Abstract
The balance between photosynthetic carbon (C) assimilation and C loss via respiration (R), emission of volatile organic compounds (VOCs), and rhizodeposition determines plant net primary production and controls to a large extent ecosystem C budgets. Compared to photosynthesis, the physiology, environmental control and ecological importance of processes involving C release from trees have been less studied; it is the purpose of this review to address these questions in oak trees with special focus on R and VOC emissions. Mass-based leaf dark R scales positively with specific leaf area, nitrogen content and photosynthetic capacity, and it is normally greater in deciduous species than evergreen sclerophyllous ones. Leaf dark R increases with temperature, and is constrained by water shortages; however, the magnitude of these responses may vary at different temporal scales. Similarly, R in woody tissues increases with temperature, although in a hysteretic manner during a diel period. On a seasonal basis, besides temperature, water availability becomes the main abiotic driver of woody tissue R as drought stress down-regulates maintenance and growth metabolic processes in stems and roots. Respiration in foliar and woody tissues is expected to account for about half of photosynthesis; nevertheless, R can largely fluctuate with ontogenetic, biotic and abiotic factors independently of C uptake. Volatile organic compounds have multiple roles in plant-environment interactions and plant-plant signalling. Oak genus is one of the strongest emitter of isoprenoids, which are the most important VOCs released from plants. Most oak species release isoprene constitutively; however, several oak species distributed around the Mediterranean (mostly evergreen) do not produce isoprene, but alternatively emit monoterpenes or lack constitutive emissions of VOCs. The rate of emission of VOCs from leaves increases with leaf temperature and irradiance, being the derived C loss relative to photosynthesis about 1%, except during heat waves when this percentage may increase up to 5%. Emission of VOCs is constrained by drought-stress to a lesser extent than leaf photosynthesis, thus the relative C loss through VOCs also increases with drought severity. Overall, the hypothesis of homeostatic ratios between plant C gain and C loss, an artefact of our better understanding of photosynthesis in comparison to all these processes that encompass tree C loss, should be revisited to better understand C cycling in oaks and to better predict oak physiological performance under climate change scenarios.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
The advance in the understanding of our environment is largely driven by the development of suitable technologies to quantify and explain the subject of study. Research in plant carbon (C) cycling clearly illustrates how methodological feasibility has driven knowledge in a particular direction (Körner 2015): As leaves can be easily enclosed in sealed chambers and gas exchange measured with an array of sophisticated systems (Hunt 2003), research on leaf C assimilation has traditionally held a predominant role in studying tree C budgets to the detriment of C efflux from other tree organs. Atmospheric CO2 is assimilated by plants through photosynthesis (P) and part of it is released back to the atmosphere and soil through respiration (R), emission of volatile organic compounds (VOCs) and rhizodeposition . The difference between C assimilation and C loss is known as net primary production (NPP) and represents the net gain of C to be invested in plant growth, maintenance, defence, reproduction or storage. Net primary production is a key output of dynamic global vegetation models to predict C exchange between terrestrial ecosystems and the atmosphere. Net primary production is commonly calculated as the difference between P and R (e.g. Waring et al. 1998; Luyssaert et al. 2007; Piao et al. 2010; Rambal et al. 2014):
In this equation, P is a well-known process mechanistically described more than 35 years ago (Farquhar et al. 1980), whereas R is a comparatively understudied C flux, despite its predominant role in ecosystem C balance (Valentini et al. 2000; Amthor 2000). Moreover, VOCs and rhizodeposits are commonly ignored in this conceptual framework despite evidence of a non-negligible contribution to tree C loss (Jones et al. 2004; Bracho-Nunez et al. 2013; Sindelarova et al. 2014). Equation (10.1) should be therefore further developed to integrate all C fluxes between the plant and its environment:
The biological significance of processes involving C release for survival and regeneration is unquestionable. Briefly, the mitochondrial oxidation of C substrates in all plant living cells produces reducing power [e.g. NAD(P)H from NAD(P)+], C skeleton intermediates, and usable energy (ATP) from ADP and inorganic phosphate to fulfil metabolic requirements. During the numerous reactions of mitochondrial respiration CO2 is formed, a fraction of which is released to the atmosphere , and another recycled within chloroplasts. VOCs are organic chemicals emitted from all plant organs that play multiple roles in plant reproduction, plant protection, and plant-plant signalling; whereas rhizodeposits consist of a wide range of compounds involved in plant nutrition, plant defence, and signalling between plant roots and surrounding organisms of the rhizosphere.
The genus Quercus comprises more than 600 species including deciduous and evergreen trees and shrubs adapted to a broad range of environmental conditions, from semi-arid Mediterranean evergreen woodlands to sub-boreal, temperate and subtropical deciduous forests (Mabberley 2008). Due to its wide distribution in the Northern hemisphere , Quercus spp. are extensively surveyed in physiological research and constitute an excellent taxonomic group to study the variability in R and the emission of VOCs among different plant functional types and across environmental conditions. In this chapter we want to draw the attention to an important side of plant C budget that has largely been overlooked in oak species: the release of C from the plant to the environment. We will focus on R and VOCs emission due to the scarce literature on oak rhizodeposition . We aim at (i) explaining the variability in R and VOCs emission among different oak species, along gradients of environmental conditions, and at different temporal scales; (ii) highlighting the relative contribution of R and VOCs emission to oak C budgets; (iii) and summarizing information on the chemical typology, mechanisms of synthesis and release, and ecophysiological significance of VOCs among oak species.
10.2 Plant Respiration
Plant R consists on the mitochondrial oxidation of C substrates to produce usable energy , reducing power, and C skeleton intermediates with the consequent release of CO2 as a reaction product. Plant R is commonly simplified by a single equation developed in the early 1970s, in which R is partitioned into growth and maintenance processes (McCree 1970; Thornley 1970):
where R is respiration rate (mol CO2 s−1), RG and RM are growth and maintenance respiration rates (mol CO2 s−1), G is growth rate (g new biomass s−1), W is living biomass (g dry mass), gR is growth respiration coefficient (mol CO2 (g new biomass )−1) and mR is maintenance respiration coefficient (mol CO2 (g dry biomass )−1 s−1). Despite the magnitude of R, ca. 35 to 80% of P (Amthor 2000), substantial improvements in the mechanistic understanding of respiration are still lacking since the early 1970s (Cannell and Thornley 2000; Amthor 2000; Thornley 2011). Respiratory processes continue to be simplified by the growth-and-maintenance-respiration paradigm, and we are far from understanding respiration at the same detail as we do for photosynthesis (Farquhar et al. 1980). Gifford (2003) stated that “plant respiratory regulation is too complex for a mechanistic representation in current terrestrial productivity models for carbon accounting and global change research”. This idea seems to be tacitly accepted and given that the rates of enzymatic reactions involved in R are temperature -dependent, plant R is commonly estimated from a single equation derived from Arrhenius kinetics (Davidson et al. 2006):
where R is the respiration rate at temperature T, Rb is the respiration rate at a basal temperature Tb, and Q10 is the relative increase in respiration rate corresponding to a 10 °C temperature rise. Hence dynamic global vegetation models often estimate plant R from temperature data—neglecting other biotic and abiotic regulators of plant R such as water availability, C and nutrient supply, and energy demand—to quantify C fluxes and pools at the global scale (Smith and Dukes 2013; Fatichi et al. 2014). Alternatively, R is sometimes assumed to be a constant fraction of P at the tree scale (Waring et al. 1998; Van Oijen et al. 2010). Nevertheless, there is growing evidence that C cycling is not uniquely driven by C assimilation (“source-driven”), but also by abiotic constraints to plant growth and cell maintenance processes (“sink-driven”) (see Körner 2015 for a review), as recently observed in Q. ilex (Lempereur et al. 2015), which challenges the assumption of homeostatic R:P ratios. Consequently, we are unable to accurately estimate plant R at different temporal and spatial scales, and to comprehensively understand the regulation of the plant respiratory physiology despite its primary role in tree C cycling, productivity and survival (Atkin and Macherel 2009).
In this section, we distinguish between leaf and woody tissues to better summarize current knowledge on oak R. The physiological functioning of an organ is determined by its particular anatomy and structure, and thus leaves and woody tissues have different energy requirements and C-related expenditures derived from respiratory processes. Moreover, dissimilar methodological approaches to quantify R in different organs have contributed to further distance R research between leaves and woody tissues.
10.2.1 Leaf Respiration
10.2.1.1 Physiology and Variability Among Species
Leaf R is mostly dependent on the availability of ADP, C substrates—lipids, amino acids and mostly carbohydrates —and the amount, position and protein content of mitochondria within leaf cells, particularly mesophyll cells, whose contribution to total leaf R in some species is >90% (Long et al. 2015). Data compilations from plant species across the globe evidence that mass-based leaf dark respiration (Rd) rates—more easily measured and typically higher than leaf R rates in light conditions (Zaragoza-Castells et al. 2007)—are positively related with specific leaf area (SLA) , leaf nitrogen (N) concentration and photosynthetic capacity (Pmax) (Reich et al. 1998; Wright et al. 2006; Reich et al. 2008; Atkin et al. 2015). In the first case, the relationship is due to the lower proportion of structural components in high-SLA leaves; in the second, to the higher amount of respiratory enzymes present in leaves with high N concentrations. Finally, the correlation of Rd with Pmax can reflect the role of Pmax in providing the mitochondria with respiratory C substrates, or the importance of N for synthesizing both respiratory and photosynthetic enzymes.
