Summary
How leaf respiration (R d) is represented in leading terrestrial biosphere models (TBMs ) is reviewed, followed by an overview of how emerging global datasets provide opportunities to improve parameterization of leaf R d in large-scale models. We first outline how TBMs have historically accounted for variations in respiratory CO2 release in mature leaves, using assumed relationships between leaf nitrogen, photosynthetic capacity and R d. The need for TBMs to account for light inhibition of R d in mature leaves is highlighted, followed by a discussion on how R d of upper canopy leaves is used to predict maintenance respiration in whole plants. We then outline how respiratory energy requirements of growth are accounted for in TBMs, pointing out that current assumptions on the costs of biosynthesis are based on theoretical calculations that may not be valid for all plant species and environments. The chapter then considers how improvements might be made to TBMs with respect to the parameterization of leaf R d. We show how recently compiled datasets provide improved capacity to predict global variations in baseline R d measured at a standard temperature, and how baseline R d likely acclimates to sustained changes in growth temperature. Application of this dataset reveals markedly higher rates of leaf R d than currently predicted by TBMs , suggesting that TBMs may be underestimating global plant respiratory CO2 release. The availability of a new, global dataset on short-term temperature responses of leaf R d is highlighted. Analysis of this dataset reveals that leaf R d does not exhibit the exponential response assumed by most TBMs; rather, the temperature-sensitivity declines as leaves warm, with convergence in the temperature-response across biomes and plant functional types . We show how equations derived from these datasets may provide the TBM community with a new framework to improve representation of mature leaf respiration in TBMs.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Over the past 30 years, increasing efforts have been put into the development of terrestrial biosphere models (TBMs ) and associated land surface components of Earth system models (ESMs) (Running and Coughlan 1988; Raich et al. 1991; Woodward et al. 1995; Haxeltine and Prentice 1996a; Ruimy et al. 1996; Cox et al. 1998; Cox 2001; Sitch et al. 2008; Clark et al. 2011; Booth et al. 2012; Prentice and Cowling 2013; Fisher et al. 2014). TBMs are used to represent carbon exchange between plants and the atmosphere, with CO2 release by plant respiration (R) being crucial for TBM predictions (King et al. 2006; Huntingford et al. 2013; Wythers et al. 2013), reflecting the fact that ~60 Pg carbon are respired by plants into the atmosphere each year (Prentice et al. 2001; Canadell et al. 2007; Denman et al. 2007; IPCC 2013). Because leaf R represents approximately half of overall respiratory CO2 release by whole-plants (Atkin et al. 2007), even small fractional changes in leaf respiration can have large impacts on net carbon uptake by plants, which in turn can affect ecosystem net carbon exchange and storage (Piao et al. 2010). Moreover, by influencing the CO2 concentration in the atmosphere, feedbacks can occur that alter the extent of future global warming (Cox et al. 2000; Huntingford et al. 2013). There is growing acceptance, however, that representation of leaf respiration in TBMs is inadequate and lags behind that of photosynthetic parameterization, leading to large uncertainties in predictions of future climates and vegetation dynamics (Gifford 2003; Leuzinger and Thomas 2011; Huntingford et al. 2013; Smith and Dukes 2013; Lombardozzi et al. 2015). In this chapter, we outline how foliar respiratory CO2 release is currently parameterized in TBMs , and identify a range of areas where improvement is now possible.
Both fully-expanded (i.e. mature) leaves and developing leaves contribute to respiratory rates of whole shoots; for both leaf tissue types, variation in the rate of respiratory CO2 release can be influenced by factors such as availability of substrate, demand for respiratory products [e.g. adenosine triphosphate (ATP), reducing equivalents and/or tricarboxylic acid (TCA) cycle intermediates] and respiratory capacity (i.e. abundance of active respiratory proteins). Importantly, demand for respiratory products differs markedly between immature (i.e. expanding) and mature (i.e. fully-expanded) leaves (Fig. 6.1; see also Chap. 8 in this volume), with energy demands for biosynthesis being dominant in expanding leaves, whereas maintenance processes (e.g. protein turn-over and maintenance of solute gradients) are central to energy demand in mature leaves (Penning de Vries 1975; Amthor 2000; Bouma 2005). Reflecting this difference, a theoretical framework was conceived (Thornley 1970, 2011, Amthor 2000) that recognized that respiratory energy is used to support both growth (R g) and maintenance (R m) processes (the so-called ‘growth-and-maintenance-respiration paradigm’–GMRP) (Amthor 2000). While the GMRP is not without its critics [e.g. respiration in mature leaves is also likely influenced by growth-linked processes such as the energy costs associated with phloem loading (Bouma et al. 1995)], it nonetheless forms the basis of how whole-plant respiration (R p) is parameterized in most TBMs (Gifford 2003; Smith and Dukes 2013; see Table 6.1 for details). For example, in the Community land surface model JULES [Joint UK Land Environment Simulator (Clark et al. 2011)], used as the land surface scheme in the UK Hadley Centre climate models, the starting point for modeling R p is to first predict rates of respiration of mature leaves at 25 oC (i.e. R d), with leaf R d then used to estimate rates of maintenance respiration in whole shoots and roots (R pm) (Fig. 6.2). Whole-plant growth respiration (R pg) is then calculated as one quarter of whole-plant gross primary productivity (GPP ) minus R pm, with overall R p being estimated from the sum of R pm plus R g (Fig. 6.2). In subsequent sections, we outline how respiratory rates of developing and mature leaves are accounted for in leading TBMs such as JULES, highlighting uncertainties and areas where our growing understanding of how genotypic and environmental variation in leaf respiration across the globe could be used to improve the predictive capacity of TBMs.
Developmental changes in leaf (R). Solid line shows R declining sharply as leaves expand (Azcón-Bieto et al. 1983; Fredeen and Field 1991; Evans et al. 2000; Armstrong et al. 2006), meeting energy demands associated with biosynthesis (i.e. cell division and expansion) & maintenance (e.g. protein turnover and maintenance of solute gradients) processes (Penning de Vries 1975; Amthor 2000; Bouma 2005). In expanding leaves, demands of biosynthesis are dominant. Maintenance costs become relatively more important as leaves expand, being dominant in mature leaves; potentially, energy demands associated with sucrose and amino acid production/export (Bouma 2005) may also influence rates of mature leaf respiration. Thus, variation in demand for respiratory products from mature leaves – such as might occur when plants vary in whole plant growth rates with concomitant changes in demand for sucrose/amino acid export from mature leaves to meristematic regions – could potentially impact on respiratory rates of mature leaves
Schematic showing progression via which whole-plant respiration rates (R p) are calculated from leaf nitrogen in the land surface model of JULES (Joint UK Land Environment Simulator) (Cox 2001; Clark et al. 2011). For individual plant functional types (PFTs), assumed foliar N concentrations are used to predict maximum rates of CO2 carboxylation by Rubisco (V cmax) at a standard temperature of 25 °C, based on assumed V cmax–[N] relationships derived from Schulze et al. (1994). Thereafter, maintenance respiration in mature leaves (R d) at the standard temperature of 25 °C is estimated based on assumed R d-V cmax relationships (Farquhar et al. 1980; Collatz et al. 1991, 1992). Then, rates of leaf R d at other temperatures are calculated assuming a R d-temperature relationship such as a fixed Q 10 approach. Whole-plant maintenance respiration (R pm) is then estimated via assumptions of how N is allocated among leaves, stems and roots, and respiration-[N] relationships in each organ. Crucially, this approach assumes a common maintenance R-N relationship in above and below-ground organs. Finally, rates of R pm are modulated by a soil-moisture correction factor, β to yield the final whole-plant R pm estimate. Whole-plant growth respiration (R pg) is then calculated as being 25% of the residual of gross primary productivity (GPP ) minus R pm
When considering how to improve representation of leaf R d in TBMs , the ideal solution would be to develop a process-based model that accurately predicts spatial and temporal variations in respiratory fluxes. For photosynthesis, a mechanistic biochemical framework is available (Farquhar et al. 1980), enabling CO2 uptake to be modeled in TBMs (e.g. Haxeltine and Prentice 1996a; Cox 2001; Clark et al. 2011; Ziehn et al. 2011; Oleson et al. 2013; Ali et al. 2015). However, while our understanding of the underlying factors that regulate leaf respiratory fluxes has improved markedly in recent years (Plaxton and Podesta 2006; Noguchi and Yoshida 2008; Buckley and Adams 2011; Kruse et al. 2011; Millar et al. 2011; Tcherkez et al. 2012; Sweetlove et al. 2013), at present there is no ‘Farquhar-model-equivalent’ for leaf R d. Recognizing this, the TBM community has – by necessity – relied on a phenomenological approach to predict variation in leaf R d in TBMs , using reported relationships between R d of mature leaves and associated traits, and chemical analyses of plant tissues to predict whole-plant growth respiration . However, when TBMs were first developed, relatively few data were available on R d of mature leaves, requiring them to rely on measurements made on a limited number of species/environments (e.g. Farquhar et al. 1980; Ryan 1991; Collatz et al. 1992; Ryan 1995; Haxeltine and Prentice 1996a; Ruimy et al. 1996; see Table 6.1 for details).
In subsequent sections, we discuss in detail how variations in leaf R d have historically been parameterized in leading TBMs , and highlight key uncertainties in assumed relationships between R d of mature leaves and associated traits. How leaf R d is scaled to whole plants is also outlined; as part of this section, we discuss the basis upon which growth respiration (R pg) is accounted for in TBMs, and discuss whether the assumption by TBMs of a single growth respiration coefficient (g R – i.e. respiratory CO2 released per unit biomass produced by growth) for all plants is valid. The chapter concludes with a section on potential ways parameterization of global variations in mature-leaf R d over different time scales could be improved in future versions of TBMs. The chapter does not provide a comprehensive update on the role of genotype and environment in determining variations in mature-leaf R d, which while important are beyond the scope of this chapter. Here, readers are encouraged to consult relevant reviews and primary literature (e.g. Atkin and Tjoelker 2003; Gonzelez-Meler et al. 2004; Atkin et al. 2005, 2015; Flexas et al. 2005; Rodríguez-Calcerrada et al. 2010; Searle et al. 2011b; Slot et al. 2013, 2014; Smith and Dukes 2013; Ayub et al. 2014; Way and Yamori 2014; Weerasinghe et al. 2014; Slot and Kitajima 2015; Vanderwel et al. 2015; Drake et al. 2016; Heskel et al. 2016b; Reich et al. 2016; Rowland et al. 2016).
Finally, a note on abbreviations used to describe leaf respiration. In most TBMs , respiration of mature leaves is designated as ‘R d’. However, this can occasionally cause confusion when interpreting precisely what the abbreviation ‘R d’ refers to. In leaf-level studies assessing variations in gas exchange rates, R d is often used to describe leaf respiration measured in darkness (e.g. Reich et al. 1998; Pons and Welschen 2002; Lee et al. 2005; Liang et al. 2013). However, in the paper that often forms the basis of TBM estimates of GPP , R d was defined as non-photorespiratory mitochondrial CO2 release in the light (Farquhar et al. 1980), with Brooks and Farquhar (1985) subsequently defining R d as being ‘day’ respiration (also designated as ‘R day’), and respiration in darkness as being ‘R n’ (i.e. ‘night’ respiration). With the exception of a few studies (Mercado et al. 2007; Clark et al. 2011; Harper et al. 2016), TBMs typically assume that R d = R n (i.e. light does not inhibit leaf respiration). For this reason, in most sections in this chapter, we use the term ‘R d’ to refer to leaf respiration taking place throughout the 24-h cycle (day and night). The only exception is a section devoted to the topic of light inhibition of leaf respiration, where we use the terms R light and R dark to define fluxes measured in the light and dark, respectively.
2 Representation of Leaf Respiration in Terrestrial Biosphere Models
As noted above, there is no single approach to estimating plant respiration in TBMs , with Schwalm et al. (2010) reporting 15 unique approaches in a survey of 21 TBMs. However, in models that explicitly represent leaf R d, a common approach is to relate R d to foliar nitrogen concentration ([N]) and/or photosynthesis (Fig. 6.2, Table 6.1). Such approaches are based, in part, on the fact that variations in foliar [N], and/or photosynthetic rates, impact on the demand for respiratory products (e.g. ATP, reducing equivalents and/or carbon skeletons) by metabolic processes such as phloem loading, N assimilation and protein turn-over (Lambers 1985; Bouma et al. 1994, 1995; Noguchi and Yoshida 2008). Photosynthesis can also impact on respiratory rates via demand for ATP associated with sucrose synthesis, exchange of excess redox equivalents and substrate supply (Krömer et al. 1988; Raghavendra et al. 1994; Krömer 1995; Hoefnagel et al. 1998). Moreover, as N is a core component of the photosynthetic system, positive relationships are often observed between light-saturated photosynthesis and [N] (Field and Mooney 1986; Evans 1989; Schulze et al. 1994) as well as between leaf R d and [N] (Ryan 1991, 1995; Reich et al. 1996, 2008; Wright et al. 2006; Atkin et al. 2015). Hence, the use of foliar [N] and/or photosynthesis to predict leaf R d in TBMs is based on a solid empirical and theoretical framework. For models that predict variation in leaf R d via assumed relationships with photosynthetic capacity, foliar [N] remains often pivotal, reflecting assumed relationships between photosynthesis and [N] (e.g. Fig. 6.2). In this section, we discuss details on how respiratory fluxes are predicted from foliar [N] and/or photosynthesis in a range of leading TBMs , highlighting, where possible, the source datasets that underpin the assumed relationships.
