Abstract
Objective
To determine whether there was a difference between epinephrine and norepinephrine in achieving a mean arterial pressure (MAP) goal in intensive care (ICU) patients.
Design
Prospective, double-blind, randomised-controlled trial.
Setting
Four Australian university-affiliated multidisciplinary ICUs.
Patients and participants
Patients who required vasopressors for any cause at randomisation. Patients with septic shock and acute circulatory failure were analysed separately.
Interventions
Blinded infusions of epinephrine or norepinephrine to achieve a MAP ≥70 mmHg for the duration of ICU admission.
Measurements
Primary outcome was achievement of MAP goal >24 h without vasopressors. Secondary outcomes were 28 and 90-day mortality. Two hundred and eighty patients were randomised to receive either epinephrine or norepinephrine. Median time to achieve the MAP goal was 35.1 h (interquartile range (IQR) 13.8–70.4 h) with epinephrine compared to 40.0 h (IQR 14.5–120 h) with norepinephrine (relative risk (RR) 0.88; 95% confidence interval (CI) 0.69–1.12; P = 0.26). There was no difference in the time to achieve MAP goals in the subgroups of patients with severe sepsis (n = 158; RR 0.81; 95% CI 0.59–1.12; P = 0.18) or those with acute circulatory failure (n = 192; RR 0.89; 95% CI 0.62–1.27; P = 0.49) between epinephrine and norepinephrine. Epinephrine was associated with the development of significant but transient metabolic effects that prompted the withdrawal of 18/139 (12.9%) patients from the study by attending clinicians. There was no difference in 28 and 90-day mortality.
Conclusions
Despite the development of potential drug-related effects with epinephrine, there was no difference in the achievement of a MAP goal between epinephrine and norepinephrine in a heterogenous population of ICU patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pharmacological support of the circulation with vasopressors is a fundamental part of critical care medicine. Drug strategies to augment and maintain adequate circulatory function have evolved from a substantial body of basic science research [1–3]. Foremost of these drugs are the catecholamines, epinephrine and norepinephrine, that have been used by clinicians either as sole agents, or in combinations with each other or other vasoactive drugs for the last 25 years. Despite their established use, there are few randomised-controlled trials to guide clinicians about their efficacy and effectiveness [4, 5]. Concerns about adverse effects, particularly with epinephrine, on vital organ perfusion [6–8] and lactic acidosis [9, 10] have led to recommendations for the preferential use of norepinephrine or dopamine for the treatment of shock [11]. However, the strength of these recommendations is limited by methodological flaws in published studies. Epinephrine continues to be widely used, particularly in low-income countries where alternative, more expensive drugs such as norepinephrine are restricted or unavailable [12, 13].
To address whether there was a difference between epinephrine and norepinephrine in achieving a prescribed mean arterial pressure (MAP) goal in patients requiring vasopressors, we conducted a prospective, double-blind, multicentred, randomised-controlled study in a heterogeneous population of intensive care unit (ICU) patients (the CAT Study) [14].
Material and methods
Ethics statement
Institutional Ethics Committee approvals were obtained from participating institutions. Informed consent was obtained from the patient whenever possible, or from a legal surrogate.
Study design and treatment protocol
Patients between the ages of 18 and 80 years admitted to multidisciplinary ICUs in four metropolitan teaching hospitals in Australia between February 2004 and June 2006 were assessed. Eligible patients were those whom the treating clinician judged to require an infusion of either epinephrine or norepinephrine for any cause at the time of enrolment. Patients undergoing resuscitation for cardiac arrest or anaphylaxis; an admission diagnosis of pheochromocytoma or hypoadrenalism; those taking monoamine oxidase inhibitors and those in whom death was considered to be likely within 24 h of randomisation were excluded.
