Abstract
In 1905, Constantin S. Mereschkowsky (1855–1921) proposed that the green organelles (chloroplasts) of algae and land plants evolved from ancient, once free-living cyanobacteria. This endosymbiotic hypothesis was based on numerous lines of evidence. In a 1910 paper, Mereschkowsky argued that the time has come to introduce a new theory on the origin of living beings; since Darwin’s era, so many new findings have accumulated that now an alternative, anti-selectionist theory of evolution has to be established. Based on the principle of symbiosis (i.e., the union of two different organisms whereby both partners mutually benefit), Mereschkowsky coined the term “symbiogenesis theory,” which is based on an analogy between the feeding process of amoebae and cellular events that may have occurred in the ancient oceans. Mereschkowsky’s symbiogenesis hypothesis explains the origin of chloroplasts from archaic cyanobacteria, with respect to plant evolution. In 1927, the Russian cytologist Ivan E. Wallin (1883–1969) proposed that the mitochondria of eukaryotic cells are descendants of ancient, once free-living bacteria. Here, I outline the origin and current status of the Mereschkowsky–Wallin concept of symbiogenesis (primary and secondary endosymbiosis) and explain why it is compatible with the Darwin–Wallace principle of natural selection, which is described in detail. Nevertheless, largely due to the work of Lynn Margulis (1938–2011), symbiogenesis is still considered today as an Anti-Darwinian research program. I will summarize evidence indicating that symbiogenesis, natural selection, and the dynamic Earth (plate tectonics) represent key processes that caused major macro-evolutionary transitions during the 3500-million-year-long history of life on Earth.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The German botanist Andreas F. W. Schimper (1856–1901) is well known for his elegant studies on the microstructure of the “chlorophyll bodies” in plant tissues, notably in green leaves of crop species. In a landmark paper of 1883, published in the Botanische Zeitung, Schimper introduced the idea of a symbiotic origination of plastids (chloroplasts) as follows:
Should it turn out definitely that the plastids are not formed de novo in the egg cells, then their relation to the organism, which contains them, would reasonably remind one of a symbiosis. It is likely that the green plants have their origin in the unification of a colourless organism with another living being, which was evenly coloured green by chlorophyll (Schimper 1883: 105).
This brief statement, consisting of only two sentences (a footnote to the main text), marks the origin of the concept of symbiogenesis, i.e., the theory that green organisms (algae, bryophytes, ferns, etc.) originated via the fusion of microbial cells, which developed a symbiotic relationship that later gave rise to a new class of organisms, i.e., the land plants (embryophytes) (Hagemann 2007).
Interestingly, this idea was already in the mind of another German botanist, before Schimper (1883) published his remark. In his famous Vorlesungen über Pflanzen-Physiologie (Lectures on the Physiology of Plants, 1882), Julius Sachs (1832–1897) referred to earlier publications of Schimper. Based on his own observations, Sachs (1882) wrote that the “chlorophyll bodies” (chloroplasts) in the moss Funaria hygrometrica multiply via divisions, as if they were independent microbes living within the protoplasmic space of foreign cells (Fig. 1). However, this conclusion was largely ignored over the following two decades. Due to the work of the Russian biologist Constantin S. Mereschkowsky (1855–1921), who published in 1905 a general hypothesis on the endosymbiotic origin of chloroplasts from once free-living cyanobacteria (Fig. 2), this idea became popular among biologists.
Illustration of chloroplast division in the green leaves of the gametophyte of the moss Catharinea undulata by Julius Sachs. Adult plantlet (gametophyte) with sporophytes (a), cytoplasm (leaf) with numerous large chloroplasts (b), and photosynthetic organelle in the process of division (organellokinesis) (adapted from Sachs 1882)
In 1890, the German physician and histologist Richard Altmann (1852–1900) proposed that subcellular particles we today call mitochondria (“bioplasts”) may be descendants of once free-living microbes (Altmann 1890). This idea was elaborated and extended by the Russian biologist Ivan Wallin (1883–1969), who published a major monograph on this topic (Wallin 1927).
The novel concept of the emergence of new body plans via the unification of archaic cell types (symbiogenesis, a term coined by Mereschkowsky in 1910) was rejected by cell biologists of the 1920s but four decades later revived and corroborated by independent evidence. Moreover, it was viewed early on as being at odds with the dominant “evolutionary force” of natural selection. Hence, the idea that emerged with Schimper, Sachs, Altmann, Mereschkowsky, and Wallin must be interpreted as an “Anti-Darwinian” concept of evolution (Mereschkowsky 1920). Accordingly, in the next section, I will outline the idea of natural selection, as envisioned by Darwin and Wallace, in order to make clear why symbiogenetic thinking became a major research agenda that claimed to be an alternative view, regarded to be incompatible with the “Darwinian (selectionist)” mode of evolution (see Margulis 2010).
2 Darwin and Wallace: Natural Selection and the Elimination of the Unfit
It has been shown repeatedly that natural selection (or the survival of the fittest) was not a “one-man idea” of Charles Darwin (1809–1882) but rather originated independently in the brains of two naturalists: Darwin, summarized in his Origin of Species (1859), and the much lesser-known Alfred Russel Wallace (1823–1913) (see Depew 2017). Hence, it is fair to describe this idea as the “Darwin–Wallace principle of natural selection” (Kutschera 2003, 2008a, b, 2009a, b) (Fig. 3). Since Wallace has written extensively on this topic, notably in books and articles published after Darwin’s death (Kutschera and Hossfeld 2013), we will summarize some of Wallace’s descriptions of this key process that brings about evolutionary change, with reference to sexual selection and related issues in humans. The quotes in the next section are adapted from Smith (2012), where the original references are listed.
In a 1866 letter to Darwin, Wallace wrote that “Natural selection … does not so much select special variations as exterminate the most unfavourable ones.” In 1877, Wallace argued, with reference to insects, as follows: “In the case of butterflies the argument becomes even stronger, because the fertility is so much greater, and the weeding out of the unfit takes place, to a great extent, in the egg and the larvae state.” In his famous monograph entitled Darwinism, Wallace (1889) argued that “Natural selection... acts perpetually and on an enormous scale in weeding out the ‘unfit’ at every stage of existence, and preserving only those which are in all respects the very best.” One year later (1890), Wallace wrote in an article that “The survival of the fittest is really the extinction of the unfit.”