2.1 Using Foliar Nitrogen As a Predictive Trait for Mature Leaf Respiration
Terrestrial biosphere models such as Biome-BGC (Thornton et al. 2005), Century (Parton et al. 1987), CLM (Oleson et al. 2013) and O-CN (Zaehle and Friend 2010) predict rates of leaf R d based on R d-[N] relationships reported by Ryan (1991; Table 6.1). Foliar N (used as a proxy for N uptake) is also used to predict leaf R d in Sheffield DGVM (Woodward et al. 1995; Woodward and Lomas 2004). In LPJ, rates of leaf R d are also predicted based on assumed R d-[N] relationships that are PFT (plant functional type) and biome specific, corrected for tissue C:N ratios and which are ultimately derived from Ryan (1991). In Ryan (1991), data from nine herbaceous and two coniferous tree species (i.e. 11 species in total) were assembled from published literature to assess the overall relationship between foliar R d (mol C mol N−1 h−1) measured at 20 °C and [N] (mol N mol C−1), yielding:
Thus, rates of R d at a set measuring temperature can be predicted in TBMs by using information on foliar [N]. TBMs using this approach assume that all PFTs and biomes follow the same R d-[N] relationship. In a subsequent comparison of 14 tree species, Ryan (1995) found that the relationship between R d and [N] did not differ between boreal and sub-alpine sites and that there was relatively little variability in N-based rates of leaf R d among species; thus, the assumption of a common R d-[N] relationship across PFTs and biomes appeared to hold. Subsequently, Amthor and Baldocchi (2001) collated published data on N-based rates of leaf R d (measured at different temperatures, depending on the study) for 23 species [including data from Ryan (1991)]. For the current chapter, we have normalized rates to 20 °C assuming a fixed Q10 (i.e. proportional change in metabolic rates per 10 °C change in temperature) of 2.2, and excluded data from soybean that exhibited particularly high R d [17.4 μmol CO2 mol−1 N s−1; (Thomas and Griffin 1994)]; analysis of the resultant dataset revealed three-fold variation in N-based rates of leaf R d (2.2 – 6.8 μmol CO2 mol−1 N s−1). Contained within this comparison were species from several PFTs (C3 grasses and forbs, broad-leaved evergreen trees, broad-leaved deciduous trees, and conifers), suggesting that R d-[N] relationships may differ among PFTs. Indeed, a cross-biome comparison revealed that rates of R d at a given [N] differ among PFTs [forbs > broad-leaved shrubs > broad-leaved trees > needle-leaved trees; (Reich et al. 1998)]. Later, Reich et al. (2008) found that the y-axis intercept of R d-[N] relationships was highest in herbs, followed by woody angiosperms, and with gymnosperms exhibiting the lowest R d-[N] intercept. PFTs also differed with respect to the slope (exponent) of the R d-[N] relationship, with the slope ranking: gymnosperms > woody angiosperms > herbs. The slope of the R d-[N] relationship was consistently >1.0 (Reich et al. 2008), likely reflecting the combined effects of higher energy requirements and greater allocation of tissue N to metabolism (relative to non-metabolic N pools) in metabolically active tissues (Poorter and Evans 1998; Reich et al. 2008).
Recently, a comparison of 899 species across 100 globally-distributed sites also found that rates of R d (at 25 °C) at a given [N] differ among PFTs [C3 herbs > shrubs > broad-leaved trees > needle-leaved trees; (Atkin et al. 2015)]. Rates of R d (at 25 °C) at a given [N] are also higher in plants growing in cold environments than their warm-grown counterparts (Tjoelker et al. 1999; Atkin et al. 2008, 2015) and higher in plants growing in high-light than low-light environments (Wright et al. 2006). In some studies, nutrient supply and/or soil moisture have also been shown to influence rates of R d at a given [N] (Meir et al. 2001; Wright et al. 2001; Atkin et al. 2013; Rowland et al. 2016). Collectively, these observations strongly suggest that there is no common leaf R d-[N] relationship across PFTs and environments. Given this, if leaf nitrogen is used as a predictor of R d in TBMs , PFT -specific equations that take growth temperature/irradiance, soil moisture , and/or nutrient availability into account are needed. Later, we discuss an example of how this approach can be implemented (Atkin et al. 2015).
Why does the rate of leaf R d at a given [N] vary among plants? There are three possible reasons, in principle: (i) differential allocation of nitrogen within leaves to metabolic and non-metabolic components; (ii) factors that might influence the metabolic flux through the respiratory system; and (iii) thermal effects (e.g. acclimation to sustained changes in growth temperature). For (i), it is now well established that the fraction of total leaf N allocated to photosynthetic processes differs among and within PFTs. For example, a greater fraction of leaf N is allocated to photosynthesis in C3 herbs than broad-leaved trees (with reduced allocation to non-photosynthetic components such as cell wall and structural proteins), leading to higher rates of photosynthesis per unit leaf N (Field and Mooney 1986; Reich et al. 1997; Poorter and Evans 1998; Hikosaka 2004; Pons and Westbeek 2004; Warren and Adams 2004). N allocation to photosynthesis is also greater in species with low leaf mass per unit leaf area (LMA, g dry mass m−2) than their high LMA counterparts (Takashima et al. 2004; Warren and Adams 2004; Harrison et al. 2009; Bahar et al. 2017). Given tight metabolic coupling between mitochondria and chloroplasts (Raghavendra et al. 1994; Hoefnagel et al. 1998), one would expect increased allocation of leaf N to photosynthetic processes to also be associated with increased allocation of leaf N to respiratory proteins. In turn, this would result in higher rates of leaf R d at a given [N], not necessarily because of higher respiratory fluxes per se, but because more of the total leaf N pool is linked to metabolism. For (ii) on factors that influence respiratory rates, variability in N allocation could also be important, reflecting the likelihood that proteins involved in metabolism exhibit higher turn-over rates than their structural N counterparts (Nelson et al. 2014), thus increasing ATP demand associated with protein synthesis and repair (Hachiya et al. 2007). Indeed, variation in energy demand is likely to be a major factor responsible for variation in rates of leaf R d at a given [N], influenced not only by protein turn-over [which likely accounts for near 20% of nocturnal respiration (Bouma et al. 1994)], but also other maintenance processes (e.g. maintenance of solute gradients) (Amthor 2000; Bouma 2005). Variation in the engagement of non-phosphorylating pathways of mitochondrial electron transport (e.g. alternative oxidase , rotenone-insensitive NADH dehydrogenase, external NAD(P)H dehydrogenase ), proton leakage through the inner mitochondrial membrane and proton flux via uncoupling proteins (PUMP) could all reduce the efficiency of ATP synthesis (Affourtit et al. 2001; Sweetlove et al. 2006; Armstrong et al. 2008; Millar et al. 2011; Searle et al. 2011a; Kornfeld et al. 2012), leading to variation in the rate of leaf R d at a given [N]. For the third point on factors affecting respiratory rates (thermal effects), see section II.C of this chapter.
2.2 Links Between Respiration and Photosynthetic Metabolism in Mature Leaves
As noted above, in TBMs that predict R d from assumed relationships with photosynthesis, the starting point can still originate with foliar [N]. Strong positive relationships are often reported between light-saturated rates of photosynthesis (A) and N, reflecting the importance of leaf N for light harvesting, photosynthetic electron transport and carboxylation capacity (Field and Mooney 1986; Evans 1989; Schulze et al. 1994; Reich et al. 1999). Similarly, maximum carboxylation capacity of Rubisco (V cmax) is often positively correlated with leaf [N] (Meir et al. 2002; Kattge et al. 2009; Domingues et al. 2010; Walker et al. 2014). Reflecting this, many TBMs predict variation in V cmax based on assumed relationships with leaf [N]. An example is the TRIFFID dynamic global vegetation model (Cox et al. 1998, 2000; Cox 2001) – implemented in JULES (Clark et al. 2011) – where V cmax (mol CO2 m−2 s−1) at 25 °C is assumed to be linearly dependent on leaf [N], n l in the JULES terminology [kg N (kg C)−1] according to:
and
with the constants being derived from a survey of 23 studies by Schulze et al. (1994), assuming that leaf dry matter is 40% C by mass and that V cmax is equal to twice light-saturated net photosynthesis for C3 plants and equal to light-saturated net photosynthesis for C4 plants.
TBMs that use V cmax to predict rates of leaf R d (Table 6.1) adopt a range of coefficients to link the two processes. For example, in BETHY, leaf R d was assumed to be 1.1% of V cmax for C3 plants (Knorr 2000; Ziehn et al. 2011), reflecting the assumed relationship reported in Farquhar et al. (1980). For C4 plants, leaf R d was assumed to be 4.2% of V cmax (Knorr 1997). In both BIOME3 (Haxeltine and Prentice 1996a) and JULES (Cox 2001; Clark et al. 2011), leaf R d was assumed to be 1.5% of V cmax for C3 plants (Collatz et al. 1991); interestingly, the latter study cited Farquhar et al. (1980) as its source for the leaf R d-V cmax relationship (although in that reference, leaf R d was assumed to be 1.1% of V cmax for C3 plants, not 1.5%). BIOME3 and JULES differ in their predicted leaf R d-V cmax relationships for C4 plants [4.2% (Knorr 1997) and 2.5% (Collatz et al. 1992), respectively], with neither estimate based on large screening of leaf respiration rates in C4 plants. Indeed, in Collatz et al. (1992), the estimate was based solely on measurements made on corn. Thus, in none of the models linking V cmax to leaf R d is the assumed relationship based on comprehensive surveys of respiratory and photosynthetic values in nature. Moreover, no allowance is made for variations in rates of leaf R d at a given V cmax, even though there is now evidence that leaf R d-V cmax relationships vary among PFTs and environments (Atkin et al. 2015). For example, at 25°C leaf R d as a percentage of V cmax is higher in C3 herbs (7.8%) than shrubs (4.5%), needle-leaved trees (3.8%) and broad-leaved trees (3.3%). Further, leaf R d at a given V cmax (at 25 °C) is greater in plants growing in cold than warm biomes (Atkin et al. 2015). From these observations, two conclusions can be made: (i) for most species, leaf R d as a percentage of V cmax is actually greater than that assumed in current TBMs ; and (ii) leaf R d-V cmax relationships vary amongst PFTs and biomes . The next generation of TBMs will have to account for these observations, if leaf R d continues to be predicted from modeled rates of V cmax.
2.3 Temperature Dependence of Mature Leaf Respiration in TBMs
In the TBMs listed in Table 6.1, rates of R d (predicted from assumed R d-[N] and/or R d-V cmax relationships) at a specified reference temperature are used to model rates of R d at other leaf temperatures. How leaf R d varies with temperature is crucial for TBM predictions, because of the importance of temperature-mediated changes in respiratory CO2 efflux in determining future carbon storage in vegetation and atmospheric CO2 concentrations (King et al. 2006; Atkin et al. 2008; Huntingford et al. 2013; Wythers et al. 2013; Lombardozzi et al. 2015; Heskel et al. 2016b). Here, consideration needs to be given to how R d responds to temperature, both over short (e.g. minutes-hours) and long (days, months, years) periods of time.
In most TBMs , leaf R d is assumed to increase exponentially with increasing temperature, with the Q 10 value (i.e. proportional increase in R d per 10 °C increase in leaf T) assumed to be fixed through time (with typical values of 2.0–2.3). Examples of TBM frameworks using a fixed Q 10 include BIOME-BGC (Running and Coughlan 1988), Century (Melillo et al. 1993), CLM4.5 (Oleson et al. 2013), JULES (Cox 2001) and TEM (Raich et al. 1991). In an earlier version of JULES (i.e. MOSES-TRIFFID) that modeled global carbon fluxes under a ‘business-as-usual’ emission scenario, the assumption of a fixed Q 10 of 2.0 resulted in positive carbon feedbacks that increased future atmospheric CO2 concentrations and global temperatures (Cox et al. 2000). Importantly, by assuming a fixed temperature response of R d, global respiratory CO2 release was predicted to exceed global GPP by the end of the century, making the land surface a net source of CO2 to the atmosphere (Cox et al. 2000). Earlier versions of MOSES-TRIFFID (Cox et al. 1998, 1999) allowed leaf R d to follow the same temperature dependency as V cmax, with the latter incorporating low and high temperature limit functions into the calculation of temperature dependence . More recently, JULES was modified to yield a bell-shaped temperature function, with peak rates of leaf R d at 32 °C (via linking leaf R d to V cmax, and assuming the latter has a peak rate at 32 °C) (Huntingford et al. 2013). Doing so results in marked increases in carbon storage in land vegetation in the tropics, when compared to model runs that assumed a fixed Q 10 of 2.0 (Huntingford et al. 2013). Thus, TBMs predictions are strongly dependent on whether or not a fixed Q 10 is used.
