Abstract
Vasopressors are extensively used to provide cardiovascular support in the critically ill by increasing vascular tone and therefore mean arterial pressure. There are a number of indications for vasopressors in critical care, but their predominant role is in treating hypotension associated with circulatory shock. This chapter presents the key principles underlying the choice and use of vasopressors in clinical practice, focussing on practical implications for the clinician. The main adrenergic and non-adrenergic agents currently available are discussed in detail, as well as novel candidates emerging alongside them. For each class and agent, the relevant pharmacology, clinical indications, advantages and pitfalls are described, supported by up-to-date medical evidence.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning ObjectivesVasopressors are a potent class of pharmacological agents used to produce vasoconstriction in critically ill patients. Since vascular resistance is an important determinant of mean arterial pressure (MAP), vasopressors have a direct effect on it and are widely used for cardiovascular support.
This chapter outlines the main vasoconstrictors used in current practice, their mechanisms of action, advantages, pitfalls and clinical applications with up-to-date medical evidence where available. This will equip the reader with the knowledge to appreciate the choice of vasopressors in the ICU, based on clinical indication, desired effect and side-effect profile.
1 Introduction
The main role of vasopressors is to improve mean arterial pressure (MAP), and thereby tissue perfusion, by increasing arterial vasomotor tone, given that:
There may be a need to increase MAP for a number of reasons including management of shock, maintenance of cerebral perfusion in brain injury and optimisation of renal perfusion in acute kidney injury (AKI) and hepato-renal syndrome.
The main distinction between vasopressors and inotropes lies in their lack of a direct effect on cardiac output (CO), though indirect ‘reflex’ cardiovascular changes often occur. In addition to this, several drugs display both inotropic and vasopressor characteristics. Vasoconstriction can be mediated by various classes of receptors, and vasopressors can be classified based on their action on adrenergic or other receptors.
Some essential concepts should be considered with all vasoactive agents:
-
Prior to initiating vasoconstrictive therapy, adequate fluid resuscitation must be carried out to restore circulating volume, improve CO and optimise peripheral perfusion.
-
Most vasopressors follow variable dose-response relationships, and their effects depend on concentration and patient factors. This is especially relevant given the complications that may ensue, which are discussed later in this chapter.
-
The dosing of vasopressors can be challenging. As a rule of thumb, it may be appropriate to administer low initial doses and gradually titrate up. This should be based on patient response, desired outcome and monitoring for side effects, rather than aiming for a predetermined infusion dose. Most vasopressors have a relatively short half-life and their dosage can be adjusted as necessary.
-
Administration is generally through centrally inserted venous access. Peripheral routes are more prone to extravasation, which can result in devastating necrosis of local tissue.
2 Adrenergic Vasopressors
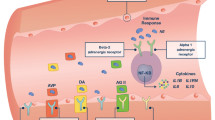
Adrenoceptors are G protein-coupled receptors found throughout the human body; the most relevant ones within the cardiovascular system include alpha- and beta-receptors. Postsynaptic alpha-1 and to a lesser extent alpha-2 receptors lead to vasoconstriction by direct stimulation of vascular smooth muscle. In the myocardium, alpha-1 receptors have also been shown to have a mild inotropic but not chronotropic effect [1, 2]. Moreover, presynaptic alpha-2 receptors are part of a negative feedback loop that inhibits the release of noradrenaline.
Beta-1 receptors are most abundant in the myocardium, where they produce both direct inotropic and chronotropic effects, without significantly affecting vascular calibre. They also lead to increased renin release and bladder relaxation. Their beta-2 counterparts are responsible for smooth muscle relaxation, with resulting bronchodilation and vasodilation. Within these receptor classes, several further subtypes have been identified, though their clinical relevance is uncertain given that no subtype-specific drugs currently exist.
Polymorphisms of adrenergic receptor genes have been characterised particularly in chronic illnesses such as hypertension, coronary artery disease and heart failure. While they’re unlikely to play a causative role in their pathogenesis, they might affect the pharmacodynamics of adrenergic drugs [3]. The importance of this in ICU patients is not yet clear and will require further studies targeted specifically at this population. In septic shock, a beta-2 polymorphism has been linked to increased mortality and noradrenaline requirements [4].
Adrenergic vasopressors include endogenous catecholamines such as noradrenaline, adrenaline and dopamine and synthetic ones, commonly phenylephrine, ephedrine and metaraminol.
