Abstract
The purpose of this chapter is to provide an overview of the main systems of microalgae production with highlights of biofuel production. The large-scale production systems (raceway ponds, horizontal tubular photobioreactors, and heterotrophic bioreactors) and small-scale photobioreactors (vertical and flat-plate photobioreactors) will be presented and discussed with a special emphasis on the main factors affecting its efficiency, biomass productivities reported in the literature, scaling-up, costs of construction and operation, and commercial applications. Besides this, the recent developments in microalgae cultivation systems will be reviewed in their main aspects. Finally, the criteria for selecting an appropriate bioreactor for microalgae cultivation will be presented, as well as the pros and cons of each system will be discussed in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Historically microalgae have been of interest since 1942, when Harder and von Witsch (1942) suggested that microalgae could be viable sources of lipids to be used as food or to produce biofuels. Since then, an increasing amount of research involving microalgae and their bioproducts has been performed. Currently, these microorganisms are considered one of the most promising sources for bioenergy production (Chisti 2016; Chew et al. 2017; Raslavičius et al. 2018).
Compared with conventional oil seeds, the biofuels produced from microalgae have several advantages that include the higher productivities, the ability to use nonarable land for microalgal cultivation and possibility to use wastewater and gas flue as source of nutrients and carbon to promote growth (Jacob-Lopes et al. 2014; Collotta et al. 2017). Microalgae also can produce different types of biofuels, such as biodiesel, bioethanol, biohydrogen, syngas, biobutanol, and bioelectricity (Chang et al. 2017; Su et al. 2017a, b). Unfortunately, until now, the majority of economic analyses conclude that microalgae biofuels cannot compete with conventional fuels (Lundquist et al. 2010; Sun et al. 2011). On the other hand, the concept of biorefinery can be explored with the aim to improve economic aspects. This is possible because of the wide variety of high-value compounds that microalgae can produce, such as carotenoids, proteins, long-chain polyunsaturated fatty acids, vitamins, and phycobilins (Chew et al. 2017).
Industrialization of microalgae products requires large-scale culture systems, which generally are raceway ponds, closed photobioreactors (PBRs), or heterotrophic bioreactors. Open systems are much cheaper and easier to operate than closed systems, however, have many operational problems, such as contamination, evaporation, susceptibility to weather conditions, and extensive land requirements. On the other hand, closed systems can eliminate these limitations, but with a high capital cost, difficulty in scaling-up, and high shear stress. However, due to the high operational control and the high productivity provided by the PBRs, researchers have been invested heavily in the development of new photobioreactors designs, in order to reduce these limitations, and thus make microalgae-based processes viable (Chang et al. 2017).
This chapter discusses the systems of microalgae production in large-scale (raceway ponds, horizontal tubular photobioreactors, and heterotrophic bioreactors) and small-scale (vertical tubular photobioreactors and flat-plate photobioreactors), with emphasis on major factors that influence their efficiency, biomass productivities, costs of biomass production, scaling-up, and commercial applications. Moreover, recent developments in microalgae cultivation systems are presented. Finally, the advantages and disadvantages of all microalgae production systems discussed are compared, and the criteria for selecting an appropriate PBR are presented.
2 Large-Scale Microalgae Biomass Production
2.1 Raceway Ponds
The raceway ponds were first developed in the 1950s for treating wastewater and, since the 1960s, outdoor open raceways have been used in commercial production of microalgae and cyanobacteria (Chisti 2016). Currently, it is the most utilized system for commercial microalgae production, accounting for more than 95% of algae production worldwide, owing to their flexibility, low cost, and easy of scaling-up (Fernandez et al. 2013; Chang et al. 2017).
A raceway pond is a closed loop recirculation channel with a typical culture depth of about 0.25–0.30 m. The circulation mixes the nutrients, and cells are provided by the paddle wheels. The ponds are usually kept shallow because the algae need to be exposed to sunlight, and sunlight can only penetrate the pond water to a limited depth (Singh and Sharma 2012; Chang et al. 2017).
These ponds can be simply constructed in compacted soil with a 1- to 2-mm-thick plastic membrane. However, although it is cheaper, it is not commonly used for biomass production due to the high risk of contamination (Singh and Sharma 2012). To produce a biomass with high added value, the ponds are often made of concrete block walls and dividers lined with a plastic membrane to prevent seepage. Depending on the end use of the biomass, special care may be required to use liners that do not leach contaminating and inhibitory chemicals into the algal broth (Borowitzka 2005; Chisti 2016).
2.1.1 Major Factors Affecting the Raceway Pond Performance
2.1.1.1 Choice of Location
The choice of location of a raceway system has the greatest impact on biomass productivity. The factors to consider in the geographic location are average annual irradiance level, prevailing temperature, rainfall, land slope, potential nutrient sources, cost of the water, and land.
In terms of illumination, a minimum solar irradiation of 4.65 kWh/m2/d is required to sustain high growth rates (Benemann et al. 1982). According to Chisti (2013), in an ideal condition, the temperature should be around 25 ℃, with a minimum of diurnal and seasonal variations. A geographic location with rainfall not more than 1000 mm of rain per year facilitates the microalgae cultivation, since that can minimize the dilution of algae stock in the ponds (Bennett et al. 2014). In an ideal situation, the land slope should not be greater than 2% to avoid significant earthmoving costs during pond construction, but the US Department of Energy (DOE) cites a 5% maximum slope (DOE 2010; Bennett et al. 2014).
Other factor that depends on the local climate is the evaporation, which is influenced by the level of irradiance, the wind velocity, the air temperature, and the absolute humidity. An average freshwater evaporation rate of 10 L/m2/d has been noted for some tropical regions. In this sense, freshwater needs to be added periodically to raceway to compensate the evaporation (Becker 1994; Chisti 2016).
2.1.1.2 Engineering Parameters
In raceways, the pond depth is one of the engineering parameters that has most influence on cultivation performance, because it is closely related to temperature control, mixing, and light utilization efficiency. In general, the biomass productivity is higher in cultivations with lower depth raceways, but this also depends on the microalgae species used and the dimensions.
In raceway ponds, the mixing serves several purposes such as periodic exposure of cells to sunlight, keeping cells into suspension, availability of the nutrient to algal cells, and removal of photosynthetically generated oxygen. In this sense, an ideal mixing supply can increase productivity by nearly 10 times. Conventionally, the mixing is conventionally measured by the Reynolds number (Re), which in an ideal situation is about 257,000, considering a 1.5 m wide channel with a broth depth of 0.3 m and a culture velocity of 0.3 m/s (Chisti 2016).
2.1.1.3 Carbon Supply and pH
The carbon is the major constituent of microalgal cells, with approximately 50% of the cell mass. All carbon is photosynthetically assimilated from CO2 and, this assimilation is closely related to the pH of the medium, since that, if CO2 is consumed rapidly and not replenished, the pH becomes alkaline. In raceway ponds, generally, the pH is instable, because the CO2 absorption from atmosphere through the surface of a raceway is insufficient to support the high photosynthesis rate for a good part of the day (Chisti 2013). An alkaline pH results in generation of toxic ammonia from dissolved ammonium salts, lowers the affinity of algae for CO2, and increases the flexibility of mother cells, delaying completion of the cell cycle (Juneja et al. 2013). For this reason, to obtain better productivities in raceway, it is necessary to engineer a supply of CO2.
Gas diffusers are used in raceways to inject CO2 in the form of fine bubbles. According to Li et al. (2014), the CO2 concentration greater than 73 µmol/L at a pH of 8.0 is optimal for the normal growth of microalgae. To produce high-value compounds, commercial pure carbon dioxide has been extensively used in microalgal cultures. However, this entails in additional economic costs and reduces the economic viability and sustainability of the process. It is estimated that the cost of the carbon source in microalgae production ranges from 8 to 27% of the daily production cost (Li et al. 2014). Furthermore, in this type of system, between 35 and 70% of the pure CO2 injected into a pond is lost to the atmosphere. As an alternative, flue gas can be used, which also could contribute to the mitigation of environmental problems (de Godos et al. 2014).
2.1.1.4 Oxygen Accumulation
The photosynthesis reaction produces stoichiometrically 1.9 tons of oxygen to produce 1 ton of microalgal biomass. So, when there is intense microalgal growth, an excess of oxygen is generated. At high concentrations of O2, the productivity of microalgae reduces considerably due to photorespiration and photoinhibition effects (Raso et al. 2012).