Eligible patients were randomly assigned to receive either infusions of epinephrine (Adrenaline®, AstraZeneca, Sydney, Australia) or norepinephrine (Levophed®, Abbot Australasia, Sydney, Australia). Randomisation was performed using a random-number generator (StatMate®, GraphPad Software, San Diego, USA) in variable block allocations, stratified by participating centre. Randomisation codes were provided to designated staff at each institution not involved in the study or the clinical care of patients. Study drug was prepared as infusions in identical appearance and volumes: 15 mg of study drug was added to 250 ml 5% dextrose water and administered in ml/h that equates to a dose in μg/min [15]. Treating clinicians prescribed a MAP goal according to each patient’s clinical status and response to treatment at any stage of the study period. Where no prescribed goal was set, a default MAP target of at least 70 mmHg was set until increased or decreased by the treating clinician. The allocated study drug was used for the duration of the ICU admission until the MAP goal was achieved for greater than 24 h without study drug, death or discharge. There was no restriction on the use of other vasopressors (apart from norepinephrine or epinephrine), inotropes, inodilators or catecholamine-sparing agents (e.g. hydrocortisone or vasopressin) during the study period. Following randomisation, patients admitted to the ICU from locations where vasopressor(s) had been commenced before admission to the ICU (e.g. emergency or operating rooms) had the study drug commenced and then titrated up to achieve the MAP goal whilst the prior infusion was titrated down over 1 h. Patients requiring subsequent episodes of vasopressor support and those readmitted to the ICU within 28 days after randomisation were assigned to the originally allocated study drug. Haemodynamic management, including fluid resuscitation, monitoring and all other aspects of patient care were performed at the discretion of the treating clinicians.
Baseline assessment and follow-up data collection
We obtained baseline information on age, sex and acute physiology and chronic health evaluation (APACHE) II score [16] calculated in the 24-h period prior to randomisation. The diagnostic criteria for sepsis [17] were used to identify patients with sepsis as baseline. Septic patients who subsequently required vasopressors or study drug were classified as having septic shock. Other categories included patients with acute circulatory failure (defined as a MAP ≤60 mmHg without vasopressors, or those requiring an infusion of >5μg/min of norepinephrine or epinephrine prior to randomisation) due to sepsis, acute coronary syndromes or hypovolemia; patients requiring vasopressors for augmentation of cerebral perfusion pressure following traumatic brain injury or aneurismal subarachnoid haemorrhage and those requiring vasopressors for post-operative hypotension.
Baseline physiological parameters included haemodynamic variables (MAP, central venous pressure and heart rate), urine output, net fluid balance (calculated as total fluid input minus total fluid output), arterial pH, lactate and intravenous insulin requirements (as an index of the intensity glycemic control rather than serial blood glucose measurements).
Organ failure(s) at baseline was defined by the cardiovascular, respiratory, renal, hematologic and hepatic components of the sequential organ failure assessment (SOFA) score [18].
Baseline interventions included co-administered vasopressors and inotropes, the requirement for mechanical ventilation, renal replacement therapy, intra-aortic counterpulsation and pulmonary artery catheterisation.
Following randomisation, initial haemodynamic and metabolic variables (MAP, heart rate, central venous pressure, pH and lactate) were recorded every 4 h for 16 h to compare the immediate effects of study drug after randomisation. Thereafter, these variables were recorded daily until ICU discharge or death.
Outcome measures
The primary outcome was the time taken to achieve a clinician-prescribed MAP goal for greater than 24 h without vasopressors, also expressed as the number of vasoactive drug-free days from randomisation.
Secondary outcomes were mortality at 28 and 90-days after randomisation.
Outcomes were also determined a priori in the subgroup of patients with severe sepsis at baseline and post hoc in those with acute circulatory failure (as defined above).
Study and data management
Database construction (Microsoft® Access, Microsoft Corporation, USA) and study management was performed in the Department of Intensive Care Medicine at St George Hospital, Sydney.
Two pre-planned interim analyses were performed following recruitment of the first 75 (25%) and 150 (50%) patients and reviewed by a Data Monitoring and Safety Committee to determine whether there was a significant difference in serious adverse events, specifically the development of significant ventricular or supraventricular tachycardia or deaths between the two drugs. Projections of sample sizes to obtain potentially significant difference between the two drugs based on the observed differences were determined, but these did not invoke stopping rules or extension of the study to a larger sample.
Independent statistical analysis was conducted at the Australian and New Zealand Intensive Care Research Centre (Monash University, Melbourne, Australia). Detailed data checks were conducted that included thorough range and logic checks, with all queries directed back to site study staff for clarification and correction after reviews of source data.