The assumption of a constant Q 10 (i.e. a Q 10 that is independent of leaf temperature) is in some ways surprising, as it has been long recognized that the temperature-response of R d is highly variable (James 1953; Forward 1960; Tjoelker et al. 2001). For example, changes in growth temperature that last several days, can alter the short-term Q 10 (Atkin et al. 2005; Armstrong et al. 2008), with Q 10 values often varying seasonally (Atkin et al. 2000b; Zaragoza-Castells et al. 2008). There is also evidence that temperature-corrected Q 10s can vary with climate of origin amongst woody species (Criddle et al. 1994) and that Q 10s are lower in tissues where substrates and/or energy demand limit respiration (Atkin and Tjoelker 2003). Moreover, Q 10 values decline as measurement temperature increases (James 1953; Forward 1960; Tjoelker et al. 2001; Atkin and Tjoelker 2003; Zaragoza-Castells et al. 2008; Heskel et al. 2016b), with the Q 10 reaching unity (i.e. Q 10 = 1.0) at high leaf temperatures as R d reaches a maximum rate (at T max). Beyond T max, further heating results in irreversible declines in R d (i.e. Q 10 < 1.0). Reflecting this, a number of models allow for decreasing temperature sensitivity of R d as leaves warm, either using a modified Q 10 (Tjoelker et al. 2001) such as in BETHY, or via application of modified Arrhenius activation energy (E a) functions (Robson 1981; Paembonan et al. 1991; Lloyd and Taylor 1994) such as in LPJ, O-CN and Sheffield DGVM.
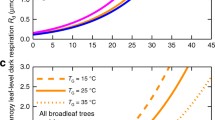
With the exception of BETHY (Knorr 2000) and LPJ (Bonan et al. 2003; Sitch et al. 2003), rates of leaf R d at a standard temperature are static within current generation TBMs , with no allowance made for potential changes in those reference values of R d in response to sustained changes in growth temperature. That is, leaf R d is not allowed to thermally acclimate despite mounting evidence that leaf R d does adjust to sustained changes in growth temperature. Acclimation can result in homeostasis of R d in plants grown at different temperatures, when measured at their respective growth temperatures (Larigauderie and Körner 1995; Atkin and Tjoelker 2003). Acclimation also results in R d (at a standard temperature) increasing upon cold acclimation and declining upon acclimation to warmer temperature. Growth temperature dependent changes in R d at a standard temperature can occur over periods of a few days (Atkin et al. 2000b; Bolstad et al. 2003; Lee et al. 2005; Zaragoza-Castells et al. 2007; Armstrong et al. 2008), suggesting that respiration metabolism acclimates to changes in growth temperature over time scales of several days and longer (Reich et al. 2016). As discussed in later sections, acclimation also manifests itself at the global scale, resulting in higher rates of leaf R d at a given measuring temperature in plants growing in cold environments compared to warm habitats (Atkin et al. 2015; Vanderwel et al. 2015). Importantly, acclimation results in a reduction in the long-term temperature-sensitivity of R d (Fig. 6.3; Larigauderie and Körner 1995); as such, accounting for thermal acclimation is likely to be important when predicting rates of respiratory CO2 release at global and regional levels, especially in response to increasing growth temperatures that are a consequence of global warming (King et al. 2006; Atkin et al. 2008; Smith and Dukes 2013; Wythers et al. 2013; Lombardozzi et al. 2015; Reich et al. 2016). Accounting for acclimation (via growth-temperature mediated changes in leaf R d at a standard temperature) is likely to increase overall estimates of leaf R d in cool habitats (e.g. arctic and boreal ecosystems), whereas it may lead to lower estimates of leaf R d in warmer habitats (Atkin et al. 2008). In recent runs of CLM4.5, Lombardozzi et al. (2015) reported that accounting for thermal acclimation of both photosynthesis and respiration has a marked impact on terrestrial carbon pools, with high latitudes gaining the most carbon under acclimation (reflecting the greater gains through photosynthesis than increased carbon losses by respiration), whereas accounting for acclimation had little impact on tropical carbon pools. Similarly, accounting for thermal acclimation of respiration alone in the PnET-CN ecosystem model was found to increase NPP by 9% when averaged across high latitude grassland and forests (Wythers et al. 2013). Thus, failure to account for thermal acclimation of leaf R d in TBMs is likely to lead to marked overestimates in the extent to which respiratory CO2 release increases as global warming heats individual ecosystems.
Diagram to illustrate the impacts of thermal acclimation on modeled rates of leaf respiration. In most TBMs , rates of leaf respiration (leaf R d) at a standard measuring temperature (here shown at 25 °C) are used to model rates of leaf R d at other temperatures assuming that respiration does not thermally acclimate; the no-acclimation scenario (in blue) shows values where leaf R d increases with temperature according to a fixed Q 10 approach. The dashed line shows calculated rates of leaf R d across a range of temperatures for a scenario where leaf R d acclimates to sustained changes in growth temperature. This is achieved via allowing the rate of leaf R d at the standard measuring temperature to increase and decrease when growth temperatures are below and above the standard temperature, respectively. Thus, when TBMs that have the standard temperature at 25 °C are initially spun-up to equilibrium, accounting for acclimation will result in increased rates of leaf R d where daily average growth temperatures are <25 °C (i.e. most environments) compared to models that do not account for acclimation. Similarly, accounting for acclimation will result in reduced rates of leaf R d in hot tropical ecosystems (Atkin et al. 2008). Thereafter, as all ecosystems warm, acclimation reduces predicted future increases in leaf R d
2.4 Light Inhibition of Leaf Respiration
As noted earlier, most TBMs typically assume that rates of leaf respiration taking place in the light are the same as those in darkness (i.e. light does not inhibit leaf respiration). Yet, there is long-standing evidence that rates of leaf respiration in the light/day (R light) are often lower than those in darkness/night (R dark) (Sharp et al. 1984; Brooks and Farquhar 1985; Pärnik and Keerberg 1995; Villar et al. 1995; Atkin et al. 1997; Pärnik et al. 2007; Way et al. 2015). Indeed, when measured at a common temperature, R light can be 80% lower than R dark (Atkin et al. 2006; Zaragoza-Castells et al. 2007). This issue has been acknowledged and addressed in the latest version of JULES (Clark et al. 2011). Failure to account for light inhibition can lead to large over-estimates of daily respiration in individual leaves (Atkin et al. 2006), and whole ecosystems [and hence by necessity net primary productivity (Janssens et al. 2001; Wohlfahrt et al. 2005; Mercado et al. 2007; Bruhn et al. 2011; Heskel et al. 2013; Wehr et al. 2016)]. It can also have important implications for our understanding of the processes controlling the rate of net CO2 assimilation in the light (A net), particularly in ecosystems exhibiting low rates of A net where assuming that leaf R takes place at similar rates in the dark and light can result in substantial errors in estimates of carboxylase (V c) and oxygenase (V o) rates of Rubisco .
Given the lack of understanding of how light inhibition varies among terrestrial biomes , attempts to account for light inhibition in TBMs have had to rely on assumed inhibition values. Lloyd et al. (1995) provided one of the earliest attempts to model light inhibition through forest canopies using R light versus irradiance curves from work on tobacco by Brooks and Farquhar (1985), where respiration in darkness was assumed to equal respiration in the light when growth irradiance <10 μmol photons m−2 s−1, but with R light < R dark when canopy irradiance >10 μmol photons m−2 s−1, the latter calculated according to the equation:
with I o being the incoming irradiance at the top of the canopy . This approach has since been applied in JULES (Mercado et al. 2007; Clark et al. 2011). Later, in a study modeling CO2 exchange in tropical forests, Lloyd et al. (2010) used data from an evergreen tree species Eucalyptus pauciflora (Atkin et al. 2000a) to formulate an equation that modeled the irradiance dependence of leaf respiration according to:
where α, β, and γ are fitted empirical constants with values of 0.9575 and 29.85 μmol m−2 s−1 and 5.114 × 10−5 μmol photons μmol−1 CO2, respectively, and I is the irradiance experienced by a leaf. Using this approach results in a 73%, 82% and 88% inhibition of leaf respiration at irradiances (I) of 100, 200 and 500 μmol photons m−2 s−1, respectively. More recently in studies using the JULES TBM, a uniform 30% inhibition of leaf respiration has been applied in conditions when irradiance >10 μmol photons m−2 s−1 (Clark et al. 2011; Harper et al. 2016). While the assumption of 30% inhibition has yet to be assessed across a range of habitats and species, a recent analysis of light inhibition in eight tropical forest species growing in North Queensland, Australia, found average light inhibition of 32% (Weerasinghe et al. 2014). Yet, we continue to lack data on patterns of light inhibition across a wider range of species.
Crucial to successful incorporation of light inhibition of leaf respiration into large scale models will be determining whether the degree of light inhibition differs systematically among plant species adapted to contrasting habitats. Whether there are systematic differences among species/biomes in the degree of light inhibition of leaf respiration will depend on: (i) the mechanisms responsible for light inhibition; and (ii) whether species differ in those traits associated with inhibition. While the factors responsible for light inhibition remain uncertain, past studies have pointed to light-dependent reductions in the activity of the pyruvate dehydrogenase (PDH) complex (Budde and Randall 1990; Gemel and Randall 1992) and malic enzyme (Hill et al. 1992) thought to play a role. Both enzymes play central roles in regulating carbon flow from glycolysis through the tricarboxylic acid (TCA) cycle in mitochondria. Further, transition to a truncated TCA cycle in the light, that results from removal of carbon skeletons to support N-assimilation (e.g. synthesis of glutamate ) and transfer of amino groups within the photorespiratory pathway, can result in reduced rates of TCA cycle CO2 release (Igamberdiev et al. 2001; Tcherkez et al. 2005, 2008, 2012). Increased use of stored organic acids can also reduce demand for TCA cycle intermediates, potentially slowing rates of R light (Gauthier et al. 2010). Metabolic modeling has also suggested that reduced rates of the oxidative pentose phosphate pathway (OPPP) in the light might also contribute to lower R light compared to R dark (Buckley and Adams 2011). Common to a number of the above factors is a link to photorespiratory metabolism, with high rates of photorespiration being linked to reduced PDH activity and increased demand for TCA intermediates. Similarly, changes in the demand for TCA intermediates by N assimilation could impact on the degree of light inhibition. Thus, it remains possible that criteria that predict variations in the degree of light inhibition might be identified via screening rates of R light, R dark, photorespiration and N assimilation in a wide range of plant species representative of PFTs used in TBMs . At present, however, such data are lacking.
Further, despite studies investigating the impacts of atmospheric CO2 , water supply , nutrient availability, growth irradiance and temperature on light inhibition (Wang et al. 2001; Pinelli and Loreto 2003; Shapiro et al. 2004; Pärnik et al. 2007; Zaragoza-Castells et al. 2007; Ayub et al. 2011; Crous et al. 2012; Heskel et al. 2012; Atkin et al. 2013; Ayub et al. 2014; Heskel et al. 2014; McLaughlin et al. 2014; Weerasinghe et al. 2014; Way et al. 2015), it remains unclear if there are systematic differences in the R light to R dark ratio along sustained differences in the growth environment. Finally, uncertainty remains as to the effects of short-term changes in leaf temperature (i.e. second-minutes-hours) on the degree of light inhibition (Way and Yamori 2014). In Bernacchi et al. (2001), R light of tobacco exhibited an activation energy (46.39 kJ mol−1) which is similar to that often reported for R dark; others have also reported little difference in the short-term temperature dependence of R light and R dark (Shapiro et al. 2004; Griffin and Turnbull 2013; McLaughlin et al. 2014; Way et al. 2015). By contrast, a number of different studies have reported the degree of light inhibition of leaf respiration to increase with rising leaf temperature (Harley et al. 1992; Atkin et al. 2000a; Loreto et al. 2001; Bruhn 2002; Pons and Welschen 2003; Atkin et al. 2006; Zaragoza-Castells et al. 2007), and decrease with increasing temperature in one study (Way and Sage 2008). Given the conflicting nature of these reports, and the importance of the temperature response of leaf respiration for TBM predictions (Huntingford et al. 2013), further work is needed to determine whether there are systematic patterns (across plant taxa and environments) in the effect of leaf temperature on the degree of light inhibition of respiration.
