CC, Holmberg KJ, Cabral K, Brugge JS (2010) Identifying single-cell molecular programs by stochastic profiling. Nat Methods 7:311–317. nature.com
Joanne P, Kitsara M, Boitard SE, Naemetalla H, Vanneaux V, Pernot M, Larghero J, Forest P, Chen Y, Menasché P, Agbulut O (2016) Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials 80:157–168
Jun HW, Yuwono V, Paramonov SE, Hartgerink JD (2005) Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv Mater 17:2612–2617
Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL (2012a) Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med 4:180–191
Jung D, Minami I, Patel S, Lee J, Jiang B, Yuan Q, Li L, Kobayashi S, Chen Y, Lee KB, Nakatsuji N (2012b) Incorporation of functionalized gold nanoparticles into nanofibers for enhanced attachment and differentiation of mammalian cells. J Nanobiotechnol 10:23
Kai D, Prabhakaran MP, Jin G, Ramakrishna S (2011) Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res Pt B Appl Biomater 98B:379–386
Kai D, Wang QL, Wang HJ, Prabhakaran MP, Zhang Y, Tan YZ, Ramakrishna S (2014) Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater 10:2727–2738
Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P (1998) Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A 95:8801–8805
Kaneko N, Matsuda R, Toda M, Shimamoto K (2011) Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am J Physiol Heart Circ Physiol 300:H754–H761
Kang BJ, Kim H, Lee SK, Kim J, Shen Y, Jung S, Kang KS, Im SG, Lee SY, Choi M, Hwang NS, Cho JY (2014) Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater 10:3007–3017
Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G (2011) Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8:228–240
Kemp CD, Conte JV (2012) The pathophysiology of heart failure. Cardiovasc Pathol 21:365–371
Khan M, Xu Y, Hua S, Johnson J, Belevych A, Janssen PML, Gyorke S, Guan J, Angelos MG (2015) Evaluation of changes in morphology and function of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) cultured on an aligned-nanofiber cardiac patch. PLoS One 10:e0126338
Kharaziha M, Shin SR, Nikkhah M, Topkaya SN, Masoumi N, Annabi N, Dokmeci MR, Khademhosseini A (2014) Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 35:7346–7354
Khil MS, Bhattarai SR, Kim HY, Kim SZ, Lee KH (2005) Novel fabricated matrix via electrospinning for tissue engineering. J Biomed Mater Res Pt B Appl Biomater 72:117–124
Khuspe GD, Chougule MA, Navale ST, Pawar SA, Patil VB (2014) Camphor sulfonic acid doped polyaniline-tin oxide hybrid nanocomposites: synthesis, structural, morphological, optical and electrical transport properties. Ceram Int 40:4267–4276
Kim S, Ahn SE, Lee JH, Lim D-S, Kim K-S, Chung H-M, Lee S-H (2007a) A novel culture technique for human embryonic stem cells using porous membranes. Stem Cells 25:2601–2609
Kim SE, Kim BK, Gil JE, Kim SK, Kim JH (2007b) Comparative analysis of the developmental competence of three human embryonic stem cell lines in vitro. Mol Cells 23:49–56
Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A (2010) Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A 107:565–570
Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Hongguang H, Loh YH, Aryee MJ, Lensch MW, Li H, Collins JJ, Feinberg AP, Daley GQ (2011) Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol 29:1117–1119
Kingham E, Oreffo ROC (2013) Embryonic and induced pluripotent stem cells: understanding, creating, and exploiting the nano-niche for regenerative medicine. ACS Nano 7:1867–1881
Kitsara M, Joanne P, Boitard SE, Ben Dhiab I, Poinard B, Menasché P, Gagnieu C, Forest P, Agbulut O, Chen Y (2015) Fabrication of cardiac patch by using electrospun collagen fibers. Microelectron Eng 144:46–50
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH (2012) Control of stem cell fate and function by engineering physical microenvironments. Integr Biol 4:1008–1018
Kumar D, Dale TP, Yang Y, Forsyth NR (2015) Self-renewal of human embryonic stem cells on defined synthetic electrospun nanofibers. Biomed Mater 10:065017
Kuppan P, Sethuraman S (2013) PCL and PCL-gelatin nanofibers as esophageal tissue scaffolds: optimization, characterization and cell-matrix interactions nanocarriers for pancreatic cancer view project working on islets transplantation view project. J Biomed Nanotechnol 9:1540–1555