Statistical analysis
At the time of study design, accurate data regarding time to the achievement of targeted MAP goals with vasopressors was not available. As such, power calculations were based on a study that determined the proportion of patients with septic shock that achieved a MAP goal using vasopressors within 48 h post-randomisation [4]. Based on these data and a clinical assumption that 70% of patients would achieve a MAP goal within 48 h following the administration of either epinephrine or norepinephrine, 280 patients were required in order to detect an absolute reduction of 15% in response to either drug with an alpha of 5% and a power of 80%.
Principal analyses were performed on an intention-to-treat basis.
Where data were missing, we report the number of available observations and make no assumptions about missing data.
The primary outcome was determined for the first episode only in patients with more than one episode. Due to the potential for competing risk for death between the intervention and primary outcome in a time-to-event analysis [19–21], patients who died whilst receiving study drug infusion or following a decision to withdraw therapy were allocated an infusion time 1 h longer than the maximum infusion time for any patient in their study hospital [22].
The primary outcome was presented using Kaplan-Meier curves, analysed using the log-rank test stratified by participating institution. Data are presented as means ± standard deviations (SD) or medians with interquartile ranges (IQR). Proportions were compared using a chi-squared test or Fisher’s exact test, and continuous variables using unpaired t-tests. Comparisons of event rates in the two groups are presented as hazard ratios (HR) with 95% confidence intervals (95% CI).
The data were exported from the study database to Intercooled Stata software (version 9.2, STATA Corporation, College Station, Texas).
Results
Study patients
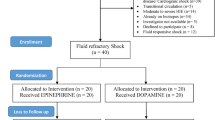
We screened a total of 636 patients who required vasopressors. Of these, 356 were excluded and 280 were randomised to receive either epinephrine or norepinephrine in equal proportions (n = 140). A total of 3 patients withdrew consent for follow-up, resulting in 139 patients (99.3%) in the epinephrine group and 138 patients (98.5%) in the norepinephrine group being included in the analysis (Fig. 1).
Baseline demographics, APACHE II scores, number of organ failures and physiological parameters were similar in the two groups (Table 1).
Treatment effects
There was no difference in the MAP achieved using epinephrine or norepinephrine during the infusion period; nor was there a difference in the maximal daily dose required to achieve the MAP goal (Fig. 2). There was no difference in mean central venous pressure, daily urine output or net fluid balance during the infusion period between the two groups.
Comparisons between maximum daily dose (μg/min) of epinephrine and norepinephrine (open labels) and the effect on maximal mean arterial pressure (MAP) (closed labels) during the initial 16 h (1–16 h) and the initial 7 days (D1–D7) of infusion period. Data are expressed as mean ± SD. Dashed line indicates default MAP goal of 70 mmHg
Epinephrine was associated with the development of significant tachycardia and lactic acidosis that developed within the initial 4 h after randomisation that was sustained for the first 24 h of study treatment, in addition to increased insulin requirements, following which there was no difference between the two drugs (Fig. 3).
Comparisons between the epinephrine and norepinephrine on heart rate (top panel) and arterial lactate (middle panel) from baseline, during the initial 16 h (1–16 h) of infusion and the maximum daily level during the initial 4 days (D1–D4) of infusion period. Bottom panel shows comparison on effect on mean daily insulin dose (as an index of intensity of glycemic control in the absence of blood glucose measurements) during initial 4 days of infusion period. * P < 0.001
A total of 22 patients were withdrawn from study treatment by the treating clinician: 18/139 (12.9%) in the epinephrine group and 4/138 (2.8%) in the norepinephrine group (P = 0.002). Lactic acidosis (7/18 for epinephrine vs. 2/4 for norepinephrine) tachycardia (4/18 vs. 1/4) and inability to achieve prescribed parameters (5/18 vs. 1/4) were cited as the most common reason for withdrawal from study treatment. All of these patients subsequently received open-labelled norepinephrine for the duration of clinical management as directed by the treating clinicians, but were included in the intention-to-treat analysis.
There was no difference in the incidence of other severe adverse events, specifically supra- or ventricular tachyarrhythmias between the two groups.
There was no difference in pre-randomisation or concomitant use of dobutamine, dopamine or milrinone during the study period. There was no difference between the epinephrine and norepinephrine groups in the proportion of patients receiving hydrocortisone (24.4 vs. 21.0%, P = 0.49) or vasopressin (13.7 vs. 10.9%, P = 0.48) during the study period.
