Abstract
The use of lignocellulosic fibers as reinforcement in polymer composites is attracting interest due to their properties such as mechanical properties and environmental benefits. Nevertheless, the hydrophilic character of lignocellulosic fibers reduces the compatibility with the hydrophobic matrices resulting in composites with poor mechanical properties. Therefore, in order to reduce the hydrophilic character of fiber and improve the fiber/matrix adhesion, is necessary to modify the fiber surface morphology. In this chapter, different lignocellulosic fiber treatments and the effect of these treatments on fiber properties as well as on composite mechanical performance were discussed. Even though chemical treatments are the most widely used, physical and biological treatments are environmentally friendly and promising alternatives.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Synthetic fiber reinforced plastics have proven to meet the structural and durability requirements of components for various applications. Nevertheless, the growing global energy crisis and ecological risks have focused more research interests in natural fiber reinforced polymer composites due to their potential (Praveen et al. 2016). Lignocellulosic fibers can be used as reinforcement in polymeric matrix composites due to their characteristics. Among other properties, lignocellulosic fibers have acceptable specific properties, low density, biodegradability, low cost, and recyclability (Yu et al. 2014; Lu et al. 2014; Arbelaiz et al. 2005c).
The mechanical properties of polymeric composites are governed by the individual properties of the components and by the interface formed between the matrix and fiber. A critical factor in reinforced polymeric composites is the strength of the bond between the fiber and polymer matrix. The load is transmitted through the fiber-matrix interface and consequently, the optimal mechanical performance of fiber reinforced composite depends, among other variables, on the interfacial bond between fiber and polymer matrix (Praveen et al. 2016).
2 Fiber-Matrix Adhesion
The adhesion between fiber and polymeric matrix is sustained by the interfacial forces. These forces can be broadly divided into primary forces and secondary forces. Primary forces, also known as short-range forces, arise from chemical bonding. Chemical bonding compromises of interlinking between molecules of the substrate by covalent, ionic or metallic bonds. The secondary or intermolecular forces, such as Van der Waals forces, are caused from the physical attraction between two substrates (Kim and Pal 2010). There is no single theory of adhesion which can satisfactorily explain the mechanism of adhesion between lignocellulosic fiber and the polymeric matrix. Many adhesion mechanisms, such as mechanical interlocking, adsorption, and diffusion theory, and boundary layer theory are developed in order to explain lignocellulosic fiber/polymeric matrix adhesion (Kim and Pal 2010). In the literature, fibers are modified using different treatments in order to improve fiber/matrix adhesion. A brief discussion of different lignocellulosic fiber treatments and the effect of these treatments on fiber properties as well as on composite mechanical performance is given in this chapter.
3 Lignocellulosic Fiber
Natural fibers can be classified according to their source; plant, animal, or mineral (Saba et al. 2014). Plant fibers or lignocellulosic fibers are mainly composed of cellulose, hemicelluloses, and lignins. Table 1 shows the chemical composition of sisal, flax, and hemp lignocellulosic fibers determined by using the standards methods of Technical Association of Pulp and Paper Industry (TAPPI).
Cellulose, the main component of lignocellulosic fibers, is the most abundant natural organic compound which performs structure-forming functions in plant-cell wall (Daintith 2000; Allaby 1998). Cellulose is a semicrystalline polysaccharide consisting of a linear chain of hundreds to thousands of β-(1,4)-glycosidic bonds linked D-glucopyranose with the presence of a large amount of hydroxyl groups (Zhou et al. 2016). The repeating unit of cellulose is shown in Fig. 1.
Hemicelluloses have a heterogeneous monosaccharide composition mainly based on pentoses (D-xylose and L-arabinose), hexoses (D-glucose, D-galactose, D-mannose, L-rhamnose) and uronic acid units (D-galacturonic acid and D-glucuronic acid) (Egües 2013). Hemicelluloses are often referred to as matrix components and may be found in the middle lamellae that bind cell walls of fibers, in the primary wall regions and in the thicker, cellulose-rich, secondary layer of the plant-cell wall (Focher 1992). The heterogeneity of hemicelluloses extends to branching polymers, thus giving new dimensions and complexities within the cell wall (Focher 1992). However, the degree of polymerization of nature cellulose is 10–100 times higher than that of hemicellulose ones (Bledzki and Gassan 1999).