It is well known that Wallace was a champion of women’s rights (Kutschera 2015b). Accordingly, he discussed this gender issue with reference to the principle of natural selection. In 1893, he argued that “The survival of the fittest is really the extinction of the unfit. … In order to cleanse society of the unfit we must give to woman the power of selection in marriage, and the means by which this most important and desirable end can be attained will be brought about by giving her such training and education as shall render her economically independent.” One year later (1894), the British naturalist expressed this idea as follows: “I believe that the unfit will be gradually eliminated from the race, and human progress secured, by giving to the pure instincts of women the selective power in marriage.”
In 1896, Wallace summarized natural selection as follows: “Accepting, then, these facts of variation, and always keeping in mind the severity of the struggle for existence, nine tenths at least of the progeny of the higher animals perishing annually before reaching maturity, thus leading to a systematic and continual weeding out of the less fit …”
In the year 1900, Wallace addressed the “women’s issue” with reference to natural selection again, in the following words: “It would operate, not as among the lower animals and plants by the actual destruction of the unfit, but by their less rapid increase, since, under equal conditions of education and mode of life, it is certain that marriage would be delayed till some industrial success had been reached by both parties.”
In an article of 1909, Wallace referred to the philosopher Herbert Spencer (1820–1903), who coined the phrase “survival of the fittest,” adapted by Darwin and used in later editions of his Origin of Species (Darwin 1872). Accordingly, Wallace wrote that “Spencer suggested the term ‘survival of the fittest’, as more closely representing what actually occurs; and it is undoubtedly this survival, by extermination of the unfit, combined with universally present variation, which brings about that marvelous adaptation to the ever-varying environment, which is an essential feature of every living creature which survives to produce offspring.”
In the year of his death, the 90-year-old biologist summarized the principles of natural and sexual selection, with reference to women’s choices in selecting a husband, in the following words: “The survival of the fittest is really the extinction of the unfit; and it is the one brilliant ray of hope for humanity that, just as we advance in the reform of our present cruel and disastrous social system, we shall set free a power of selection in marriage that will steadily and certainly improve the character, as well as the strength and the beauty of our race” (Wallace 1913).
These quotes (Smith 2012) document that, around the time when the concept of symbiogenesis emerged, natural selection was interpreted to be largely a “destructive” process, i.e., a form of species “extinction” (see the equation evolution = speciation – extinction in Fig. 3). However, due to the work of Wallace (1889), and notably that of August Weismann (1834–1914), it is shown that purifying selection removes phenotypes that are not well adapted to a stable environment, whereas dynamic natural selection under gradually changing conditions “creates” new forms of life, via the emergence and propagation of those variants that are adapted to novel environments for survival and reproduction (Weismann–Schmalhausen principle of dynamic selection; see Kutschera 2009a, b). In the next section, we will explore in detail how the anti-(neo)Darwinian concept of symbiogenesis emerged and spread among scientists and philosophers in Europe.
3 Historical Roots and Elaboration of Symbiogenesis
In excellent review articles on the origin of symbiogenetic theorizing in biology and philosophy, Carrapiço (2010, 2015) summarized the achievements of several eminent thinkers not mentioned above. Beginning with Anton de Bary (1831–1888), who introduced, in 1878, the term “symbiosis” at the 51st Congress of German Naturalists and Physicians in Kassel, Germany (Kutschera 2011a, b), the following key figures should be recognized.
In 1902, the Russian author, anarchist, and politician Peter Kropotkin (1842–1921) published a book entitled Mutual Aid. A Factor of Evolution. In this monograph, he argued that, contrary to the Darwin–Wallace concept of natural selection, cooperation between organisms rather than competition should be viewed as the key factor that has driven biological evolution. However, since Kropotkin (1902) was no biologist and argued from a purely philosophical perspective, his work has been largely ignored.
Three years later (1905), Constantin Mereschkowsky published his landmark paper on the “Nature and Origin of Chromatophores in the Plant Kingdom” that will be discussed in detail below.
In 1915, the British biologist Hermann Reinheimer (1872–1950s?) published a monograph entitled Symbiogenesis: The Universal Law of Progressive Evolution. In this book, the term symbiogenesis was used, but without reference to Mereschkowsky’s papers on this topic (notably his article of 1910) (Fig. 2). It is likely that Reinheimer was not aware of Mereschkowsky’s works, which were published in German. On the other hand, Reinheimer understood the German language—unfortunately, we cannot reconstruct anymore whether or not he had read Mereschkowsky’s article. In his first book (Symbiogenesis), Reinheimer (1915) defined “symbiosis” as a physiological partnership between individuals of different species, exactly as Anton de Bary had introduced this key term into the biological sciences some decades earlier. Reinheimer’s definition of “symbiogenesis” reads as follows:
By symbiogenesis I mean the production and increase of values throughout organic life by means of a symbiotic principle of co-operation or reciprocity between different organs of the individual, by evolved and complex body, as well as between different organisms in a species or different species, genera, orders, etc., even in the last and most fundamental way between plant and animal in the web of life (Reinheimer 1915: 156).
It is obvious that this very broad and inclusive definition of “symbiogenesis” did not impress the biologists of Reinheimer’s time, because, in the natural sciences, only concepts and ideas that have an unequivocal meaning are taken seriously and are discussed openly in the peer-reviewed literature. Nevertheless, it is important to acknowledge that a first book with this key term in its title was published before Mereschkowsky had written the last of his three major contributions on this topic (Mereschkowsky 1905, 1910, 1920).
In the year when Mereschkowsky’s last symbiogenesis article appeared in print, Reinheimer (1920) published a monograph entitled Symbiosis. A Socio-Physiological Study of Evolution. In this work, the interaction of organisms during development and evolution is described in detail. The author regarded all organisms in combination as a kind of “world society,” composed of many species and families of plants and animals that represent individuals of this collection of living beings. Again, this work was more of a philosophical than a scientific nature, so that Reinheimer (1920) was largely ignored by evolutionary researchers of his time. A short note on Reinheimer is not out of place here: he was born in 1872 in Germany (Hesse) and became a British citizen in 1901. Reinheimer lived in London as a self-employed stock broker and died during the 1950s (the exact date of his death is unknown). Reinheimer published his books via Editors and Companies that were associated with alternative-esoteric views of life, such as vegetarianism, theosophy, anarchism, or metaphysics. Based on our limited knowledge, it is likely that he had never been affiliated with any academic institution in England or Germany and may have been (like Alfred R. Wallace) a self-educated private person working in the area of organismic biology.