Outcomes
There was no difference in the median time to achieve the MAP goal between epinephrine (35.1 h; IQR 13.8–70.4 h) and norepinephrine (40.0 h; IQR 14.5–120.0 h) (HR 0.88; 95% CI 0.69–1.12; P = 0.26) (Fig. 4). There was no difference in the median number of vasopressor-free days between epinephrine (26.0 days; IQR 19.2–27.3) and norepinephrine (25.4 days; IQR 13.8–27.3) (P = 0.31).
In the a priori subgroup of patients with severe sepsis at baseline (158/277), there was no difference in the median time to achieve the MAP goal between epinephrine (35.1 h; IQR 16.7–75 h; n = 76) and norepinephrine (50.0 h; IQR 18.2–127.5 h; n = 82) (HR 0.81; 95% CI 0.59–1.12; P = 0.18). There was no difference in the number of vasopressor-free days between epinephrine (26.3 days; IQR 17.2–27.3) and norepinephrine (24.2 days; IQR 7.7–26.5) (P = 0.13).
Similarly, there were no differences in the median time to achieve the MAP goals in the post hoc subgroup of patients with acute circulatory failure (128/277) between epinephrine (38.6 h; IQR 18.0–85.7; n = 64) and norepinephrine (40.0 h; IQR 15.1–122.8; n = 64) (HR 0.89; 95% CI 0.62–1.27; P = 0.49). There was no difference in the number of vasopressor-free days between epinephrine (25.7 days; IQR 17.2–27.3) and norepinephrine (25.7 days; IQR 9.7–27.3) (P = 0.80).
There was no significant difference in 28 or 90-day mortality between the two drugs in the overall group or in the two subgroups (Table 2).
Per-protocol analyses excluding patients withdrawn by clinicians, those who did not receive study drug and censoring those patients who died 45 days or longer did not influence the primary outcomes both for the entire patient cohort and in the subgroup of patients with severe sepsis.
Discussion
We conducted a study comparing the haemodynamic effects of epinephrine and norepinephrine in a heterogeneous population of critically ill patients who required vasopressors. We found no statistically significant differences in the time to achievement of a target MAP and other haemodynamic resuscitation endpoints between the two drugs. In subgroups of patients with severe sepsis and acute circulatory failure, there was no difference in the time to the achievement of target MAP, nor any difference in the number of vasopressor-free days between the two drugs.
The use of epinephrine was associated with significant, but transient metabolic effects and tachycardia that prompted clinicians to withdraw a number of patients receiving epinephrine from the study.
Our study was designed to determine the relative effectiveness of two commonly used vasopressors under “real life” clinical conditions. Our study has a number of methodological strengths. It was a prospective, multicentred, double-blind, randomised-controlled study conducted over a short inception period with a completion rate in excess of 98%. We recruited a study population in excess of the total number of patients included a systematic review of high-quality randomised rials of vasopressors [23]. We selected a patient-centred primary outcome used in daily clinical practice that was reliably determined. All data were analysed on an intention-to-treat basis.
Our study also has a number of limitations. Firstly, due to a paucity of phase II and III studies from which to determine accurate power calculations, our study sample size was based on time to resolution of shock at 48 h. Extrapolation of our findings from a heterogeneous population of ICU patients requiring vasopressors to specific conditions including septic and cardiogenic shock and neurological patients requires caution.
Secondly, the large number of patients withdrawn by clinicians from the epinephrine group may have potentially introduced bias further limiting the robustness of our conclusions. However, sensitivity analyses confirmed that the primary outcome was minimally influenced by the withdrawal of these patients.
Thirdly, the interdependence between the primary outcome and death may further introduce bias due to competing risks. However, additional sensitivity analyses did not influence the primary outcomes.
Most of the published literature on the clinical use of vasopressors in the ICU consists of studies with limited methodological strengths, predominated by non-blinded, case-control studies using various dosing regimens of vasopressors, often in combination with synthetic drugs, on surrogate endpoints.