Noradrenaline
An endogenous catecholamine and neurotransmitter, noradrenaline is primarily a direct alpha-1 agonist with modest beta-1 and beta-2 action in the cardiovascular system. Overall, it produces significant systemic vasoconstriction with minimal impact on heart rate; its mild beta-agonist effect is counteracted by a reflex bradycardia from increased afterload. The rise in MAP and consequently in diastolic pressure is thought to improve coronary artery blood flow, which together with beta-1 activation can result in a modest increase in stroke volume. However, cardiac output is variable and might actually decrease because of greater afterload and reflex bradycardia. The half-life of exogenous noradrenaline is 1.5 min [5].
Noradrenaline’s principal application is in septic shock, where current international guidelines advocate for its use as a first-line vasopressor [6]. Noradrenaline is associated with lower mortality rates and lower incidence of tachyarrhythmia compared to dopamine [7, 8], while adrenaline is more likely to lead to tachycardia [9]. Interestingly, a national noradrenaline shortage period in the United States was met by a rise in phenylephrine use for the treatment of septic shock at affected centres, corresponding with greater inpatient mortality [10].
Noradrenaline may also be used in other causes of shock. In cardiogenic shock, vasoactive therapy can help maintain MAP and improve coronary blood flow as a supportive bridge to definitive diagnosis and treatment. Noradrenaline might often need to be used in combination with dobutamine in this situation [11]. It is associated with a lower incidence of tachycardia, hyperlactataemia and arrhythmia compared to adrenaline [12] and lower mortality than dopamine [13].
Noradrenaline also has a role in critical hypotension from traumatic haemorrhagic shock, while volume replacement is being achieved: in addition to its systemic effect, venoconstriction particularly at the splanchnic level is thought to help divert more volume into the arterial circulation [14, 15].
Additionally, it can be used in type 1 hepato-renal syndrome (HRS) if terlipressin is contraindicated [16] and in acute brain injury to achieve a target MAP for the desired cerebral perfusion pressure (CPP) [17]. Hypertensive therapy with noradrenaline can be trialled as part of haemodynamic augmentation in the treatment and prevention of vasospasm following subarachnoid haemorrhage (SAH).
Adrenaline
The pharmacology of adrenaline is broader than that of its immediate precursor noradrenaline, with preferential activation of beta-1 and beta-2 receptors over alpha-1 receptors.
At lower doses, it exerts predominantly a beta-1 agonist function with minimal change to vascular tone, as beta-2 and alpha-1 stimulation in vascular smooth muscle counteract one another. This results in direct inotropy and chronotropy, thus increasing cardiac output. At higher doses, its alpha-agonist properties prevail, resulting in vasoconstriction. The half-life of adrenaline is 2–3 min given intravenously.
The use of adrenaline is currently recommended in international resuscitation guidelines for cardiac arrest [18, 19], mainly because of its alpha-adrenergic effect. However, evidence is limited and largely drawn from out-of-hospital events, and while some studies have found a greater likelihood of return of spontaneous circulation with adrenaline, it may not improve survival nor neurological outcome at discharge [20]. Moreover, early administration (<2 min from first defibrillation) in shockable rhythms is associated with worse prognosis [21]. Its role in out-of-hospital cardiac arrest is being investigated in the PARAMEDIC-2 trial [22].
Adrenaline remains a suitable second-line agent in septic shock if noradrenaline alone is not sufficient to achieve the target MAP [6]. However, it may cause hyperlactataemia which may not be related to any adverse effect but can complicate the use of serum lactate as a resuscitation target.
In cardiogenic shock, the combination of noradrenaline and dobutamine is preferred to adrenaline because of the greater risk of tachycardia and the hyperlactataemia associated with the latter [11, 12].
Intramuscular/intravenous adrenaline is considered the first-line agent for use in anaphylactic shock [23], with its benefits being linked to beta-2-mediated bronchodilation in addition to vasoconstriction.
Dopamine
Dopamine is a direct precursor to noradrenaline, and, unlike the other endogenous catecholamines, its agonist action extends beyond adrenergic receptors. It is a potent activator of dopamine receptors, as well as beta- and alpha-adrenoceptors. Dopaminergic receptors too are G protein-coupled and various subtypes exist; these can be grouped into D1- and D2-like, though dopamine is an unselective agonist of both. Their activation in myocardial tissue produces a degree of inotropy and chronotropy, less pronounced than that from adrenoceptors. In the vascular system, the overall effect is of vasodilation particularly in the renal, mesenteric and splanchnic circulations [24].