In raceway ponds, the only mechanism commonly used for removal of oxygen from the medium is the agitation by the paddle wheel and is not particularly effective. Even with high surface areas, the oxygen removal is insufficient during periods of maximum photosynthetic activity. At these photosynthesis peaks, the performance of the system can decrease up to 35% due to the excess dissolved oxygen in the medium, which can reach 300% of the air saturation value. Moreover, the biochemical composition of microalgae biomass can be influenced by the oxygen level in the pond (Richmond 1990; Chisti 2013).
2.1.1.5 Culture Contamination
As the raceway ponds are open to the environment, they are easily contaminated by bacteria, viruses, fungi, and other microalgae species and by predators. An alternative to control the contamination is to place the lakes inside greenhouses with controlled environmental conditions, but for the production of biofuels on a large scale, this is economically unfeasible.
However, it is known that not many contaminants can survive under extreme conditions. In this way, the contamination can be avoided by cultivating some highly resistant microalgal strains at high pH or high salinity. The species with the best performance in commercial cultivation in raceways includes Chlorella sp., Spirulina sp., and Dunaliella sp., which are cultivated under stringent conditions that inhibit the growth of other microorganisms or other species of microalgae (Chang et al. 2017).
2.1.2 Biomass Production in Raceway Ponds
Although biomass productivities of 0.40 g/L/d or higher have already been reported (Wen et al. 2016), values much lower than these are typically found, as shown in Table 1. The reported productivities are specific for the reactor designs, operating conditions, local weather conditions, and algae species. For this reason, the productivities obtained with a specific system cannot be simply extrapolated to other growth conditions. The biomass productivities in raceways are considered low, but generally are compensated by high product prices and low construction and operating costs.
2.1.3 Cost of Construction and Operation of Raceway Ponds
In terms of cost of construction, the plastic-lined earthen are the raceway ponds with the best cost-benefit, as unlined earth ponds are not generally considered satisfactory for producing algal biomass (Chisti 2013). According to Chisti (2016), the cost estimated to produce a 100-ha plastic-lined pond of compacted earth was about US$ 144,830 per ha in 2014. This cost data can be corrected for inflation and thus provides a reasonable estimate of the current cost (Chisti 2013). In this way, the capital cost estimated for 2017 is of US$ 149,598 per ha. This estimate includes the earthworks, the plastic lining, the carbon dioxide supply tubing, inlets and outlets, the baffles, the paddle wheel, and motor.
To produce dry microalgae biomass in outdoor commercial raceway ponds, Nosker et al. (2011) and Chisti (2007) estimated a cost of € 4.95 and US$ 3.80 per kg of dry weight, respectively. According to Nosker et al. (2011), the factors which influence production costs are irradiation conditions, mixing, photosynthetic efficiency of systems, culture media, and carbon dioxide costs. Thus, by optimizing these factors, the production cost can reduce up to € 0.68 per kg.
2.1.4 Scaling-up in Raceway Ponds
The microalgae cultivation in open ponds is already a consolidated and widely practiced method for large-scale cultivation, since that are easily scaled up. There have been records of large-scale cultivation in raceways since 1987, where two 1000 m2 raceway ponds were used as a test facility between 1987 and 1990 in New Mexico. These tests were conducted to verify the potential of microalgal biomass production for low-cost biodiesel production and were considered technically feasible (Rawat et al. 2013).
Although already a widely used technology, cultivations in raceway ponds still pose many challenges in terms of economic viability for large-scale biofuel production. This is mainly due to the low biomass productivity presented in these systems the need for extensive areas of land and substantial costs for harvesting (Scott et al. 2010).
2.1.5 Commercial Microalgae Cultivation in Raceway Ponds
The commercialization of biofuel from microalgae is still in its early gestation and has lot of challenges to achieve cost-competitive fuels. Currently, the industrial microalgae biomass production is restricted to high value.
Raceway ponds are under operation worldwide to produce a diverse range of products. For example, Cyanotech Corporation, in Hawaii, has cultivations in raceways of Spirulina platensis and Haematococcus pluvialis, to produce Spirulina Pacifica® and BioAstin® Natural Astaxanthin, respectively. Nikken Sohonsha Corporation (Japan) produces more than 40 different products (healthcare products, medical products, cosmetics, dietary supplements, fertilizers, and animal feeds) from microalgae as Chlorella, Dunaliella, Monodus, and Isochlysis. Tianjin Norland Biotech (China) cultivates Spirulina, Chlorella, and H. pluvialis to produce Spirulina tablets, Chlorella tablets, astaxanthin, astaxanthin oil, and phycocyanin.
However, it is important to point out that many companies are working with pilot plant tests for biofuels production. Examples of companies that are using open systems in their tests are the LiveFuels (USA), OriginOil Inc. (USA), PatroSun (USA), Neste Oil (FI), Ingrepo (NL), and Aquaflow Bionomics (NZ) (Su et al. 2017a, b).
2.2 Tubular Photobioreactor
Recently, closed PBRs, especially tubular photobioreactors have been successfully used for commercial microalgal biomass production. Unlike open raceways, tubular photobioreactors permit a good control of culture conditions and high solar radiation availability and, consequently, a high biomass productivity, which makes this type of system potential for biofuel production and compounds of high commercial value (Kunjapur and Eldridge 2010; Abomohra et al. 2016).
A tubular photobioreactor consists of an array of straight transparent tubes that are usually made of plastic or glass and have a diameter of 0.1 m or less. These transparent tubes can be arranged in different patterns (e.g., straight, bent, or spiral) and orientations (e.g., horizontal, inclined, vertical, or helical) in order to maximize the sunlight capture (Huang et al. 2017). However, to increase the scale, the tubes are usually arrayed in a horizontal fence-like, which improves the land utilization, and also have a better angle for incident light (Junying et al. 2013).
Besides the solar array for algae growth, a tubular photobioreactor is also composed of a harvesting unit to separate algae from the suspension, a degassing column for gas exchange and cooling (heating) and a circulation pump (Wang et al. 2012). The microalgal culture flows through solar collector tubing and is recirculated by maintaining highly turbulent flow, which is produced using either a mechanical pump or a gentler airlift pump (Abomohra et al. 2016; Chang et al. 2017).
This type of photobioreactor can be illuminated by artificial or natural light. The artificial illumination is technically possible, but expensive compared with outdoor cultivations, which is just viable for commercial production of high added value products.
2.2.1 Major Factors Affecting the Tubular Photobioreactor Performance
2.2.1.1 Light Supply
In autotrophic microalgae production, the light availability is the most important factor that influences the cell productivity and is one of the most difficult to control in outdoor cultures, due to the variation in solar radiation during the day and during the change of season (Fernandez et al. 1997).
In terms of design, the light capture is influenced by the transparency of the materials and the surface/volume ratio. The most common materials used for PBR construction are glass, plexiglass, polyvinyl chloride (PVC), acrylic-PVC, and polyethylene. All these materials have transparency suitable for the microalgae cultivations. However, they all have their pros and cons and need to be evaluated according to the type of process and desired product. Glass is strong and transparent and very good material for the construction of laboratory-scale PBRs. However, it requires many connection parts for the construction of large-scale PBRs, which could be costly. For this reason, the plastic type is most suitable for large-scale tubular photobioreactor, mainly of polyethylene (Wang et al. 2012).
2.2.1.2 Temperature
As already mentioned, the optimal temperature for microalgae cultures is generally around 25 ℃, and most microalgae species can tolerate temperatures between 16 ℃ and 35 ℃. In closed PBR, generally, the volume is small because a thin optical thick mixing ness is applied for the sake of light transfer. Therefore, variations in temperature during the day/night cycle and the seasons’ changes have significant effects on microalgal cultivation. In this sense, it is necessary to set up a cost-effective cooling system (Huang et al. 2017).
Several methods have been tested to prevent overheating of the microalgae cultivation. Among them are as follows: (i) shading of the tubes with dark-colored sheets (Torzillo 1997), (ii) cooling of the culture by spraying water on the surface of the photobioreactor (Becker 1994), (iii) submerging part of the photobioreactor or the entire culture on a large body of water (Becker 1994), and (iv) installing a heat exchanger for the photobioreactor (Watanabe et al. 2011). However, shading the PBR is inefficient because it greatly reduces the illumination and consequently in the yield of biomass. Water spraying is efficient for cooling, but entails an increase in the cultivation costs. On the other hand, the method of submersion besides efficient in the control of the temperature has been demonstrated to promote the average light intensity in the culture (Huang et al. 2017).