A systematic review of vasopressors for the treatment of shock identified only 8 high-quality randomised-controlled trials using patient-centred outcomes from 120 relevant articles that included a total of 172 patients [23]. The authors concluded that although no drug(s) were shown to be superior in controlled trials, clinicians are left with uncertainty about patient-centred benefits or harm attributable to a particular vasopressor and therefore have to rely on clinical experience to guide patient management.
Similarly, the limitations for recommendations for the use of vasopressors in shock is recognised in evidence-based guidelines [11] that call for the need for high-quality randomised-controlled studies, which until recently, have not been published.
A multicentred, randomised-controlled French trial, conducted between 1999 and 2004, demonstrated no difference in 28-day mortality between patients with septic shock treated using a prescribed haemodynamic management algorithm including vasopressor therapy with epinephrine or a combination of norepinephrine and dobutamine [5]. In this study, epinephrine was associated with the development of a transient lactic acidosis, but no differences in organ failure scores, ICU or hospital stay or adverse events between the two groups were demonstrated.
In contrast to our study, few patients were withdrawn by clinicians due to metabolic side effects associated with epinephrine. This may reflect persistent concerns by clinicians about epinephrine-induced lactic acidosis that have previously been described in patients with severe sepsis [13, 24] and following cardiac surgery [10], particularly once serum lactate was routinely incorporated into point-of-care blood gas analysers. Our study and the French study demonstrate that the epinephrine-induced lactic acidosis was not associated with loss of haemodynamic efficacy or the development of new organ dysfunction. This phenomenon may be attributed to epinephrine-specific β-receptor stimulation, including activation of pyruvate dehydrogenase resulting in hyperlactatemia and hyperglycemia [25] that is not associated with tissue dysoxia [26]. These observations therefore do not represent a compelling contraindication to the use of epinephrine, although norepinephrine should be available as an alternative when epinephrine is used as a preferential agent.
The challenges in conducting a definitive mortality-based study of vasopressors in shock states are highlighted by our study and the French study, both of which were relatively underpowered. The observed 28-day mortality rates in the two studies in patients with septic shock (approximately 26 and 36% respectively) suggest that a study population in excess of 4,000 patients would be required to determine an absolute reduction in mortality of 5%. Such a study is unlikely to be conducted or funded given the established role that these drugs have in clinical practice.
Therefore, in conjunction with the French study, our study provides substantive data to inform clinicians about the pharmacodynamic and metabolic effects of these two commonly used vasopressors. Ultimately, the selection of a vasopressor for the augmentation of mean arterial pressure will depend on the treating clinician’s experience and on the individual patient’s response to the treatment.
We demonstrated no difference in the haemodynamic responses to epinephrine and norepinephrine and suggest that either of these drugs may be used effectively in the ICU. Whilst both drugs are off-patent and substantially cheaper than synthetic catecholamines, the use of epinephrine presents a cost-effective alternative to norepinephrine, particularly in low-income countries where the use of norepinephrine is unavailable or restricted due to cost.
CAT study investigators
Writing Committee
John A Myburgh (Chair), Alisa Higgins, Jeffrey Lipman, Naresh Ramakrishnan, John Santamaria
The Writing Committee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Statistical analysis
Alisa Higgins (Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne)
Data Monitoring and Safety Committee
Rinaldo Bellomo (Chair) (Austin Medical Centre, Melbourne), Sing Kai Lo (The George Institute for International Health, Sydney)
Site investigators
Royal Brisbane Hospital, Queensland, Australia—Renae Deans, Greg Comadira, Melissa Lassig-Smith, Jeffrey Lipman, Janine Stuart
Royal North Shore Hospital, Sydney, Australia—Arina Dan, Ann O’Connor, Julie Potter, Naresh Ramakrishnan, Ray Raper
St George Hospital, Sydney, Australia—Warren Eather, Kathryn Girling, Marie Hodgetts, Theresa Jacques, Alina Jovanovska, Kerry Munsie, Francesca Munster, Alan McKeag, John A Myburgh, Sally Newton, Michael O’Leary, George Skowronski, Jane Treloggen