Because oak species encompass a wide gradient of leaf functional characteristics, primarily abridged in the separation of broad-leaf evergreen and deciduous oaks, variability in leaf Rd rates among oak species is also large and partly related with climate conditions. Taking as example the 13 oak species included in the global database of respiration GlobResp (Kattge et al. 2011; Atkin et al. 2015), both area- and mass-based leaf Rd varied by approximately sevenfold. Global patterns across broad climatic gradients evidence that plant species from cold sites exhibit higher rates of leaf Rd than those from warmer sites at a comparable measurement temperature , a pattern that holds for oak species (Kattge et al. 2011; Atkin et al. 2015). Further, plant species from dry sites tend to have higher leaf Rd rates than those from mesic sites (Atkin et al. 2015), with this difference being only partly explained by differences in SLA or Pmax (Wright et al. 2006). Some adaptations to stress, such as high rates of metabolite turnover can result in high respiratory costs in processes of cell maintenance and repair in cold , high-light or arid environments (Wright et al. 2006; Atkin et al. 2015). Similarly, high N investment in repair compounds could also explain why individuals from colder and drier populations of Q. ilex exhibited higher leaf maintenance respiratory costs than those from warmer and wetter populations when grown under the same conditions (Laureano et al. 2008). However, different evolutionary selection pressures, including abiotic and biotic stress factors and competition for resources have shaped the respiratory metabolism in different ways, which precludes a straightforward relationship of leaf Rd with stress resistance . As such, some leaf structural and chemical adaptations to stress, such as low N concentrations and low biomass allocation to metabolic components, can result in low leaf Rd rates in long-lived, sclerophyllous leaves of some oaks growing in nutrient -poor or drought -prone sites. At a comparable temperature of 25 °C, mass-based leaf Rd was lowest in the Mediterranean evergreen Q. ilex (4.0 nmol CO2 g−1 s−1) and highest in the deciduous Q. alba and Q. rubra (25–27 nmol CO2 g−1 s−1). This is consistent with low Pmax of sclerophyllous leaves, and the positive relationship between Pmax and leaf Rd (Wright et al. 2006).
10.2.1.2 Response to Environmental Changes at Different Time Scales
Within species, Rd varies in relation to ontogeny and environmental changes. As oak leaves progressively stop growing, both in lamina area and thickness, Rd decreases rapidly and reaches a phase in which fluctuations are not due to ontogenetic changes but mostly to climatic shifts; eventually, when leaves start to senesce, Rd starts to decline again markedly due to remobilization of leaf N and degradation of the respiratory machinery (Collier and Thibodeau 1995; Miyazawa 1998; Xu and Baldocchi 2003; Rodríguez-Calcerrada et al. 2012). Ontogenetic variations over the leaf life span make that, for comparative purposes, measurements of Rd are typically made in non-senescent mature leaves that have fully expanded. However, the responses of leaf physiology and Rd to climatic shifts can vary as affected by ontogeny and age, something that clearly merits more research to improve C balance models (Niinemets 2014).
The plasticity of Rd to air temperature , irradiance or water and nutrient availability is considerable in oak species. Multiple changes occur in the respiratory metabolism in response to the need of the tree to adjust the production of respiratory products to shifting demands imposed by environmental changes. This plasticity in the respiratory metabolism—reflected in varying rates of Rd—allows trees to orchestrate whole-plant plasticity and overcome periods of sub-optimal growing conditions. Two of the most important drivers of leaf physiology over the leaf life span are temperature and water availability.
Changes in temperature elapsed over hours, days, months or years affect the rates of Rd. However, the magnitude of the change in Rd rates depends on the time scale of temperature changes. Short-term raises in temperature provoke an exponential increase in leaf Rd that is typically higher than that occurred over longer term warming periods due to the thermal acclimation of the respiratory metabolism (Reich et al. 2016). This process of acclimation involves a reversible decline in the activity of respiratory enzymes. Frequently, the respiratory capacity (i.e. intercepts of respiratory temperature response curves) differs across temporal scales, probably due to shifts in the amount of mitochondria or mitochondrial enzymes. The consequence of this is that Rd measured at prevailing ambient temperature barely changes over broad, long-term changes in temperature (see Fig. 10.1a for an illustration of this phenomenon, and Slot and Kitajima 2015 for a recent review of the process across biomes and experimental conditions). The thermal acclimation of leaf R (more easily and frequently examined in dark than light conditions, i.e. Rd) is a general response of healthy, non-growing oak leaves (e.g. Bolstad et al. 2003; Lee et al. 2005; Zaragoza-Castells et al. 2008; Rodríguez-Calcerrada et al. 2011) that may accompany different thermal photosynthetic adjustments to balance leaf net C gain with C needs in the new environment (Way and Yamori 2014; Slot and Kitajima 2015). The extent of the thermal acclimation of Rd varies among species and with leaf developmental status, magnitude of temperature change (Slot and Kitajima 2015) and interaction with other abiotic factors such as irradiance (Bolstad et al. 1999) or soil water availability (Turnbull et al. 2001; Rodríguez-Calcerrada et al. 2011).
Theoretical representation of the plant respiration (R) response to shifts in temperature (a, b) and water availability (c) at different temporal scales. Respiration exponentially increases with temperature at any temporal scale; however, thermal acclimation (commonly observed in leaves) leads to lower respiratory capacity of tissues grown under warm conditions (a, adapted from Atkin and Tjoelker 2003). Hysteresis between R and temperature over diel cycles has been observed for stem R, but not for leaf dark R (even if leaf R at a given temperature is usually lower in light than dark conditions) (b, adapted from Salomón et al. 2016b). Improved water status at night-time increases stem R under constant temperature on a diel basis, whereas stem R progressively decreases when water availability becomes limiting on a seasonal basis, as theoretically illustrated during the drought event (the beginning of the drought is represented by the vertical arrow) (c, adapted from Saveyn et al. 2007b). Shaded areas indicate night-time
Despite the importance of C losses for leaf and plant C balance , very few studies have examined the impacts of temporal soil water fluctuations on Rd in oak species. Most of these studies have been conducted on the drought -tolerant, widespread Mediterranean oak Q. ilex (e.g. Rodríguez-Calcerrada et al. 2011; Varone and Gratani 2015). The results of these studies are consistent with the drought -induced decline in leaf Rd and increase in leaf Rd/P ratio that is generally reported for other plant species (see review of Atkin and Macherel 2009). The main reasons behind this short-term decline in leaf Rd are: (i) a reduction in the amount of mitochondrial protein and (ii) a reduction in enzymatic activity due to limited turnover of ATP to ADP (associated to down-regulation of energy consumption processes) or limited flow of triose phosphate from chloroplasts into mitochondria (associated to impaired P). The complex regulation of leaf R makes that, as it happens in response to temperature , leaf Rd does not necessarily exhibit the same response to drought over short- and long-term time scales. However, few studies have examined how long-term decreases in soil water availability affect leaf R and C balance in oak trees (Turnbull et al. 2001; Rodríguez-Calcerrada et al. 2011; Sperlich et al. 2016). In southwestern Europe , two parallel throughfall-reduction experiments have been set up in two Q. ilex forest stands to study the long-term effects of increased drought on foliar respiratory rates. Rodríguez-Calcerrada et al. (2011) observed that leaf Rd decreased in response to seasonal decline of leaf water potential similarly in trees subjected to normal and 7-year reduced throughfall, so that leaf Rd was lower in the trees that had experienced a reduction in throughfall and a greater decline of leaf water potential during the dry season, but did not differ between treatments at optimal soil water conditions. These results and those of Limousin et al. (2010) on the nature of photosynthetic limitations in the same species and experimental site suggested that 7 years of increased drought had not modified the physiology of leaf mesophyll cells. In another Q. ilex stand subjected to a longer period of rain reduction (14 years) of similar intensity, Sperlich et al. (2016) found the same lack of treatment effect on leaf Rd, but a significant increase in leaf R during daytime in trees receiving less rain, suggesting that the reorganization of the respiratory metabolism depends on the duration of increased drought . Collectively, the results suggest that drought -induced declines in leaf R over short time periods seemingly change to drought -induced increases in leaf R as trees adapt to increased drought (Turnbull et al. 2001; Rodríguez-Calcerrada et al. 2011; Atkin et al. 2015; Sperlich et al. 2016), however, more studies are needed to understand long-term responses of leaf R to drought .
10.2.2 Stem and Root Respiration
10.2.2.1 How to Estimate It? Methodological Constrains to Measure Woody Respiration
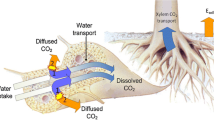
Although stems and roots constitute the largest fraction of biomass in woody species, especially in large trees (Poorter et al. 2012), our knowledge of woody R is by far less advanced compared with that of leaf R. The main obstacle to understand R in woody tissues remains in the difficulty to accurately measure it. Radial CO2 efflux to the atmosphere (EA) from stems and roots, which can be measured with cuvettes surrounding the monitored organ, is commonly assumed to equal the rate of R of these organs. Nevertheless, locally respired CO2 in roots and stems can either diffuse to the soil or the atmosphere, respectively, or alternatively accumulate within woody tissues due to substantial barriers to radial gas diffusion offered by outer tissues. Accordingly, concentrations of internal CO2 in xylem (xylem [CO2 ]) range from <1 to 26%, values one to two orders of magnitude higher than atmospheric [CO2 ]. As xylem [CO2 ] builds up inside the tree, it dissolves in the sap solution until equilibrium between gaseous and liquid phases is reached according to Henry´s law; respired CO2 moves upward in the transpiration stream, and eventually diffuses to the soil or the atmosphere elsewhere (see Teskey et al. 2008; Rodríguez-Calcerrada et al. 2015b for reviews). Internal transport of respired CO2 has therefore resulted in significant misestimation of woody R from measurements of EA, as consistently observed in several oak species (McGuire and Teskey 2002; Teskey and McGuire 2002; Bloemen et al. 2014; Salomón et al. 2016b).
An additional constraint that hinders direct measurements of woody R is the re-assimilation of internal CO2 by chloroplast -containing woody tissues (see Ávila et al. 2014 for a review). For instance, recycling of respired CO2 transported through the xylem offset 19 and 70% of C respiratory losses in branches of Q. alba and stems of Q. robur, respectively (Coe and McLaughlin 1980; Berveiller et al. 2007). To solve this issue, woody P is commonly disabled by using opaque cuvettes; nevertheless, woody P above and below the cuvette might induce axial diffusion of internal CO2 that would decrease EA within the monitored segment, as observed in Q. robur stems during the dormant season (Saveyn et al. 2008). Additional difficulties arise when measuring root R due to inaccessibility of root systems and the unclear discrimination between autotrophic and heterotrophic respiration from measurements of soil CO2 efflux (Hanson et al. 2000). All these limitations hinder the establishment of a widely accepted methodological approach to systematically measure woody R.