In 1923, the Russian biologist Ivan Wallin (1883–1969) published an article on the origin of mitochondria, and 4 years later, his important monograph Symbionticism and the Origin of Species appeared in print (1927), wherein the work of Altmann (1890) was acknowledged. Like Mereschkowsky, Wallin (who had emigrated to the United States) was a “hands-on biologist” who published original work on several biological topics (see below).
Finally, it is worth mentioning the book of the Russian biologist Boris N. Kozo-Polyansky (1890–1957), who published, around the time when Wallin released his most important work, a monograph entitled Symbiogenesis: A New Principle of Evolution (in Russian). In this work, Kozo-Polyansky (1924) argued that symbiogenesis, defined sensu Mereschkowsky (1910), must be regarded as an important driving force during evolution; in contrast to most of his contemporaries, Kozo-Polyansky accepted the Darwinian principle of natural selection (Fig. 3). In addition to these basic insights, the Russian biologist introduced the ecological concept of the organism as a “consortium” (Kozo-Polyansky 1924). Recently, Margulis (2010) argued that the work of Kozo-Polyansky was more important than previously assumed and that this biologist should be credited with being one of the founding fathers of this anti-Darwinian research agenda.
In summary, this historic review documents that the basic ideas of symbiosis and symbiogenesis (Figs. 1 and 2), respectively, were very popular at a time when the Darwinian principle of natural selection (Fig. 3) was eclipsed by the erroneous theory of “Mutationism,” i.e., the hypothesis that new species emerge as a result of macro-mutations in populations of parental organisms, without any role of natural selection (Kutschera and Niklas 2004). It should be stressed again that most of the authors cited above were “anti-selectionists”; they did not accept the Darwin–Wallace principle as a positive force in the “creation” of new species and body plans.
In the next section, we will summarize the contributions of Mereschkowsky and other scientists that shaped our current view of the evolutionary process. Finally, I will address the work of Lynn Margulis (1938–2011) and Margaret Dayhoff (1925–1983) and provide an integrative general scheme of macroevolution (see Figs. 6 and 8).
4 The Mereschkowsky–Wallin Principle of Symbiogenesis
As noted, the work of the lesser-known symbiogenesis theorists referred to above was of limited significance. In this section, the key insights published by the leading theorists Mereschkowsky and Wallin are summarized. In his first “symbiogenesis paper” published in 1905, entitled Über Natur und Ursprung der Chromatophoren im Pflanzenreiche (On the nature and origin of chromatophores in the plant kingdom), C. S. Mereschkowsky concluded that the chloroplasts (plastids) of green algae and land plants (embryophytes) were once free-living cyanobacteria (Cyanophyceae) (Figs. 2 and 4; the structure of a major chloroplast is shown in Fig. 5). This endosymbiotic concept explaining the origin of plant organelles was based on several lines of evidence—data from the scientific literature, and novel microscopic observations by the Russian biologist (Mereschkowsky 1905). In his second article entitled Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen (Theory of two species of plasmas as basis of symbiogenesis, a new concept of origin of organisms), Mereschkowsky (1910) wrote that his intention was to publish a new theory on the evolutionary development of living beings on Earth. In this key publication (Fig. 2), Mereschkowsky stated that the attempts of Charles Darwin, Ernst Haeckel (1834–1919), and others to solve this problem have been without success, because not all pertinent facts were available when these naturalists published their most influential books (Darwin 1859, 1872; Haeckel 1866, 1877). The work of Alfred Russel Wallace on natural selection, notably his popular monograph Darwinism (Wallace 1889), was ignored by Mereschkowsky, which may be due to the fact that the British naturalist usually played down his true achievements, compared to those of his mentor Charles Darwin (Kutschera 2008a, b). In this paper, Mereschkowsky (1910) argued that many novel findings in the areas of biochemistry, cytology, and physiology have accumulated since the time of Darwin and Haeckel, notably with respect to unicellular organisms that occur in aquatic as well as terrestrial habitats. Accordingly, Mereschkowsky (1910) boldly claimed that it is now necessary to propose a new theory on the origin of species, with a focus on plants.
Transmission electron micrograph of a mature, fully developed chloroplast in the mesophyll of a green leaf (bean, Phaseolus vulgaris) s starch, st stroma (adapted from Kleinig and Sitte 1986)
With reference to the de Baryan concept of symbiosis (i.e., the union of two different organisms whereby both partners mutually benefit), Mereschkowsky (1910) introduced the term “symbiogenesis theory.” The basic idea of symbiogenesis, as envisioned by him, can be interpreted as an analogy between the uptake of small particles or bacteria (i.e., phagocytosis) of amoebae, which are eukaryotic unicellular microbes, and hypothetical processes that may have occurred millions of years ago in the oceans of the young Earth. Mereschkowsky’s symbiogenesis hypothesis attempted to account for the origin of the chloroplasts from ancient cyanobacteria and hence provided insight into the first steps in the evolution of the Kingdom Planta, notably that of the land plants (embryophytes) (Kutschera and Niklas 2005, 2008). In his third and less influential symbiogenesis paper, Mereschkowsky (1920) published a tentative scheme illustrating his idea as to how land plants may have evolved from green algae (Sapp et al. 2002; Geus and Höxtermann 2007).
Six years after Mereschkowsky’s death, the Russian cytologist Ivan E. Wallin proposed that the mitochondria of eukaryotic cells may be descendants of ancient, once free-living bacteria (Wallin 1927). In addition, the author suggested that the primary source of genetic novelty for speciation events may have been a periodic, repeated fusion of bacterial endosymbionts with eukaryotic host cells. However, this second hypothesis of Wallin, which was, decades later, elaborated by Margulis and Sagan (2002) is not supported by convincing data (Kutschera and Niklas 2005, 2008).
5 Evolutionary Origin of Multicellular Organisms
As mentioned above, two scientists, Lynn Margulis and Margaret Dayhoff, have greatly contributed to our understanding of symbiogenesis and cell evolution. Whereas the work of Margulis has been acknowledged in many details (see Carrapiço 2010, 2015; Cavalier-Smith 2013), the key insights of Dayhoff remained less popular. In a recent article, Martin and Cerff (2017) summarized the elegant molecular work of Dayhoff (DNA-sequence analyses, reconstruction of phylogenetic trees, etc.) (Figs. 4, 5), which led to the definitive proof that chloroplasts and mitochondria descended, with modification, from once free-living cyanobacteria and alpha-proteobacteria, respectively. In the following section, we summarize the pertinent cellular events that led to the emergence of eukaryotic cells (eukaryogenesis).