St Vincent’s Hospital, Melbourne, Australia—Nicole Groves, Jenny Holmes, Gabrielle Hanlon, John Santamaria
References
Magder S, Rastepagarnah M (1998) Role of neurosympathetic pathways in the vascular response to sepsis. J Crit Care 13:169–176
Hein L (2006) Adrenoceptors and signal transduction in neurons. Cell Tissue Res 326:541–551
Myburgh JA (2002) The systemic and cerebrovascular effects of inotropes and vasopressors. In: Gullo A (ed) APICE: anaesthesia. Pain intensive care and emergency medicine. Springer, Milan, pp 283–299
Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, Stoll C, Peter K (1999) Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med 27:723–732
Annane D, Vignon P, Renault A, Bollaert PE, Charpentier C, Martin C, Troche G, Ricard JD, Nitenberg G, Papazian L, Azoulay E, Bellissant E (2007) Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 370:676–684
Meier-Hellmann A, Reinhart K, Bredle DL, Specht M, Spies CD, Hannemann L (1997) Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med 25:399–404
Levy B, Bollaert PE, Lucchelli JP, Sadoune LO, Nace L, Larcan A (1997) Dobutamine improves the adequacy of gastric mucosal perfusion in epinephrine-treated septic shock. Crit Care Med 25:1649–1654
De Backer D, Creteur J, Silva E, Vincent JL (2003) Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med 31:1659–1667
Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hien TT, White NJ (1996) The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet 348:219–223
Totaro RJ, Raper RF (1997) Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med 25:1693–1699
Dellinger RP, Vincent JL (2005) The Surviving Sepsis Campaign sepsis change bundles and clinical practice. Crit Care 9:653–654
Lipman J, Roux A, Kraus P (1991) Vasoconstrictor effects of adrenaline in human septic shock. Anaesth Intensive Care 19:61–65
Wilson W, Lipman J, Scribante J, Kobilski S, Lee C, Krause P, Cooper J, Barr J (1992) Septic shock: does adrenaline have a role as a first-line inotropic agent? Anaesth Intensive Care 20:470–474
Myburgh JA, Higgins A, Jovanovska A, Lipman J, Santamaria J, Ramakrishnan N, the CAT Study Investigators (2007) A comparison of epinephrine and norepinephrine on reversal of shock. Intensive Care Med 33:S197
Myburgh JA (2003) Inotropic agents. In: Oh TE, Bersten AD, Soni N (eds) Intensive care manual. Butterworths, London, pp 841–855
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Moeschberger ML, Klein JP (1995) Statistical methods for dependent competing risks. Lifetime Data Anal 1:195–204
Satagopan JM, Ben Porat L, Berwick M, Robson M, Kutler D, Auerbach AD (2004) A note on competing risks in survival data analysis. Br J Cancer 91:1229–1235
Minini P, Chavance M (2004) Sensitivity analysis of longitudinal normal data with drop-outs. Stat Med 23:1039–1054
Clark DE, Ryan LM (1997) Modeling injury outcomes using time-to-event methods. J Trauma 42:1129–1134
Mullner M, Urbanek B, Havel C, Losert H, Waechter F, Gamper G (2004) Vasopressors for shock. Cochrane Database Syst Rev:CD003709
Day NP, Phu NH, Mai NT, Bethell DB, Chau TT, Loc PP, Chuong LV, Sinh DX, Solomon T, Haywood G, Hien TT, White NJ (2000) Effects of dopamine and epinephrine infusions on renal hemodynamics in severe malaria and severe sepsis. Crit Care Med 28:1353–1362
Watt M, Howlett KF, Febbraio MA, Spriet LL, Hargreaves M (2001) Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol 534:269–278
Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE (2005) Relation between muscle Na + K + ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365:871–875
Acknowledgments
We wish to acknowledge funding for statistical analysis of this study from the Australian and New Zealand College of Anaesthetists (Project grant: 06/024). We also acknowledge the financial contribution from participating institutions that provided substantial support from internal funds. We acknowledge Gordon Doig for assistance with initial study design, Andrew Forbes for assistance with the statistical analysis plan, and Rinaldo Bellomo and Simon Finfer for editorial comment. We also thank the nursing and medical staff of the four ICUs of the participating institutions whose enthusiasm and hard work made the CAT study possible.
Conflict of interest statement
The investigators declare no conflicts of interest relating to this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Myburgh, J.A., Higgins, A., Jovanovska, A. et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med 34, 2226–2234 (2008). https://doi.org/10.1007/s00134-008-1219-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1219-0