10.2.2.2 Response to Environmental Changes at Different Time Scales
Due to the few studies on woody R in oaks, the influence of abiotic drivers (mainly temperature and water availability) on woody R is summarized independently of any intrageneric classification . In a study with seven oak species grown under uniform conditions, Martinez et al. (2002) did not find any intrinsic difference in root R attributable to the evergreen or deciduous character of the species. Likewise, potential differences in woody R ascribed to the ring-porous or diffuse-porous wood anatomy of oaks remain untested, despite their differential wood phenology and growth (Pérez-de-Lis et al. 2016).
The temperature sensitivity of woody R, expressed as the change in R that occurs over 10 °C (Q10) ranges from 1.4 to 3.1 in oak species, with mean values close to two (i.e., R rates double for an increase in temperature of 10 °C, Table 10.1), as similarly observed for a variety of species in leaves and roots (see Atkin and Tjoelker 2003 and references therein). On a diel basis, woody R increases along the day with increasing temperatures and decreases at night-time exhibiting a characteristic hysteresis (Table 10.1; Fig. 10.1b). Several factors have been suggested to cause the day-time depression in temperature -normalized stem R observed in oaks: (i) internal transport of respired CO2 with the transpiration stream at day-time (Negisi 1982; McGuire and Teskey 2002; Teskey et al. 2008), (ii) enhanced metabolic activity of woody tissues owing to improved water status at night-time (Negisi 1982; Saveyn et al. 2007a; Salomón et al. 2016b), (iii) lagged temperature transmission and/or delayed radial CO2 diffusion due to physical barriers presented by peripheral tissues (Rodríguez-Calcerrada et al. 2014), and (iv) refixation of respired CO2 nearby the darkened monitored stem segment at day-time (Saveyn et al. 2008). At a seasonal scale, the down-regulation of temperature -normalized R with increasing temperatures across the year (Atkin and Tjoelker 2003) has the potential to reduce C loss through woody R. The thermal acclimation of leaf R (Fig. 10.1a) is a well-documented phenomenon in oaks (see previous sub-section) that has been less studied in woody tissues. A meta-analysis across 44 forested ecosystems, six of them dominated by oak species, supports the hypothesis of thermal acclimation of R in roots (Burton et al. 2008): An attenuated rate of temperature -driven increase in root R across ecosystems (Q10 = 1.6) was observed in comparison to short-term fluctuations within individual stands (Q10 = 2–3). Likewise, lower rates of temperature -normalized root R were registered in experimentally heated plots in mixed hardwood forests co-dominated by Q. velutina, although the concomitant effect of soil drying along with soil heating could not be discarded as a driver of R reductions (Burton et al. 2008). Thermal acclimation of R rates in stems may also occur in oak species such as Q. ilex (Rodríguez-Calcerrada et al. 2014), but literature is much scarcer in this regard. Again, the concomitant increase in temperature with summer drought in some ecosystems hinders to unequivocally ascribe seasonal reductions in stem R to thermal acclimation, given that water shortages constrain stem growth and associated respiratory costs in this widespread Mediterranean oak (Lempereur et al. 2015). The different temperature sensitivity of metabolic processes involved in maintenance and growth R (Amthor 2000) complicates the study of acclimation of maintenance R to abiotic stress in sites where secondary growth varies amply throughout the year. Accordingly, higher Q10 values observed in stems of Q. accutisima during the colder non-growing season relative to the warmer growing season were attributed to the greater temperature sensitivity of maintenance processes (Yang et al. 2012b) rather than to the potential effect of thermal acclimation of R.
Oak species subjected to drought stress commonly show reduced R as a consequence of constrained growth and metabolic activity. This effect has been observed at the ecosystem (Reichstein et al. 2002; Unger et al. 2009; Rambal et al. 2014), organ (Saveyn et al. 2007b; Rodríguez-Calcerrada et al. 2014), and cellular level (Saveyn et al. 2007a). During a diel cycle, night-time reduction in the vapour pressure deficit and transpiration lead to replenishment of water reservoirs within woody tissues (Steppe et al. 2006), as observed in Q. ilex (Salomón et al. 2017). Increase in cell turgor facilitates cell expansion and growth (Lockhart 1965), which in turn may lead to enhanced rates of overall R. This hypothesis of growth respiration mainly confined to night-time hours is supported by substantial night-time increases in both EA and xylem [CO2 ] observed in Q. robur stems under relatively constant temperature across 24 h (Fig. 10.1c; Saveyn et al. 2007a, b). During the course of a year, progressive soil drying in summer was found to constrain fine root turnover (López et al. 2001) and stem growth (Lempereur et al. 2015) in Q. ilex . Impeded growth and down-regulation of maintenance processes likely explains typical reductions in woody R when water becomes limiting (Fig. 10.1c), as observed in roots of Q. cerris (Rey et al. 2002), Q. robur (Molchanov 2009), and mixed oak stands (Burton et al. 2002), as well as in stems of Q. ilex (Rodríguez-Calcerrada et al. 2014) and Q. robur (Saveyn et al. 2007b). Drought -induced reductions in R suggest a threshold in soil water content below which woody R becomes largely driven by water availability and independent of temperature (e.g. Reichstein et al. 2002; Rey et al. 2002). Likewise, sharp increases in xylem [CO2 ] observed in Q. robur and Q. pyrenaica stems after rain events following dry periods (Saveyn et al. 2007b; Salomón et al. 2016a) provide further evidence of drought -driven constraints to woody R. On the other hand, reduced resistance to radial CO2 diffusion due to reduced water content of peripheral woody tissues as the soil dries out (Teskey et al. 2008; Salomón et al. 2016b) may partially explain increases in stem and root CO2 efflux during mild drought in oak trees (e.g. Edwards and Hanson 1996; Dannoura et al. 2006; Molchanov 2009). Such conflicting results evidence our deficient understanding of drought effects on woody R. At an inter-annual timescale, acclimation of stem R to long-term increased drought was not observed in Q. ilex after eight years of experimental throughfall-reduction (Rodríguez-Calcerrada et al. 2014). Further research in this line would be necessary to test potential down-regulation of woody R to prolonged drought in order to better predict C cycling at the whole-tree level under changing climate regimes.
10.2.3 Relative Importance of R for Tree Carbon Budgets
Ecosystem R (RECO) determines ecosystem C balance in a wide range of environmental conditions (Valentini et al. 2000). Ecosystem R can be biometrically partitioned into leaf, stem and soil R by measuring samples of each component in chambers and upscaling the measurements to the stand level. The broad range of variation in the contribution of each component to RECO is partly due to stand structure, composition and age, but also to uncertainties in calculations. First, estimations of annual leaf respiratory C losses at the stand scale range from 3 to 37% of RECO in oak forests (Table 10.2). At an intra-annual scale, the largest contribution of leaf R to RECO generally occurs when new leaves expand; the lowest contribution can occur at peak summer drought in some evergreen Mediterranean forests (Rodríguez-Calcerrada et al. 2012). Second, the high proportion of parenchyma in the wood of oak species can make C losses from stems potentially important in oak -dominated ecosystems. The contribution of stem R to RECO in pure and mixed oak stands ranges between 5 and 38% on an annual basis, with mean values of ca. 15% (Table 10.2). The highest contribution of stem R to RECO occurs in spring coincident with high stem growth rates in temperate and continental climates (Curtis et al. 2005; Miyama et al. 2006), and before water becomes limiting in the case of drought -prone Mediterranean regions (Rodríguez-Calcerrada et al. 2014). Third, soil R is the largest respiratory C flux to the atmosphere (Valentini et al. 2000) and accounts for 48–85% of RECO in oak forests (Table 10.2). Ambiguous discrimination between heterotrophic (microbes and soil fauna) and autotrophic sources of soil CO2 efflux hinders accurate estimations of root R. Assuming an average contribution of soil R to RECO of 67% (Table 10.2), and a mean contribution of root R to soil R of 50% (Hanson et al. 2000; Burton et al. 2008), root R would account for 33% of RECO. More conservative contributions of root R to soil R observed in oak stands—ranging from 15 to 40% (Reichstein et al. 2002; Rey et al. 2002; Unger et al. 2009; Luan et al. 2011)—would reduce the contribution of root R to RECO to 10–27%, respectively. Furthermore, it is worth noting that neglecting internal fluxes of root respired CO2 through xylem results in substantial underestimation of root R rates when these are estimated via soil CO2 efflux measurements; underestimation ranges from 2 to 18% in Quercus species (Bloemen et al. 2014; Salomón et al. 2015) and reach up to 50% in other taxa (Aubrey and Teskey 2009). Overall, these estimates evidence the important role of autotrophic R in plant and ecosystem C budgets and further highlight the need of more experimental research on plant R to improve the accuracy of dynamic global vegetation models.
10.3 Volatile Organic Compounds (VOCs)
Plants produce a large array of metabolites whose vapor pressures are high enough (approx. ≥ 0.01 kPa) to become volatilized under ambient temperature conditions. All plant organs, namely flowers and fruits, foliage , stem and roots can release VOCs. Flower and leaf emissions are by far the best investigated ones. However, in the last decade increasing research has been afforded to root emissions, whose ecological roles in soil biotic interactions are only in the beginning to be appreciated (see e.g. Weissteiner et al. 2012; Delory et al. 2016). VOCs are emitted from plant organs either constitutively or temporarily following induction by stress factors. This classification is however not straightforward, because the emissions of constitutive VOCs are also up and down-regulated by environmental factors including stress events (Peñuelas and Staudt 2010).
Phytogenic VOCs are mainly composed of C and hydrogen plus occasionally other elements such as oxygen, nitrogen and sulphur, or more rarely halogens. Once emitted the C skeleton of VOCs reacts gradually with oxidants in the atmosphere to form ultimately CO2 , thus closing the carbon cycle. However, a substantial portion of intermediate products may be removed from the atmosphere via dry and wet deposition. Products from VOC oxidation can condense with each other and other atmospheric constituents and contribute to the formation of secondary organic aerosols, which have important impacts on climate forcing and human health (Hallquist et al. 2009).