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473:326–335
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE (2007) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25:1015–1024
Lahti A, Kujala V et al (2012) Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Model Mech 5(2):220–230
Lang S, Loibl M, Herrmann M (2018) Platelet-rich plasma in tissue engineering: hype and hope. Eur Surg Res 59:265–275
Lecault V, Vaninsberghe M, Sekulovic S, Knapp DJHF, Wohrer S, Bowden W, Viel F, McLaughlin T, Jarandehei A, Miller M, Falconnet D, White AK, Kent DG, Copley MR, Taghipour F, Eaves CJ, Humphries RK, Piret JM, Hansen CL (2011) High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat Methods 8:581–589
Lee S, Serpooshan V, Tong X, Venkatraman S, Lee M, Lee J, Chirikian O, Wu JC, Wu SM, Yang F (2017) Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials 131:111–120
Lemoine MD, Mannhardt I, Breckwoldt K, Prondzynski M, Flenner F, Ulmer B, Hirt MN, Neuber C, Horváth A, Kloth B, Reichenspurner H, Willems S, Hansen A, Eschenhagen T, Christ T (2017) Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci Rep 7:5464
Leung M, Cooper A, Jana S, Tsao C-T, Petrie TA, Zhang M (2013) Nanofiber-based in vitro system for high myogenic differentiation of human embryonic stem cells. Biomacromolecules 14:4207–4216
Li J, Minami I, Yu L, Tsuji K, Nakajima M, Qiao J, Suzuki M, Shimono K, Nakatsuji N, Kotera H, Liu L, Chen Y (2016) Extracellular recordings of patterned human pluripotent stem cell-derived cardiomyocytes on aligned fibers. Stem Cells Int 2016:2634013
Li J, Minami I, Shiozaki M, Yu L, Yajima S, Miyagawa S, Shiba Y, Morone N, Fukushima S, Yoshioka M, Li S, Qiao J, Li X, Wang L, Kotera H, Nakatsuji N, Sawa Y, Chen Y, Liu L (2017a) Human pluripotent stem cell-derived cardiac tissue-like constructs for repairing the infarcted myocardium. Stem Cell Rep 9:1546–1559
Li J, Zhang F, Yu L, Fujimoto N, Yoshioka M, Li X, Shi J, Kotera H, Liu L, Chen Y (2017b) Culture substrates made of elastomeric micro-tripod arrays for long-term expansion of human pluripotent stem cells. J Mater Chem B 5:236–244
Li X, Chen YY, Wang XM, Gao K, Gao YZ, Cao J, Zhang ZL, Lei J, Jin ZY, Wang YN (2017c) Image-guided stem cells with functionalized self-assembling peptide nanofibers for treatment of acute myocardial infarction in a mouse model. Am J Transl Res 9:3723–3731
Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP (2012) Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 109:E1848–E1857
Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP (2013) Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc 8:162–175
Liang G, Zhang Y (2013) Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell 13:149–159
Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC (2013) Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 127:1677–1691
Lim S et al (2009) Electrospun scaffolds for stem cell engineering. Elsevier, Amsterdam
Lin CC, Anseth KS (2009) PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res 26:631–643
Lin YD, Ko MC, Wu ST, Li SF, Hu JF, Lai YJ, Harn HI-C, Laio IC, Yeh ML, Yeh HI, Tang MJ, Chang KC, Su FC, Wei EIH, Lee ST, Chen JH, Hoffman AS, Wu WT, Hsieh PCH (2014) A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater Sci 2:567–580
Liu X, Wang X, Horii A, Wang X, Qiao L, Zhang S, Cui FZ (2012) In vivo studies on angiogenic activity of two designer self-assembling peptide scaffold hydrogels in the chicken embryo chorioallantoic membrane. Nanoscale 4:2720–2727
Liu L, Yoshioka M, Nakajima M, Ogasawara A, Liu J, Hasegawa K, Li S, Zou J, Nakatsuji N, Ichiro KK, Chen Y (2014) Nanofibrous gelatin substrates for long-term expansion of human pluripotent stem cells. Biomaterials 35:6259–6267
Liu L, Ichiro KK, Yoshioka M, Nakajima M, Li J, Fujimoto N, Terada S, Tokunaga Y, Koyama Y, Sato H, Hasegawa K, Nakatsuji N, Chen Y (2017) Nano-on-micro fibrous extracellular matrices for scalable expansion of human ES/iPS cells. Biomaterials 124:47–54
Liyang Y, Zhang H, Feng QS, Cai MB, Deng W, Qin D, Yun JP, Tsao GSW, Kan T, Esteban AM, Pei D, Zeng YX (2013) The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin J Cancer 32:205–212
Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y (2009) Heart disease and stroke statistics—2009 update. Circulation 119:e21
Lu WN, Lü SH, Bin WH, Li DX, Duan CM, Liu ZQ, Hao T, He WJ, Xu B, Fu Q, Song YC, Xie XH, Wang CY (2009) Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng Pt A 15:1437–1447
Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, Conklin BR, Grigoropoulos CP, Healy KE (2014) Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials 35:1367–1377
Macrí-Pellizzeri L, Pelacho B, Sancho A, Iglesias-García O, Simón-Yarza AM, Soriano-Navarro M, González-Granero S, García-Verdugo JM, De-Juan-Pardo EM, Prosper F (2015) Substrate stiffness and composition specifically direct differentiation of induced pluripotent stem cells. Tissue Eng Pt A 21:1633–1641
Mahlstedt MM, Anderson D, Sharp JS, McGilvray R, Barbadillo Muñoz MD, Buttery LD, Alexander MR, Rose FRAJ, Denning C (2010) Maintenance of pluripotency in human embryonic stem cells cultured on a synthetic substrate in conditioned medium. Biotechnol Bioeng 105:130–140
Martins AM, Vunjak-Novakovic G, Reis RL (2014) The current status of iPS cells in cardiac research and their potential for tissue engineering and regenerative medicine. Stem Cell Rev Rep 10:177–190
Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE (2016) In vitro cardiac tissue models: current status and future prospects. Adv Drug Deliv Rev 96:203–213
Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C (2011) Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur Heart J 32:952–962
Matsa E, Burridge PW, Wu JC (2014) Human stem cells for modeling heart disease and for drug discovery. Sci Transl Med 6:239ps6
Meiry G, Reisner Y, Feld Y, Goldberg S, Rosen M, Ziv N, Binah O (2001) Evolution of action potential propagation and repolarization in cultured neonatal rat ventricular myocytes. J Cardiovasc Electrophysiol 12:1269–1277
Mihardja SS, Gonzales JA, Gao D, Sievers RE, Fang Q, Stillson CA, Yu J, Peng M, Lee RJ (2013) The effect of a peptide-modified thermo-reversible methylcellulose on wound healing and LV function in a chronic myocardial infarction rodent model. Biomaterials 34:8869–8877
Minami I, Yamada K, Otsuji TG, Yamamoto T, Shen Y, Otsuka S, Kadota S, Morone N, Barve M, Asai Y, Tenkova-Heuser T, Heuser JE, Uesugi M, Aiba K, Nakatsuji N (2012) A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep 2:1448–1460
Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12:459–465
Mohammadi Amirabad L, Massumi M, Shamsara M, Shabani I, Amari A, Mossahebi Mohammadi M, Hosseinzadeh S, Vakilian S, Steinbach SK, Khorramizadeh MR, Soleimani M, Barzin J (2017) Enhanced cardiac differentiation of human cardiovascular disease patient-specific induced pluripotent stem cells by applying unidirectional electrical pulses using aligned electroactive nanofibrous scaffolds. ACS Appl Mater Interfaces 9:6849–6864
Moon JJ, Hahn MS, Kim I, Nsiah BA, West JL (2009) Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng Pt A 15:579–585
Mordwinkin NM, Lee AS, Wu JC (2013) Patient-specific stem cells and cardiovascular drug discovery. J Am Med Assoc 310:2039–2040
Moyes KW, Sip CG, Obenza W, Yang E, Horst C, Welikson RE, Hauschka SD, Folch A, Laflamme MA (2013) Human embryonic stem cell-derived cardiomyocytes migrate in response to gradients of fibronectin and Wnt5a. Stem Cells Dev 22:2315–2325
Müller-Ehmsen J, Peterson KL, Kedes L, Whittaker P, Dow JS, Long TI, Laird PW, Kloner RA (2002) Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation 105:1720–1726
Mummery C, Ward D, Van Den Brink CE, Bird SD, Doevendans PA, Opthof T, Brutel De La Riviere A, Tertoolen L, Van Der Heyden M, Pera M (2002) Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat 200:233–242
Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, Van den Brink S, Hassink R, Van der Heyden M, Opthof T, Pera M, Brutel de la Riviere A, Passier R, Tertoolen L (2003) Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107:2733–2740
Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT (2006) Differentiation potential of mesenchymal stem cells of different origin. Bull Exp Biol Med 141:147–151
Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med 366:54–63
Nakayama KH, Joshi PA, Lai ES, Gujar P, Joubert LM, Chen B, Huang NF (2015) Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Regen Med 10:745–755