Ancient endosymbiotic processes (i.e., symbiogenesis) that occurred ca. 2100–1600 million years ago (mya) in the oceans (i.e., after the Great Oxygenation Event, ca. 2300 mya) gave rise to the first eukaryotic cells. Today, these key events in the history of life are explained within the framework of the “serial primary endosymbiosis theory” for cell evolution, which is supported by a solid body of empirical data (see Kleinig and Sitte 1986; Margulis 1993 for a classic review, and Kutschera and Niklas 2004, 2005, 2008; Zimorski et al. 2014; Archibald 2014; Speijer et al. 2015; Martin et al. 2015; Martin and Cerff 2017 for more recent accounts).
The capture of an ancient alpha-proteobacterium by a host cell that resembled an extant (a-mitochondriate) Archaeon occurred probably only once during evolution (Fig. 6). Evidence for this major conclusion is largely based on the finding that the protein import machineries (TIM/TOM in mitochondria, TIC/TOC for plastids) of these organelles are uniform in all Kingdoms of life. After subsequent intracellular domestication events, the once free-living alpha-proteobacterium was reduced to an organelle, which produces and exports energy-rich adenosine triphosphate (ATP, intra-cytoplasmatic concentration ca. 5 mM). This “energy currency of the cell” has not only the well-known function to permit biochemical processes to occur but also to stabilize proteins in the “crowded” protoplasm (Rice and Rosen 2017).
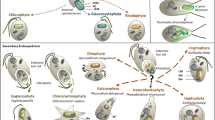
Symbiogenesis and the origin of green algae/land plants (embryophytes) and the phytoplankton of freshwater/marine ecosystems, respectively. Primary endosymbiosis gave rise to the unicellular chlorophytes, which evolved into land plants. Secondary endosymbiotic events led to the origination of planktonic organisms (red and green lineages, respectively) that are important primary producers in the oceans. mya million of years ago (adapted from Kutschera 2015a)
In a subsequent primary endosymbiotic event, an ancient cyanobacterium was engulfed, domesticated-incorporated, and finally reduced to a photosynthetic, green organelle (chloroplast). After the domestication of these ancient microbes, horizontal gene transfer to the nucleus occurred in both mitochondria and plastids, so that today these “enslaved” organelles contain a “miniaturized” genome (Zimorski et al. 2014). These alpha-proteobacterial and a cyanobacterial endosymbionts (i.e., the ancestral mitochondrion and chloroplast, respectively) multiply in the cytoplasm by binary fission, like their free-living ancestors (Fig. 4). In most organisms, they are inherited, during sexual reproduction, via the egg cell.
All multicellular organisms consist of eukaryotic cells, which are much more complex than prokaryotes (archaea, bacteria, cyanobacteria). From an energetic point of view, the ATP level per gene is at least 1000-fold higher in eukaryotic cells, due to mitochondrial activity, compared to prokaryotes. As detailed by Martin and Cerff (2017), under aerobic conditions, heterotrophic eukaryotic cells can produce (due to the presence of mitochondria) theoretically 30–40 ATP per glucose. Under real-world conditions, the number is ca. 32 ATP per metabolized glucose molecule. Prokaryotic microbes, on the other hand, create only about 4 ATP per glucose metabolized, using fermentation under oxygen-limiting conditions.
Without the emergence of mitochondria- and chloroplast-containing (photosynthetic) complex cells via serial primary endosymbiosis (i.e., symbiogenesis), the extant biosphere would exclusively be inhabited by prokaryotes, but no heterotrophic protists, chlorophytes (green algae), and their multicellular descendants would be present. As a result, animals (including humans), fungi, and plants evolved as a consequence of ancient invasions of prokaryotes into an Arachaean host (Figs. 6 and 8), a concept also known as the “two primary domains of life model” (Martin et al. 2015; Kutschera 2015a, 2016; McInnerney and O’Connell 2017). This merger of two cell types to create novel unicellular organisms (the Mereschkowsky–Wallin principle of symbiogenesis) was a key macro-evolutionary process leading to the development of complex organisms on Earth (Archibald 2014; Niklas 2016; Kutschera and Niklas 2005, 2008; Kutschera 2015a, 2016, 2017).
In addition, at least three independent ancient secondary endosymbiotic events, i.e., the incorporation and enslavement of unicellular algae by heterotrophic eukaryotic host cells, resulted in chimeric “monster organisms” (such as euglenids and dinoflagellates). Today, these photosynthetic protists represent the majority of extant phytoplankton in marine and freshwater ecosystems of the Earth (Figs. 6 and 7). They are the dominant photosynthetic primary producers in the oceans and account for ca. 40–50% of primary photosynthetic activity in the biosphere (Cavalier-Smith 2000, 2013; Knoll 2003; Kutschera and Niklas 2008; Martin and Quigg 2012).
Scanning electron micrograph of the diatom Thalassiosira eccentrica, a photosynthetically active member of the marine phytoplankton (the two solid shells of the microorganism are shown). This eukaryotic microbe originated via secondary endosymbiosis (adapted from Kleinig and Sitte 1986)
According to Mereschkowsky (1905, 1910, 1920), Margulis (1993, 2010), Margulis and Sagan (2002), and other symbiogenesis researchers (see Carrapiço 2010, 2015), primary and secondary endosymbiosis is an evolutionary process incompatible with natural selection. For a discussion of this argument, we briefly recapitulate some achievements of Alfred Russel Wallace.
In his popular book Darwinism, Wallace (1889) discussed the “problem of the Origin of Species” and coined the phrase “the Darwinian theory of natural selection.” Moreover, he wrote that “I am the advocate of pure Darwinism” (p. 12). His own significant contributions to the development of the “Darwin–Wallace principle of natural selection” (Fig. 3) are only briefly described in his book. However, Wallace (1889) rejected the Lamarckian–Darwinian concept of an inheritance of acquired characteristics and incorporated the discoveries and theoretical principles of the German zoologist August Weismann into his theoretical concepts. Hence, Wallace became one of the founding fathers of the Neo-Darwinian theory of biological evolution, which later gave rise to the Synthetic Theory (Mayr 1984, 2001; Kutschera and Niklas 2004, 2008; Kutschera and Hossfeld 2013; Kutschera 2015a; 2017).