The physiological effects of dopamine on various receptor classes are largely dose-dependent [25, 26] and at some concentrations can act almost purely as a vasopressor. This must be considered when selecting the initial dose and especially before up-titrating it.
-
Low-dose dopamine (<3 μg/kg/min) exerts mainly a dopaminergic effect, with consequent reduction in vascular tone and mild increase in cardiac output. Moreover, in the kidneys it acts as a natriuretic hormone reducing sodium reabsorption in the proximal convoluted tubule and increasing water excretion [27].
-
Intermediate doses (<10 μg/kg/min) lead to activation of beta-1 receptors and greater inotropic effect, usually accompanied by an increase in heart rate.
-
At higher doses (>10 μg/kg/min), dopamine is more akin to a vasoconstrictor with predominantly alpha-1 effects.
The plasma concentration of dopamine can be very variable and is often not reflected by the infusion rates described above, particularly in the critically ill where its clearance is less predictable. The titration of dopamine should therefore be guided by its desired use and clinical effect. Its half-life given intravenously is approximately 2 min.
The applications of dopamine in the ICU setting have become somewhat limited. In septic shock, because of greater mortality and incidence of tachyarrhythmia, it has been superseded by noradrenaline [7, 8]. Its role in this population is confined to those with bradycardia and low risk of arrhythmia [6]. Moreover, the concept of ‘renal-dose’ dopamine in critical care has been largely abandoned, as there is no proven benefit to renal function in this group of patients [28, 29]. The increase in diuresis sometimes reported with low-dose dopamine is likely to be mediated by its natriuretic effect rather than any improvement in glomerular filtration, given its unselective vasodilation of both afferent and efferent arterioles. In cardiogenic shock, dopamine is associated with increased mortality and arrhythmic events compared to noradrenaline [13].
Phenylephrine
This synthetic selective alpha-1 agonist has virtually no beta-activity. Because of this, the resulting increase in afterload can lead to unopposed reflex bradycardia and reduction in cardiac output.
Phenylephrine can be used as a bolus in the rapid correction of hypotension of abrupt onset or in case of concomitant pre-existing tachycardia. Further applications include severe hypotension in fixed output states such as aortic stenosis and hypertrophic obstructive cardiomyopathy to reduce the left ventricular outflow tract gradient. As detailed above, the rise in mortality seen with increased phenylephrine use during a national noradrenaline shortage would suggest caution against more widespread use [10].
Ephedrine
Similar to adrenaline, ephedrine is a direct alpha- and beta-agonist, though a weaker one. Its main effect is instead via an indirect mechanism, acting on peripheral sympathetic neurons as a noradrenaline-releasing agent and to inhibit its reuptake [30]. This can lead to pronounced tachyphylaxis, limiting its usefulness particularly in critically ill patients with a generally depleted pool of catecholamines. It is used in boluses to correct transient hypotension in anaesthetic practice.
Metaraminol
Though mostly a vasoconstrictor through alpha-agonist effects, metaraminol too is a modest noradrenaline-releasing agent. In the ICU setting, bolus doses can help reverse or prevent hypotension during endotracheal intubation. It can also be administered peripherally during the initial stabilisation of an unwell patient, such as in the emergency department, or before central venous access is obtained.
3 Non-adrenergic Vasopressors
Catecholamines can be associated with an increase in myocardial oxygen demand and tachyarrhythmia; thus non-adrenergic compounds have gained attention as possible adjunctive vasopressor agents. However, it is unclear whether they confer any improvement in overall mortality [31]. The main non-adrenergic vasopressors are discussed here.
Vasopressin
Vasopressin is an endogenous stress hormone released by the posterior pituitary gland mainly in response to increased serum osmolality, hypovolaemia and hypotension. Stimulation of vasopressin receptors leads to several effects, including vasoconstriction especially in the muscle, skin and splanchnic vessels (V1a receptors) and vasodilation in pulmonary and coronary circulation; water retention (V2 receptors); and release of ACTH from the anterior pituitary (V1b receptors) [32]. The use of vasopressin in critical care stems from evidence that its levels are significantly decreased in patients with septic shock receiving catecholamines [33]. In health, the effects of vasopressin on the circulatory system are not dramatic. However, in shock states it leads to vasoconstriction, an effect reinforced by its blockade of potassium-dependent ATP channels [34].