2.2.1.3 CO2/O2 Balance and Mixing
As explained in Sects. 2.1.1.3 and 2.1.1.4, the carbon dioxide and oxygen must be maintained in equilibrium and in moderate concentrations, since the excess of both causes damage to the cells. In this sense, the photobioreactor must contain a space for exhaust gases and an efficient mixing system, where promote turbulence and therefore mass transfer between the gas and liquid phases inside a photobioreactor (Wang et al. 2012).
In addition to its key role in the balance of gases and pH of the system, the mixing also is necessary to prevent sedimentation of algal cells, ensure that all cells of the population have uniform average exposure to light and nutrient and facilitate heat transfer and avoid thermal stratification. In tubular photobioreactor, the mixing is usually provided by aeration with CO2-enriched gas bubbles or pumping, mechanical agitation, or a combination of these means. The choice depends on the scale of the system and the microalga species used, because some do not tolerate vigorous agitations (Suh and Lee 2003; Wang et al. 2012; Huang et al. 2017).
2.2.2 Biomass Productivity in Tubular Photobioreactor
The high biomass productivity is the greatest advantage of tubular photobioreactors, especially if compared to raceway ponds. Table 2 shows the biomass productivities in different tubular photobioreactors. The values range from 0.05 to 1.9 g/L/d. This variation is due to the type of geometric configuration used, microalgae species and operating and environmental conditions used in each study. If we compare these productivities values with those found in raceway ponds (Table 1), it is possible to see that except to the values found by Olaizola (2000), all the other productivities in tubular PRBs are greater than found in raceways.
2.2.3 Costs of Construction and Operation of Tubular Photobioreactors
The cost of PBRs has a major influence on production cost for large-scale biomass. The company AlgaeLink NV (Yerseke, The Netherlands) commercializes a horizontal serpentine PBR made of large-diameter transparent plastic tubes. For a system of 97 m3, 1200 m2 of occupied area, made of 2000 m long, 25 cm diameter PMMA tubes, according to Zitelli et al. (2013), the price was about € 194,000 in 2012. Through inflation calculation described in Chisti (2013), this price in 2017 is about € 202,798.
Norsker et al. (2011) calculated the cost for outdoor production of microalgae biomass in tubular photobioreactor and concluded that € 4.15 is the price for producing 1 kg of dry weight biomass, in a 100-ha plant. On the other hand, Grima (2009) found a cost of € 25 per kg of dry weight, in a horizontal tubular PBR of 4000 L. However, according to these authors, it is possible to reduce this cost up to € 0.5 per kg, through of process optimization.
2.2.4 Scaling-up
The scale-up of a tubular photobioreactor is not so simple in an open system, because it requires scaling-up of both the solar receiver and the airlift device. In principle, the volume of the solar receiver may be increased by increasing the diameter and the length of the tube. However, an increase in tube length can result in unacceptable concentrations of dissolved oxygen along the tubes. For this reason, in practice, only the tube diameter may be varied (Grima et al. 2001). Any change in tube diameter would imply a change in the light/dark cycle inside of photobioreactor. These cycles can improve the biomass productivity due to the ability of some species to store light energy to maintain their metabolism in the absence of light (Maroneze et al. 2016). Thus, the geometry of the PBR must be optimized according to the species used.
Grima et al. (2001) concluded that for Phaeodactylum tricornutum, the optimal photobioreactor (0.2 m3) configuration and operations conditions were as follows: a solar receiver tube of 0.06 m diameter, 80 m long, connected to a 4 m tall airlift. Although not having a simple scaling-up, the tubular PBR is already quite widespread in large scale.
2.2.5 Commercial Applications of Horizontal Tubular PBRs
Currently, many biotech companies around the world are using tubular photobioreactors to produce microalgae biomass and several bioproducts. Among them are the Algaelink in the Netherlands that use horizontal and tubular photobioreactors for biomass and jet fuel production. The Heliae (USA) is using spiral tubular PBR to produce astaxanthin from H. pluvialis. In Cadiz, Spain, the Fitoplancton Marino SL uses a horizontal serpentine PBR cooled by immersion in a water pool to produce lyophilized microalgae biomass and slurries of several microalgae for aquaculture use (Torzillo et al. 2015; Chang et al. 2017).
2.3 Microalgal Heterotrophic Bioreactors
The heterotrophic bioreactors are a feasible alternative to overcome the light energy dependency that limits the scale-up and significantly complicates the design of photobioreactors (Vieira et al. 2012). Although restricted to a few microalgal species, the heterotrophic cultivation can be conducted in conventional reactor configurations such as stirred tank and bubble column reactors, which are relatively cheap, easily scalable, and generally present high kinetic performance (Queiroz et al. 2011; Perez-Garcia et al. 2011).
Moreover, to reduce the cost related to microalgae biofuel production, the organic carbon source and nutrients for the microalgae cultivation can be obtained from agro-industrial wastes (Queiroz et al. 2011; Francisco et al. 2015; Katiyar et al. 2017). In addition to meeting the demand for organic carbon, the use of wastewater in these cultivations also contributes to agro-industrial waste management (Maroneze et al. 2014).
On the other hand, the major limitations of these types of cultivation are the contamination and competition with other microorganisms that grow faster than the microalgae, inability to produce light-induced metabolites and inhibition of growth by excess organic substrate.
2.3.1 Major Factors Affecting Bioreactors in Heterotrophic Cultivations
2.3.1.1 Oxygen Supply
In aerobic bioprocesses, oxygen is a critical substrate for a cell metabolism that needs continuous supply, as it can easily become rate limiting due to its low solubility in water. According to Griffiths et al. (1960), independent of the organic substrate or the microalgae species, the biomass productivity is enhanced by higher levels of aeration.
In contrast, the aeration system energy requirement is a significant cost in bioreactors and also contributes to the carbon footprint of heterotrophic cultivations. So, for a viable biofuel production, a trade-off between the operating costs related to energy required for aeration and the productivity of the bioprocess (Santos et al. 2015). In this sense, Santos et al. (2015) concluded that for a heterotrophic bubble column bioreactor, the aeration of 0.5 VVM (volume of air per volume of medio per minute) is an equilibrium between kinetic performance and power requirements in bioreactor.
2.3.1.2 Mixing and Viscosity
Like in the cultivation systems already discussed, mixing is one of the most important operations in heterotrophic microalgal cultivation. This operation is necessary for uniformly distributing nutrients and for gas exchange. The adequate mixing can be provided by impellers and baffles or by aeration with airlift or bubble column systems (Perez-Garcia and Bashan 2015).
The viscosity of the medium is closely related to the mixing, where high viscosity in cultures requires higher impeller speed or airflow, which increases power consumption and operational costs. The viscosity comes mainly from the exogenous carbon source used, but is also increased with the high cell concentration and/or with the production of viscous cellular material.
2.3.2 Biomass Productivity in Heterotrophic Bioreactors
In terms of biomass production, the heterotrophic cultivations can present higher values of productivity, when compared to the other large-scale systems discussed in this chapter, as shown in Table 3, which summarizes the microalgal biomass productivities in heterotrophic cultivations with different carbon sources, bioreactors type, and microalgae species reported in the literature.
2.3.3 Costs in Heterotrophic Bioreactors
The production costs of the heterotrophic microalgae production depend on variables such as bioreactor, carbon source, microalgae strain, downstream processing operations, type and quality of the end product, among others. Tabernero et al. (2012) evaluated the production of microalgal biodiesel from C. protothecoides biomass grown heterotrophically. The cost estimated to produce one kilogram of biomass was US$ 1.29 per kg (corrected for 2017). This value was estimated for a biorefinery producing biomass in 465 continuously stirred bioreactors each of 150,000 L and producing 10 million/L/year of biodiesel.
A value below this (US$ 0.06/kg, corrected to 2017) was found by Roso et al. (2015) to produce P. autumnale biomass, in a techno-economic analysis of a simulated large-scale process to produce bulk oil and lipid-extracted algae in an agro-industrial biorefinery. These authors also found values of US$ 0.40/kg and US$ 0.07/kg (corrected to 2017) to produce bulk oil and lipid-extracted algae, respectively.
2.3.4 Scaling-up in Heterotrophic Bioreactors
Another differential of heterotrophic cultivation is the scaling-up relatively easy, and the bioreactors are available commercially for cultivation of several microorganisms with working volumes up to 100,000 L. Li et al. (2007) investigated the scale-up from 250 mL flasks to 11,000 L bioreactors of a heterotrophic cultivation with C. protothecoides to biodiesel production. The authors were successful in scaling-up and suggested that it is feasible to expand heterotrophic Chlorella cultivation for biodiesel production at the industry level.