Nakayama KH, Alcazar C, Yang G, Quarta M, Paine P, Doan L, Davies A, Rando TA, Huang NF (2018) Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. NPJ Regen Med 3:16
Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, Jan T, Wilson KD, Leong D, Rosenberg J, Yao M, Robbins RC, Wu JC (2011) Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest 121:1217–1221
Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge PW, Patlolla B, Lee AS, Wu H, Beygui RE, Wu SM, Robbins RC, Bers DM, Wu JC (2013) Screening drug-induced arrhythmia events using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 128:S3
Nguyen PK, Riegler J, Wu JC (2014) Stem cell imaging: from bench to bedside. Cell Stem Cell 14:431–444
Nishikawa T, Nonomura M, Arai K, Hayashi J, Sawadaishi T, Nishiura Y, Hara M, Shimomura M (2003) Micropatterns based on deformation of a viscoelastic honeycomb mesh. Langmuir 19:6193–6201
Noseda M, Peterkin T, Simões FC, Patient R, Schneider MD (2011) Cardiopoietic factors extracellular signals for cardiac lineage commitment. Circ Res 108:129–152
Okita K, Yamanaka S (2011) Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci 366:2198–2207
Olson EN (2006) Gene regulatory networks in the evolution and development of the heart. Science (80- ) 313:1922–1927
Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P (2003) Bone marrow stem cells regenerate infarcted myocardium. In: Pediatric transplantation, pp 86–88
Orlova Y, Magome N, Liu L, Chen Y, Agladze K (2011) Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 32:5615–5624
Parker KK, Ingber DE (2007) Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc B Biol Sci 362:1267–1279
Parrag IC, Zandstra PW, Woodhouse KA (2012) Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol Bioeng 109:813–822
Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MHC, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgard PO, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA (2015) Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol 17:994–1003
Pekkanen-Mattila M, Häkli M, Pölönen RP, Mansikkala T, Junnila A, Talvitie E, Koivisto JT, Kellomäki M, Aalto-Setälä K (2019) Polyethylene terephthalate textiles enhance the structural maturation of human induced pluripotent stem cell-derived cardiomyocytes. Materials (Basel) 12:1805
Pick M (2009) Directed differentiation of embryonic stem cells. In: Emerging technology platforms for stem cells, pp 203–233
Plowright AT, Engkvist O, Gill A, Knerr L, Wang QD (2014) Heart regeneration: opportunities and challenges for drug discovery with novel chemical and therapeutic methods or agents. Angew Chem Int Ed 53:4056–4075
Pouget J-P, Pougeta JP, Hsub C-H, Mac AG, Epsteind DAJ (1995) Structural investigation of metallic PAN-CSA and some of its derivatives anion effects in organic conductors view project structural investigation of metallic PAN-CSA and some of its derivatives. Artic Synth Met 69:119–120
Prabhakaran M et al (2011) Electrospun biocomposite nanofibrous patch for cardiac tissue engineering. Biomed Mater 6:055001
Prabhakaran MP, Mobarakeh LG, Kai D, Karbalaie K, Nasr-Esfahani MH, Ramakrishna S (2014) Differentiation of embryonic stem cells to cardiomyocytes on electrospun nanofibrous substrates. J Biomed Mater Res Pt B Appl Biomater 102:447–454
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction. Circ Res 119:91–112
Puig-Sanvicens VAC, Semino CE, zur Nieden NI (2015) Cardiac differentiation potential of human induced pluripotent stem cells in a 3D self-assembling peptide scaffold. Differentiation 90:101–110
Qazi TH, Rai R, Boccaccini AR (2014) Tissue engineering of electrically responsive tissues using polyaniline based polymers: a review. Biomaterials 35:9068–9086
Radisic M, Christman KL (2013) Materials science and tissue engineering: repairing the heart. In: Mayo clinic proceedings, pp 884–898
Rana P, Anson B, Engle S, Will Y (2012) Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: bioenergetics and utilization in safety screening. Toxicol Sci 130:117–131
Rao C, Prodromakis T, Kolker L, Chaudhry UAR, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM (2013) The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 34:2399–2411
Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4:446–456
Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, Domogatskaya A, Xiao Z, Damdimopoulou P, Sheikhi M, Inzunza J, Nilsson AS, Baker D, Kuiper R, Sun Y, Blennow E, Nordenskjöld M, Grinnemo KH, Kere J, Betsholtz C, Hovatta O, Tryggvason K (2014) Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun 5:3195
Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ (2014) Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J Biomech Eng 136:051005
Rowland TJ, Miller LM, Blaschke AJ, Doss EL, Bonham AJ, Hikita ST, Johnson LV, Clegg DO (2010) Roles of integrins in human induced pluripotent stem cell growth on matrigel and vitronectin. Stem Cells Dev 19:1231–1240
Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE (2016) Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 134:1557–1567
Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP (2011) Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol 31:e72
Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, Wu JC (2014) Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol 64:436–448
Sapir Y, Kryukov O, Cohen S (2011) Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials 32:1838–1847
Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T (2008) Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118:S145–S152
Şenel Ayaz HG, Perets A, Ayaz H, Gilroy KD, Govindaraj M, Brookstein D, Lelkes PI (2014) Textile-templated electrospun anisotropic scaffolds for regenerative cardiac tissue engineering. Biomaterials 35:8540–8552
Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, Vunjak-Novakovic G (2009) Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp Cell Res 315:3611–3619
Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T (2006) Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J 20:708–710
Sill TJ, von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006
Smallwood SA, Lee HJ, Angermueller C, Krueger F, Saadeh H, Peat J, Andrews SR, Stegle O, Reik W, Kelsey G (2014) Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods 11:817–820
Song JH, Yoon BH, Kim HE, Kim HW (2008) Bioactive and degradable hybridized nanofibers of gelatin-siloxane for bone regeneration. J Biomed Mater Res Pt A 84:875–884
Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L (1998) Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 82:482–495
Stehbens WE, Martin BJ (1993) Ultrastructural alterations of collagen fibrils in blood vessel walls. Connect Tissue Res 29:319–331
Sun Y, Chen CS, Fu J (2012) Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys 41:519–542
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Tang Y, Liu L, Li J, Yu L, Severino FPU, Wang L, Shi J, Tu X, Torre V, Chen Y (2016a) Effective motor neuron differentiation of hiPSCs on a patch made of crosslinked monolayer gelatin nanofibers. J Mater Chem B 4:3305–3312
Tang Y, Liu L, Li J, Yu L, Wang L, Shi J, Chen Y (2016b) Induction and differentiation of human induced pluripotent stem cells into functional cardiomyocytes on a compartmented monolayer of gelatin nanofibers. Nanoscale 8:14530–14540
Tao ZW, Favreau JT, Guyette JP, Hansen KJ, Lessard J, Burford E, Pins GD, Gaudette GR (2017) Delivering stem cells to the healthy heart on biological sutures: effects on regional mechanical function. J Tissue Eng Regen Med 11:220–230
Tate MC, Shear DA, Hoffman SW, Stein DG, Archer DR, LaPlaca MC (2002) Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplant 11:283–295
Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, Fukuda K (2013) Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12:127–137
Torabi M, Abazari MF, Zare Karizi S, Kohandani M, Hajati-Birgani N, Norouzi S, Nejati F, Mohajerani A, Rahmati T, Mokhames Z (2020) Efficient cardiomyocyte differentiation of induced pluripotent stem cells on PLGA nanofibers enriched by platelet-rich plasma. Polym Adv Technol 32:1168–1175
Trollinger DR, Rivkah Isseroff R, Nuccitelli R (2002) Calcium channel blockers inhibit galvanotaxis in human keratinocytes. J Cell Physiol 193:1–9
Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK (2011) Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One 6:e23657
Van Dijk A, Niessen HWM, Ursem W, Twisk JWR, Visser FC, Van Milligen FJ (2008) Accumulation of fibronectin in the heart after myocardial infarction: a putative stimulator of adhesion and proliferation of adipose-derived stem cells. Cell Tissue Res 332:289–298