10.3.1 Metabolic Origins and Ecological Importance
Of the thousands existing plant VOCs, the majority belongs to three biosynthetic classes, fatty acid derived volatiles, volatile aromatic compounds and volatile terpenoids (isoprenoids ). The most prominent fatty acid-derived VOCs are Green Leaf Volatiles (GLVs) that are formed from the breakdown of free fatty acids by lipoxygenase and hydroperoxide-lyase enzymes (Matsui 2006). GLVs comprise mainly mono-unsaturated C6 alcohols, aldehydes and acetate esters that are potentially emitted from all plant species. GLV are emitted in high amounts only after exposure to stresses such as wounding, herbivore attack, extreme temperatures or acute ozone exposure. Upon stress GLVs are formed and emitted within seconds to minutes and rapidly disappear when the stress ceases (e.g. Staudt et al. 2010). Volatile aromatic compounds derive from the shikimate pathway (benzenoids and phenylpropanoids). They are most common in flower scents (Schiestl 2010). However, recent studies made at plant and canopy levels emphasize that considerable amounts of benzenoids are also emitted from vegetative tissues in particular under stress conditions (Misztal et al. 2015). Volatile isoprenoids are classified according to the number of C5 units they have: C5 hemiterpenes, C10 monoterpenoids and C15 sesquiterpenoids. In planta, isoprenoids are synthesized within two distinct pathways, the 2-methyl-erythritol-4-phosphate pathway operating in plastids and the mevalonate pathway operating in the cytosol, endoplasmic reticulum and peroxisomes (Lu et al. 2016 and references therein). Volatile isoprenoids can also be secondarily formed from the breakdown of higher isoprenoids such as homoterpenes (Tholl et al. 2011) and apocarotenoids (Walter et al. 2010). The quantity and quality of emitted isoprenoids and aromatic compounds inherently differ among plant taxa. The hemiterpene isoprene is the most important VOC released from terrestrial vegetation. Globally ca. 600 Tg (1012 g) of isoprene are annually emitted, which is about 2/3 of the total biogenic VOC release (Sindelarova et al. 2014). However, only about 30% of vascular plants emit isoprene from their foliage (Fineschi et al. 2013). Oaks, poplars and willows figure among the strongest isoprene emitters (Kesselmeier and Staudt 1999).
In addition to the volatiles of these three major classes, a number of short-chain oxygenated volatiles (collectively called OVOCs) are frequently observed in plant emissions such as methanol, formaldehyde, formic acid, ethanol, acetaldehyde, acetic acid, methyl acetate, acetone, ethylene and methylglyoxal. These have different metabolic origins and occur rather universally in plants. For instance methanol is formed during cell wall formation and is therefore particularly released during the growing period (Bracho-Nunez et al. 2011; Brilli et al. 2016). With an estimated annual emission of ca. 130 Tg, methanol is the second important phytogenic VOC worldwide (Sindelarova et al. 2014). Emissions of ethanol and the equivalent C2 aldehyde and acid are mostly associated with fermentation during hypoxia (Kreuzwieser and Rennenberg 2013). Many of these OVOCs are also secondarily formed during the oxidation of primary emitted higher VOCs such as terpenes (e.g. Gaona-Colmán et al. 2016).
Diverse ecological functions have been attributed to VOC production in plants. The attraction of pollinators and seed dispersers by flower and fruit scents is an essential driver of sexual reproduction and evolution in many plant species (Schiestl 2010). Further, numerous studies have shown that plant volatiles induced by herbivore or pathogen attacks have toxic, repellent or aposematic effects, or attract predators and parasitoids of the attackers and thereby contribute to limit damages to plants (see e.g. Van Loon et al. 2000). So far, such functions are largely unknown for oaks. Oaks are wind pollinated and a potential role of volatiles in sustaining acorn dispersal has rarely been documented (e.g. Verdú et al. 2007). Only few studies have reported that herbivory or pathogen attack affect quantitatively and/or qualitatively the volatile production from oaks (Staudt and Lhoutellier 2007; Paris et al. 2010; Copolovici et al. 2014). A study with Q. robur suggests potential beneficial effects of herbivory-induced VOCs against the European oak leaf roller (Ghirardo et al. 2012). On the other hand, Vuts et al. (2016) demonstrated that volatiles of Q. robur can attract the two-spotted oak buprestid, a bark beetle that causes severe damages in European oak forests.
The possible function of the constitutive isoprene production in chloroplasts (or analog monoterpenes ) is still a matter of debate. With regard to biotic stress, a study using transgenic isoprene emitting Arabidopsis plants has shown that isoprene can disturb the attraction of a parasitic wasp to volatiles from herbivore-infested plants. Thus, by troubling VOC-mediated trophic interactions of neighbouring species, isoprene emitters could promote their interspecific competitiveness (Loivamäki et al. 2008). However, since isoprene is one of the most common background-VOC in the atmosphere , insects may rapidly adapt to avoid such interferences in host recognition (Müller et al. 2015). The major function of isoprene is thought to reinforce the resistance to abiotic stresses, in particular to oxidative and high temperature stress (see e.g. Loreto and Schnitzler 2010). The most pertinent results supporting these hypotheses were achieved with transgenic plants, either isoprene synthase over-expressing mutants of the non-isoprene emitting species Arabidopsis (Sasaki et al. 2007; Velikova et al. 2012) and tobacco (Vickers et al. 2009b; Tattini et al. 2014) or isoprene synthase silenced mutants of the isoprene emitting species poplar (Behnke et al. 2007, 2010). However, several studies using the same or other transgenic mutants reported conflicting results with respect to these hypotheses (Behnke et al. 2009, 2012; Palmer-Young et al. 2015). Further, it is uncertain that the improved resistance to abiotic stress in isoprene emitters is, as initially hypothesized, due to direct effect of isoprene by scavenging oxidants or stabilizing membrane structure. In fact, isoprene dissolves too little in bio-membranes to efficiently change membrane conformation (Harvey et al. 2015) and is only fairly suitable to scavenge oxidants due to its moderate reactivity (Atkinson and Arey 2003; Palmer-Young et al. 2015) and the high toxicity of reaction products (Cappellin et al. 2017; Matsui 2016 and references therein). More likely, the physiological effects of isoprene are indirect by taking part in the plant’s stress signalling network (Vickers et al. 2009a; Vanzo et al. 2016). In any case, the genetically engineered absence or presence of isoprene synthase in mutants causes transcriptomic, proteomic and metabolic changes in various metabolic pathways even under non-stress conditions, including the biosynthesis of phenyl-propanoids and lipids that affects the composition and ultrastructure of chloroplast membranes (Velikova et al. 2015; Harvey and Sharkey 2016). Numerous other VOCs than isoprene have shown to be involved in within-plant and between-plant stress signalling, among which diverse GLVs, aromatic compounds and higher isoprenoids (Havaux 2014; Delory et al. 2016; Matsui 2016).
10.3.2 Diversity of VOCs in Oak Species
The great majority of oak species hitherto screened for VOC emission has been found to release isoprene constitutively at high rates from its foliage (up to several tenths of nmol m−2 s−1). However, several oak species with distribution around the Mediterranean do not produce isoprene , but produce either monoterpenes in high amounts or shown no constitutive VOC emissions. These exceptions are mostly but not exclusively evergreen oaks and belong all to the two very closely groups Cerris and Ilex (Welter et al. 2012; Monson et al. 2013). Diversification of isoprene emission in oaks has been mainly observed at species and higher taxon levels and more rarely at population level. So far, inherent intra and/or inter population variability in the quantity or quality of constitutively produced VOC has not been reported within isoprene emitting oak species (Welter et al. 2012; Steinbrecher et al. 2013). By contrast in monoterpene emitting species, compositional diversification (i.e. chemotypes) has been frequently observed within and among populations (Staudt et al. 2001b, 2004; Loreto et al. 2009; Welter et al. 2012). In addition, a few low or non-emitting individuals were detected in some monoterpene emitting populations. Conversely, dual isoprene /monoterpene emitting oak individuals seem to be extremely rare in natural oak populations (Staudt et al. 2004). This is somewhat surprising, because many oaks can hybridize with each other resulting in widespread genetic introgression or rise of new species (e.g. Valbuena-Carabaña et al. 2007). For example, the endemic oak Q. afares (Algerian oak ) is considered to be a stabilized hybrid between Q. suber and Q. canariensis. Yet, Q. afares produces exclusively monoterpenes (Welter et al. 2012) as one of its parent species (Q. suber), but seems to have completely lost or suppressed the VOC production capacity of its second parent Q. canariensis, which produces only isoprene . These findings indicate that qualitative diversification in the monoterpene production capacity occurs frequently and has no or only minor consequences for the competitiveness of the trees. By contrast, the loss or gain of isoprene synthesis happens more rarely during species evolution , possibly because it requires co-evolving compensatory mutations to overcome failures in metabolic homeostasis.
10.3.3 Response to Environmental Changes at Different Time Scales
Unlike for CO2 and water vapor, the foliar exchange of VOCs is less constrained by stomatal conductance. This is due to their generally low gas phase concentrations inside the leaves, which allow changes in the diffusive resistance by stomata movements to be compensated by concomitant changes in the concentration gradients between the substomatal cavity and the outer atmosphere. Nevertheless, many OVOCs are at least partly under stomatal control either because these VOCs are transported with the transpiration stream and/or because these VOCs dissolve efficiently in liquid phase, which in turn delays the re-equilibrium of the gaseous concentration in the apoplast in response to stomata movements (Niinemets et al. 2002). In any case, all VOC emissions are strongly modulated by external factors, above all temperature , which governs the VOC’s vapor pressures and diffusion velocities, in addition to metabolic processes involved in the VOC release (Staudt et al. 2017b). In oaks, the major bulk of the emitted VOCs (constitutive isoprene and monoterpenes ) is directly regulated by their synthesis rate inside the chloroplasts, which in turn depends on the availability of primary substrates coming from light-dependent photosynthetic processes. Recent studies on poplar suggest that most of the short-term variation of isoprene emission is due to limitations in the availability of reduction power from photosynthetic electron transport (Rasulov et al. 2016 and references therein). Thus, constitutive isoprene and monoterpene emissions from oaks, and perhaps also stress-induced de novo synthesized emissions of sesquiterpenes, are strongly and almost instantaneously modulated by both temperature and light (e.g. Staudt and Lhoutellier 2011). The shape of the emission response to light resembles that of net photosynthesis ; i.e. a rectangular hyperbola approaching an asymptote at high light values (Staudt and Bertin 1998). By contrast, the temperature response exhibits a shape typical for the temperature dependence of enzyme-catalyzed reactions (Fig. 10.2). In addition to these fast responses, light and temperature modulates the oak ’s overall emission capacity over the seasons with changing weather conditions (e.g. Pier and McDuffie 1997; Staudt et al. 2002, 2003, 2017a) mostly via the expression of genes of rate-limiting enzymes (Schnitzler et al. 1997; Fischbach et al. 2002; Lavoir et al. 2009). Constitutive isoprenoid emissions from oaks are also influenced by other environmental factors such as ozone (Velikova et al. 2005), atmospheric CO2 concentration (Loreto and Sharkey 1990; Staudt et al. 2001a) or shortage and excess of soil water (Bertin and Staudt 1996; Staudt et al. 2002, 2008; Rodríguez-Calcerrada et al. 2013; Bourtsoukidis et al. 2014; Saunier et al. 2017).