According to Perez-Garcia and Bashan (2015), the practical aspects required for large-scale biofuel production from heterotrophic cultivation of microalgae are as follows: (i) the species must be robust and able to grow in the absence of light and under extreme conditions, such as high or low pH, high temperatures, or high salinity; (ii) the microalgae strain must also have a rapid growth to be able to compete with other heterotrophic microorganisms and thus avoid contamination; (iii) the exogenous carbon source must be inexpensive and easily found; and (iv) the biofuel generated must present a quality standard required by the legislation and in quantity that makes the process economically viable.
2.3.5 Commercial Applications of Heterotrophic Bioreactors
The commercial production of microalgae via heterotrophic metabolism has been made by the Solazyme Inc., in Moema, São Paulo (Brazil). Solazyme’s technology enables it to successfully convert a range of low-cost plant-based sugars into biofuels. The biofuels that this company has produced and tested are biodiesel (SoladieselBD®), renewable diesel (SoladieselRD®), aviation turbine fuel (Solajet™), and renewable jet fuel. Furthermore, Solazyme is producing renewable oils for the chemicals, nutrition and skin and personal care space utilizing today’s existing industrial scale fermentation capacity.
3 Small-Scale Photobioreactors
3.1 Vertical Photobioreactors
Vertical reactors were among the first algal mass culture systems described in the literature (Cook 1950). These systems are compact, user-friendly bioreactors with a high ratio of surface area/volume, low contamination risk, and high biomass productivity. However, at present, these systems are not used as photobioreactors, except for investigational purposes, due to their difficult of scaling-up (Mirón et al. 1999).
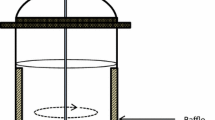
Vertical photobioreactors consist of vertical tubes constructed with a transparent material (polyethylene or glass tubes) to allow the penetration of light. An air diffuser is located at the bottom of the reactor, where the sparged gas is converted into tiny bubbles. This sparging with gas mixture provides the constant agitation of the medium, mass transfer of CO2 and also removes O2 produced during photosynthesis. Based on their mode of liquid flow, vertical tubular photobioreactors can be divided into bubble column and airlift PBRs (Singh and Sharma 2012; Chang et al. 2017).
Bubble column photobioreactors are cylindrical vessel with height greater than twice the diameter, simply agitated by bubbling CO2 and air from a sparger at the photobioreactor bottom without any special internal constructions and completely lack any moving parts (Singh and Sharma 2012; Koller 2015).
Airlift photobioreactors are cylindrical tubes with two interconnecting zones. One of the zones is called riser where gas mixture is sparged, whereas the other zone, the downcomer, does not receive the gas. The system can be with internal loop and external loop. In the first option, regions are separated either by a draft tube or a split cylinder. On the other hand, in the external loop, riser and downcomer are separated physically by two different tubes.
3.1.1 Major Factors Affecting the Vertical PBRs Performance
3.1.1.1 Light Available
In vertical photobioreactors, the illumination is accomplished externally, which can be natural or artificial. The light available plays an essential role for the good performance of any photosynthetic culture. To obtain sufficient illumination, both the airlift and the bubble column photobioreactors cannot exceed about 0.2 m in diameter; otherwise, the light availability will be reduced severely, mainly in the center of cylinder. Additionally, it must also be considered that the height of the cylinder should not exceed 4 m due to structural reasons (Huang et al. 2017). Furthermore, in a vertical tubular photobioreactor, the light availability also is influenced by aeration rates, gas holdup, and superficial velocity (Mirón et al. 1999).
3.1.1.2 Aeration Rate, Gas Holdup, and Superficial Gas Velocity
As in horizontal photobioreactors, the agitation of the system gives only by pneumatic path, the aeration is responsible for culture mixing. In an ideal aeration rate, the microalgae are kept in suspension, the light/dark cycle is minimized, the CO2 is diffused homogeneously, and its excess is removed, thus maintaining the pH stable and the produced oxygen is removed. In general, in airlift photobioreactors, the mixing is better than bubble column and thus can sustain better biomass production of different microalgae (Fernandes et al. 2014).
Gas holdup is one of the most important parameters characterizing airlift and tubular photobioreactors. It is necessary to the hydrodynamic design in different industrial processes because it governs gas phase residence time and gas–liquid mass transfer. The gas holdup is defined as the volume of the gas phase divided by the total volume. This parameter is influenced mainly by the superficial gas velocity and the type of gas diffuser (Mirón et al. 1999).
Superficial gas velocity is the ratio of the volumetric gas flow rate and cross-sectional area of the reactor. The photosynthetic efficiency of the culture is affected by the dark zone that may exist at the center of the photobioreactor. This dark zone is totally dependent on the gas superficial velocity, which is the ratio of the volumetric gas flow rate and cross-sectional area of the reactor. According to Janssen et al. (2003), high gas velocity (>0.05 m/s) is recommended for increasing the photosynthetic efficiency.
3.1.2 Biomass Productivity in Vertical PBR
Productivities of microalgal biomass in vertical photobioreactors vary with the type of mode of liquid flow, dimensions, microalgae species, and operating and environmental conditions implemented. The values of productivities in these systems of production reported in the literature vary between 0.031 and 0.77 g/L/d, as shown in Table 4.
3.1.3 Costs in Vertical Photobioreactors
According to Wang et al. (2014), for a 20 L indoor bubble column PBR, the cost of biomass production was about US$ 431.39 per kg. On the other hand, in a 200 L outdoor bubble column photobioreactor, the cost to produce 1 kg of dry weight biomass was US$ 58.69. The estimated cost by the methodology proposed by Chisti (2013) in 2017 for biomass production is of approximately US$ 445.58 and US$ 60.63 per kg of dry weight biomass in a 20 L indoor bubble column photobioreactor and a 200 L outdoor bubble column photobioreactor, respectively.
3.1.4 Scaling-up in Vertical Photobioreactors
The vertical tubular photobioreactors are limited to laboratory and pilot scales, which is attributed to fragility of the material, gas transfer at the top regions of the system, temperature control, gas holdup, and a limited surface for illumination especially at up-scaled devices in case of algal species with high demands for illumination (Koller 2015). However, Mirón et al. (1999) affirm that such perceptions have never been substantiated and, according to their results, both bubble column and airlift photobioreactors are more suitable to scaling-up that horizontal PBRs.
A practical example of the difficulty of scale-up is the failure case of GreenFuel Technologies Corporation, at Arizona in 2007. Firstly, GreenFuel designs vertical inclined closed photobioreactors and installed pilot plant to recycle CO2 emissions into microalgal biomass for biofuels production. With the success of the pilot plant, months later the company installed a photobioreactor at the same plant, but 100 times larger than its earlier test models. Due to incorrect scaling-up, the project of millions of dollars failed, its photobioreactors turned out to be twice as expensive as expected and the company had to fire nearly half its staff (Waltz 2009).
3.2 Flat-Plate Photobioreactors
Flat-plate photobioreactors have received much attention for microalgae biomass production due to their large illumination surface area (Ugwu et al. 2008). In this type of photobioreactor, a thin layer of culture is passed across a flat panel made of a transparent material, as glass, plexiglass, or polycarbonate (Faried et al. 2017). They can be oriented at different angles so as to modify the light intensity and use diffused and reflected light. Agitation can be provided either by bubbling air from its one side through perforated tube or by rotating it mechanically using a motor (Chang et al. 2017).
Flat-panel photobioreactors feature important advantages for biomass production of photoautotrophic microorganisms and may become a standard reactor type for the mass production of several algal species (Sierra et al. 2008). However, the capital and operational cost of such systems are still too high to produce microalgae biomass as feedstock for biofuels or other low-value products with currently available technologies (Li et al. 2014).
The construction of flat-plate reactors dates back to the early 1950s (Burlew 1953), since then, many different designs have been developed. Tredici and collaborators developed a rigid alveolar panel photobioreactor (Tredici et al. 1991; Tredici and Materassi 1992). Pulz and Scheibenbogen (1998) proposed a flat-plate PBR inner walls arranged to promote an ordered horizontal culture flow that was forced by a mechanical pump. Recently, Li et al. (2014) developed a flat-panel photobioreactor with internal bulk liquid flow and an external airlift with the purpose of developing a scalable industrial photobioreactor.