In septic shock, vasopressin can be added to noradrenaline as a second-line agent for hypotension unresponsive to catecholamine therapy or to reduce the dose of noradrenaline [6]. In a large randomised controlled trial, vasopressin reduced mortality in less severe shock (those that required lower noradrenaline doses initially) [35]. Early administration was also associated with a decreased requirement for renal replacement therapy (although there was no impact on renal failure outcomes), and a sparing effect on noradrenaline dose is consistently seen [36, 37].
Terlipressin
Terlipressin is a synthetic prodrug and vasopressin analogue with a longer duration of action and slightly greater selectivity for V1a receptors [38]; its longer half-life means that it can be administered as intravenous boluses.
In upper GI variceal haemorrhage, terlipressin is used as complementary treatment at presentation until definitive haemostasis is achieved [39]. It is more effective than vasopressin and similar to balloon tamponade in controlling the bleeding [40, 41], with mortality rates similar to octreotide and somatostatin [39].
In addition to this, terlipressin may be of benefit in hepato-renal syndrome (HRS), given in combination with albumin, to counteract splanchnic vasodilation and improve renal function [16]. The evidence for this is largely confined to type 1 HRS and without concurrent sepsis, and it has no advantage over noradrenaline when the patient is managed in an area where the latter can be safely administered.
Angiotensin II
The renin-angiotensin-aldosterone system is one of several physiological rescue mechanisms activated in response to hypotension. The vasoactive properties of angiotensin II are mediated largely via AT-1 receptors; they include increased vascular tone, aldosterone secretion, salt and water retention and vasopressin release. It also has roles in coagulation and the pro-inflammatory response [42]. Angiotensin-converting enzyme in the pulmonary circulation converts angiotensin I into angiotensin II, and it is thought that vasodilatory shock can impair this process due to insults to the pulmonary vasculature.
In vasodilatory shock, exogenous angiotensin II in addition to high-dose vasopressors can achieve an early (in the first 3 h) improvement in MAP with a sparing effect on background vasopressor dose and no significant adverse events [43]. However, more evidence is required to fully understand its effect on important clinical outcomes.
4 Adverse Events
Therapy with vasopressors must be monitored closely, not least because their cardiovascular effects can produce serious adverse events, including tissue hypoperfusion, tachyarrhythmia and myocardial infarction.
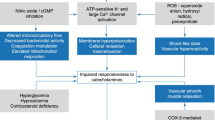
Excessive vasoconstriction can impair circulation to the peripheries, particularly skin and digits. While this typically develops gradually, rarely sudden arterial occlusion may occur and threaten limb or bowel perfusion. It is unclear whether vasopressors are exclusively responsible for this, since advanced circulatory shock itself will significantly impair peripheral blood flow. Therefore, maintaining a satisfactory MAP is still likely to be of greater benefit overall. The incidence of such events in cardiovascular shock has been reported at 6.5% with noradrenaline and 9.2% with dopamine, the majority being mild skin ischaemia [13]. While it is true that adding vasopressin has a sparing effect on noradrenaline, it is still a vasoconstrictor and does not alter the incidence of these events [35]. If peripheral hypoperfusion is suspected, it is important to reassess the patient thoroughly and establish whether they are appropriately fluid resuscitated or excessively vasoconstricted or perhaps have developed a superimposed element of cardiogenic shock.
Vasopressors can also be associated with tachyarrhythmia, likely due to a combination of changes to vascular physiology and myocardial excitability. Agents that act directly on the myocardium via beta-1 stimulation are generally thought to be more prone to this, with significantly higher rates of arrhythmias seen, for instance, with dopamine (24.1%) compared to noradrenaline (12.4%) [13]. The cause for a tachyarrhythmia should be investigated as usual before attributing it to vasopressor therapy. In addition to treating the abnormal rhythm, fluid and electrolyte status should be optimised and consideration given to switching to a less arrhythmogenic alpha-selective or vasopressin agent.
The haemodynamic changes produced by vasoactive agents can increase myocardial oxygen demand [12], especially if tachycardia ensues. In the context of a critically ill patient, this can be associated with myocardial infarction. It is therefore important to obtain continuous cardiac monitoring when administering vasoconstrictors and perform 12-lead electrocardiograms (ECGs) in case of deterioration.