Effects of temperature (left) and light intensity (photosynthetic photon flux density (PPFD), right) on monoterpene emission (upper graphs), photosynthesis (middle graphs) and the resulting relative carbon loss from monoterpene emission (C loss , lower graphs) in Q. ilex leaves. Temperature effect was measured at a PPFD of about 315, 615 and 2000 µmol m−2 s−1. Light effect was measured at a temperature of about 15, 25, 30 and 41 °C. Data were compiled from Staudt and Bertin (1998)
10.3.4 Relative Importance for Tree Carbon Budgets
Given that in oaks almost all primary C substrate used in the biosynthesis of volatile isoprenoids comes from ongoing photosynthesis , the C loss associated with emission can be expressed as the ratio of mol emitted C to mol assimilated C. As shown in Fig. 10.2, the instantaneous C loss varies much with changing temperature but little with light. Under most conditions the instantaneous C loss of assimilated C by constitutive isoprenoid emissions will be less than 1%. However, during heat spells, the C loss can substantially increase, especially when combined with drought events. In fact, isoprenoid emissions from oaks are less sensitive to water shortage than photosynthesis . The emissions decrease only when drought is severe while under mild drought they remain stable or even increase (Fig. 10.3). As a result, VOC emissions from oaks may significantly drain photosynthetic C and energy during conditions in which other sinks associated with growth are inhibited. In addition to constitutively produced isoprenoids , new volatiles induced by abiotic or biotic stresses may further exhaust the tree’s C resources. The exact C loss by these emissions is difficult to assess since stress usually induces a wealth of high-molecular, very reactive trace compounds (many of which remain undetected) that are emitted sporadically. Generally, the emission rates reported for stress-induced VOCs are lower than those for strong constitutive isoprenoid emissions. For instance, in Q. ilex leaves VOC emissions induced by gypsy moth infestation (mainly sesquiterpenes and homoterpenes) accounted for about 10% of the total foliar VOC release (Staudt and Lhoutellier 2007).
Plot of isoprenoid emissions, photosynthesis and resulting relative C losses from isoprenoid emissions against predawn water potential during two drought experiments on Quercus pubescens (a, isoprene emitter) and Quercus suber (b, monoterpene emitter). Oak saplings were exposed to one or two consecutive drying cycles for Q. pubescens and Q. suber, respectively. Gas exchange measurements were made under the same standard light and temperature conditions (1000 µmol m−2 s−1 PPFD; 30 °C). Lines are best fits on C-loss data assuming an exponential relationship for Q. pubescens (R2 = 0.92; Rodríguez-Calcerrada et al. 2013) and Q. suber (R2 = 0.67; Staudt et al. 2008)
The emissions of OVOCs from foliage have often been neglected when estimating C losses by VOC emission, because their accurate measurement requires different techniques to that of common VOCs. However, as mentioned above, many of them are ubiquitous plant volatiles that can be emitted at quite high rates, such as methanol. By combining PTR-MS technique with classical GC-MS, Bracho-Nunez et al. (2013) determined a large range of VOC exchange in 28 plant species, among which three oak species. At standard light and temperature conditions, mean C loss of net-photosynthesis by VOC emission ranged between 1.4 and 3.7%, with methanol and acetone contributing between 5 and 66% to the total VOC release. The quantities of VOCs released from roots and stem tissues are only poorly known and to our knowledge have never been reported for oaks. Nevertheless, Weissteiner et al. (2012) identified more than 60 VOCs in the headspace of washed healthy and damaged roots of young Q. petraea × Q. robur trees, of which 13 compounds were consistently released. Asensio et al. (2007) investigated the VOC exchange of soil in a Q. ilex forest and concluded that it represented 0.003% of the total C emitted by soil as CO2 .
On the other hand, there is increasing awareness that VOC exchanges can be bidirectional at least for some OVOCs and hence compensation points in VOC concentration exist, above which VOCs are taken up by plants (Niinemets et al. 2014; Matsui 2016 and references therein). For example, Staudt et al. (2000) observed bidirectional exchanges of acetic acid from diverse plant species, with net emissions dominating during day-time and net depositions dominating during night-time. For Q. ilex , the average deposition rate observed in darkness was more than half of the average emission rate observed under illumination (0.41 and 0.72 ng C m−2 s−1, respectively). Furthermore, secondary VOCs produced during atmospheric oxidation can be taken up by the vegetation and metabolized, thus possibly recovering parts of the C lost by emission (Karl et al. 2010; Park et al. 2013). Bidirectional above-canopy VOC fluxes have been recently measured by Schallhart et al. (2016) in a mixed oak forest during the early summer season. These authors observed an average net VOC efflux to the atmosphere of 41.8 nmol C m−2 s−1 which accounted for a bit less than 2% of the net uptake of CO2 . This number likely represented the upper limit of VOC-related C loss in that study site, because the measurements were conducted during the season when the emission capacity for constitutive isoprenoids reaches usually its maximum. Continuous online year-long measurements of total VOC exchanges at plant or canopy levels are still sparse due to methodological constraints. As an exception, Brilli et al. (2016) have monitored by eddy covariance both VOC and CO2 exchanges over a temperate poplar plantation throughout a whole growing season. Although poplar is, similar to most temperate oak species, a strong isoprene emitter, they observed a relatively small net VOC flux of ca. 1 g m−2 per growing season accounting for about 0.8% of the net ecosystem CO2 exchange. Earlier studies extrapolated discontinuous VOC emission/flux measurements by means of generic emission models to assess their weight relative to annual ecosystem C budget . For example Kesselmeier et al. (2002) estimated the annual C loss by VOC emission from an evergreen oak Mediterranean forest being 0.45% of their annual GPP.
10.4 Rhizodeposition
The rhizosphere is a highly populated environment. There are thousands of non-volatile organic compounds released by roots that mainly consist of carbohydrates , amino acids, vitamins, lipids, and a wide variety of secondary metabolites and proteins. Rhizodeposits can alter the physico-chemical soil properties and play important roles in the interactions of the plant with microbes or competing plant species (Bais et al. 2004; Bashir et al. 2016). As example, phenolic compounds help roots to deter the attack of pathogens (Lanoue et al. 2010); flavonoids facilitate the mutualistic symbiosis with mycorrhizal fungi (Nagahashi et al. 2010); the synthesis and release of some enzymes increase the availability of phosphorus forms that are absorbable by the roots (Dakora and Phillips 2002); while the exudation of phospholipids by root tips can reduce the surface tension of the soil solution and enhance the uptake of water and nutrients (Read et al. 2003). Similar interactions might exist in the rhizosphere of oak trees. However, the function and chemical profiling of rhizodeposits have been rarely studied within this genus. One of the scarce studies documents the effect of herbivory on C rhizodeposition in 2-year-old Q. rubra seedlings (Frost and Hunter 2008): It was observed that foliar herbivory reduced C allocation to fine roots whilst root exudation was actively regulated to maintain constant rates of C rhizodeposition , likely to sustain nutrient supply to microbes.
Rhizodeposition is affected by edaphic and environmental factors (reviewed by Nguyen 2009). The abundance of soil microorganisms substantially enhances the allocation of C assimilates to the rhizosphere. Besides, soil texture affects microbial activity, nutrient cycling, and soil physical properties, so that rhizodeposition increases in loam and clay soils due to their higher fertility and the smaller size of soil pores that facilitate the flow of organic compounds. Regarding climatic conditions, rhizodeposition is expected to increase during drought stress (Henry et al. 2007), and seems to be unaffected by changes in atmospheric [CO2 ] (Nguyen 2009).
For experimental simplicity, research on rhizodeposition has focussed on herbaceous plants and young tree seedlings (<2 months old; reviewed by Jones et al. 2009), so that any extrapolation to large trees would be biased by the potential effect of plant age on C allocation patterns (Nguyen 2009). Assessments on the contribution of C rhizodeposits to plant C budgets is an experimentally elusive task, mainly because of the technical limitation that the soil imposes for the quantification of C flow through the rhizosphere, and the natural abundance of soil microorganisms that promptly assimilate rhizodeposits. We are aware of only one study in which the contribution of C rhizodeposits to tree C budgets has been surveyed in oak species. In mature trees of Q. serrata and Q. glauca, C loss via root exudation was proportional to that of root R (10%) on a root-weight basis, and accounted for 3% of NPP (Sun et al. 2017). Similarly, studies on annual herbs and tree seedlings using isotopic tracers estimate that the portion of C assimilated through photosynthesis and lost via rhizodeposition ranges between 2 and 11% (Jones et al. 2009; Preece and Peñuelas 2016). However, these rough estimates should be taken with caution due to the uncertain origin and fate of C within the rhizosphere (Jones et al. 2009). Finally, there is increasing evidence that roots of autotrophic plants can take up amino-acids hence assimilating organic C and nutrients (reviewed by Schmidt et al. 2013). This mixotrophic behaviour has been observed in Q. petraea roots during spring, when the strong C demand for growth before budburst cannot completely rely in new assimilates (Bréda et al. 2013), thus adding further complexity to the estimation of net C loss belowground.