2.5 Whole-Plant Maintenance Respiration – Accounting for Soil Moisture
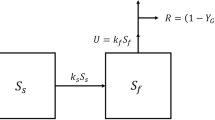
A range of approaches are used in TBMs to estimate rates of whole-plant maintenance respiration (R pm), with leaf R d of upper-canopy leaves forming the starting point of these calculations in most TBMs (Table 6.1). Here, we focus on scaling up from leaves to whole-plants in JULES – in that model, upper-canopy leaf R d is used to predict equivalent rates of maintenance respiration in whole canopies (R dc) via multiplication of leaf R d by parameters that account for light attenuation through the canopy . Thereafter, canopy -level R dc is used to calculate whole-plant R pm according to the following equation (Cox 2001):
where N l, N s and N r are the N contents of leaves, stems (sapwood ) and roots, and the factor of 0.012 is a unit conversion term. Hence, in JULES, whole-plant R pm is calculated on the basis of nitrogen partitioning in above and below-ground organs, with scaling between respiration and tissue N being assumed to be constant, an assumption that is challenged by more recent studies (Reich et al. 2008). Canopy-level R dc is adjusted to account for low soil moisture contents via multiplication by a moisture stress factor (β).
In Equation (6.6), the moisture stress factor (β) is a function of soil moisture content (θ) in the rootzone, taking a value of zero if θ is below wilting point (θ w ), a value of unity if θ is greater than critical moisture concentration (θ c ), and having a linear decrease between θ c and θ w (Cox et al. 1998). Variable θ c is a value below which physiological performance of plants is reduced, and so the ‘β’ approach similarly can be modeled as impacting on photosynthesis during drought periods. This approach, which was suggested by Cox et al. (1998), therefore assumes that drought reduces canopy -level R dc by the same proportion as whole-canopy net photosynthesis (A) in all plant species; that is, the R dc/A ratio remains identical in well-watered and moisture stressed plants. However, a growing body of empirical data shows that R d/A ratios increase markedly under drought (Flexas et al. 2006; Atkin and Macherel 2009; Ayub et al. 2011; Crous et al. 2011; Rodríguez-Calcerrada et al. 2011), reflecting the greater sensitivity of photosynthesis than R d to drought. Incorrectly accounting for drought-mediated changes in canopy-level R dc and/or A is likely to result in large errors in rates of predicted net ecosystem CO2 exchange (Flexas et al. 2006), and so a new ‘β’ but specific for respiration will be considered for future JULES model versions. This may be expected to be particularly important for low productivity ecosystems where leaf R d represents a large proportion of overall carbon exchange (Zaragoza-Castells et al. 2008). It is important, therefore, that ecosystem gas exchange models be developed to the state where drought-mediated changes in R dc are taken into account when simulating ecosystem carbon fluxes and when interfaced with TBMs to predict the impacts of global climate change on carbon exchange in terrestrial ecosystems (Ryan 2002). The impact of drought on temperature responses of leaf R d also needs to be considered, with recent literature pointing to drought accentuating downward adjustments (i.e. acclimation ) of leaf R d in response to rising temperatures in summer (Rodríguez-Calcerrada et al. 2010; Crous et al. 2011).
2.6 Estimating Whole-Plant Respiration – Importance of Growth Respiration
In models that use the ‘growth-and- maintenance -respiration paradigm’ (GMRP) (Amthor 2000) to estimate rates of whole-plant respiration (R p), rates of growth respiration in whole plants (R pg) need to be estimated. In most of the models listed in Table 6.1, R pg is calculated on the basis that the energy costs of growth are a fixed fraction of GPP (e.g. BIOME-BGC & Century), or a fixed fraction of GPP minus R pm (e.g. BETHY, BIOME3, JULES, LPJ, ORCHIDEE and TEM). For the latter, 0.20–0.25 of GPP-R pm (herein termed the ‘growth respiration coefficient’ – GRC ESM) is assumed to represent R pg for all plant functional types and biomes , according to the following equation:
In this section, we discuss the origins of the fixed GRC ESM values used in some leading TBMs .
According to the GMRP, the rate of respiration in whole plants is the sum of R pm and R pg, according to:
where W is the dry mass of mature tissues, m R is the maintenance respiration coefficient (i.e. respiratory CO2 associated with maintenance of existing biomass per unit time, with units of mol CO2 (g biomass)−1 s−1), G is the growth rate (e.g. g new biomass time−1), and g R is the respiratory CO2 released per unit biomass produced by growth. Thus, R pg can be viewed as linked not only to G, but also to the efficiency of biosynthetic pathways, reflected in the variable of central interest, g R. This parameter is central to calculating R pg as part of the GMRP. Using a biosynthesis ‘pathway analysis method’ to analyze growth costs in several crop species, Penning de Vries et al. (1983) reported an overall average g R value of 0.33; in such cases, 33% of the carbon retained in growth is released to the atmosphere by respiration in the production of that growth. Importantly, there was greater than three-fold variation in g R [0.13 in carbohydrate -rich tubers to 0.43 in lipid-rich tissues (Penning de Vries et al. 1983)]. Variation in g R could have profound implications for estimates of R pg of individual organs and whole-plants.
The GMRP can also be viewed from a growth (G) rate perspective, according to:
where A is the rate of gross photosynthesis with the same units as respiration above, and Y g is the yield of growth processes (i.e. fraction of substrate inputs retained in the products of growth). For example, if Y g = 0.8, then for every 100 units of C fixed by photosynthesis, 80 units are retained in the resultant biomass while 20 units are released back to the atmosphere by respiration. In Penning de Vries et al. (1983), Y g varied from 0.70 in lipid-rich palm nuts to 0.89 in starch -rich tubers, with an average value of 0.75 (Penning de Vries et al. 1983; Thornley and Cannell 2000). That is, 70–89% of the carbon in substrates was retained in tissue biomass (Amthor 2000), with the variability in this parameter further suggesting that no single growth efficiency parameter can be applied universally across plant tissues, organs and/or species. Yet, inspection of Table 6.1 shows that several TBMs assume that respiratory costs associated with growth are indeed constant, both across plant functional types (PFTs) and biomes .
In JULES and LPJ, the ‘growth respiration coefficient’ (GRC ESM) used to calculate R pg is a single value (i.e. 0.25). To understand the origins of this fixed GRC ESM, it is necessary to show the inverse relationship between Y g and g R, where increases in the CO2 requirements associated with biosynthesis (g R) reduce the growth yield (Y g), according to (Thornley 1970; Amthor 2000):
Similarly, increases in the growth yield are associated with a lower g R according to, re-writing equation (6.1) as:
When combined with information on rates of GPP and R pm, variation in Y g and g R influence rates of R pg according to:
If one assumes that all species exhibit g R and Y g values of 0.33 and 0.75, respectively, then the GRC ESM used to calculate R pg in equation (6.8) will be 0.25 (i.e. GRC ESM = 0.33 × 0.75). Thus, the ‘universal’ GRC ESM (i.e. 0.25) used in JULES and LPJ turns to be:
In JULES and LPJ, GRC ESM is based on the average g R and Y g values of several crop species (Penning de Vries et al. 1983; Thornley and Cannell 2000), rather than a comprehensive assessment of GRC ESM values across PFTs and/or biomes; here, we note that the reliance on crop species data was necessary because there were no wider surveys of GRC ESM at the time JULES and LPJ were developed. In any case, assuming a fixed GRC ESM of 0.25 gives undue weight to high-lipid tissues (Penning de Vries et al. 1983). While high lipid concentrations are common in seeds of some species, leaves, stems and roots of plants growing in natural ecosystems are more likely to be dominated by carbohydrates , and therefore exhibit lower GRC ESM [e.g. akin to a GRC ESM = 0.13 × 0.89 = 0.12 of carbohydrate-rich tubers (Penning de Vries et al. 1983)]. Given this, relying on average crop-derived g R and Y g values (Penning de Vries et al. 1983) to parameterize the land surface component of TBMs may result in an over-estimation of global R pg.
To illustrate the potential impact of different GRC ESM values, we present the following example where the global average GRC ESM is closer to 0.12 rather than 0.25, reflecting carbohydrate -rich leaves rather than lipid-rich seeds (Penning de Vries et al. 1983; Amthor 2000). If we assume that global GPP − R pm = 50 Gt C yr−1, then assuming a GRC ESM of 0.12 would mean that global R pg is ~6.0 Gt C yr−1 as opposed to ~12.5 Gt C yr−1 when GRC ESM = 0.25. Thus, application of GRC ESM values across the range reported by (Penning de Vries et al. 1983) could result in a change in predicted R pg that is of similar magnitude to annual anthropogenic C emissions (IPCC 2013). Given the wide range of R pg values and the magnitude of the resultant C-fluxes, it is not surprising that parameterization of R pg represents one of the greatest single uncertainties in TBM model predictions (Dietze et al. 2014). Quantifying variation in GRC ESM thus represents a challenge that needs to be urgently addressed.
There are a number of reasons to suspect that the GRC ESM might not be static across genotypes & environments. Firstly, given that tissue chemical compositions vary among environments (Dahlin et al. 2013; Asner et al. 2014; Niinemets et al. 2015) and species/PFTs (Poorter and Bergkotte 1992; Van Arendonk and Poorter 1994; Cornelissen et al. 1997; Wright et al. 2004), and that the energy costs of building tissues of contrasting chemical composition are variable (Nagel et al. 2002; Poorter et al. 2006; Villar et al. 2006; Díaz et al. 2016), it seems unlikely that GRC ESM will be invariant. Secondly, factors such as developmental and/or environment-mediated increases in the engagement of non-phosphorylating pathways of mitochondrial electron transport (e.g. alternative oxidase , rotenone-insensitive NADH dehydrogenase, external NAD(P)H dehydrogenase ), proton leakage through the inner mitochondrial membrane and proton flux via uncoupling proteins (PUMP) could all reduce the efficiency of ATP synthesis (Rasmusson et al. 2004; Sweetlove et al. 2006; Armstrong et al. 2008; Millar et al. 2011; Searle et al. 2011a; Kornfeld et al. 2012), which in turn could increase g R and decrease Y g, thus altering GRC ESM . Given these issues, it seems extremely unlikely that GRC ESM is universally constant amongst species and environments. Only by understanding how GRC ESM values – and thus g R and Y g – vary among PFTs and biomes can we predict the dynamic changes in growth respiration of terrestrial ecosystems across the Earth’s surface, and in doing so, predict future changes in the land carbon sink and future biogeography of land plants.
3 Global Surveys of Leaf Respiration and Its Temperature Dependence
In earlier sections, we outlined how a range of TBMs represent variation in leaf respiration rates (Table 6.1) using previously reported relationships between respiration, photosynthesis and/or leaf nitrogen concentrations. Importantly, each TBM has had to rely on a relatively small data-set linking respiration to other traits (Farquhar et al. 1980; Collatz et al. 1991, 1992; Ryan 1991; Knorr 1997). Such data-sets have been used to predict variation in leaf R d at a standard temperature (typically 20 or 25 °C). In the absence of global analyses of the temperature dependence of leaf R d, assumptions have also been made about how to model rates of leaf R d as leaves cool and warm over both short timescales (i.e. near-instantaneous variation in imposed micrometeorology, and over long periods which could include acclimation to anthropogenic-induced global warming). To address these deficiencies, two global databases are now available that provide equations that predict global variability in baseline values of leaf R d [the ‘GlobResp’ database of R d at 25°C and associated leaf traits (Atkin et al. 2015), that brings together new and previously reported data (Wright et al. 2006 and references cited therein)] and the short-term temperature dependence of leaf R d (Heskel et al. 2016b). In this section, we describe each data-set, and outline how those datasets can be incorporated into TBMs .