Activation of beta-1 receptors can reduce insulin sensitivity leading to hyperglycaemia [26], while the absorption of exogenous insulin and other subcutaneous drugs may be diminished because of local vasoconstriction. Uncontrolled plasma glucose is associated with worse outcomes in the critically ill and should be closely monitored.
Locally, extravasation of vasopressors leads to excessive vasoconstriction and tissue necrosis. The risk of this is reduced by administration via centrally sited venous access, which should be positioned and secured appropriately before use. If extravasation does occur, the vasopressor infusion should be moved to a different, centrally located venous port. Local administration of subcutaneous phentolamine (a selective alpha-antagonist) may help reverse the excessive vasoconstriction, and plastic surgery input should be sought.
Practical Implications
The main vasopressors for specific clinical situations (◘ Table 30.1) and commonly encountered adult doses (◘ Table 30.2) are listed below. This is only an indicative summary, and in medical practice these decisions will be influenced by several aspects including clinical indication, experience, patient factors and relevant up-to-date guidelines and evidence.
Take-Home Messages
-
Vasopressors are used predominantly to increase mean arterial pressure by increasing vascular tone.
-
Appropriate fluid resuscitation should be ensured when starting vasopressor therapy.
-
Establishing the cause for haemodynamic instability will help guide the choice of vasopressor (e.g. noradrenaline in septic shock).
-
Serious complications can occur with vasopressors, and close monitoring is essential to detect and act on them early.
Conclusions
Vasopressors are an important therapy for cardiovascular support in the ICU.
Catecholamines are widely used for this purpose, and their action is mediated predominantly via alpha-adrenergic vasoconstriction and beta-agonist inotropy and chronotropy.
Non-adrenergic agents are gaining interest in the hope to reduce complications associated with catecholamines and provide further support when catecholamine doses rise. They have been shown to have an adrenergic-sparing action and in certain circumstances comparable effects. As their function is further investigated, they are likely to find increasing applications in the ICU alongside adrenergic vasopressors.
In addition to this, genetic polymorphisms can affect response to vasopressors, and a better understanding will help tailor individual therapy in the future.
References
Landzberg JS, Parker JD, Gauthier DF, Colucci WS. Effects of myocardial alpha 1-adrenergic receptor stimulation and blockade on contractility in humans. Circulation. 1991;84:1608–14.
Williamson AP, Seifen E, Lindemann JP, Kennedy RH. WB4101- and CEC-sensitive positive inotropic actions of phenylephrine in rat cardiac muscle. Am J Phys. 1994;266:H2462–7.
Brodde O-E. Beta1- and beta2-adrenoceptor polymorphisms and cardiovascular diseases. Fundam Clin Pharmacol. 2008;22:107–25.
Nakada T, Russell JA, Boyd JH, Aguirre-Hernandez R, Thain KR, Thair SA, Nakada E, McConechy M, Walley KR. Beta2-Adrenergic receptor gene polymorphism Is associated with mortality in septic shock. Am J Respir Crit Care Med. 2010;181:143–9.
Benedict CR, Fillenz M, Stanford C. Changes in plasma noradrenaline concentration as a measure of release rate. Br J Pharmacol. 1978;64:305–9.
Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign. Crit Care Med. 2017;45:486–552.
Avni T, Lador A, Lev S, Leibovici L, Paul M, Grossman A. Vasopressors for the treatment of septic shock: systematic review and meta-analysis. PLoS One. 2015;10:1–17.
Gamper G, Havel C, Arrich J, Losert H, Pace NL, Müllner M, Herkner H. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2016; https://doi.org/10.1002/14651858.CD003709.pub4.
Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34:2226–34.
Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317:1433.
Levy B, Bastien O, Karim B, et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015;5:17.
Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011;39:450–5.
De Backer DP, Biston P, Devriendt J, Madl C. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779–89.
Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100.
Gelman S, Mushlin P. Catecholamine-induced changes in the splanchnic circulation affecting systemic hemodynamics. Anesthesiology. 2004;100:434–9.
Ginès P, Angeli P, Lenz K, et al. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Bratton SL, Chestnut RM, Ghajar J, et al. Cerebral perfusion thresholds. J Neurotrauma. 2007;24:S-59–64.