10.5 Conclusions
Carbon assimilation traditionally occupies a predominant role in the study of tree C cycling, whereas processes involved in the C release from the plant to the atmosphere are comparatively understudied. Respiration , emission of VOCs and rhizodeposition constitute therefore the overlooked side of tree C budgets, included those of oak trees. The ecological importance, physiology and environmental control of R and VOC emissions in oak species have been reviewed; a simplified schematic of the tree C budget in oak —dominated stands is presented in Fig. 10.4. Autotrophic R is expected to consume half of the assimilated C, with respiration —to—photosynthesis ratios ranging between 35 and 80%. The relative contribution of leaves, stems and roots to the overall C respiratory expenditure largely fluctuates according to stand structure, composition and age (Table 10.2). The C loss associated with VOC emissions accounts for about 1% of gross P. This percentage may increase up to 5% during heat waves and under drought stress (Figs. 10.2 and 10.3), and even more if VOCs emitted from flowers, fruits, stems and roots are taken into account. Rhizodeposits represent an additional and non-negligible source of tree C loss . However, scarce literature on oak rhizodeposition discourages attempts to provide a rough quantitative estimation. Furthermore, environmental—induced fluctuations in oak R and VOC emissions are not necessarily proportional to fluctuations in C assimilation . Thus, the assumption of homeostatic ratios between C loss and C gain should be revisited as it might lead to erroneous predictions on the strength of oak stands as C sinks in a climate changing world, conclusion that could be extrapolated to other tree taxa. A comprehensive understanding of oak C loss comparable to that of photosynthesis would be therefore necessary to accurately assess oak C cycling in scenarios of climate change .
Simplified schematic of tree carbon (C) budget in an oak forest. Net primary production can be estimated as the difference between photosynthesis (P) and overall C loss . Tree C loss occurs via emission of volatile organic compounds (VOCs), autotrophic respiration (RA), and rhizodeposition of organic compounds. Autotrophic respiration is partitioned into leaf, stem and root respiration (RLEAF, RSTEM and RROOT, respectively) and is expressed as a fraction of ecosystem respiration (RECO), which additionally integrates heterotrophic respiration (RH) of living organisms in the soil
References
Amthor J (2000) The McCree–de wit-penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot 86:1–20
Asensio D, Penuelas J, Ogaya R, Llusià J (2007) Seasonal soil VOC exchange rates in a Mediterranean holm oak forest and their responses to drought conditions. Atmos Environ 41:2456–2466
Atkin OK, Macherel D (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot 103:581–597
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Atkin OK, Bloomfield KJ, Reich PB et al (2015) Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol 206:614–636
Atkinson R, Arey J (2003) Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos Environ 37:197–219
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184:35–40
Ávila E, Herrera A, Tezara W (2014) Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species. Photosynthetica 52:3–15
Bais HP, Park SW, Weir TL et al (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9:26–32
Bashir O, Khan K, Hakeem K et al (2016) Soil microbe diversity and root exudates as important aspects of rhizosphere ecosystem. In: Hakeem K, Akhtar M (eds) Plant, soil and microbes. Springer International Publishing, Switzerland, pp 337–357
Behnke K, Ehlting B, Teuber M et al (2007) Transgenic, non-isoprene emitting poplars don’t like it hot. Plant J 51:485–499
Behnke K, Kleist E, Uerlings R et al (2009) RNAi-mediated suppression of isoprene biosynthesis in hybrid poplar impacts ozone tolerance. Tree Physiol 29:725–736
Behnke K, Loivamäki M, Zimmer I et al (2010) Isoprene emission protects photosynthesis in sunfleck exposed Grey poplar. Photosynth Res 104:5–17
Behnke K, Grote R, Brüggemann N et al (2012) Isoprene emission-free poplars—a chance to reduce the impact from poplar plantations on the atmosphere. New Phytol 194:70–82
Bertin N, Staudt M (1996) Effect of water stress on monoterpene emissions from young potted holm oak (Quercus ilex L.) trees. Oecologia 107:456–462
Berveiller D, Kierzkowski D, Damesin C (2007) Interspecific variability of stem photosynthesis among tree species. Tree Physiol 27:53–61
Bloemen J, Agneessens L, Van Meulebroek L et al (2014) Stem girdling affects the quantity of CO2 transported in xylem as well as CO2 efflux from soil. New Phytol 201:897–907
Bolstad P, Mitchell K, Vose J (1999) Foliar temperature-respiration response functions for broad-leaved tree species in the southern Appalachians. Tree Physiol 19:871–878
Bolstad PV, Reich P, Lee T (2003) Rapid temperature acclimation of leaf respiration rates in Quercus alba and Quercus rubra. Tree Physiol 23:969–976
Bourtsoukidis E, Kawaletz H, Radacki D et al (2014) Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees Struct Funct 28:193–204
Bracho-Nunez A, Welter S, Staudt M, Kesselmeier J (2011) Plant-specific volatile organic compound emission rates from young and mature leaves of Mediterranean vegetation. J Geophys Res 116:D16304
Bracho-Nunez A, Knothe NM, Welter S et al (2013) Leaf level emissions of volatile organic compounds (VOC) from some Amazonian and Mediterranean plants. Biogeosciences 10:5855–5873
Bréda N, Maillard P, Montpied P et al (2013) Isotopic evidence in adult oak trees of a mixotrophic lifestyle during spring reactivation. Soil Biol Biochem 58:136–139
Brilli F, Gioli B, Fares S et al (2016) Rapid leaf development drives the seasonal pattern of volatile organic compound (VOC) fluxes in a “coppiced” bioenergy poplar plantation. Plant Cell Environ 39:539–555
Burton A, Pregitzer KS, Ruess R et al (2002) Root respiration in North American forests: effects of nitrogen concentration and temperature across biomes. Oecologia 131:559–568
Burton AJ, Melillo JM, Frey SD (2008) Adjustment of forest ecosystem root respiration as temperature warms. J Integr Plant Biol 50:1467–1483
Cannell MGR, Thornley JHM (2000) Modelling the components of plant respiration: some guiding principles. Ann Bot 85:45–54
Cappellin L, Algarra Alarcon A, Herdlinger-Blatt I et al (2017) Field observations of volatile organic compound (VOC) exchange in red oaks. Atmos Chem Phys 17: 4218-4207
Coe JM, McLaughlin SB (1980) Winter season corticular photosynthesis in Cornus florida, Acer rubrum, Quercus alba, and Liriodendron tulipifera. For Sci 26:561–566
Collier DE, Thibodeau BA (1995) Changes in respiration and chemical content during autumnal senescence of Populus tremuloides and Quercus rubra leaves. Tree Physiol 15:759–764
Copolovici L, Vaartnou F, Estrada MP, Niinemets U (2014) Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol 34:1399–1410
Curtis PS, Vogel CS, Gough CM et al (2005) Respiratory carbon losses and the carbon-use efficiency of a northern hardwood forest, 1999–2003. New Phytol 167:437–456
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
Dannoura M, Kominami Y, Tamai K et al (2006) Development of an automatic chamber system for long-term measurements of CO2 flux from roots. Tellus 58:502–512
Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob Change Biol 12:154–164
Delory BM, Delaplace P, Fauconnier ML, du Jardin P (2016) Root-emitted volatile organic compounds: can they mediate belowground plant-plant interactions? Plant Soil 402:1–26
Edwards NT, Hanson PJ (1996) Stem respiration in a closed-canopy upland oak forest. Tree Physiol 16:433–439
Farquhar GD, Von Caemmerer S, Berry JA (1980) A biochemical model of photosynthestic CO2 assimilation in leaves of C3 species. Planta 90:78–90
Fatichi S, Leuzinger S, Korner C (2014) Moving beyond photosynthesis: from carbon source to sink-driven vegetation modeling. New Phytol 201:1086–1095
Fineschi S, Loreto F, Staudt M, Peñuelas J (2013) Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives. In: Niinemets Ü, Monson RK (eds) Biology, controls and models of tree volatile organic compound emissions. Tree Physiol vol 5. Springer, Dordrecht, pp 1–20
Fischbach RJ, Staudt M, Zimmer I et al (2002) Seasonal pattern of monoterpene synthase activities in leaves of the evergreen tree Quercus ilex. Physiol Plant 114:354–360
Frost CJ, Hunter MD (2008) Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol 178:835–845
Gaona-Colmán E, Blanco MB, Barnes I, Teruel MA (2016) Gas-phase ozonolysis of β-ocimene: temperature dependent rate coefficients and product distribution. Atmos Environ 147:46–54
Ghirardo A, Heller W, Fladung M et al (2012) Function of defensive volatiles in pedunculate oak (Quercus robur) is tricked by the moth Tortrix viridana. Plant, Cell Environ 35:2192–2207
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171
Goulden ML, Munger W, Fan S et al (1996) Measurements of carbon sequestration by long-term eddy covariance: methods and a critical evaluation of accuracy. Glob Change Biol 2:169–182
Hallquist M, Wenger JC, Baltensperger U et al (2009) The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys 9:5155–5236
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146
Harvey CM, Sharkey TD (2016) Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell Environ 39:1251–1263
Harvey CM, Li Z, Tjellström H et al (2015) Concentration of isoprene in artificial and thylakoid membranes. J Bioenerg Biomembr 47:419–429
Havaux M (2014) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Henry A, Doucette W, Norton J, Bugbee B (2007) Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J Environ Qual 36:904–912
Hunt S (2003) Measurements of photosynthesis and respiration in plants. Physiol Plant 117:314–325
Ito A, Inatomi M, Mo W et al (2007) Examination of model-estimated ecosystem respiration using flux measurements from a cool-temperate deciduous broad-leaved forest in central Japan. Tellus 59:616–624
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Karl T, Harley P, Emmons L et al (2010) Efficient atmospheric cleansing of oxidized organic trace gases by vegetation. Science 330:816–819
Kattge J, Díaz S, Lavorel S et al (2011) TRY—a global database of plant traits. Glob Change Biol 17:2905–2935
Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33:23–88
Kesselmeier J, Ciccioli P, Kuhn U et al (2002) Volatile organic compound emissions in relation to plant carbon fixation and the terrestrial carbon budget. Global Biogeochem Cycles 16:1–9