3.1 Global Dataset of Baseline Leaf Respiration Rates – ‘GlobResp’
‘GlobResp’ contains data on upper canopy leaf respiration and associated leaf traits (e.g. photosynthetic capacity, area:mass relationships, nitrogen/phosphorus concentrations) on 899 species from 100 sites distributed around the globe (arctic to equatorial tropics); it also provides climate information (Hijmans et al. 2005) for each site. Several woody and non-woody PFTs are represented in the dataset, with those PFTs covering a majority of the standard vegetation types used within TBMs (e.g. C3 grasses/herbs, shrubs, broad-leaf trees and needle-leaf trees). Both deciduous and evergreen vegetation types are represented within the dataset. Importantly, however, ‘GlobResp’ does not contain data on R d of C4 grasses. Analysis of ‘GlobResp’ area-based rates of leaf R d (at a standard temperature of 25 °C) revealed on average, three-fold higher rates of baseline respiration in the Arctic than the tropics (Fig. 6.4; Atkin et al. 2015), suggesting an acclimation effect. As a result of these global patterns in baseline R d (Fig. 6.4), rates measured at the prevailing average daily growth temperature of each site were only two-fold higher in the hot tropics than the cold Arctic, despite a 20 °C difference in growth temperature (8–28 °C). Subsequent analysis by Vanderwel et al. (2015) has shown that the ‘GlobResp’ patterns of leaf R d are consistent with thermal acclimation responses, whereby cold-grown plants exhibit higher rates of leaf respiration at a standard temperature than their warm grown counterparts (Atkin and Tjoelker 2003; Kruse et al. 2011; Slot and Kitajima 2015; Reich et al. 2016). Importantly, when measured at the standard temperature of 25 °C, species and PFTs at the cold sites exhibited higher R d at a given photosynthetic capacity (V cmax) or leaf [N]) than species from warmer sites. PFTs also differed in rates of R d at a given V cmax or leaf [N], being higher in C3 herbs than woody plants.
Latitudinal variation in baseline leaf respiration measured in darkness, for area-based rates of leaf R d normalized to a standard temperature of 25 °C. Data points are for individual measurements, with site:species means of the same dataset being reported in ‘GlobResp’ (Atkin et al. 2015)
Using mixed-effects statistical models, Atkin et al. (2015) provided a set of PFT -specific equations that predicted mean rates of R d across the globe. The equations captured a substantial amount of species variation across diverse sites, with the equations offering a reasonable first approximation for the purposes of modeling . Noting that TBMs often predict variation in leaf R d via assumed relationships to leaf N (either directly, or via assumed leaf N-V cmax-R d relationships; Table 6.1), equations are available that link leaf R d with leaf [N]. In a PFT-dependent matter, area-based rates of leaf R d at 25°C were found to vary with area-based values of leaf N concentration (n l , a with units gN (m2 leaf)−1). Moreover, leaf R d at 25 °C varied with the prevailing growth temperature (T G ), in a consistent manner for all PFTs. The availability of equations for each PFT [Table 6.2 – assembled using ESM#3 in Table S4 of Atkin et al. (2015)] creates the opportunity to alter representation of leaf R d at 25 °C from that assumed in the standard version of JULES to one which uses current, globally-relevant estimates of leaf R d at 25 °C derived from ‘GlobResp’. Thus, the general form of the above equations is:
Using equation (6.14) not only changes the scaling between leaf R d at 25 °C and leaf [N] from that currently assumed in standard runs of JULES, but also results in leaf R d at 25 °C changing in response to changes in T G , with rates of leaf R d at 25 °C decreasing by ~0.4 μmol CO2 m−2 s−1 for every temporal 10 °C rise in T G . That is, leaf R d at 25 °C is allowed to acclimate to sustained changes in T G at each site. This approach assumes that the global spatial patterns in leaf R d at 25 °C are consistent with temporally-based adjustments in leaf R d at 25 °C (i.e. acclimation ). There is now strong support for this assumption (Slot and Kitajima 2015; Vanderwel et al. 2015). Importantly, any TBM runs made using equation (6.14) do not allow for adaptive changes in the baseline rate of respiration as the world warms over coming decades. Current data points to little adaptive difference in short-term temperature responses of respiration (Heskel et al. 2016b), while the work of Slot and Kitajima (2015) suggests that the ability to acclimate does not differ among biomes or plant functional types . Thus, assuming no adaptive changes in the temperature dependence (short or long term) is supported by available data. Of course, if PFT representation at any site changes in a future, warmer world, the basal rates of respiration could be altered at such sites, reflecting the PFT-dependent nature of leaf R d at 25 °C (Atkin et al. 2015).
Fig. 6.5 illustrates the consequences of shifting from current PFT -specific estimates of leaf R d at 25 °C in JULES (Cox et al. 1998; Cox 2001; Clark et al. 2011) to PFT-specific rates predicted from analysis of the ‘GlobResp’ dataset (Atkin et al. 2015). In the original version of JULES, rates of leaf R d at 25 °C were assumed to remain constant irrespective of T G , but vary among PFTs based on reported R d–V cmax-[N] relationships (Farquhar et al. 1980; Collatz et al. 1991; Schulze et al. 1994) and PFT-specific leaf [N]. By contrast, leaf R d at 25 °C varies with T G when applying equations from ‘GlobResp’ (Atkin et al. 2015), with rates normalised to 25 °C being greater in cold than warm habitats. Importantly, replacement of the existing JULES parameterization with ‘GlobResp’ (Atkin et al. 2015) results in marked increases in predicted leaf R d at 25 °C for all of the four PFTs for which data are available (C3 grasses, shrubs, broad-leaf trees and needle-leaf trees). As a result, model predictions of global leaf R d are likely to be much higher when using ‘GlobResp’. Assessing the consequences of this for predicted global net primary productivity will be an important stimulus for developing the next generation of TBMs .
Consequences of shifting from current estimates of leaf R d at 25 °C in JULES (Cox et al. 1998; Cox 2001; Clark et al. 2011) to rates predicted from analysis of the ‘GlobResp’ dataset (Atkin et al. 2015). Shown are values for four plant functional types for which data are available in ‘GlobResp’. In the original version of JULES, rates of leaf R d at 25 °C were assumed to remain constant irrespective of growth temperature, but vary among PFTs based on reported R d–V cmax-[N] relationships (Farquhar et al. 1980; Collatz et al. 1991; Schulze et al. 1994) and PFT -specific leaf [N]. By contrast, leaf R d at 25 °C varies with growth temperature when applying equations from ‘GlobResp’ (Atkin et al. 2015), with rates at 25 °C being greater in cold than warm habitats. See equation (6.14) for details of how the new leaf R d at 25 °C is calculated within each PFT
3.2 Convergence in Temperature Response Curves of Leaf Respiration
As noted earlier, how leaf R d responds to short-term variations in temperature will be crucial for TBM predictions, reflecting the importance of temperature-mediated changes in respiratory CO2 efflux in determining future carbon storage in vegetation and atmospheric CO2 concentrations (King et al. 2006; Atkin et al. 2008; Huntingford et al. 2013; Wythers et al. 2013; Lombardozzi et al. 2015; Heskel et al. 2016b). In several TBMs (Table 6.1), leaf R d is assumed to increase with rising temperature such that respiration doubles for each 10 °C increase in temperature (i.e. Q 10 = 2.0). However, as indicated above, the true Q 10 is rarely a fixed value. Instead, the temperature coefficient of leaf R d decreases as leaves warm. Reductions in the Q 10 with increasing leaf temperature have been linked to substrate and/or adenylate limitations at high measuring temperatures (Atkin and Tjoelker 2003).
It is thus important that TBMs be able to model the dynamic nature of the temperature response of leaf R d. Acknowledging this, alternative models have been developed that allow for declines in the temperature sensitivity of leaf respiration as leaves warm. These model variants adopt modified Arrhenius formulations (Lloyd and Taylor 1994; Kruse and Adams 2008; Zaragoza-Castells et al. 2008; Noguchi et al. 2015), universal temperature dependence (UTD) (UTD; Gillooly et al. 2001) and temperature-dependent Q 10 functions (Tjoelker et al. 2001). Recently, high-resolution measurement of the temperature response of leaf R d has enabled comparison of different model types, with three-parameter formulations (Kruse and Adams 2008; O’Sullivan et al. 2013; Adams et al. 2016; Heskel et al. 2016a, b) providing superior fits. Using this approach, Heskel et al. (2016b) analyzed 673 temperature responses of leaf R d from 231 species across 18 globally distributed sites spanning 7 biomes that ranged from Arctic tundra to tropical rainforests. Their analysis confirmed that leaf R d does not exhibit an exponential response to temperature. Tjoelker et al. (2001) reported similar findings, albeit using a smaller (minimal data from tropical ecosystems), lower resolution data set. Importantly, Heskel et al. (2016b) found convergence in the short-term temperature response of leaf R d across biomes and PFTs , suggesting that a single empirical model can be used to predict the short-term temperature dependence of leaf R d for global vegetation . The best such model describes this temperature dependence as log-polynomial rather than log-linear, with the Q 10 continuously declining with increasing leaf temperature in a manner distinct from earlier observations (Tjoelker et al. 2001) and models (Lloyd and Taylor 1994; Gillooly et al. 2001), but similar to that reported by Kruse and Adams (2008). Discussion of the merits of the three-component models of Heskel et al. (2016b) and Kruse and Adams (2008) can be found in recent reports (Adams et al. 2016; Heskel et al. 2016a).
Heskel et al. (2016b) used a derivation of their global log-polynomial model (GPM) to predict values of leaf R d at any given ambient temperature (T a), according to:
where R d , 25 is the rate of leaf R d at a standard temperature of 25 °C, ‘b’ is the slope of log R d versus temperature curves at 0°C, and ‘c’ describes how the slope of log R d versus temperature curves declines with increasing temperature. Heskel et al. (2016b) found there were no significant differences in ‘b’ or ‘c’ parameters among biomes or PFTs, suggesting that temperature response curves of global vegetation can be modeled using a single polynomial function, where b = 0.1012 and c = –0.0005. The convergence in the temperature sensitivity of leaf R d suggests that there are universally applicable controls on the temperature response of leaf respiratory metabolism across the globe (Heskel et al. 2016b).
Fig. 6.6 shows how replacing a fixed Q 10 = 2.0 with the GPM (Heskel et al. 2016b) impacts on predicted rates of leaf R d at any given temperature, using rates of leaf R d at 25 °C as the reference point. Adopting the GPM results in reduced estimates of leaf R d at low temperatures, with little change in rates at temperatures >25 °C. The consequences of shifting from a fixed Q 10 to the GPM results in 28% lowering of predicted daily respiration in cold sites, such as those in the Arctic (Heskel et al. 2016b). Such changes are likely to impact predicted net primary productivity values of colder sites.
Comparison of theoretical temperature responses curves of leaf respiration in darkness, calculated assuming rates of leaf R d at 25 °C of 1.0 μmol CO2 m−2 s−1, with rates at other leaf temperatures predicted assuming a fixed Q 10 (i.e. proportional change in leaf R d per 10 °C change in temperature) value of 2.0 (i.e. a common Q 10 assumed in TBMs ; Table 6.1), and assuming that respiration varies with temperature according to that predicted by the global polynomial model (GPM; Heskel et al. 2016b). The structure of the GPM is similar that of the 3-component model approaches based on Arrhenius theory (Kruse and Adams 2008; Kruse et al. 2016). Adopting the GPM results in reduced estimates of leaf R d at low temperatures, with little change in rates a temperatures >25 °C
3.3 Merging ‘GlobResp’ with the Global Polynomial Model
By merging equations derived from ‘GlobResp’ (Atkin et al. 2015) with those of the GPM (Heskel et al. 2016b), we suggest that TBMs will be able to predict variations in R d in upper canopy leaves, taking into account: (i) PFT -specific baseline respiration rates at 25 °C; (ii) nitrogen-dependent variations in baseline respiration rates at 25 °C; (iii) growth-temperature variations in baseline respiration rates at 25 °C (i.e. thermal acclimation ); (iv) responses of leaf R d to short-term (e.g. diurnal) changes in temperature. An example of this integrative approach is shown in Fig. 6.7, where equations (6.14) and (6.15) are combined to model the temperature response of leaf R d to short-term changes in temperature, for plants acclimated to growth temperatures of 15, 25 and 35 °C. Also shown is the temperature response curve of leaf R d in the standard form of JULES, assuming no thermal acclimation and a fixed Q 10 of 2.0 (Cox et al. 1998; Cox 2001; Clark et al. 2011). Adopting the ‘GlobResp’ approach allows for acclimation -dependent changes in baseline values of leaf R d, resulting in higher rates at any given leaf temperature in cold-grown plants compared to their warm-grown counterparts. Importantly, ‘GlobResp’ predicts much higher rates at any given temperature than was previously assumed in the standard form of JULES, particularly in cold habitats. When incorporated into TBMs , this is likely to result in significant increases in leaf R d and overall plant R (R p) and reduced net primary productivity (NPP) at regional and global scales, depending on how future iterations of TBMs will model gross primary productivity (GPP ), noting that NPP = GPP− R p.