The key concept of this theory of the 1940s, the “Darwin–Wallace principle of natural selection,” which may be interpreted as a process resulting from biological differences among individuals in expanding populations, has been confirmed in numerous field and laboratory studies, ranging from bacteria to humans and plants (Endler 1986; Bell 1997; Mayr 1984, 2001; Kutschera and Niklas 2004; Carroll 2006; Gregory 2008; Niklas 2016). Natural selection also operates in variable populations of unicellular eukaryotic microbes that originated from primary and secondary endosymbiotic events (i.e., via symbiogenesis), such as diatoms (Fig. 7). Hence, the argument that symbiogenesis and natural selection are contradictory, mutually exclusive processes is invalid. In the next section, we briefly discuss geologic events that were responsible for the long-term creation of new environments and hence major selection pressures over millions of years of organismic evolution.
6 The Snider–Wegener Concept of Shifting Continents
In 1669, Nicolaus Steno (1638–1686) established some of the fundamental principles of paleontology and stratigraphy by identifying fossils as remnants of once-living organisms, and the proposal that rock strata are analogous to the pages in a history book. Accordingly, Steno concluded that the surface of the Earth is not static, but dynamic, and that the fossil record represents a chronology of living beings that inhabited our planet in different eras of Earth’s history (Cutler 2003).
Despite Steno’s early insights, which indicated that the surface of our planet may be in slow motion, the idea of a static Earth prevailed. In 1858, when Darwin and Wallace published their papers on natural selection (Fig. 3), Antonio Snider-Pellegrini (1802–1885) proposed that identical plant fossils found in European and North American coal deposits may be explained by the idea that the two continents were once connected together during the Pennsylvanian period. In his book The Creation and its Mysteries Unveiled, Snider-Pellegrini (1858) published two maps of the Earth, depicting the continents before and after separation. Although the author referred to fossils with reference to continental drift, Snider-Pellegrini’s primary source of inspiration was the book of Genesis in the Bible (LeGrand 1988).
As expected, Snider-Pellegrini’s (1858) fanciful maps did not convince the geologists of his time. Accordingly, the idea of a static Earth prevailed again. Due to the work of the German scientist Alfred Wegener (1880–1930), convincing evidence for a purely naturalistic theory of continental drift was proposed that later revolutionized geology. Like Snider-Pellegrini (1858), Wegener examined the maps of the globe and suggested that most of the extant continents may fit together like a puzzle. For instance, the West African coastline fits into the east coast of South America and the Caribbean Sea; a similar fit is apparent across the Pacific. Even more striking results were obtained when the submerged continental shelves were compared rather than the continents. In 1915, the first edition of his book was published. Subsequently, Wegener revised and considerably extended the text, so that the 4th edition of 1929 represents the definitive version of this important monograph.
In his book, The Origin of the Continents and Oceans (1929), Wegener proposed that the current, isolated continents were once compressed into a single proto- or supercontinent: Pangaea (“all lands”), which covered about half of the Earth’s surface, was surrounded by one giant ocean called Panthalassa (“universal sea”). Wegener’s drift theory provided a novel explanation for the formation of mountains via the compression and upward folding of the edges of moving continents. Moreover, he postulated that earthquakes and volcanism are definitively caused by continental drift (Wegener 1929).
Wegener’s inability to provide an adequate explanation for the physical forces responsible for the possible drift of the continental land masses, and the prevailing assumption that the Earth was immovable (static), resulted in the ignorance and dismissal of his theory. In the late 1960s, Snider’s and Wegener’s forgotten ideas were rediscovered, supported by independent lines of evidence from geology and paleobiology, and expanded into the concept of the dynamic Earth, also known as the theory of plate tectonics (LeGrand 1988; Irving 2005; Nield 2007; Kutschera 2009a; Mallard et al. 2016) (Fig. 8).
General scheme of organismic macroevolution during the history of life on Earth, with special reference to symbiogenesis (primary endosymbiosis). Chemical evolution resulted in the Last Universal Common Ancestor (LUCA). Via directional natural selection, under changing environmental conditions (dynamic Earth), members of all five Kingdoms of life evolved (Monera, Protoctista, Animalia, Fungi, Plantae) (adapted from Kutschera 2015a)
Independent lines of evidence document that the dynamic Earth has not only created and modified most terrestrial and aquatic habitats through the eons of geological time but also destroyed entire groups of organisms via volcanic eruptions and the associated release of poisonous gases (SO2, SO3, CO2, etc.) leading to mass extinctions. Hence, plate tectonics has been responsible for the creation of new ecological niches, as well as the destruction of populations, and therefore naturally selected those individuals in variable populations that propagated “their kind” under new environmental conditions (survival-reproduction of the most suitable individuals) (Kutschera 2017). In 1915, when these ideas were proposed for the first time by Wegener, the Russian biologist Mereschkowsky was working on his last symbiogenesis paper that was published 5 years later. In this major contribution, Mereschkowsky (1920) further expanded the organismic view of evolutionary theory by outlining a multi-kingdom perspective that is summarized below.
7 Constantin Mereschkowsky and the Five Kingdoms of Life
In the nineteenth century, when Darwin (1859, 1872) published his Origin of Species, and Wallace (1889) summarized and extended these revolutionary ideas, systematic biology (taxonomy) was not very well developed. Throughout these great works, which provided the organizing principle of modern biology—descent with modification (organismic evolution)—Darwin and Wallace referred to animals and plants, i.e., multicellular macroorganisms. Only at the end of the last chapter, Darwin (1859, 1872) briefly mentioned “lower algae.” From such animal-plant-like intermediate forms of life (freshwater flagellates of the genus Euglena), all organic beings may have descended (Darwin 1872). It has been shown that Darwin’s classical “Proto-Euglena hypothesis” is no longer acceptable (Kutschera and Niklas 2008). Hence, in Darwin’s and Wallace’s time, animals, plants, and very few “infusoria” were the model organisms of choice to explain the principle of evolution via natural selection. Bacteria, amoebae, and many other microorganisms described by nineteenth-century naturalists are not mentioned by Darwin (1859, 1872) and only briefly addressed by Wallace (1889, 1913). It was Ernst Haeckel who introduced the “Protista” and “Bacteria,” microbes he studied from a taxonomic point of view (Haeckel 1866, 1877). However, his concepts concerning their mode of evolution remained speculative and unconvincing (Hossfeld 2010; Kutschera 2011a, b, 2016).