Körner C (2015) Paradigm shift in plant growth control. Curr Opin Plant Biol 25:107–114
Kosugi Y, Tanaka H, Takanashi S et al (2005) Three years of carbon and energy fluxes from Japanese evergreen broad-leaved forest. Agric For Meteorol 132:329–343
Kreuzwieser J, Rennenberg H (2013) Flooding-driven emissions from trees. In: Niinemets Ü, Monson RK (eds) Biology, controls and models of tree volatile organic compound emissions. Springer, Dordrecht, pp 237–252
Lanoue A, Burlat V, Henkes GJ et al (2010) De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol 185:577–588
Laureano RG, Lazo YO, Linares JC et al (2008) The cost of stress resistance: construction and maintenance costs of leaves and roots in two populations of Quercus ilex. Tree Physiol 28:1721–1728
Lavoir AV, Staudt M, Schnitzler JP et al (2009) Drought reduced monoterpene emissions from the evergreen Mediterranean oak Quercus ilex: results from a throughfall displacement experiment. Biogeosciences 6:1167–1180
Lee TD, Reich PB, Bolstad PV (2005) Acclimation of leaf respiration to temperature is rapid and related to specific leaf area, soluble sugars and leaf nitrogen across three temperate deciduous tree species. Funct Ecol 19:640–647
Lempereur M, Martin-StPaul NK, Damesin C et al (2015) Growth duration is a better predictor of stem increment than carbon supply in a Mediterranean oak forest: implications for assessing forest productivity under climate change. New Phytol 207:579–590
Li Q, Chen J, Moorhead DL (2012) Respiratory carbon losses in a managed oak forest ecosystem. For Ecol Manage 279:1–10
Limousin JM, Misson L, Lavoir AV et al (2010) Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant Cell Environ 33:863–875
Lockhart JA (1965) An analysis of irreversible plant cell elongation. J Theor Biol 8:264–275
Loivamäki M, Mumm R, Dicke M, Schnitzler J-P (2008) Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc Natl Acad Sci 105:17430–17435
Long BM, Bahar NHA, Atkin OK (2015) Contributions of photosynthetic and non-photosynthetic cell types to leaf respiration in Vicia faba L. and their responses to growth temperature. Plant Cell Environ 38:2263–2276
López B, Sabate S, Gracia CA (2001) Annual and seasonal changes in fine root biomass of a Quercus ilex L. forest. Plant Soil 230:125–134
Loreto F, Schnitzler J-P (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15:154–166
Loreto F, Sharkey TD (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182:523–531
Loreto F, Bagnoli F, Fineschi S (2009) One species, many terpenes: matching chemical and biological diversity. Trends Plant Sci 14:416–420
Lu X, Tang K, Li P (2016) Plant metabolic engineering strategies for the production of pharmaceutical terpenoids. Front Plant Sci. https://doi.org/10.3389/fpls.2016.0164
Luan J, Liu S, Wang J et al (2011) Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol Biochem 43:503–512
Luyssaert S, Inglima I, Jung M et al (2007) CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob Change Biol 13:2509–2537
Mabberley DJ (2008) Mabberley’s plant book: a portable dictionary of plants, their classifications and uses. Cambridge University Press, UK
Martinez F, Lazo YO, Fernandez-Galiano RM, Merino JA (2002) Chemical composition and construction cost for roots of Mediterranean trees, shrub species and grassland communities. Plant Cell Environ 25:601–608
Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9:274–280
Matsui K (2016) A portion of plant airborne communication is endorsed by uptake and metabolism of volatile organic compounds. Curr Opin Plant Biol 32:24–30
McCree KJ (1970) An equation for the rate of respiration of white clover plants grown under controlled conditions. In: Prediction and measurement of photosynthetic productivity. Proceedings of the IBP/PP technical meeting, Trebon, 14–21 Sept 1969, pp 221–229
McGuire MA, Teskey RO (2002) Microelectrode technique for in situ measurement of carbon dioxide concentrations in xylem sap of trees. Tree Physiol 22:807–811
Misztal PK, Hewitt CN, Wildt J et al (2015) Atmospheric benzenoid emissions from plants rival those from fossil fuels. Sci Rep 5:12064
Miyama T, Kominami Y, Tamai K et al (2006) Components and seasonal variation of night-time total ecosystem respiration in a Japanese broad-leaved secondary forest. Tellus 58:550–559
Miyazawa S (1998) Slow leaf development of evergreen broad-leaved tree species in Japanese warm temperate forests. Ann Bot 82:859–869
Molchanov AG (2009) Effect of moisture availability on photosynthetic productivity and autotrophic respiration of an oak stand. Russ J Plant Physiol 56:769–779
Monson RK, Jones RT, Rosenstiel TN, Schnitzler JP (2013) Why only some plants emit isoprene. Plant Cell Environ 36:503–516
Müller A, Kaling M, Faubert P et al (2015) Isoprene emission by poplar is not important for the feeding behaviour of poplar leaf beetles. BMC Plant Biol 15:1
Nagahashi G, Douds D, Ferhatoglu Y (2010) Functional categories of root exudate compounds and their relevance to AM fungal growth. In: Koltai H, Kapulnik Y (eds) Arbuscular mycorrhizas: physiology and function. Springer, Berlin, pp 33–56
Negisi K (1982) Diurnal fluctuations of the stem bark respiration in relationship to the wood temperature in standing young Pinus densiflora, Chamaecyparis obtusa and Quercus myrsinaefolia trees. J Japanese For Soc 64:315–319
Nguyen C (2009) Rhizodeposition of organic C by plant: mechanisms and controls. In: Lichtfouse E, Navarrete M, Debaeke P et al (eds) Sustainable agriculture. Springer, Netherlands, pp 97–123
Niinemets U (2014) Cohort-specific tuning of foliage physiology to interacting stresses in evergreens. Tree Physiol 34:1301–1304
Niinemets U, Reichstein M, Staudt M et al (2002) Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol 130:1371–1385
Niinemets Ü, Fares S, Harley P, Jardine KJ (2014) Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition. Plant Cell Environ 37:1790–1809
Palmer-Young EC, Veit D, Gershenzon J, Schuman MC (2015) The Sesquiterpenes(E)-ß-Farnesene and (E)-α-Bergamotene quench ozone but fail to protect the wild tobacco Nicotiana attenuata from Ozone, UVB, and drought stresses. PLoS ONE 10:e0127296
Paris CI, Llusia J, Peñuelas J (2010) Changes in monoterpene emission rates of Quercus ilex infested by aphids tended by native or invasive Lasius ant species. J Chem Ecol 36:689–698
Park J-H, Goldstein AH, Timkovsky J et al (2013) Active atmosphere-ecosystem exchange of the vast majority of detected volatile organic compounds. Science 341:643–647
Peñuelas J, Staudt M (2010) BVOCs and global change. Trends Plant Sci 15:133–144
Pérez-de-Lis G, García-González I, Rozas V, Olano JM (2016) Feedbacks between earlywood anatomy and non-structural carbohydrates affect spring phenology and wood production in ring-porous oaks. Biogeosci Discuss 35:1–19
Piao S, Luyssaert S, Ciais P et al (2010) Forest annual carbon cost: a global-scale analysis of autotrophic respiration. Ecology 91:652–661
Pier PA, McDuffie C (1997) Seasonal isoprene emission rates and model comparisons using whole-tree emissions from white oak. J Geophys Res 102:23963–23971
Poorter H, Niklas KJ, Reich PB et al (2012) Biomass allocation to leaves, stems and roots: meta-analysis of interspecific variation and environmental control. New Phytol 193:30–50
Preece C, Peñuelas J (2016) Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 409:1–17
Rambal S, Lempereur M, Limousin JM et al (2014) How drought severity constrains gross primary production (GPP) and its partitioning among carbon pools in a Quercus ilex coppice? Biogeosciences 11:6855–6869
Rasulov B, Talts E, Niinemets Ü (2016) Spectacular oscillations in plant isoprene emission under transient conditions explain the enigmatic CO2 response. Plant Physiol 172:2275–2285
Read DB, Bengough AG, Gregory PJ et al (2003) Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol 157:315–326
Reich PB, Walters MB, Ellsworth DS et al (1998) Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114:471–482
Reich PB, Tjoelker MG, Pregitzer KS et al (2008) Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett 11:793–801
Reichstein M, Tenhunen J, Roupsard O et al (2002) Ecosystem respiration in two Mediterranean evergreen Holm Oak forests: drought effects and decomposition dynamics. Funct Ecol 16:27–39
Reich et al (2016) Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531(7596): 633–636
Rey A, Pegoraro E, Tedeschi V et al (2002) Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob Change Biol 8:851–866
Rodríguez-Calcerrada J, Jaeger C, Limousin JM et al (2011) Leaf CO2 efflux is attenuated by acclimation of respiration to heat and drought in a Mediterranean tree. Funct Ecol 25:983–995
Rodríguez-Calcerrada J, Limousin JM, Martin-Stpaul NK et al (2012) Gas exchange and leaf aging in an evergreen oak: causes and consequences for leaf carbon balance and canopy respiration. Tree Physiol 32:464–477
Rodríguez-Calcerrada J, Buatois B, Chiche E et al (2013) Leaf isoprene emission declines in Quercus pubescens seedlings experiencing drought—any implication of soluble sugars and mitochondrial respiration? Environ Exp Bot 85:36–42
Rodríguez-Calcerrada J, Martin-StPaul NK, Lempereur M et al (2014) Stem CO2 efflux and its contribution to ecosystem CO2 efflux decrease with drought in a Mediterranean forest stand. Agric For Meteorol 195–196:61–72
Rodríguez-Calcerrada J, López R, Salomón R et al (2015a) Stem CO2 efflux in six co-occurring tree species: underlying factors and ecological implications. Plant Cell Environ 38:1104–1115
Rodríguez-Calcerrada J, Salomón R, Gil L (2015b) Transporte y reciclaje de CO2 en el interior del árbol: factores que complican la estimación de la respiración leñosa a través de la emisión radial de CO2. Bosque 36:5–14
Salomón R, Valbuena-Carabaña M, Rodríguez-Calcerrada J et al (2015) Xylem and soil CO2 fluxes in a Quercus pyrenaica Willd. coppice: root respiration increases with clonal size. Ann For Sci 72:1065–1078
Salomón R, Valbuena-Carabaña M, Teskey R et al (2016a) Seasonal and diel variation in xylem CO2 concentration and sap pH in sub-Mediterranean oak stems. J Exp Bot 67:2817–2827
Salomón RL, Valbuena-Carabaña M, Gil L et al (2016b) Temporal and spatial patterns of internal and external stem CO2 fluxes in a sub-Mediterranean oak. Tree Physiol 36:1409–1421
Salomón RL, Limousin JM, Ourcival JM et al (2017) Stem hydraulic capacitance decreases with drought stress: implications for modelling tree hydraulics in the Mediterranean oak Quercus ilex. Plant Cell Environ 40:1379–1391