3.2.1 Major Factors Affecting the Flat-Plate Photobioreactor Performance
3.2.1.1 Light Supply
The flat-plate photobioreactors can be illuminated artificially or through sunlight. However, as the use of sunlight is much more economically feasible, and these systems have an excellent setting to capture sunlight, this is the most commonly used option.
The light absorption is totally dependent on the length of light path. In general, the biomass productivity is highest at the smallest light path and smallest at the longest light path PBR (Richmond and Cheng-Wu 2001). Other configuration that has an influence on light capture is the tilt angle of flat-plate photobioreactor. Throughout the year, the optimal tilt of the PBR that allows maximal incident light will change due to the position of the sun (Wang et al. 2012). Hu et al. (1998) described that as a general rule, the optimal angle for year-round biomass production is equal to the geographic latitude of the location.
3.2.1.2 Gas Balance and Mixing
A great advantage of flat-panel reactors is that they have a much shorter oxygen path than tubular reactors, so the accumulation of dissolved oxygen is low (Sierra et al. 2008; Chang et al. 2017). According to Sierra et al. (2008), in a flat-panel photobioreactor, an aeration of 0.25 VVM (volume of air per volume of liquid per minute) and a power supply of 53 W/m3 are sufficient to maintain the balance of gases, mixing is ideally suited for most microalgal culture. Other authors reported even much higher aeration rates up to 2.0 VVM with positive effects (Alias et al. 2004; Wang et al. 2005).
3.2.1.3 Temperature
Microalgae cultivations in outdoor PBRs are exposed to seasonal and diurnal variation of temperature. These variations have a direct influence on the cellular growth and the chemical composition of the biomass, and therefore, for the development of an efficient and controlled process, the temperature must be maintained with the least possible variation.
Particularly, flat-plate photobioreactors are very susceptible to overheating due to its thin layer of cultivation and high light exposure. For this reason, the PBRs must have an efficient temperature control system. This control is usually done by water spraying (evaporative cooling) or alternatively, by using internal heat exchangers (Chang et al. 2017).
3.2.2 Biomass Productivity in Flat-Plate Photobioreactor
Some values of biomass productivity reported in the literature are shown in Table 5, which range from 0.16–4.3 g/L/d. These values vary according to the species and parameters used for photobioreactor construction and cultivation. Due to the large light exposure surface area, high biomass productivity is found in these systems, however, are still limited to laboratory scale and pilot scale.
3.2.3 Costs in Flat-Plate PBRs
Tredici et al. (2016) evaluated the production cost of the microalga Tetraselmis suecica in a 1-ha plant made of “Green Wall Panel-II” (GWP®-II) photobioreactors located in Tuscany-Italy. The GWP® is flat disposable photobioreactor, designed and patented in 2004 and commercialized by Fotosintetica & Microbiologica S.r.l. Through a techno-economic analysis, they conclude that, for a 1-ha, the total capital investment is about € 1,661,777 and the total fixed capital per annum is of € 101,260. Also in this analysis, they found a cost of € 12.4 to produce 1 kg of biomass (dry weight). This cost can be reduced when the plant is installed in a region with more favorable climatic conditions. The authors related that in Tunisia, the cost of biomass production is of € 6.2 kg/in a 1-ha plant with the same PBR. Lower production costs (€ 5.96/kg) in a vertical flat-panel photobioreactor of commercial scale were found by Norsker et al. (2011), but if we update this value by calculating the inflation correction described in Chisti (2013), this value is of about € 6.36/kg.
3.2.4 Scaling-up in Flat-Plate Photobioreactors
The scale-up in flat-plate photobioreactors presents some challenges, which are usually caused by the large surface area of the photobioreactor. This type of design requires many modules and supports materials, shows difficulty in controlling culture temperature and is very susceptible to the fouling, which is the phenomena that occur when cells attach to the plastic walls, causing a reduction in light availability and an increased risk of contamination (Carvalho et al. 2014; Chang et al. 2017).
Despite the limitations, several commercial large-scale flat-plate photobioreactors have been developed. One example is the Green Wall Panel (GWP®) that has a concept of ‘disposable panels’ for large-scale applications. This system commercialized by Fotosintetica & Microbiologica S.r.l consists of vertical PBRs of 100-litre bags, made of a polyethylene foil enclosed in a rigid framework (Tredici et al. 2016). Other systems available commercially are the flat-plate airlift patented and produced by Subitec GmbH, in Germany. In this case, the photobioreactors are produced on scales varying from 6 to 180 L per unit.
4 Recent Developments in Microalgae Cultivation Systems
Recently, biofilm cultivation of microalgae emerged as a new biomass production strategy. These systems consist of a densely packed layer of microalgae that grow attached to a solid surface, which should be illuminated and should be frequently exposed to water containing nutrients. Among the advantages of the biofilm-based microalgae cultivation are the cost reduction related to microalgae harvesting, reduced light limitation, low footprint, low water consumption, and efficient CO2 mass transfer. In contrast, the limitations of the system are the formation of gradients over the biofilm for pH, nutrients, and light (Gross et al. 2015; Hoh et al. 2015).
Another photobioreactor configuration that has attracted attention in recent years is the membrane photobioreactor, mainly for the cultivation of microalgae using wastewater. The membrane photobioreactor is a technology that integrates a conventional enclosed PBR with a submerged or side-stream membrane filtration process using microfiltration or ultrafiltration membranes for solid–liquid separation. These systems can operate in continuous mode, which increases the microalgal biomass production, they produce a high quality treated effluent with low levels of organic substances, pathogen, and suspended solids and are easy to operate and scale-up. However, only limited studies exist about these techniques and for a large-scale implementation, techno-economic analyses and environmental performance assessment are required to assess their viability (Billad et al. 2015; Luo et al. 2016).
Finally, hybrid photobioreactors have proved to be a promising technology for the mass production of microalgae compared with single PBRs. Hybrid photobioreactors are systems that combine different growth stages in two types of PBRs, closed and open, in which the disadvantage of one PBR is complemented by the other (Brennan and Owende 2010). These configurations aim to compensate the drawbacks caused by the limitation of surface/volume ratio and scale-up of open and closed conventional photobioreactors. These systems are based on a proper height/diameter ratio, generating configurations of reactors with heavy workloads in contrast to very long tubes or shallow ponds. The main advantages of these photobioreactors include low use of land area with high culture volume, low operating costs and are potential to scaling-up. On the other hand, this type of configuration is limited to the cultivation of microalgae species with the ability to store energy to sustain cell growth for periods in the dark, without affecting the rate of photosynthetic metabolism (Ramírez-Mérida et al. 2017).
The biomass productivities found in microalgae cultivation with these photobioreactors are shown in Table 6. All these systems are relatively new, and therefore, only a limited number of studies are found in the literature and are restricted to laboratory scale.
5 Criteria for the Selection of Microalgae Cultivation System
According to Chang et al. (2017), the main criteria to be considered in the choice of an ideal photobioreactor are as follows: (i) type and quality of the target product; (ii) tolerance of microalgal strains; and (iii) scale and performance versus cost.
The first criterion to be considered is the type and quality of the desired product. For the biofuel production, it is essential to produce a biomass rich in lipid or carbohydrate with a low cost to be competitive with conventional fossil fuels. In this case, a heterotrophic bioreactor integrated into a biorefinery system can be a good choice due to the high productivity, low cost, and low-land demand. Additionally, these systems can operate in parallel in wastewater treatment, when they are used as a source of carbon and nutrients for algal growing. On the other hand, to produce light-induced metabolites and high-value products intended for human consumption, a closed PBR is more advisable (Li et al. 2014; Chang et al. 2017).
The characteristics of the microalgae strain that will be used must also be considered at the moment of the choice. Mainly in terms of adaptability and tolerance under outdoor conditions and shear forces and oxygen buildup generated by PBRs. In open ponds, strains must be able to compete with other microorganisms for nutrients and must have the ability to tolerate photoperiods and climate changes. In the case of closed photobioreactors, the strains must withstand strong shear forces generated by pumping or aeration and must be able to tolerate a possible excess of oxygen in the system (Brennan and Owende 2010; Chang et al. 2017).