Lignins are phenolic complex, cross-linked polymer, comprising both aliphatic and aromatic constituents, that is found in plant-cell walls. Its function appears to be cement together and anchor cellulose fibers, to stiffen the cell wall and provide protection against microbial attacks, external agents, moisture, and weathering (Egües 2013). Structures of the three major precursors of lignin are shown in Fig. 2.
4 Surface Modification of Lignocellulosic Fiber
The use of natural fibers as reinforcement in polymer composites is attracting much interest due to its potential, however, the hydrophilic character of the fibers reduces the compatibility with the hydrophobic matrices resulting in composites with poor mechanical properties (Lu et al. 2014; Arbelaiz et al. 2005c). Most of the research related to lignocellulosic fibers and polyolefin matrices showed that the adhesion between the lignocellulosic fiber and polymers such as polypropylene (PP) or polyethylene (PE) was poor (Arbelaiz et al. 2005c; Merkel et al. 2014; Ranganathan et al. 2016). Other polymers such as poly(lactic acid) (PLA) presents moderate polarity induced by the presence of ester bonds (Orue et al. 2015; Jiang et al. 2012) and could form hydrogen bonds with the hydroxyl groups of lignocellulosic fibers. It was observed that the surface tension value of PLA is close to sisal fibers resulting in good fiber wettability with PLA matrix (Orue et al. 2015). Even though the wettability of lignocellulosic fiber with PLA polymer is good, the adhesion between them was not good enough to improve PLA strength after fiber addition (Orue et al. 2016b).
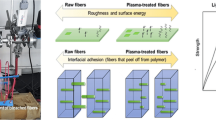
The adhesion between lignocellulosic fiber and polymer matrix can be improved (i) modifying the surface of fibers, (ii) modifying the polymer or (iii) modifying both of them at the same time. Surface modification is essential to reduce the hydrophilic character of the lignocellulosic fibers, improve the wettability between the fiber and matrix and to improve fiber/polymer matrix adhesion (Bledzki and Gassan 1999; Bledzki et al. 1996). In the literature, lignocellulosic fibers are subjected to several surface treatments such as chemical treatments (Arbelaiz et al. 2005c; Orue et al. 2015; Zou et al. 2012; Goriparthi et al. 2012; Chen et al. 2012; Sun et al. 2016; Datta and Kopczynska 2015; Paul et al. 2010b), physical treatments (Hou et al. 2014; Bozaci et al. 2013; Li et al. 2014), and biological treatments (Pickering et al. 2007; George et al. 2014). Chemical treatments provide the means of permanently modifying the nature of fiber cell walls by grafting polymer onto the fibers, crosslinking of the fiber cell walls, or by using coupling agents (Zhou et al. 2016; Xie et al. 2010). Physical treatments change the structural and surface properties of the lignocellulosic fiber by introducing surface crosslinking, modifying the surface energy and/or generating reactive free radicals and groups, and thereby influence the mechanical bonding to the matrix (Zhou et al. 2016). Biological treatments employ microorganisms, mainly white and soft-rot fungi, actinomycetes, and enzymes which degrade lignin through the action of lignin-degrading enzymes, such as peroxidases and laccases (Saritha et al. 2012). The main lignocellulosic surface treatments are summarized in Fig. 3.
4.1 Chemical Treatments
4.1.1 Alkali Treatment
Alkali treatment is a simple and efficient method to improve lignocellulosic fiber/polymer matrix adhesion (Orue et al. 2016b; Raj et al. 2011). Usually, in alkali treatments, lignocellulosic fibers were treated with NaOH or KOH solutions for a specified time to remove noncellulosic compounds, such as lignin, hemicelluloses and other organics (Orue et al. 2015, 2016b). The removal of noncellulosic compounds could create voids in the fiber structure (Orue et al. 2016b), and alkali treatment could depolymerize the native cellulose structure and expose cellulose crystallites (Li et al. 2007; Mohanty et al. 2001). Figure 4 presents the schematic view of the cellulose fiber structure, before and after an alkali treatment. The effect of alkali treatment on the properties of lignocellulosic fibers depends on the alkali treatment conditions, such as NaOH concentration and temperature (Gassan and Bledzki 1999). For example, severe alkali conditions can damage the fiber structure decreasing the properties of lignocellulosic fibers (Li et al. 2007; Wang et al. 2007). Therefore, all these conditions are important factors that must be optimized for each fiber type.