The Russian botanist and cytologist Mereschkowsky (1905, 1910, 1920) was one of the first to integrate, in addition to animals and plants, bacteria, cyanophytes (i.e., cyanobacteria), green algae, amoebae (Protists), fungi, and other “lower organisms” into an evolutionary scenario that he called symbiogenesis—the origination of new forms of life by the combination of two or several unicellular living beings which enter into symbiosis. Hence, Mereschkowsky—notably in his paper of 1920—was one of the founding fathers of a “numerous Kingdoms principle” that incorporated all known forms of life into an evolutionary framework. Today, the organisms on Earth are classified according to the “Five-Kingdom System” (Barnes 1998; Margulis and Schwartz 1998):
-
1.
Monera (Bacteria or Prokaryotae)
-
2.
Protoctista (protists, like diatoms [Fig. 7], algae, and amoebae)
-
3.
Animalia (animals, including humans)
-
4.
Fungi (molds, yeasts, and mushrooms)
-
5.
Plantae (bryophytes, ferns, and seed plants)
According to this classification scheme of the living world, we distinguish between prokaryotic microbes, unicellular microorganisms that consist of small bacterial cells (Kingdom 1), and the eukaryotes (Kingdoms 2–5). These micro- and macroorganisms are composed of much larger eukaryotic cells, which are defined by the presence of a nucleus and mitochondria (see Fig. 8).
8 Evolution of Life in a Bacterial World: Animals and Plants as Superorganisms
During the 1950s, it became obvious that bacteria dominate the biosphere. At that time, it was estimated that about 50% of protoplasmic biomass on Earth may be composed of prokaryotic microbes (essentially aquatic cyanobacteria, plus archaea and eubacteria). Decades later, this concept solidified so that it is now generally accepted that we live on a “planet of the microbes” (Whitman et al. 1998; Kutschera 2009a, b, 2011a, b, 2015a). The “unseen majority” of bacteria inhabit, for instance, the gut of animals/humans, where they are important symbionts for digestion and health of the eukaryotic host organism (Charbonneau 2016).
However, it is not widely recognized that the growth of land plants (embryophytes), Mereschkowsky’s model organisms, is regulated and modified to some extent by microbes: plant growth-promoting rhizobacteria (PGPRs) and pink-pigmented facultative methylotrophic bacteria (PPFMs, also called, “methylobacteria”). Among the PGPRs, we distinguish between symbiotic microbes that live inside the plant body and free-living bacteria that inhabit the rhizosphere (region around the roots) of their host organism. The most prominent PGPRs are bacteria of the genus Rhizobium that induce symbiotic root nodules in leguminous crop plants, such as pea, lupines, etc., and fix atmospheric nitrogen (N2). Of similar importance are free-living bacteria of the genera Azobacter, Azotobacter, Bacillus, Phyllobacterium, Pseudomonas, and Streptomyces. These root-associated rhizobacteria promote the growth of crop plants (cucumber, wheat, rice, sunflower, maize, strawberries, potato, Indian lilac, etc.) by the production/secretion of phytohormones (auxins, cytokinins), the solubilization of mineral nutrients (potassium, phosphate, etc.), or the production of antibiotics (prevention of plant diseases). Since, for instance, sugarcane plants harbor in their intercellular spaces large populations of endophytic bacteria (Beijerinckia, Herbaspirillum, etc.), and, in addition to the PGPRs, the PPFMs or methylobacteria (genus Methylobacterium) likewise live attached to these green organisms (from the flowers via the leaves/stem down to the root tips), it is fair to interpret land plants as superorganisms. The well-known soil-borne mycorrhizas (fungi associated with the root system) should also be mentioned in this context, since Mereschkowsky (1905, 1910, 1920) discussed these organisms in some detail and published a scheme illustrating their possible evolutionary development (Kutschera 2007; Kutschera and Khanna 2016).
9 Conclusions: Symbiogenesis as the “Big Bang” in Organismic Evolution
The most ancient traces of microbial life on Earth are about 3800 mya old (Allwood 2016).
After the emergence of living units via the occurrence of hypothetical proto-cells about 4000 mya (LUCA, i.e., the last universal common ancestor), bacteria, and later cyanobacteria, dominated the aquatic habitats on the young Earth (Knoll 2003; Schopf 2006; Kutschera 2017). As a result of the evolutionary “invention” of oxygenic photosynthesis, ancient cyanobacteria created the O2-containing atmosphere that emerged about 2200 mya (Knoll 2003; Zimorski et al. 2014). Symbiogenesis (i.e., primary endosymbiosis) was the key macro-evolutionary event (or the “big bang”) that gave rise to the first eukaryotic microorganisms via the fusion of an archaeon (host) and a bacterium (guest). This “two primary domains of life” model is in accordance with Ernst Haeckel’s (1866) idea that all living beings on planet Earth originated from bacteria (Kutschera 2016; McInnerney and O’Connell 2017). The first cell chimeras were heterotrophic, mitochondria-containing units without photosynthetic organelles; later, cells with photoautotrophic microbes, i.e., domesticated/enslaved cyanobacteria (which later became chloroplasts), evolved (Figs. 6 and 8).
Natural selection of those individuals best adapted to the corresponding environment in growing populations of pro- and eukaryotic micro- and macroorganisms not only “shaped” the evolving phenotypes but was also responsible for the diversification of life (Dobzhansky 1955; Mayr 1984, 2001; Bell 1997; Klingsolver and Pfennig 2007; see also Pigliucci 2017). The dynamic Earth (i.e., plate tectonics) resulted in the formation of mountains and deep oceans and caused volcanism (Mallard et al. 2016). Hence, via these geological processes, new habitats and niches for evolving populations of organisms in all five Kingdoms of life were created. In addition, the climate of the planet has been modified via changes in oceanic and atmospheric chemistry, as well as global topography. Mass extinctions were to a large extent caused by plate tectonics/volcanism, although extraterrestrial causes, such as meteorite impacts, may also have elicited these global catastrophes.