Sasaki K, Saito T, Lämsä M et al (2007) Plants utilize isoprene emission as a thermotolerance mechanism. Plant Cell Physiol 48:1254–1262
Saunier A, Ormeño E, Wortham H et al (2017) Chronic drought decreases anabolic and catabolic BVOC emissions of Quercus pubescens in a Mediterranean Forest. Front Plant Sci 8:71
Saveyn A, Steppe K, Lemeur R (2007a) Daytime depression in tree stem CO2 efflux rates: is it caused by low stem turgor pressure? Ann Bot 99:477–485
Saveyn A, Steppe K, Lemeur R (2007b) Drought and the diurnal patterns of stem CO2 efflux and xylem CO2 concentration in young oak (Quercus robur). Tree Physiol 27:365–374
Saveyn A, Steppe K, Lemeur R (2008) Report on non-temperature related variations in CO2 efflux rates from young tree stems in the dormant season. Trees 22:165–174
Schallhart S, Rantala P, Nemitz E et al (2016) Characterization of total ecosystem-scale biogenic VOC exchange at a Mediterranean oak-hornbeam forest. Atmos Chem Phys 16:7171–7194
Schiestl FP (2010) The evolution of floral scent and insect chemical communication. Ecol Lett 13:643–656
Schmidt S, Raven JA, Paungfoo-Lonhienne C (2013) The mixotrophic nature of photosynthetic plants. Funct Plant Biol 40:425–438
Schnitzler J, Lehning A, Steinbrecher R (1997) Seasonal pattern of isoprene synthase activity in Quercus robur leaves and its significance for modeling isoprene emission rates. Bot Acta 110:240–243
Sindelarova K, Granier C, Bouarar I et al (2014) Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos Chem Phys 14:9317–9341
Slot M, Kitajima K (2015) General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 177:885–900
Smith NG, Dukes JS (2013) Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Glob Change Biol 19:45–63
Sperlich D, Barbeta A, Ogaya R et al (2016) Balance between carbon gain and loss under long-term drought: impacts on foliar respiration and photosynthesis in Quercus ilex L. J Exp Bot 67:821–833
Staudt M, Bertin N (1998) Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak Quercus ilex L. leaves. Plant Cell Environ 21:385–395
Staudt M, Lhoutellier L (2007) Volatile organic compound emission from holm oak infested by gypsy moth larvae: evidence for distinct responses in damaged and undamaged leaves. Tree Physiol 27:1433–1440
Staudt M, Lhoutellier L (2011) Monoterpene and sesquiterpene emissions from Quercus coccifera exhibit interacting responses to light and temperature. Biogeosciences 8:2757–2771
Staudt M, Wolf A, Kesselmeier J (2000) Influence of environmental factors on the emissions of gaseous formic and acetic acids from orange (Citrus sinensis L.) foliage. Biogeochemistry 48:199–216
Staudt M, Joffre R, Rambal S, Kesselmeier J (2001a) The effect of elevated CO2 on terpene emission, leaf structure and related physiological parameters of young Quercus ilex. Tree Physiol 21:437–445
Staudt M, Mandl N, Joffre R, Rambal S (2001b) Intraspecific variability of monoterpene composition emitted by Quercus ilex leaves. Can J For Res 31:174–180
Staudt M, Rambal S, Joffre R (2002) Impact of drought on seasonal monoterpene emissions from Quercus ilex in southern France. J Geophys Res 107:4602
Staudt M, Joffre R, Rambal S (2003) How growth conditions affect the capacity of Quercus ilex leaves to emit monoterpenes. New Phytol 158:61–73
Staudt M, Mir C, Joffre R et al (2004) Isoprenoid emissions of Quercus spp. (Q. suber and Q. ilex) in mixed stands contrasting in interspecific genetic introgression. New Phytol 163:573–584
Staudt M, Ennajah A, Mouillot F, Joffre R (2008) Do volatile organic compound emissions of Tunisian cork oak populations originating from contrasting climatic conditions differ in their responses to summer drought? Can J For Res 38:2965–2975
Staudt M, Jackson B, El-aouni H et al (2010) Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol 30:1320–1334
Staudt M, Morin X, Chuine I (2017a) Contrasting direct and indirect effects of warming and drought on isoprenoid emissions from Mediterranean oaks. Reg Environ Change 17:2121–2133
Staudt M, Bourgeois I, Al Halabi R et al (2017b) New insights into the parametrization of temperature and light responses of mono-and sesquiterpene emissions from Aleppo pine and rosemary. Atmos Environ 152:212–221
Steinbrecher R, Contran N, Gugerli F et al (2013) Inter- and intra-specific variability in isoprene production and photosynthesis of Central European oak species. Plant Biol 15:148–156
Steppe K, De Pauw DJW, Lemeur R, Vanrolleghem A (2006) A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol 26:257–273
Sun et al (2017) Relationship between fine-root exudation and respiration of two Quercus species in a Japanese temperate forest. Tree Physiol 37(8): 1011–1020
Tattini M, Velikova V, Vickers C et al (2014) Isoprene production in transgenic tobacco alters isoprenoid, non-structural carbohydrate and phenylpropanoid metabolism, and protects photosynthesis from drought stress. Plant Cell Environ 37:1950–1964
Teskey RO, McGuire MA (2002) Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant Cell Environ 25:1571–1577
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Tholl D, Sohrabi R, Huh J-H, Lee S (2011) The biochemistry of homoterpenes—common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 72:1635–1646
Thornley JHM (1970) Respiration, growth and maintenance in plants. Nature 227:304–305
Thornley JHM (2011) Plant growth and respiration re-visited: maintenance respiration defined—it is an emergent property of, not a separate process within, the system—and why the respiration: photosynthesis ratio is conservative. Ann Bot 108:1365–1380
Turnbull MH, Whitehead D, Tissue DT et al (2001) Responses of leaf respiration to temperature and leaf characteristics in three deciduous tree species vary with site water availability. Tree Physiol 21:571–578
Unger S, Máguas C, Pereira JS et al (2009) Partitioning carbon fluxes in a Mediterranean oak forest to disentangle changes in ecosystem sink strength during drought. Agric For Meteorol 149:949–961
Valbuena-Carabaña M, González-Martínez SC, Hardy OJ, Gil L (2007) Fine-scale spatial genetic structure in mixed oak stands with different levels of hybridization. Mol Ecol 16:1207–1219
Valentini R, Matteucci G, Dolman AJ et al (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865
Van Loon JJA, De Boer JG, Dicke M (2000) Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol Exp Appl 97:219–227
Van Oijen M, Schapendonk A, Hoglind M (2010) On the relative magnitudes of photosynthesis, respiration, growth and carbon storage in vegetation. Ann Bot 105:793–797
Vanzo E, Merl-Pham J, Velikova V et al (2016) Modulation of protein S-nitrosylation by isoprene emission in poplar. Plant Physiol 170:1945–1961
Varone L, Gratani L (2015) Leaf respiration responsiveness to induced water stress in Mediterranean species. Environ Exp Bot 109:141–150
Velikova V, Tsonev T, Pinelli P et al (2005) Localized ozone fumigation system for studying ozone effects on photosynthesis, respiration, electron transport rate and isoprene emission in field-grown Mediterranean oak species. Tree Physiol 25:1523–1532
Velikova V, Sharkey TD, Loreto F (2012) Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal Behav 7:139–141
Velikova V, Müller C, Ghirardo A et al (2015) Knocking down of isoprene emission modifies the lipid matrix of thylakoid membranes and influences the chloroplast ultrastructure in poplar. Plant Physiol 168:859-870
Verdú JR, Lobo JM, Numa C et al (2007) Acorn preference by the dung beetle, Thorectes lusitanicus, under laboratory and field conditions. Anim Behav 74:1697–1704
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009a) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291
Vickers CE, Possell M, Cojocariu CI et al (2009b) Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ 32:520–531
Vuts J, Woodcock CM, Sumner ME et al (2016) Responses of the two-spotted oak buprestid, Agrilus biguttatus (Coleoptera: Buprestidae), to host tree volatiles. Pest Manag Sci 72:845–851
Walter MH, Floss DS, Strack D (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232:1–17
Wang M, Guan D-X, Han S-J, Wu J-L (2010) Comparison of eddy covariance and chamber-based methods for measuring CO2 flux in a temperate mixed forest. Tree Physiol 30:149–163
Waring R, Landsberg J, Williams M (1998) Net primary production of forests: a constant fraction of gross primary production? Tree Physiol 18:129–134
Way DA, Yamori W (2014) Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth Res 119:89–100
Weissteiner S, Huetteroth W, Kollmann M et al (2012) cockchafer larvae smell host root scents in soil. PLoS One. doi:10.1371/journal.pone.0045827
Welter S, Bracho-Nuñez A, Mir C et al (2012) The diversification of terpene emissions in Mediterranean oaks: lessons from a study of Quercus suber, Quercus canariensis and its hybrid Quercus afares. Tree Physiol 32:1082–1091
Wright IJ, Reich PB, Atkin OK et al (2006) Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytol 169:309–319
Xu L, Baldocchi DD (2003) Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol 23:865–877
Yang JY, Teskey RO, Wang CK (2012a) Stem CO2 efflux of ten species in temperate forests in Northeastern China. Trees 26:1225–1235
Yang Q, Xu M, Chi Y et al (2012b) Temporal and spatial variations of stem CO2 efflux of three species in subtropical China. J Plant Ecol 5:229–237
Zaragoza-Castells J, Sánchez-Gómez D, Valladares F et al (2007) Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long-lived leaves. Plant Cell Environ 30:820–833
Zaragoza-Castells J, Sánchez-Gómez D, Hartley IP et al (2008) Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of acclimation in both high-light and shaded habitats. Funct Ecol 22:172–184
Acknowledgements
This book chapter has received funding from the FWO and the European Union´s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 665501, and from the “Legado de González-Esparcia” granted to RLS. J R-C acknowledges the support of the Spanish Ministry of Economy and Competitiveness via the “Ramón y Cajal” programme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Salomón, R.L., Rodríguez-Calcerrada, J., Staudt, M. (2017). Carbon Losses from Respiration and Emission of Volatile Organic Compounds—The Overlooked Side of Tree Carbon Budgets. In: Gil-Pelegrín, E., Peguero-Pina, J., Sancho-Knapik, D. (eds) Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.. Tree Physiology, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-319-69099-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-69099-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69098-8
Online ISBN: 978-3-319-69099-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)