When a biofuel is the target product, the most important issue is the cost of the biomass which will be processed to yield the fuel. For this, the systems must present a high kinetic performance at large-scale production. It is known that closed systems are significantly more efficient in biomass production compared with open systems. At the same time, most closed systems have a difficult and expensive scaling-up, and open systems can be scaled up easily and inexpensively to accommodate larger production rates. So, the choice of cultivation system must be based on the best trade-off between biomass productivity and production cost (Chang et al. 2017).
In addition to considering all these factors, it is important to know all the advantages and limitations of each microalgae cultivation system. In this sense, Table 7 shows the pros and cons of all the systems presented in this chapter.
6 Final Considerations
The biofuels production from microalgae has been demonstrated to have broad potential of application, but these currently still remain at the exploratory stage. This chapter underlines several aspects involved in the microalgal production systems in order to help the development of biofuels from microalgae. Despite that a great deal of work has been done to develop systems for microalgae production, to date, there is no system without limitations. The main difficulties are related to the cost of construction and operation, scaling-up, contamination, and to a limited knowledge about the new cultivation systems. Therefore, to choose a system, trade-offs among productivity, costs, scaling-up, and value of final product should be carefully made.
References
Abomohra, A., Jin, W., Tu, R., Han, S., Eid, M., & Eladel, H. (2016). Microalgal biomass production as a sustainable feedstock for biodiesel: Current status and perspectives. Renewable and Sustainable Energy Reviews, 64, 596–606.
Alias, C. B., Lopez, M. C. G. M., Fernández, F. G. A., Sevilla, J. M. G., Sanchez, J. L. G., & Grima, E. M. (2004). Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnology and Bioengineering, 87, 723–733.
Becker, E. W. (1994). Microalgae-biotechnology and microbiology (1st ed.). Cambridge: Cambridge University Press.
Benemann, J. R., Goebel, R. P., Augenstein, D. C., & Weissman, J. C. (1982). Microalgae as a source of liquid fuels. Final technical Report to U.S. DOE BER, viewed August 24, 2016, <https://www.osti.gov/scitech/biblio/6374113>.
Bennett, M. C., Turn, S. Q., & Chan, W. Y. (2014). A methodology to assess open pond, phototrophic, algae production potential: A Hawaii case study. Biomass and Bioenergy, 66, 168–75.
Bergmann, P., & Trösch, W. (2016). Repeated fed-batch cultivation of Thermosynechococcus elongatus BP-1 in flat-panel airlift photobioreactors with static mixers for improved light utilization: Influence of nitrate, carbon supply and photobioreactor design. Algal Research, 17, 79–86.
Billad, M. R., Arafat, H. A., & Vankelecom, I. F. J. (2015). Membrane technology in microalgae cultivation and harvesting: A review. Biotechnology Advances, 32, 1283–1300.
Borowitzka, M. A. (2005). Culturing microalgae in outdoor ponds. In R. A. Andersen (Ed.), Algal culturing techniques (pp. 205–218). Amsterdam: Elsevier Academic Press.
Brennan, L., & Owende, P. (2010). Biofuels from microalgae: A review of technologies for production, processing, and extractions of biofuels and co products. Renewable and Sustainable Energy Reviews, 14, 557–577.
Burlew, J. S. (1953). Algal culture: From laboratory to pilot plant (1st ed.). Washington: Carnegie Institution of Washington.
Camacho, R. F., Fernández, F. G. A., Pérez, J. A. S., Camacho, F. G., & Grima, E. M. (1999). Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnology and Bioengineering, 62, 71–86.
Carvalho, J. C. M., Matsudo, M. C., Bezerra, R. P., Ferreira-Camargo, L. S., & Sato, S. (2014). Microalgae bioreactors. In R. Bajpai, A. Prokop, & M. Zappi (Eds.), Algal biorefineries (Vol. 1, pp. 83–126). Switzerland: Springer International Publishing.
Chang, J. S., Show, P. L., Ling, T. C., Chen, C. Y., Ho, S. H., Tan, C. H., et al. (2017). Photobioreactors. In C. Larroche, M. Sanroman, G. Du, & A. Pandey (Eds.), Current developments in biotechnology and bioengineering: Bioprocesses, bioreactors and controls (pp. 313–352). Atlanta: Elsevier.
Cheng-Wu, Z., Zmora, O., Kopel, R., & Richmond, A. (2001). An industrialsize flat glass reactor for mass production of Nannochloropsis sp. (Eustigmatophyceae). Aquaculture, 195, 35–49.
Chew, K. W., Yap, J. Y., Show, P. L., Suan, N. H., Juan, J. C., Ling, T. C., et al. (2017). Microalgae biorefinery: High value products perspectives. Bioresource Technology, 229, 53–62.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Chisti, Y. (2013). Raceways-based production of algal crude oil In C. Posten & C. Walter (Eds.), Microalgal biotechnology: Potential and production (pp. 197–216). Berlin: de Gruyter.
Chisti, Y. (2016). Large-scale production of algal biomass: Raceway ponds. In F. Bux & Y. Chisti (Eds.), Algae biotechnology: Products and processes (pp. 21–40). New York: Springer.
Chiu, S. Y., Tsai, M. T., Kao, C. Y., Ong, S. C., & Lin, C. S. (2009). The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Engineering in Life Sciences, 9, 254–260.
Collotta, M., Champagne, P., Busi, L., & Alberti, M. (2017). Comparative LCA of flocculation for the harvesting of microalgae for biofuels production. Procedia CIRP, 61, 756760.
Cook, P. M. (1950). Some problems in the large-scale culture of Chlorella (pp. 53–75). Yellow Springs, OH: The Culture Foundation.
Crowe, B., Attalah, S., Agrawal, S., Waller, P., Ryan, R., Van Wagenen, J., et al. (2012). A comparison of Nannochloropsis salina growth performance in two outdoor pond designs: Conventional raceways versus the arid pond with superior temperature management. International Journal of Chemical Engineering and Applications, 2012, 9–21.
Cuaresma, M., Janssen, M., Vílchez, C., & Wijffels, R. H. (2009). Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnology and Bioengineering, 104, 352–359.
de Godos, I., Mendoza, J. L., Acién, F. G., Molina, E., Banks, C. J., Heaven, S., et al. (2014). Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresource Technology, 153, 307–314.
Department of Energy (DOE). (2010). National algal biofuels technology roadmap, viewed August 24, 2016, <https://www1.eere.energy.gov/bioenergy/pdfs/algal_biofuels_roadmap.pdf>.
Eustance, E., Badvipour, S., Wray, J. T., & Sommerfeld, M. R. (2015). Biomass productivity of two Scenedesmus strains cultivated semi-continuously in outdoor raceway ponds and flat-panel photobioreactors. Journal of Applied Phycology, 28, 1471–1483.
Faried, M., Samer, M., Abdelsalam, E., Yousef, R. S., Attia, Y. A., & Ali, A. S. (2017). Biodiesel production from microalgae: Processes, technologies and recent advancements. Renewable and Sustainable Energy Reviews, 79, 893–913.
Fernandes, B. D., Mota, A., Ferreira, A., Dragone, D., Teixeira, J. A., & Vicente, A. A. (2014). Characterization of split cylinder airlift photobioreactors for efficient microalgae cultivation. Chemical Engineering Science, 117, 445–454.
Fernandez, F. G. A., Camacho, A. C., Pérez, J. A. S., Sevilla, J. M. F., & Grima, E. M. (1997). A model for light distribution and average solar irradiance inside outdoor tubular photobioreactors for the microalgal mass culture. Biotechnology and Bioengineering, 55, 701–714.
Fernandez, F. G. A., Sevilla, J. M. F., & Grima, E. M. (2013). Photobioreactors for the production of microalgae. Reviews in Environmental Science and Bio/Technology, 12, 131–151.
Fernández, F. G. A., Sevilla, J. M. F., Pérez, J. A. S., Grima, E. M., & Chisti, Y. (2001). Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: Assessment of design and performance. Chemical Engineering Science, 56, 2721–2732.
Francisco, E. C., Franco, T. T., Wagner, R., & Jacob-Lopes, E. (2014). Assessment of different carbohydrates as exogenous carbon source in cultivation of cyanobacteria. Bioprocess and Biosystems Engineering, 37, 1497–505.
Francisco, E. C., Franco, T. T., Zepka, L. Q., & Jacob-Lopes, E. (2015). From waste-to-energy: The process integration and intensification for bulk oil and biodiesel production by microalgae. Journal of Environmental Chemical Engineering, 3, 482–487.
Gao, F., Yang, Z. H., Li, C., Wang, Y. J., Jin, W. H., & Deng, Y. B. (2014). Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresource Technology, 167, 441–446.