Van Tomme SR, Storm G, Hennink WE (2008) In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm 355:1–18
Vasita R, Katti DS (2006) Nanofibers and their applications in tissue engineering. Int J Nanomedicine 1:15–30
Wang H, Hao J, Hong CC (2011) Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem Biol 6:192–197
Wang B, Tu X, Wei J, Wang L, Chen Y (2019) Substrate elasticity dependent colony formation and cardiac differentiation of human induced pluripotent stem cells. Biofabrication 11:015005
Wanjare M, Hou L, Nakayama KH, Kim JJ, Mezak NP, Abilez OJ, Tzatzalos E, Wu JC, Huang NF (2017) Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater Sci 5:1567–1578
Wanjare M, Kawamura M, Hu C, Alcazar C, Wang H, Woo YJ, Huang NF (2019) Vascularization of engineered spatially patterned myocardial tissue derived from human pluripotent stem cells in vivo. Front Bioeng Biotechnol 7:208
Warren L, Bryder D, Weissman IL, Quake SR (2006) Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci U S A 103(47):17807–17812
Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M (2004) Fibronectin promotes VEGF-induced CD34+ cell differentiation into endothelial cells. J Vasc Surg 39:655–660
Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M (2011) Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res 109:360–364
Willerth SM, Vasconcelos S, Huang NF, Wanjare M, Kawamura M, Hu C, Alcazar C, Wang H, Woo YJ (2019) Vascularization of engineered spatially patterned myocardial tissue derived from human pluripotent stem cells in vivo. Front Bioeng Biotechnol 7:208
Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero-Nieto FJ, Sánchez Castillo M, Oedekoven CA, Diamanti E, Schulte R, Ponting CP, Voet T, Caldas C, Stingl J, Green AR, Theis FJ, Göttgens B (2015) Combined single-cell functional and gene expression analysis resolves heterogeneity within stem cell populations. Cell Stem Cell 16:712–724
Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M (2014) Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 14:869–882
Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y (2009) The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30:354–362
Yoon BS, Yoo SJ, Lee JE, You S, Lee HT, Yoon HS (2006) Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation 74:149–159
Yu J, Gu Y, Du KT, Mihardja S, Sievers RE, Lee RJ (2009) The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials 30:751–756
Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ (2010) The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31:7012–7020
Yu L, Li J, Minami I, Qu X, Miyagawa S, Fujimoto N, Hasegawa K, Chen Y, Sawa Y, Kotera H, Liu L (2019) Clonal isolation of human pluripotent stem cells on nanofibrous substrates reveals an advanced subclone for cardiomyocyte differentiation. Adv Healthc Mater 8:1900165
Zamani M, Karaca E, Huang NF (2018) Multicellular interactions in 3D engineered myocardial tissue. Front Cardiovasc Med 5:147
Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ (2012) Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 111:1125–1136
Zhao M, Pu J, Forrester JV, McCaig CD (2002) Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field. FASEB J 16:857–859
Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E (2005) Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 26:5330–5338
Acknowledgment
This work was supported by the Science and Engineering Research Board (SERB) research grants (CRG/2020/000113) and Council of Scientific & Industrial Research (CSIR) sponsored project (22(0846)/20/EMR-II).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khan, R.S., Wani, T.U., Rather, A.H., Beigh, M.A., Sheikh, F.A. (2021). Using Nanofiber Scaffolds for the Differentiation of Induced Pluripotent Stem Cells into Cardiomyocytes: The Latest Approaches in Tissue Engineering. In: Sheikh, F.A. (eds) Engineering Materials for Stem Cell Regeneration. Springer, Singapore. https://doi.org/10.1007/978-981-16-4420-7_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-4420-7_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4419-1
Online ISBN: 978-981-16-4420-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)