Figure 8 illustrates that symbiogenesis, natural selection, and the dynamic Earth were the key processes or dominant “evolutionary factors” that caused the origination as well as extinction of organisms on this ever-changing planet. This integrative “synade model” of macroevolution, which is a general theory of organismic evolution that consists of a set of fundamental biogeological principles, does not make specific predictions as to the phylogeny of any group of organisms. Neither Darwin (1859, 1872) and Wallace (1889, 1913) nor the architects of the Synthetic Theory of the 1950s had incorporated symbiogenesis (and plate tectonics) into their corresponding explanatory framework of evolutionary change (Dobzhansky 1955; Mayr 1984, 2001, 2004; Gould 2002; Haffer 2007; see also Depew 2017; Pigliucci 2017). These “driving forces” of biological evolution were rediscovered and refined during the post-synthesis era of evolutionary thought (the modern theory of biological evolution as an expanded synthesis; see Kutschera and Niklas 2004; Kutschera 2008a, b, 2009a, b, 2011a, b, 2017). The implications of this extended view of the evolving geo-biosphere can be summarized as follows.
Without the internal heat in the center of the Earth (Fig. 8), which is driven primarily by radioactive decay of heavy, naturally occurring elements such as Uranium, no continental land masses would have been created via plate tectonic events. It follows that without the dynamic Earth, life would probably still be unicellular and restricted to the oceans—no land plants and terrestrial animals would ever have had a chance to evolve. Other processes, notably natural selection under changing environmental conditions, were likewise of major importance during the about 3500 million years of history of life on Earth. All organisms produce more progeny than the environment can support. Nevertheless, symbiogenesis (primary endosymbiosis) was the “big bang” in cell evolution that gave rise to all eukaryotic organisms on Earth, from amoeba to animals and land plants. Later in the history of life, secondary endosymbiotic events led to the origin of the eukaryotic phytoplankton that represents the dominant organismic component of the oceans (Figs. 6 and 8).
To sum up, symbiogenesis and the corresponding focus on cell evolution considerably broadened our perspective of the modes and mechanisms of organismic evolution. As a result, an integrative view is emerging that goes far beyond what Darwin and Wallace, as well as the architects of the synthetic theory, ever have imagined when they published their groundbreaking monographs on the origin and phylogenetic development of life on Earth (Darwin 1859, 1872; Wallace 1889, 1913; Dobzhansky 1955; Gould 2002; Mayr 1984, 2001, 2004).
References
Allwood AC (2016) Geology: evidence of life in Earth’s oldest rocks. Nature 537:500–501
Altmann R (1890) Die Elementarorganismen und ihre Beziehungen zu den Zellen. Verlag von Veit, Leipzig
Archibald JA (2014) One plus one equals one: symbiosis and the evolution of complex life. Oxford University Press, Oxford
Barnes RSK (ed) (1998) The diversity of living organisms. Blackwell, Oxford
Bell G (1997) Selection: the mechanism of evolution. Chapman and Hall, New York
Carrapiço F (2010) How symbiogenic is evolution? Theory Biosci 129:135–139
Carrapiço F (2015) Can we understand evolution without symbiogenesis? In: Gontier N (ed) Reticulate evolution. Interdisciplinary evolution research, vol 3. Springer, Cham, pp 81–105
Carroll SB (2006) The making of the fittest. DNA and the ultimate forensic record of evolution. WW Norton, New York
Cavalier-Smith T (2000) Membrane heredity and early chloroplast evolution. Trends Plant Sci 5:174–182
Cavalier-Smith T (2013) Symbiogenesis: mechanisms, evolutionary consequences and systematic implications. Annu Rev Ecol Evol Syst 44:145–172
Charbonneau MR (2016) A microbial perspective of human developmental biology. Nature 535:48–55
Cutler A (2003) The seashell on the mountaintop. Dutton, New York
Darwin C (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London
Darwin C (1872) The origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 6th edn. John Murray, London
Depew DJ (2017) Darwinism in the 20th century: productive encounters with saltation, acquired characteristics, and development. In: Delisle RG (ed) The Darwinian tradition in context: research programs in evolutionary biology. Springer, Cham, pp 61–88
Dobzhansky T (1955) Evolution, genetics, and man. Wiley, New York
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton, NJ
Geus A, Höxtermann E (Hg) (2007) Evolution durch Kooperation und Integration. Zur Entstehung der Endosymbiosetheorie in der Zellbiologie. Basilisken-Presse, Marburg
Gould SJ (2002) The structure of evolutionary theory. Harvard University Press, Cambridge
Gregory TR (2008) Evolution as fact, theory and path. Evol Educ Outreach 1:46–52
Haeckel E (1866) Generelle Morphologie der Organismen, vol I/II. Georg Reimer, Berlin
Haeckel E (1877) Anthropogenie oder Entwicklungsgeschichte des Menschen. W. Engelmann, Leipzig
Haffer J (2007) Ornithologie, evolution, and philosophy. The life and science of Ernst Mayr 1904–2005. Springer, Berlin
Hagemann R (2007) The reception of the Schimper-Mereschkowsky endosymbiont hypothesis on the origin of plastids—between 1883 and 1960—many negative, but a few relevant positive reactions. Ann Hist Philos Biol 12:41–59
Hossfeld U (2010) Ernst Haeckel. Biographienreihe absolute. Orange, Freiburg i. Br
Irving E (2005) The role of latitude in mobilism debates. Proc Natl Acad Sci USA 102:1821–1828
Kleinig H, Sitte P (1986) Zellbiologie. Ein Lehrbuch. 2. Auflage. Verlag Gustav Fischer, Stuttgart
Klingsolver JG, Pfennig D (2007) Patterns and power of phenotypic selection in nature. Bioscience 57:561–572
Knoll AH (2003) Life on a young planet. The first three billion years of evolution on earth. Princeton University Press, Princeton, NJ
Kozo-Polyansky BM (1924) Symbiogenesis: a new principle of evolution. Marshall University, Huntington, WV
Kropotkin P (1902) Mutual aid. A factor of evolution. Free Press, London
Kutschera U (2003) A comparative analysis of the Darwin-Wallace papers and the development of the concept of natural selection. Theory Biosci 122:343–359
Kutschera U (2007) Plant-associated methylobacteria as co-evolved phytosymbionts: a hypothesis. Plant Signal Behav 2:74–78