Griffiths, D. J., Thresher, C. L., & Street, H. E. (1960). The heterotrophic nutrition of Chlorella vulgaris (brannon no. 1 strain). Annals of Botany, 24, 1–11.
Grima, E. M. (2009). Algae biomass in Spain: A case study. In First European Algae Biomass Association Conference & General Assembly, Florence.
Grima, E. M., Fernández, J., Acién, F. G., & Chisti, Y. (2001). Tubular photobioreactor design for algal cultures. Journal of Biotechnology, 92, 113–131.
Gross, M., Jarboe, D., & Wen, Z. (2015). Biofilm-based algal cultivation systems. Applied Microbiology and Biotechnology, 99, 5781–5789.
Harder, R., & von Witsch, H. (1942). Ueber Massenkultur von Diatomeen. Ber. Dtsch. Bot. Ges., 60, 14–153.
Heidari, M., Kariminia, H. R., & Shayegan, J. (2016). Effect of culture age and initial inoculum size on lipid accumulation and productivity in a hybrid cultivation system of Chlorella vulgaris. Process Safety and Environmental Protection, 104, 111–122.
Hoh, D., Watson, S., & Kan, E. (2015). Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chemical Engineering Journal, 287, 466–473.
Hu, Q., Fairman, D., & Richmond, A. (1998). Optimal tilt angles of enclosed reactors for growing photoautotrophic microorganisms outdoors. Journal of Fermentation and Bioengineering, 85, 230–236.
Hu, Q., Guterman, H., & Richmond, A. (1996). A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnology and Bioengineering, 51, 51–60.
Hu, Q., & Richmond, A. (1994). Optimizing the population density in Isochrysis galbana grown outdoors in a glass column photobioreactor. Journal of Applied Phycology, 6, 391–396.
Huang, Q., Jiang, F., Wang, L., & Yang, C. (2017). Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering, 3, 318–329.
Jacob-Lopes, E., Scoparo, C. H. G., Lacerda, L. M. C. F., & Franco, T. T. (2009). Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chemical Engineering and Processing: Process Intensification, 48, 306–310.
Jacob-Lopes, E., Zepka, L. Q., Merida, L. G. R., Maroneze, M. M., & Neves, C. (2014). Bioprocesso de conversão de dióxido de carbono de emissões industriais, bioprodutos, seus usos e fotobiorreator híbrido. BR n. PI2014000333.
Janssen, M., Tramper, J., Mur, L., & Wijffels, R. H. (2003). Enclosed outdoor photobioreactors: Light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnology and Bioengineering, 81, 193–210.
Jiménez, C., Cossío. B. R., & Niell, F. X. (2003). Relationship between physicochemical variables and productivity in open ponds for the production of Spirulina: A predictive model of algal yield. Aquaculture, 221, 331–45.
Juneja, A., Ceballos, R. M., & Murthy, G. S. (2013). Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Enegies, 6, 4607–4638.
Junying, Z., Junfeng, R., & Baoning, Z. (2013). Factors in mass cultivation of microalgae for biodiesel. Chinese Journal of Catalysis, 34, 80–100.
Katiyar, R., Gurjar, B. R., Bharti, R. Q., Kumar, A., Biswas, S., & Pruthi, V. (2017). Heterotrophic cultivation of microalgae in photobioreactor using low cost crude glycerol for enhanced biodiesel production. Renewable Energy, 113, 1359–1365.
Koller, M. (2015). Design of closed photobioreactors for algal cultivation. In A. Prokop, R. K. Bajpai, & M. E. Zappi (Eds.), Algal biorefineries volume 2: Products and refinery design (pp. 139–186). Switzerland: Springer International Publishing.
Kunjapur, A. M., & Eldridge, R. B. (2010). Photobioreactor design for commercial biofuel production from microalgae. Industrial and Engineering Chemistry Research, 49, 3516–3526.
Lal, A., & Das, D. (2016). Biomass production and identification of suitable harvesting technique for Chlorella sp. MJ 11/11 and Synechocystis PCC 6803. 3 Biotech, 6, 41–51.
Li, J., Stamato, M., Velliou, E., Jeffryes, C., & Agathos, S. N. (2014). Design and characterization of a scalable airlift flat panel photobioreactor for microalgae cultivation. Journal of Applied Phycology, 27, 75–86.
Li, X., Xu, H., & Wu, Q. (2007). Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnology and Bioengineering, 98, 764–771.
Lin, Q., & Lin, J. (2011). Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresource Technology, 102, 1615–1621.
Lopez, M. C. G., Del Rio Sanchez, E., Lopez, J. L. C., Fernandez, F. G. A., Sevilla, J. M. F., Rivas, J., et al. (2006). Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. Journal of Biotechnology, 123, 329–42.
López, C. V. G., Fernández, F. G. A., Sevilla, J. M. F., Fernández, J. F. S., García, M. C. F., & Grima, E. M. (2009). Utilization of the cyanobacteria Anabaena sp. ATCC 33047 in CO2 removal processes. Bioresource Technology, 100, 5904–5910.
Lu, Y., Zhai, Y., Liu, M., & Wu, Q. (2010). Biodiesel production from algal oil using cassava (Manihot esculenta Crantz) as feedstock. Journal of Applied Phycology, 22, 573–578.
Lundquist, T. J., Woertz, I. C., Quinn, N. W. T., & Benemann, A. (2010). Realistic technology and engineering assessment of algae biofuel production. Berkeley: Energy Biosciences Institute, University of California.
Luo, Y., Le-Clech, P., & Henderson, R. K. (2016). Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: A review. Algal Research, 24, 425–437.
Marbella, L., Bilad, M. R., Passaris, I., Discart, V., Bañadme, D., Beuckels, A., et al. (2014). Membrane photobioreactors for integrated microalgae cultivation and nutrient remediation of membrane bioreactors effluent. Bioresource Technology, 163, 228–235.
Maroneze, M. M., Barin, J. S., Menezes, C. R., Queiroz, M. I., Zepka, L. Q., & Jacob-Lopes, E. (2014). Treatment of cattle-slaughterhouse wastewater and the reuse of sludge for biodiesel production by microalgal heterotrophic bioreactors. Scientia Agricola, 71, 521–524.
Maroneze, M. M., Siqueira, S. F., Vendruscolo, R. G., Wagner, R., Menezes, C. R., Zepka, L. Q., et al. (2016). The role of photoperiods on photobioreactors—a potential strategy to reduce costs. Bioresource Technology, 219, 493–499.
Mirón, A. S., Gómez, A. C., Camacho, F. G., Grima, E. M., & Chisti, Y. (1999). Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae. Journal of Biotechnology, 70, 249–270.
Münkel, R., Schmid-Staiger, U., Werner, A., & Hirth, T. (2013). Optimization of outdoor cultivation in flat panel airlift reactors for lipid production by Chlorella vulgaris. Biotechnology and Bioengineering, 110, 2882–2893.
Norsker, N. H., Barbosa, M. J., Vermuë, M. H., & Wijffels, R. H. (2011). Microalgal production-a close look at the economics. Biotechnology Advances, 29, 24–27.
Olaizola, M. (2000). Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. Journal of Applied Phycology, 12, 499–506.
Olguín, E., Galicia, S., Mercado, G., & Pérez, T. (2003). Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. Journal of Applied Phycology, 15, 249–257.
Perez-Garcia, O., & Bashan, Y. (2015). Microalgal heterotrophic and mixotrophic culturing for bio-refining: From metabolic routes to techno-economics. In A. Prokop, R. K. Bajpai, & M. E. Zappi (Eds.), Algal biorefineries volume 2: Products and refinery design (pp. 61–132). Switzerland: Springer International Publishing.
Perez-Garcia, O., Escalante, F. M. E., de-Bashan, L. E., & Bashan, Y. (2011). Heterotrophic cultures of microalgae: Metabolism and potential products. Water Research, 45, 11–36.
Pleissner, D., Lam, W. C., Sun, Z., & Lin, C. S. K. (2013). Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresource Technology, 137, 139–146.
Pruvost, J., Le Borgne, F., Artu, A., & Legrand, J. (2017). Development of a thin-film solar photobioreactor with high biomass volumetric productivity (AlgoFilm©) based on process intensification principles. Algal Research, 21, 120–137.
Pulz, O., & Scheibenbogen, K. (1998). Photobioreactors: Design and performance with respect to light energy input. Advances in Biochemical Engineering/Biotechnology, 59, 123–152.