Integration of equations emerging from the ‘GlobResp’ dataset (Atkin et al. 2015) and Global Polynomial Model (GPM) from Heskel et al. (2016b) to predict the shape of the temperature response curve of leaf R d for broad-leaf trees, acclimated to three growth temperatures (T a; 15, 25 and 35 °C). Also shown is the temperature response curve of leaf R d for broad-leaf trees in the standard form of JULES, assuming no thermal acclimation and a fixed Q 10 of 2.0 (Cox et al. 1998; Cox 2001; Clark et al. 2011). See equations (6.14) and (6.15) in the main text for descriptions of the components in the integrated equation shown above, and Table 6.2 for parameter constants for broad-leaf trees
4 Conclusions
Research into leaf R d – both in terms of describing variability in rates among genotypes and environments – has advanced markedly over the past two decades, as manifested by a growing inventory of data that is providing new insights into how rates of leaf R d vary. There are also advances being made to clarify individual and collective mechanistic controls of respiration (through models and experiments). Armed with these advances, the TBM community can now more accurately predict spatial and temporal variations in leaf respiratory CO2 release across the globe under current climatic conditions. Yet, we are not ‘there’ yet, since the research community continues to lack a process-based model to account for the complexity of taxa- and environment-driven variations in leaf R d, thereby limiting the ability of TBMs to predict the impacts of future climate regimes. Ideally, a truly mechanistic approach will emerge in the future that meets the TBM integration requirements of being parsimonious, scalable and spatially robust. Mechanistic models can be expected to have better predictive capability compared to those that are heavily parameterized, and that is important when trying to assess how the global carbon cycle will evolve within a climatic system perturbed through human burning of fossil fuels. Achieving a more mechanistically complete description will be a major challenge, requiring new insights into the processes regulating energy metabolism of leaves, both during the day and night, in terrestrial ecosystems across the globe. We believe this is a challenge worth addressing, and especially if such models can be made applicable across spatial scales and thus appropriate for implementation in climate simulations, thereby characterizing leaf respiratory metabolism changes in the global carbon cycling.
References
Adams MA, Rennenberg H, Kruse J (2016) Different models provide equivalent predictive power for cross-biome response of leaf respiration to temperature. Proc Natl Acad Sci USA 113:E5993–E5995
Affourtit C, Krab K, Moore AL (2001) Control of plant mitochondrial respiration. Biochim Biophys Acta – Bioenerg 1504:58–69
Ali AA, Xu C, Rogers A, McDowell NG, Medlyn BE, Fisher RA et al (2015) Global-scale environmental control of plant photosynthetic capacity. Ecol Appl 25:2349–2365
Amthor JS (1989) Respiration and crop productivity. Springer, New York
Amthor JS (2000) The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot 86:1–20
Amthor JS, Baldocchi DD (2001) Terrestrial higher plant respiration and net primary production. In: Roy J, Saugier B, Mooney HA (eds) Terrestrial global productivity, physiological ecology. Academic, San Diego, pp 33–59
Armstrong AF, Logan DC, Atkin OK (2006) On the developmental dependence of leaf respiration: responses to short- and long-term changes in growth temperature. Am J Bot 93:1633–1639
Armstrong AF, Badger MR, Day DA, Barthet MM, Smith PMC, Millar AH, Whelan J, Atkin OK (2008) Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration. Plant Cell Environ 31:1156–1169
Asner GP, Martin RE, Tupayachi R, Anderson CB, Sinca F, Carranza-Jiménez L, Martinez P (2014) Amazonian functional diversity from forest canopy chemical assembly. Proc Natl Acad Sci USA 111:5604–5609
Atkin OK, Macherel D (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot 103:581–597
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Atkin OK, Westbeek MHM, Cambridge ML, Lambers H, Pons TL (1997) Leaf respiration in light and darkness. A comparison of slow- and fast-growing Poa species. Plant Physiol 113:961–965
Atkin OK, Evans JR, Ball MC, Lambers H, Pons TL (2000a) Leaf respiration of snow gum in the light and dark. Interactions between temperature and irradiance. Plant Physiol 122:915–923
Atkin OK, Holly C, Ball MC (2000b) Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant Cell Environ 23:15–26
Atkin OK, Bruhn D, Hurry VM, Tjoelker MG (2005) The hot and the cold: unraveling the variable response of plant respiration to temperature. Funct Plant Biol 32:87–105
Atkin OK, Scheurwater I, Pons TL (2006) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol 12:500–515
Atkin OK, Scheurwater I, Pons TL (2007) Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol 174:367–380
Atkin OK, Atkinson LJ, Fisher RA, Campbell CD, Zaragoza-Castells J, Pitchford J, Woodward FI, Hurry V (2008) Using temperature-dependent changes in leaf scaling relationships to quantitatively account for thermal acclimation of respiration in a coupled global climate-vegetation model. Global Change Biol 14:2709–2726
Atkin OK, Turnbull MH, Zaragoza-Castells J, Fyllas NM, Lloyd J, Meir P, Griffin KL (2013) Light inhibition of leaf respiration as soil fertility declines along a post-glacial chronosequence in New Zealand: an analysis using the Kok method. Plant Soil 367:163–182
Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D et al (2015) Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol 206:614–636
Ayub G, Smith RA, Tissue DT, Atkin OK (2011) Impacts of drought on leaf respiration in darkness and light in Eucalyptus saligna exposed to industrial-age atmospheric CO2 and growth temperature. New Phytol 190:1003–1018
Ayub G, Zaragoza-Castells J, Griffin KL, Atkin OK (2014) Leaf respiration in darkness and in the light under pre-industrial, current and elevated atmospheric CO2 concentrations. Plant Sci 226:120–130
Azcón-Bieto J, Lambers H, Day DA (1983) Respiratory properties of developing bean and pea leaves. Aust J Plant Physiol 10:237–245
Bahar NH, Ishida FY, Weerasinghe LK, Guerrieri R, O’Sullivan OS, Bloomfield KJ et al (2017) Leaf-level photosynthetic capacity in lowland Amazonian and high-elevation Andean tropical moist forests of Peru. New Phytol 214:1002–1018
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco- limited photosynthesis. Plant Cell Environ 24:253–259
Berry JA, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Ann Rev Plant Physiol 31:491–543
Bolstad PV, Reich P, Lee T (2003) Rapid temperature acclimation of leaf respiration rates in Quercus alba and Quercus rubra. Tree Physiol 23:969–976
Bonan GB, Levis S, Sitch S, Vertenstein M, Oleson KW (2003) A dynamic global vegetation model for use with climate models: concepts and description of simulated vegetation dynamics. Glob Change Biol 9:1543–1566
Booth BBB, Jones CD, Collins M, Totterdell IJ, Cox PM, Sitch S et al (2012) High sensitivity of future global warming to land carbon cycle processes. Environ Res Lett 7:024002
Bouma T (2005) Understanding plant respiration: separating respiratory components versus a process-based approach. In: Lambers H, Ribas-Carbó M (eds) Plant respiration from cell to ecosystem. Springer, Dordrecht, pp 177–194
Bouma TJ, Devisser R, Janssen JHJA, Dekock MJ, Vanleeuwen PH, Lambers H (1994) Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to assess its effect. Physiol Plant 92:585–594
Bouma TJ, De VR, Van LPH, De KMJ, Lambers H (1995) The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to leaf dark respiration. J Exp Bot 46:1185–1194
Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-biphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas exchange measurements on spinach. Planta 165:397–406
Bruhn D (2002) Plant respiration and climate change effects. University of Copenhagen, Copenhagen
Bruhn D, Mikkelsen TN, Herbst M, Kutsch WL, Ball MC, Pilegaard K (2011) Estimating daytime ecosystem respiration from eddy-flux data. Biosystems 103:309–313
Buckley TN, Adams MA (2011) An analytical model of non-photorespiratory CO2 release in the light and dark in leaves of C3 species based on stoichiometric flux balance. Plant Cell Environ 34:89–112
Budde RJA, Randall DD (1990) Pea leaf mitochondrial pyruvate dehydrogenase complex is inactivated in vivo in a light-dependent manner. Proc Natl Acad Sci USA 87:673–676
Canadell JG, Le Quere C, Raupach MR, Field CB, Buitenhuis ET, Ciais P et al (2007) Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl Acad Sci USA 104:18866–18870
Chung H-H, Barnes RL (1977) Photosynthate allocation in Pinus taeda. I. Substrate requirements for synthesis of shoot biomass. Can J For Res 7:106–111
Clark DB, Mercado LM, Sitch S, Jones CD, Gedney N, Best MJ et al (2011) The Joint UK Land Environment Simulator (JULES), model description – Part 2: Carbon fluxes and vegetation dynamics. Geosci Mod Dev 4:701–722
Collatz GJ, Ball JT, Grivet C, Berry JA (1991) Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiratio: a model that includes a laminar boundary layer. Agric For Met 54:107–136
Collatz GJ, Ball JT, Grivet C, Berry JA (1992) Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Agric For Met 54:107–136
Cornelissen JHC, Werger MJA, Castrodiez P, Van Rheenen JWA, Rowland AP (1997) Foliar nutrients in relation to growth, allocation and leaf traits in seedlings of a wide range of woody plant species and types. Oecologia 111:460–469
Cox P (2001) Description of the “TRIFFID” dynamic global vegetation model. Hadley Centre, Met Office, Bracknell
Cox PM, Huntingford C, Harding RJ (1998) A canopy conductance and photosynthesis model for use in a GCM land surface scheme. J Hydrol 212:79–94
Cox PM, Betts RA, Bunton CB, Essery RLH, Rowntree PR, Smith J (1999) The impact of new land surface physics on the GCM simulation of climate and climate sensitivity. Clim Dynam 15:183–203
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Criddle RS, Hopkin MS, McArthur ED, Hansen LD (1994) Plant distribution and the temperature coefficient of metabolism. Plant Cell Environ 17:233–243
Crous KY, Zaragoza-Castells J, Low M, Ellsworth DS, Tissue DT, Tjoelker MG et al (2011) Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: impacts of elevated atmospheric CO2 and summer drought. Glob Change Biol 17:1560–1576
Crous KY, Zaragoza-Castells J, Ellsworth DS, Duursma RA, Low M, Tissue DT, Atkin OK (2012) Light inhibition of leaf respiration in field-grown Eucalyptus saligna in whole-tree chambers under elevated atmospheric CO2 and summer drought. Plant Cell Environ 35:966–981
Dahlin KM, Asner GP, Field CB (2013) Environmental and community controls on plant canopy chemistry in a Mediterranean-type ecosystem. Proc Natl Acad Sci USA 110:6895–6900
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE et al (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York, pp 499–587
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S et al (2016) The global spectrum of plant form and function. Nature 529:167–171
Dietze MC, Serbin SP, Davidson C, Desai AR, Feng XH, Kelly R et al (2014) A quantitative assessment of a terrestrial biosphere model’s data needs across North American biomes. J Geophys Res Biogeosci 119:286–300
Domingues TF, Meir P, Feldpausch TR, Saiz G, Veenendaal EM, Schrodt F et al (2010) Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ 33:959–980
Drake JE, Tjoelker MG, Aspinwall MJ, Reich PB, Barton CVM, Medlyn BE, Duursma RA (2016) Does physiological acclimation to climate warming stabilize the ratio of canopy respiration to photosynthesis? New Phytol 211:850–863
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evans JR, Schortemeyer M, McFarlane N, Atkin OK (2000) Photosynthetic characteristics of 10 Acacia species grown under ambient and elevated atmospheric CO2. Aust J Plant Physiol 27:13–25
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Field CB, Mooney HA (1986) The photosynthetic-nitrogen relationship in wild plants. In: Givnish T (ed) On the economy of form and function. Cambridge University Press, Cambridge, pp 22–55
Fisher JB, Huntzinger DN, Schwalm CR, Sitch S (2014) Modeling the terrestrial biosphere. Ann Rev Env Res 39:91–123
Flexas J, Galmés J, Ribas-Carbó M, Medrano H, Lambers H (2005) The effects of water stress on plant respiration. In: Govindjee (ed) Volume 18 plant respiration: from cell to ecosystem. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 85–94
Flexas J, Bota J, Galmes J, Medrano H, Ribas-Carbo M (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plant 127:343–352
Forward DF (1960) Effect of temperature on respiration. In: Ruhland W (ed) Encyclopedia of plant physiology, vol 12. Springer, Berlin, pp 234–258
Fredeen AL, Field CB (1991) Leaf respiration in Piper species native to a Mexican rainforest. Physiol Plant 82:85–92