Soar J, Nolan JP, Böttiger BW, et al. European Resuscitation Council Guidelines for Resuscitation 2015. Section 3. Adult advanced life support. Resuscitation. 2015;95:100–47.
Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S444–64.
Long B, Koyfman A. Emergency medicine myths: epinephrine in cardiac arrest. J Emerg Med. 2017;52:809–14.
Andersen LW, Kurth T, Chase M, Berg KM, Cocchi MN, Callaway C, Donnino MW. Early administration of epinephrine (adrenaline) in patients with cardiac arrest with initial shockable rhythm in hospital: propensity score matched analysis. BMJ. 2016;353:i1577.
Perkins GD, Quinn T, Deakin CD, et al. Pre-hospital assessment of the role of adrenaline: measuring the effectiveness of drug administration in cardiac arrest (PARAMEDIC-2): trial protocol. Resuscitation. 2016;108:75–81.
Truhlář A, Deakin CD, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2015. Section 4. Cardiac arrest in special circumstances. Resuscitation. 2015;95:148–201.
Bangash MN, Kong ML, Pearse RM. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165:2015–33.
Calabrese EJ. Dopamine: biphasic dose responses. Crit Rev Toxicol. 2001;31:563–83.
Jentzer JC, Coons JC, Link CB, Schmidhofer M. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther. 2015;20:249–60.
Denton MD, Chertow GM, Brady HR. “Renal-dose” dopamine for the treatment of acute renal failure: scientific rationale, experimental studies and clinical trials. Kidney Int. 1996;50:4–14.
Marik PE. Low-dose dopamine: a systematic review. Intensive Care Med. 2002;28:877–83.
Kellum JA, Decker JM. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526–31.
Kobayashi S, Endou M, Sakuraya F, Matsuda N, Zhang X-H, Azuma M, Echigo N, Kemmotsu O, Hattori Y, Gando S. The sympathomimetic actions of l-ephedrine and d-pseudoephedrine: direct receptor activation or norepinephrine release? Anesth Analg. 2003;97:1239–45.
Belletti A, Musu M, Silvetti S, et al. Non-adrenergic vasopressors in patients with or at risk for vasodilatory shock. A systematic review and meta-analysis of randomized trials. PLoS One. 2015;10:1–13.
Russell JA. Bench-to-bedside review: Vasopressin in the management of septic shock. Crit Care. 2011;15:226.
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–5.
Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989–1002.
Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–87.
Gordon AC, Mason AJ, Thirunavukkarasu N, et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2017;316:509–18.
Polito A, Parisini E, Ricci Z, Picardo S, Annane D. Vasopressin for treatment of vasodilatory shock: An ESICM systematic review and meta-analysis. Intensive Care Med. 2012;38:9–19.
Bernadich C, Bandi JC, Melin P, Bosch J. Effects of F-180, a new selective vasoconstrictor peptide, compared with terlipressin and vasopressin on systemic and splanchnic hemodynamics in a rat model of portal hypertension. Hepatology. 1998;27:351–6.
National Institute for Health and Clinical Excellence. Management of acute upper gastrointestinal bleeding. (Clinical guideline 141.) 2012. http://guidance.nice.org.uk/CG141.
Freeman JG, Cobden I, Lishman AH, Record CO. Controlled trial of terlipressin (‘Glypressin’) versus vasopressin in the early treatment of oesophageal varices. Lancet. 1982;2:66–8.
Fort E, Sautereau D, Silvain C, Ingrand P, Pillegand B, Beauchant M. A randomized trial of terlipressin plus nitroglycerin vs. balloon tamponade in the control of acute variceal hemorrhage. Hepatology. 1990;11:678–81.
Corrêa TD, Takala J, Jakob SM. Angiotensin II in septic shock. Crit Care. 2015;19:98.
Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 European Society of Intensive Care Medicine
About this chapter
Cite this chapter
Fiorini, F., Antcliffe, D., Gordon, A.C. (2019). Lessons from the ICU: Choosing the Right Vasopressor. In: Pinsky, M.R., Teboul, JL., Vincent, JL. (eds) Hemodynamic Monitoring. Lessons from the ICU. Springer, Cham. https://doi.org/10.1007/978-3-319-69269-2_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-69269-2_30
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69268-5
Online ISBN: 978-3-319-69269-2
eBook Packages: MedicineMedicine (R0)