Queiroz, M. I., Hornes, M. O., Silva-Manetti, A. G., & Jacob-Lopes, E. (2011). Single-cell oil production by cyanobacterium Aphanothece microscopica Nägeli cultivated heterotrophically in fish processing wastewater. Applied Energy, 88, 3438–3443.
Ramírez-Mérida, L. G. R., Zepka, L. Q., & Jacob-Lopes, E. (2017). Current production of microalgae at industrial scale. In J. C. M. Pires (Ed.), Recent advances in renewable energy (pp. 242–260). Sharjah: Bentham Science Publishers.
Raslavičius, L., Striūgas, N., & Felneris, M. (2018). New insights into algae factories of the future. Renewable and Sustainable Energy Reviews, 81, 643–654.
Raso, S., van Genugten, B., Vermuë, M., & Wijffels, R. H. (2012). Effect of oxygen concentration on the growth of Nannochloropsis sp. at low light intensity. Journal of Applied Phycology, 24, 863–871.
Rawat, I., Kumar, R. R., Mutanda, T., & Bux, F. (2013). Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Applied Energy, 103, 444–467.
Richmond, A. (1990). Large scale microalgal culture and applications. In F. E. Round & D. J. Chapman (Eds.), Progress in phycological research (pp. 269–330). Britol: Biopress Ltd.
Richmond, A., & Cheng-Wu, Z. (2001). Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. Journal of Biotechnology, 85, 259–269.
Roso, G. R., Santos, A. M., Zepka, L. Q., & Jacob-Lopes, E. (2015). The econometrics of production of bulk oil and lipid extracted algae in an agroindustrial biorefinery. Current Biotechnology, 4, 547–553.
San Pedro, A., González-López, C. V., Acién, F. G., & Grima, E. M. (2014). Outdoor pilot-scale production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in tubular photobioreactors. Bioresource Technology, 169, 667–676.
Santos, A. M., Deprá, M. C., Santos, A. M., Zepka, L. Q., & Jacob-Lopes, E. (2015). Aeration energy requirements in microalgal heterotrophic bioreactors applied to agroindustrial wastewater treatment. Current Biotechnology, 4, 249–254.
Scott, S. A., Davey, M. P., Dennis, J. S., Horst, O., Howe, C. J., Lea-Smith, D. J., et al. (2010). Biodiesel from algae: Challenges and prospects. Current Opinion in Biotechnology, 21, 277–286.
Sierra, E., Acién, F. G., Fernández, J. M., García, J. L., González, C., & Molina, E. (2008). Characterization of a flat plate photobioreactor for the production of microalgae. Chemical Engineering Journal, 138, 136–147.
Singh, R. N., & Sharma, S. (2012). Development of suitable photobioreactor for algae production—a review. Renewable and Sustainable Energy Reviews, 16, 2347–2353.
Su, H., Zhou, X., Xia, X., Sun, Z., & Zhang. Y. (2017a). Progress of microalgae biofuel’s commercialization. Renewable and Sustainable Energy Reviews, 74, 402–411.
Su, Y., Song, K., Zhang, P., Su, Y., Cheng, J., & Chen, X. (2017b). Progress of microalgae biofuel’s commercialization. Renewable and Sustainable Energy Reviews, 74, 402–411.
Suh, I. S., & Lee, C. G. (2003). Photobioreactor engineering: Design and performance. Biotechnology and Bioprocess Engineering, 8, 313–321.
Sun, A., Davis, R., Starbuck, M., Ben-Amotz, A., Pate, R., & Piencos, P. T. (2011). Comparative cost analysis of algal oil production for biofuels. Energy, 36, 5169–5179.
Tabernero, A., Martín del Valle, E. M., & Galán, M. A. (2012). Evaluating the industrial potential of biodiesel from a microalgae heterotrophic culture: Scale-up and economics. Biochemical Engineering Journal, 63, 104–115.
Tao, Q., Gao, F., Qian, C. Y., Guo, X. Z., Zheng, Z., & Yang, Z. H. (2017). Enhanced biomass/biofuel production and nutrient removal in an algal biofilm airlift photobioreactor. Algal Research, 21, 9–15.
Torzillo, G. (1997). Tubular bioreactors. In A. Vonshak (Ed.), Spirulina platensis (Arthrospira): Phisiology, cell-biology and biotechnology (1st ed., pp. 101–115). London: Taylor and Francis.
Torzillo, G., Zittelli, G. C., & Chini Zittelli, G. (2015). Tubular photobioreactors. In A. Prokop, R. K. Bajpai, & M. E. Zappi (Eds.), Algal biorefineries volume 2: Products and refinery design (pp. 187–212). Switzerland: Springer International Publishing.
Tredici, M. R., Carlozzi, P., Zittelli, G. C., & Materassi, R. (1991). A vertical alveolar panel (VAP) for outdoor mass cultivation of microalgae and cyanobacteria. Bioresource Technology, 38, 153–159.
Tredici, M. R., & Materassi, R. (1992). From open ponds to vertical alveolar panels: The Italian experience in the development of reactors for the mass cultivation of photoautotrophic microorganisms. Journal of Applied Phycology, 4, 221–231.
Tredici, M. R., Rodolfi, L., Biondi, N., Bassi, N., & Sampietro, G. (2016). Techno-economic analysis of microalgal biomass production in a 1-há Green Wall Panel (GWP®) plant. Algal Research, 19, 253–263.
Tuantet, K., Temmink, H., Zeeman, G., Janssen, M., Wijffels, R. H., & Buisman, C. J. N. (2014). Nutrient removal and microalgal biomass production on urine in a short light-path photobioreactor. Water Research, 55, 162–174.
Ugwu, C. U., Aoyagi, H., & Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technology, 99, 4021–4028.
Ugwu, C. U., Ogbonna, J. C., & Tanaka, H. (2002). Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Applied Microbiology and Biotechnology, 58, 600–607.
Vieira, J. G., Manetti, A. G. S., Jacob-Lopes, E., & Queiroz, M. I. (2012). Uptake of phosphorus from dairy wastewater by heterotrophic cultures of cyanobacteria. Desalination and Water Treatment, 40, 224–230.
Waltz, E. (2009). Biotech’s green gold? Nature Biotechnology, 27, 15–18.
Wang, B., Lan, C. Q., & Horsman, M. (2012). Closed photobioreactors for production of microalgal biomasses. Biotechnology Advances, 30, 904–912.
Wang, S. K., Hu, Y. R., Wang, F., Stiles, M. R., & Liu, C. Z. (2014). Scale-up cultivation of Chlorella ellipsoidea from indoor to outdoor in bubble column bioreactors. Bioresource Technology, 156, 117–122.
Wang, C. H., Sun, Y. Y., Xing, R. L., & Sun, L. Q. (2005). Effect of liquid circulation velocity and cell density on the growth of Parietochloris incisa in flat plate photobioreactors. Biotechnology and Bioprocess Engineering, 10, 103–108.
Watanabe, Y., de la Noue, J., & Hall, D. O. (2011). Photosynthetic performance of a helical tubular photobioreactor incorporating the cyanobacterium Spirulina platensis. Biotechnology and Bioengineering, 47, 261–269.
Wen, X., Du, K., Wang, Z., Peng, X., Luo, L., Tao, H., et al. (2016). Effective cultivation of microalgae for biofuel production: A pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnology for Biofuels, 9, 123–135.
Xiong, W., Li, X., Xiang, J., & Wu, Q. (2008). High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbial-diesel production. Applied Microbiology and Biotechnology, 78, 29–36.
Xu, Z., Baicheng, Z., Yiping, Z., Zhaoling, C., Wei, C., & Fan, O. (2002). A simple and low-cost airlift photobioreactor for microalgal mass culture. Biotechnology Letters, 24, 1767–1771.
Zitelli, G. C., Rodolfi, L., Bassi, N., Biondi, N., & Tredici, M. R. (2013). Photobioreactors for biofuel production. In M. A. Borowitzka & N. R. Moheimani (Eds.), Algae for biofuels and energy (pp. 115–131). Dordrecht: Springer.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Maroneze, M.M., Queiroz, M.I. (2018). Microalgal Production Systems with Highlights of Bioenergy Production. In: Jacob-Lopes, E., Queiroz Zepka, L., Queiroz, M. (eds) Energy from Microalgae . Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-69093-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-69093-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69092-6
Online ISBN: 978-3-319-69093-3
eBook Packages: EnergyEnergy (R0)