Fukai S, Silsbury JH (1977) Responses of subterranean clover communities to temperature. II. Effects of temperature on dark respiration rate. Aust J Plant Physiol 4:159–167
Gauthier PPG, Bligny R, Gout E, Mahe A, Nogues S, Hodges M, Tcherkez GGB (2010) In folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current CO2 assimilation in illuminated leaves of Brassica napus. New Phytol 185:988–999
Gemel J, Randall DD (1992) Light regulation of leaf mitochondrial pyruvate dehydrogenase complex. Role of photorespiratory carbon metabolism. Plant Physiol 100:908–914
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171–186
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Gonzelez-Meler MA, Taneva L, Trueman RJ (2004) Plant respiration and elevated atmospheric CO2 concentration: Cellular responses and global significance. Ann Bot 94:647–656
Griffin KL, Turnbull MH (2013) Light saturated RuBP oxygenation by Rubisco is a robust predictor of light inhibition of respiration in Triticum aestivum L. Plant Biol 1:1438–8677
Hachiya TAKU, Terashima ICHI, Noguchi KO (2007) Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia x hybrida petals. Plant Cell Environ 30:1269–1283
Harley PC, Thomas RB, Reynolds J (1992) Modeling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282
Harper AB, Cox PM, Friedlingstein P, Wiltshire AJ, Jones CD, Sitch S et al (2016) Improved representation of plant functional types and physiology in the Joint UK Land Environment Simulator (JULES v4.2) using plant trait information. Geosci Model Dev 9:2415–2440
Harrison MT, Edwards EJ, Farquhar GD, Nicotra AB, Evans JR (2009) Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ 32:259–270
Haxeltine A, Prentice IC (1996a) BIOME3: an equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant functional types. Glob Biogeochem Cycles 10:693–709
Haxeltine A, Prentice IC (1996b) A general model for the light-use efficiency of primary production. Funct Ecol 10:551–561
Hay RKM, Walker AJ (1989) An introduction to the physiology of crop yield. Longman Scientific and Technical, White Plains
Heskel MA, Anderson OR, Atkin OK, Turnbull MH, Griffin KL (2012) Leaf- and cell-level carbon cycling responses to a nitrogen and phosphorus gradient in two arctic tundra species. Am J Bot 99:1702–1714
Heskel MA, Atkin OK, Turnbull MH, Griffin KL (2013) Bringing the Kok effect to light: a review on the integration of daytime respiration and net ecosystem exchange. Ecosphere 4:art98
Heskel MA, Bitterman D, Atkin OK, Turnbull MH, Griffin KL (2014) Seasonality of foliar respiration in two dominant plant species from the Arctic tundra: response to long-term warming and short-term temperature variability. Funct Plant Biol 41:287–300
Heskel MA, Atkin OK, O’Sullivan OS, Reich P, Tjoelker MG, Weerasinghe LK et al (2016a) Reply to Adams et al.: Empirical versus process-based approaches to modeling temperature responses of leaf respiration. Proc Natl Acad Sci USA 113:E5996–E5997
Heskel MA, O’Sullivan OS, Reich PB, Tjoelker MG, Weerasinghe LK, Penillard A et al (2016b) Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proc Natl Acad Sci USA 113:3832–3837
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hikosaka K (2004) Interspecific difference in the photosynthesis-nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hill SA, Bryce JH, Lambers H, van der Plas LHW (1992) Malate metabolism and light-enhanced dark respiration in barley mesophyll protoplasts. In: Molecular, biochemical and physiological aspects of plant respiration. SPB Academic Publishing BV, The Hague, pp 221–230
Hoefnagel MHN, Atkin OK, Wiskich JT (1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta-Bioenerg 1366:235–255
Huntingford C, Zelazowski P, Galbraith D, Mercado LM, Sitch S, Fisher R et al (2013) Simulated resilience of tropical rainforests to CO2-induced climate change. Nature Geosci 6:268–273
Igamberdiev AU, Romanowska E, Gardeström P (2001) Photorespiratory flux and mitochondrial contribution to energy and redox balance of barley leaf protoplasts in the light and during light-dark transitions. J Plant Physiol 158:1325–1332
IPCC (2013) Climate change 2013: the physical science basis. Contribution of working Group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York
James WO (1953) Plant respiration. Clarendon Press, Oxford
Janssens IA, Lankreijer H, Matteucci G, Kowalski AS, Buchmann N, Epron D et al (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol 7:269–278
Kattge J, Knorr W (2007) Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ 30:1176–1190
Kattge J, Knorr W, Raddatz T, Wirth C (2009) Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob Change Biol 15:976–991
King AW, Gunderson CA, Post WM, Weston DJ, Wullschleger SD (2006) Plant respiration in a warmer world. Science 312:536–537
Knorr W (1997) Satellite remote sensing and modeling of the global CO2 exchange of land vegetation: a synthesis study. Max-Planck-Institut fur Meteorologie, Hamburg
Knorr W (2000) Annual and interannual CO2 exchanges of the terrestrial biosphere: process-based simulations and uncertainties. Glob Ecol Biogeog 9:225–252
Kornfeld ARI, Horton TW, Yakir DAN, Searle SY, Griffin KL, Atkin OK, Subke J-A, Turnbull MH (2012) A field-compatible method for measuring alternative respiratory pathway activities in vivo using stable O2 isotopes. Plant Cell Environ 35:1518–1532
Krinner G, Viovy N, de Noblet-Ducoudré N, Ogée J, Polcher J, Friedlingstein P et al (2005) A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Glob Biogeochem Cy 19:GB1015
Krömer S (1995) Respiration during photosynthesis. Ann Rev Plant Physiol Plant Mol Biol 46:45–70
Krömer S, Stitt M, Heldt HW (1988) Mitochondrial oxidative phosphorylation participating in photosynthetic metabolism of a leaf cell. FEBS Lett 226:352–356
Kruse J, Adams MA (2008) Three parameters comprehensively describe the temperature response of respiratory oxygen reduction. Plant Cell Environ 31:954–967
Kruse J, Rennenberg H, Adams MA (2011) Steps towards a mechanistic understanding of respiratory temperature responses. New Phytol 189:659–677
Kruse J, Alfarraj S, Rennenberg H, Adams M (2016) A novel mechanistic interpretation of instantaneous temperature responses of leaf net photosynthesis. Photosyn Res 129:43–58
Lambers H (1985) Respiration in intact plants and tissues: its regulation and dependence on environmental factors, metabolism and invaded organisms. In: Douce R, Day DA (eds) Encyclopedia of plant physiology, higher plant cell respiration, vol 18. Springer, New York, pp 417–473
Larcher W (2004) Physiological plant ecology. Ecophysiology and stress physiology of functional groups. Springer, Berlin
Larigauderie A, Körner C (1995) Acclimation of leaf dark respiration to temperature in alpine and lowland plant species. Ann Bot 76:245–252
Lechowicz MJ, Hellens LE, Simon JP (1980) Latitudinal trendds in the responses of growth respiration and maintenance respiration to temperature in the beach pea, Lathyrus japonicus. Can J Bot 58:1521–1524
Lee TD, Reich PB, Bolstad PV (2005) Acclimation of leaf respiration to temperature is rapid and related to specific leaf area, soluble sugars and leaf nitrogen across three temperate deciduous tree species. Funct Ecol 19:640–647
Leuzinger S, Thomas RQ (2011) How do we improve Earth system models? Integrating Earth system models, ecosystem models, experiments and long-term data. New Phytol 191:15–18
Liang J, Xia J, Liu L, Wan S (2013) Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J Plant Ecol 6:437–447
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Lloyd J, Wong S, Styles J, Batten D, Priddle R, Turnbull C, Mcconchie C (1995) Measuring and modeling whole-tree gas exchange. Funct Plant Biol 22:987–1000
Lloyd J, Patiño S, Paiva RQ, Nardoto GB, Quesada CA, Santos AJB et al (2010) Optimisation of photosynthetic carbon gain and within-canopy gradients of associated foliar traits for Amazon forest trees. Biogeosciences 7:1833–1859
Lombardozzi DL, Bonan GB, Smith NG, Dukes JS, Fisher RA (2015) Temperature acclimation of photosynthesis and respiration: a key uncertainty in the carbon cycle-climate feedback. Geophys Res Lett 42:8624–8631
Loreto F, Velikova V, Di Marco G (2001) Respiration in the light measured by CO2-12C emission in CO2-13C atmosphere in maize leaves. Aust J Plant Physiol 28:1103–1108
McCree KJ (1974) Equations for the rate of dark respiration of white clover and grain sorghum, as functions of dry weight, photosynthetic rate, and temperature. Crop Sci 14:509–514
McGuire AD, Melillo JM, Joyce LA, Kicklighter DW, Grace AL, Moore B, Vorosmarty CJ (1992) Interactions between carbon and nitrogen dynamics in estimating net primary productivity for potential vegetaion in North America. Glob Biochem Cycles 6:101–124
McLaughlin BC, Xu CY, Rastetter EB, Griffin KL (2014) Predicting ecosystem carbon balance in a warming Arctic: the importance of long-term thermal acclimation potential and inhibitory effects of light on respiration. Glob Change Biol 20:1901–1912
Meir P, Grace J, Miranda AC (2001) Leaf respiration in two tropical rainforests: constraints on physiology by phosphorus, nitrogen and temperature. Funct Ecol 15:378–387
Meir P, Kruijt B, Broadmeadow M, Barbosa E, Kull O, Carswell F, Nobre A, Jarvis PG (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ 25(3):343–357
Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Mercado LM, Huntingford C, Gash JHC, Cox PM, Jogireddy V (2007) Improving the representation of radiation interception and photosynthesis for climate model applications. Tellus Ser B-Chem Phys Meteor 59:553–565
Metherell AK, Harding LA, Cole CV, Parton WJ (1996) CENTURY Soil Organic Matter Model Environment. Technical Documentation. Agroecosystem Version 4.0. Great Plains System Research Unit Technical Report No. 4. USDA-ARS. Accessed 15 Sept 2016
Millar AH, Whelan J, Soole KL, Day DA (2011) Organization and regulation of mitochondrial respiration in plants. Ann Rev Plant Biol 62:79–104
Nagel JM, Griffin KL, Schuster WS, Tissue DT, Turnbull MH, Brown KJ, Whitehead D (2002) Energy investment in leaves of red maple and co-occurring oaks within a forested watershed. Tree Physiol 22:859–867
Nelson CJ, Alexova R, Jacoby RP, Millar AH (2014) Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol 166:91–108
Niinemets U, Keenan TF, Hallik L (2015) A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol 205:973–993
Noguchi K, Yoshida K (2008) Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8:87–99
Noguchi K, Yamori W, Hikosaka K, Terashima I (2015) Homeostasis of the temperature sensitivity of respiration over a range of growth temperatures indicated by a modified Arrhenius model. New Phytol 207:34–42
O’Sullivan OS, Weerasinghe KWLK, Evans JR, Egerton JJG, Tjoelker MG, Atkin OK (2013) High-resolution temperature responses of leaf respiration in snow gum (Eucalyptus pauciflora) reveal high-temperature limits to respiratory function. Plant Cell Environ 36:1268–1284
Oleson KW, Lawrence DM, Bonan GB, Drewniak B, Huang M, Kovan CD, …, Yang Z-L (2013) Technical Description of version 4.5 of the Community Land Model (CLM). NCAR Technical Note NCAR/TN-503+STR. Boulder, CO
Paembonan SA, Hagihara A, Hozumi K (1991) Long-term measurement of CO2 release from the above-ground parts of a Hinoki forest tree in relation to air temperature. Tree Physiol 8:399–405
Pärnik T, Keerberg O (1995) Decarboxylation of primary and end-products of photosynthesis at different oxygen concentrations. J Exp Bot 46:1439–1447
Pärnik T, Ivanova H, Keerberg O (2007) Photorespiratory and respiratory decarboxylations in leaves of C3 plants under different CO2 concentrations and irradiances. Plant Cell Environ 30:1535–1544
Parton WJ, Schimel DS, Cole CV, Ojima DS (1987) Analysis of factors controlling soil organic-matter levels in great-plains grasslands. Soil Sci Soc Am J 51:1173–1179
Penning de Vries FWT (1975) The cost of maintenance processes in plant cells. Ann Bot 39:77–92
Penning de Vries FWT, Van Laar HH, Chardon MCM (1983) Bioenergeties of growth of seeds, fruits, and storage organs. In: Proceedings of the symposium on potential productivity of field crops under different environments, 23–26 September 1980, IRRI, Manila, Phillipines. International Riee Researeh Institute, Los Banos, pp 37–59
Piao SL, Luyssaert S, Ciais P, Janssens IA, Chen AP, Cao C et al (2010) Forest annual carbon cost: a global-scale analysis of autotrophic respiration. Ecology 91:652–661
Pinelli P, Loreto F (2003) (CO2)-C-12 emission from different metabolic pathways measured in illuminated and darkened C3 and C4 leaves at low, atmospheric and elevated CO2 concentration. J Exp Bot 54:1761–1769
Plaxton WC, Podesta FE (2006) The functional organization and control of plant pespiration. Crit Rev Plant Sci 25:159–198
Pons TL, Welschen RAM (2002) Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis. Plant Cell Environ 25:1367–1372
Pons TL, Welschen RAM (2003) Midday depression of net photosynthesis in the tropical rainforest tree Eperua grandiflora : contributions of stomatal and internal conductances, respiration and Rubisco functioning. Tree Physiol 23:937–947