Kutschera U (2008a) Darwin-Wallace principle of natural selection. Nature 453:27
Kutschera U (2008b) From Darwinism to evolutionary biology. Science 321:1157–1158
Kutschera U (2009a) Symbiogenesis, natural selection, and the dynamic earth. Theory Biosci 128:191–203
Kutschera U (2009b) Charles Darwin’s Origin of Species, directional selection, and the evolutionary sciences today. Naturwissenschaften 96:1247–1263
Kutschera U (2011a) Darwiniana Nova. Verborgene Kunstformen der Natur. LIT, Berlin
Kutschera U (2011b) From the scala naturae to the symbiogenetic and dynamic tree of life. Biol Direct 6(33):1–20
Kutschera U (2015a) Evolutionsbiologie. Ursprung und Stammesentwicklung der Organismen. 4. Auflage. Verlag Eugen Ulmer, Stuttgart
Kutschera U (2015b) A prescient view of women in evolution. Nature 523:35
Kutschera U (2016) Haeckel’s 1866 tree of life and the origin of eukaryotes. Nat Microbiol 1:16114
Kutschera U (2017) Evolution. Reference module in life sciences. Article 06399. Elsevier, pp 1–5
Kutschera U, Hossfeld U (2013) Alfred Russel Wallace (1823−1913): the forgotten co-founder of the Neo-Darwinian theory of biological evolution. Theory Biosci 132:207–214
Kutschera U, Khanna R (2016) Plant gnotobiology: epiphytic microbes and sustainable agriculture. Plant Signal Behav 11(12):e1256529, 1–4
Kutschera U, Niklas KJ (2004) The modern theory of biological evolution: an expanded synthesis. Naturwissenschaften 91:255–276
Kutschera U, Niklas KJ (2005) Endosymbiosis, cell evolution, and speciation. Theory Biosci 124:1–24
Kutschera U, Niklas KJ (2008) Macroevolution via secondary endosymbiosis: a Neo-Goldschmidtian view of unicellular hopeful monsters and Darwin’s primordial intermediate form. Theory Biosci 127:277–289
LeGrand HE (1988) Drifting continents and shifting theories. Cambridge University Press, Cambridge
Mallard C, Coltice N, Seton M, Muller RD, Tackley PJ (2016) Subduction controls the distribution and fragmentation on Earth’s tectonic plates. Nature 353:140–143
Margulis L (1993) Symbiosis in cell evolution. Microbial communities in the Archean and Proterozoic eons, 2nd edn. WH Freeman, New York
Margulis L (2010) Symbiogenesis. A new principle of evolution rediscovery of Boris Mikhaylovich Kozo-Polyansky (1890–1957). Paleontol J 44:1525–1539
Margulis L, Sagan D (2002) Acquiring genomes: a theory of the origin of species. Basic Books, New York
Margulis L, Schwartz KV (1998) Five kingdoms. An illustrated guide to the phyla of life on earth, 3rd edn. WH Freeman, New York
Martin WF, Cerff R (2017) Physiology, phylogeny, early evolution and GAPDH. Protoplasma 254:1823–1834
Martin RE, Quigg A (2012) Evolving phytoplankton stoichiometry fuelled diversification of the marine biosphere. Geosciences 2:130–146
Martin WF, Garg S, Zimorski V (2015) Endosymbiotic theories for eukaryote origin. Philos Trans R Soc B 370:20140330
Mayr E (1984) The growth of biological thought. Diversity, evolution and inheritance. Harvard University Press, Cambridge
Mayr E (2001) What evolution is. Basic, New York
Mayr E (2004) What makes biology unique? Considerations on the autonomy of a scientific discipline. Cambridge University Press, Cambridge
McInnerney JO, O’Connell MJ (2017) Microbiology: mind the gaps in cellular evolution. Nature 541:297–299
Mereschkowsky C (1905) Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Biol Centralbl 25:593–604, 689–691
Mereschkowsky C (1910) Theorie der zwei Plasmaarten als Grundlage der Symbiogenese, einer neuen Lehre von der Entstehung der Organismen. Biol Centralbl 30:278–303, 321–347, 353–367
Mereschkowsky C (1920) La plante considerée comme un complexe symbiotique. Bull Soc Sci Nat Fr 6:17–98
Nield T (2007) Supercontinent. Ten billion years in the life of our planet. Harvard University Press, Cambridge
Niklas KJ (2016) Plant evolution. An introduction to the history of live. University of Chicago Press, Chicago, IL
Pigliucci M (2017) Darwinism after the modern synthesis. In: Delisle RG (ed) The Darwinian tradition in context: research programs in evolutionary biology. Springer, Cham, pp 89–104
Reinheimer H (1915) Symbiogenesis: the universal law of progressive evolution. Knapp, Drewett, London
Reinheimer H (1920) Symbiosis: a socio-physiological study of evolution. Headley, London
Rice AM, Rosen MK (2017) Perspective: ATP controls the crowd. Science 356:701–702
Sachs J (1882) Vorlesungen über Pflanzen-Physiologie. Wilhelm Engelmann, Leipzig
Sapp J, Carrapico F, Zolotonosov M (2002) Symbiogenesis: the hidden face of Constantin Merezhkowsky. Hist Philos Life Sci 24:413–440
Schimper ATW (1883) Über die Entwicklung der Chlorophyllkörner und der Farbkörper. Botanische Zeitung 41:105–114, 121–131, 137–146, 153–162
Schopf WJ (2006) Fossil evidence of Archaean life. Philos Trans R Soc B 361:869–885
Smith CH (2012) Alfred Russel Wallace and the elimination of the unfit. J Biosci 37:203–205
Snider-Pellegrini A (1858) La Création et ses mystères dévoilés. Franck et Dentu, Paris
Speijer D, Lukes J, Elias M (2015) Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc Natl Acad Sci USA 112:8827–8834
Wallace AR (1889) Darwinism. An exposition of the theory of natural selection with some of its applications. MacMillan, London
Wallace AR (1913) Social environment and moral progress. Cassell, London
Wallin IE (1927) Symbionticism and the origin of species. Bailliere, Tindall & Cox, London
Wegener A (1929) Die Entstehung der Kontinente und Ozeane. 4. Auflage. F. Vieweg & Sohn, Braunschweig
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95:6578–6583
Zimorski V, Ku C, Martin WF, Gould SB (2014) Endosymbiotic theory for organelle origins. Curr Opin Microbiol 22:38–48
Acknowledgements
I thank two anonymous reviewers for helpful comments on an earlier version of the manuscript and the Alexander von Humboldt-Stiftung (Bonn, Germany) for financial support (AvH-fellowship 2013, Stanford, CA, USA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kutschera, U. (2017). Symbiogenesis and Cell Evolution: An Anti-Darwinian Research Agenda?. In: Delisle, R. (eds) The Darwinian Tradition in Context. Springer, Cham. https://doi.org/10.1007/978-3-319-69123-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-69123-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69121-3
Online ISBN: 978-3-319-69123-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)