Pons TL, Westbeek MHM (2004) Analysis of differences in photosynthetic nitrogen-use efficiency between four contrasting species. Physiol Plant 122:68–78
Poorter H, Bergkotte M (1992) Chemical composition of 24 wild species differing in relative growth rate. Plant Cell Environ 15:221–229
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116:26–37
Poorter H, Pepin S, Rijkers T, de Jong Y, Evans JR, Korner C (2006) Construction costs, chemical composition and payback time of high- and low-irradiance leaves. J Exp Bot 57:355–371
Prentice IC, Cowling SA (2013) Dynamic global vegetation models. In: Levin SA (ed) Encyclopedia of biodiversity, 2nd edn. Academic, Waltham, pp 670–689
Prentice IC, Farquhar GD, Fasham MJR, Goulden ML, Heimann M, Jaramillo VJ et al (2001) The carbon cycle and atmospheric carbon dioxide. In: JTHe a (ed) Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 183–237
Raghavendra AS, Padmasree K, Saradadevi K (1994) Interdependence of photosynthesis and respiration in plant cells – interactions between chloroplasts and mitochondria. Plant Sci 97:1–14
Raich JW, Rastetter EB, Melillo JM, Kicklighter DW, Steudler PA, Peterson BJ, Grace AL, Moore B, Vorosmarty CJ (1991) Potential net primary productivity in South America – application of a global model. Ecol Appl 1:399–429
Rasmusson AG, Soole KL, Elthon TE (2004) Alternative NAD(P)H dehydrogenases of plant mitochondrial. Ann Rev Plant Biol 55:23–39
Reich PB, Oleksyn J, Tjoelker MG (1996) Needle respiration and nitrogen concentration in scots pine populations from a broad latitudinal range – a common garden test with field-grown trees. Funct Ecol 10:768–776
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Walters MB, Ellsworth DS, Vose JM, Volin JC, Gresham C, Bowman WD (1998) Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114:471–482
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969
Reich PB, Tjoelker MG, Pregitzer KS, Wright IJ, Oleksyn J, Machado JL (2008) Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett 11:793–801
Reich PB, Sendall KM, Stefanski A, Wei X, Rich RL, Montgomery RA (2016) Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531:633–636
Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P et al (2005) On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob Change Biol 11:1424–1439
Robson MJ (1981) Respiratory efflux in relation to temperature of simulated swards of perennial ryegrass with contrasting soluble carbohydrate contents. Ann Bot 48:269–273
Rodríguez-Calcerrada J, Atkin OK, Robson TM, Zaragoza-Castells J, Gil L, Aranda I (2010) Thermal acclimation of leaf dark respiration of beech seedlings experiencing summer drought in high and low light environments. Tree Physiol 30:214–224
Rodríguez-Calcerrada J, Shahin O, del Rey MD, Rambal S (2011) Opposite changes in leaf dark respiration and soluble sugars with drought in two Mediterranean oaks. Funct Plant Biol 38:1004–1015
Rowland L, Zaragoza-Castells J, Bloomfield KJ, Turnbull MH, Bonal D, Burban B et al (2016) Scaling leaf respiration with nitrogen and phosphorus in tropical forests across two continents. New Phytol doi. https://doi.org/10.1111/nph.13992
Ruimy A, Dedieu G, Saugier B (1996) TURC: a diagnostic model of continental gross primary productivity and net primary productivity. Glob Biogeochem Cycles 10:269–285
Running SW, Coughlan JC (1988) A general model of forest ecosystem processes for regional applications I. Hydrologic balance, canopy gas exchange and primary production processes. Ecol Mod 42:125–154
Ryan MG (1991) Effects of climate change on plant respiration. Ecol Appl 1(2):157–167
Ryan MG (1995) Foliar maintenance respiration of subalpine and boreal trees and shrubs in relation to nitrogen content. Plant Cell Environ 18:765–772
Ryan MG (2002) Canopy processes research. Tree Physiol 22:1035–1043
Schulze ED, Kelliher FM, Körner C, Lloyd J, Leuning R (1994) Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition – a global ecology scaling exercise. Ann Rev Ecol System 25:629–660
Schwalm CR, Williams CA, Schaefer K, Anderson R, Arain MA, Baker I et al (2010) A model-data intercomparison of CO2 exchange across North America: results from the North American Carbon Program site synthesis. J Geophys Res Biogeosci 115. https://doi.org/10.1029/2009JG001229
Searle SY, Bitterman DS, Thomas S, Griffin KL, Atkin OK, Turnbull MH (2011a) Respiratory alternative oxidase responds to both low- and high-temperature stress in Quercus rubra leaves along an urban–rural gradient in New York. Funct Ecol 25:1007–1017
Searle SY, Thomas S, Griffin KL, Horton T, Kornfeld A, Yakir D, Hurry V, Turnbull MH (2011b) Leaf respiration and alternative oxidase in field-grown alpine grasses respond to natural changes in temperature and light. New Phytol 189:1027–1039
Shapiro JB, Griffin KL, Lewis JD, Tissue DT (2004) Response of Xanthium strumarium leaf respiration in the light to elevated CO2 concentration, nitrogen availability and temperature. New Phytol 162:377–386
Sharp RE, Matthews MA, Boyer JS (1984) Kok effect and the quantum yield of photosynthesis: light partially inhibits dark respiration. Plant Physiol 75:95–101
Sitch S, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer W et al (2003) Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob Change Biol 9:161–185
Sitch S, Huntingford C, Gedney N, Levy PE, Lomas M, Piao SL et al (2008) Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs). Glob Change Biol 14:2015–2039
Slot M, Kitajima K (2015) General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 177:885–900
Slot M, Wright SJ, Kitajima K (2013) Foliar respiration and its temperature sensitivity in trees and lianas: in situ measurements in the upper canopy of a tropical forest. Tree Physiol 33:505–515
Slot M, Rey-Sánchez C, Winter K, Kitajima K (2014) Trait-based scaling of temperature-dependent foliar respiration in a species-rich tropical forest canopy. Funct Ecol 28:1074–1086
Smith NG, Dukes JS (2013) Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Glob Change Biol 19:45–63
Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR (2006) Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 103:19587–19592
Sweetlove LJ, Williams TCR, Cheung CYM, Ratcliffe RG (2013) Modeling metabolic CO2 evolution – a fresh perspective on respiration. Plant Cell Environ 36:1631–1640
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J (2005) In vivo respiratory metabolism of illuminated leaves. Plant Physiol 138:1596–1606
Tcherkez G, Bligny R, Gout E, Mahe A, Hodges M, Cornic G (2008) Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proc Natl Acad Sci USA 105:797–802
Tcherkez G, Boex-Fontvieille E, Mahe A, Hodges M (2012) Respiratory carbon fluxes in leaves. Curr Opin Plant Biol 15(3):308–314
Thomas RB, Griffin KL (1994) Direct and indirect effects of atmospheric carbon dioxide enrichment on leaf respiration of Glycine max (L.) merr. Plant Physiol 104:355–361
Thornley JHM (1970) Respiration, growth and maintenance in plants. Nature 227:304–305
Thornley JHM (2011) Plant growth and respiration re-visited: maintenance respiration defined – it is an emergent property of, not a separate process within, the system – and why the respiration : photosynthesis ratio is conservative. Ann Bot 108:1365–1380
Thornley JHM, Cannell MGR (2000) Modeling the components of plant respiration: representation and realism. Ann Bot 85:55–67
Thornton PE, Law BE, Gholz HL, Clark KL, Falge E, Ellsworth DS et al (2002) Modeling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agric For Met 113:185–222
Thornton PE, Running SW, Hunt ER (2005) Biome-BGC: Terrestrial Ecosystem Process Model, Version 4.1.1. ORNL Distributed Active Archive Center
Tjoelker MG, Reich PB, Oleksyn J (1999) Changes in leaf nitrogen and carbohydrates underlie temperature and CO2 acclimation of dark respiration in five boreal tree species. Plant Cell Environ 22:767–778
Tjoelker MG, Oleksyn J, Reich PB (2001) Modeling respiration of vegetation: evidence for a general temperature-dependent Q 10. Glob Change Biol 7:223–230
Van Arendonk JJCM, Poorter H (1994) The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate. Plant Cell Environ 17:963–970
Vanderwel MC, Slot M, Lichstein JW, Reich PB, Kattge J, Atkin OK et al (2015) Global convergence in projected leaf respiration from estimates of thermal acclimation across time and space. New Phytol 207:1026–1037
Vertregt N, Penning de Vries FWT (1987) A rapid method for determining the efficiency of biosynthesis of plant biomass. J Theor Biol 128:109–119
Villar R, Held AA, Merino J (1995) Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiol 107:421–427
Villar RAFA, Robleto JR, De Jong YVON, Poorter HEND (2006) Differences in construction costs and chemical composition between deciduous and evergreen woody species are small as compared to differences among families. Plant Cell Environ 29:1629–1643
Walker AP, Beckerman AP, Gu L, Kattge J, Cernusak LA, Domingues TF et al (2014) The relationship of leaf photosynthetic traits – V cmax and J max – to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecol Evol 4:3218–3235
Wang XZ, Lewis JD, Tissue DT, Seemann JR, Griffin KL (2001) Effects of elevated atmospheric CO2 concentration on leaf dark respiration of Xanthium strumarium in light and in darkness. Proc Natl Acad Sci USA 98:2479–2484
Warren CR, Adams MA (2004) What determines rates of photosynthesis per unit nitrogen in Eucalyptus seedlings? Funct Plant Biol 31:1169–1178
Way DA, Sage RF (2008) Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) B.S.P.] Glob Change Biol 14:624–636
Way DA, Yamori W (2014) Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosyn Res 119:89–100
Way DA, Holly C, Bruhn D, Ball MC, Atkin OK (2015) Diurnal and seasonal variation in light and dark respiration in field-grown Eucalyptus pauciflora. Tree Physiol 35:840–849
Weerasinghe LK, Creek D, Crous KY, Xiang S, Liddell MJ, Turnbull MH, Atkin OK (2014) Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in Far North Queensland. Tree Physiol 34:564–584
Wehr R, Munger JW, McManus JB, Nelson DD, Zahniser MS, Davidson EA, Wofsy SC, Saleska SR (2016) Seasonality of temperate forest photosynthesis and daytime respiration. Nature 534:680–683
White MA, Thornton PE, Running SW, Nemani RR (2000) Parameterization and sensitivity analysis of the BIOME–BGC Terrestrial Ecosystem Model: net primary production controls. Earth Interact 4:1–85
Williams K, Percival F, Merino J, Mooney HA (1987) Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ 10:725–734
Wohlfahrt G, Bahn M, Haslwanter A, Newesely C, Cernusca A (2005) Estimation of daytime ecosystem respiration to determine gross primary production of a mountain meadow. Agric For Met 130:13–25
Woodward FI, Lomas MR (2004) Vegetation dynamics – simulating responses to climatic change. Biol Rev 79:643–670
Woodward FI, Smith TM, Emanuel WR (1995) A global land primary productivity and phytogeography model. Glob Biogeochem Cycles 9:471–490
Wright IJ, Reich PB, Westoby M (2001) Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol 15:423–434
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Atkin OK, Lusk CH, Tjoelker MG, Westoby M (2006) Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytol 169:309–319
Wythers KR, Reich PB, Bradford JB (2013) Incorporating temperature-sensitive Q10 and foliar respiration acclimation algorithms modifies modeled ecosystem responses to global change. J Geophys Res Biogeosci 118:77–90
Zaehle S, Friend AD (2010) Carbon and nitrogen cycle dynamics in the O-CN land surface model: 1. Model description, site-scale evaluation, and sensitivity to parameter estimates. Glob Biogeochem Cycles 24:GB1005
Zaragoza-Castells J, Sanchez-Gomez D, Valladares F, Hurry V, Atkin OK (2007) Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long-lived leaves. Plant Cell Environ 30:820–833
Zaragoza-Castells J, Sanchez-Gomez D, Hartley IP, Matesanz S, Valladares F, Lloyd J, Atkin OK (2008) Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of acclimation in both high-light and shaded habitats. Funct Ecol 22:172–184
Ziehn T, Kattge KW, Scholze M (2011) Improving the predictability of global CO2 assimilation rates under climate change. Geophys Res Lett 38. https://doi.org/10.1029/2011gl047182
Acknowledgements
This work was funded the Australian Research Council grants/fellowships (DP130101252, CE140100008) to OKA, and USA National Science Foundation International Polar Year Grant to KLG. AM and CH acknowledge the CEH National Capability fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Atkin, O.K. et al. (2017). Leaf Respiration in Terrestrial Biosphere Models. In: Tcherkez, G., Ghashghaie, J. (eds) Plant Respiration: Metabolic Fluxes and Carbon Balance. Advances in Photosynthesis and Respiration, vol 43. Springer, Cham. https://doi.org/10.1007/978-3-319-68703-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-68703-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68701-8
Online ISBN: 978-3-319-68703-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)