Typical structure of (a) untreated and (b) alkali treated cellulose fiber. Reprinted from (Mwaikambo and Ansell 2002) with permission from John Wiley and Sons
In previous works (Orue et al. 2015, 2016b) sisal fibers were pretreated with 2 wt% NaOH solution at 20 °C for overnight and after with 7.5 wt% NaOH solution under reflux at 100 °C for 90 min. The density values of alkali treated sisal fibers were higher than untreated fiber ones suggesting that the alkali treatment removed noncellulosic compounds increasing the cellulose fraction in sisal fiber. Although cellulose is the compound that gives the strength to the lignocellulosic fibers, Fig. 5 showed that the tensile strength and modulus values of alkali treated fibers were lower than untreated ones. A possible explanation could be that the removal of noncellulosic compounds could create voids in the sisal fiber structure resulting in lower tensile strength and modulus values (Orue et al. 2016b).
The removal of noncellulosic compounds was corroborated by Fourier Infrared Spectroscopy (FTIR) analysis in Fig. 6. After alkali treatment, the chemical composition of sisal fibers changed since the prominent bands of the raw fiber around 1740 and 1250 cm−1 were disappeared almost completely indicating that alkali treatment removed noncellulosic compounds. The band around 1740 cm−1 was ascribed to the acetyl and ester groups of hemicelluloses and aromatic components of lignin (Mondragon et al. 2014; De Rosa et al. 2010; Haiping et al. 2007) while the band at 1250 cm−1 was related to the C–O stretching vibration of hemicelluloses component (De Rosa et al. 2011) or aryl-alkyl ether compounds present in lignin (Mondragon et al. 2014). Moreover, thermogravimetric analysis (TGA) results indicated that the removal of noncellulosic compounds resulting in a better thermal stability of alkali treated fibers (Orue et al. 2015).
The optical microscopy images of untreated and alkali treated sisal fibers are shown in Fig. 7. It was observed that untreated sisal fibers showed a smooth surface, whereas, after alkali treatment, sisal fibers surface roughness was considerably increased and created fibers with smaller diameters probably due to the removal of noncellulosic compounds. Moreover, after treating sisal fibers with NaOH solution, slightly higher contact angles with a drop of water were obtained. Untreated sisal fibers showed a contact value of 68.2°, whereas alkali treated sisal fibers showed a value 75.4° suggesting that the alkali treated fibers were more hydrophobic than untreated ones thus improving the wettability with hydrophobic polymer matrices (Orue et al. 2015, 2016a).
Optical images of (a) untreated and (b) alkali treated sisal fibers. Reprinted from (Orue et al. 2015), Copyright (2015) with permission from Elsevier
In a previous work (Orue et al. 2016b), PLA/sisal fibers composites were compounded varying the loading of fibers from 20 to 40 wt%. Even though the tensile properties of alkali treated sisal fibers was lower than untreated ones, Table 2 showed that composites based on the alkali treated sisal fibers showed higher tensile strength values than neat PLA matrix. This fact suggested that alkali treatment improved fiber/PLA matrix adhesion. One possible reason could be that in the injection process, the viscosity of melted polymer decreased considerably filling fiber voids and improving the mechanical interlocking adhesion between sisal fiber and PLA polymer matrix.
Scanning electron microscopy (SEM) micrographs of the fractured surface of composites based on the untreated sisal fibers showed pulled-out fibers and holes suggesting a poor interfacial adhesion between fibers and PLA. However, in SEM micrographs of composite based on alkali treated fibers hardly pulled-out fibers can be observed and it was difficult to distinguish fibers from the matrix suggesting that sisal fibers were coated with PLA polymer matrix and consequently fiber/matrix adhesion was improved.
In previous works (Arbelaiz et al. 2005a, b) flax fibers were soaked in a 20 wt% aqueous solution of sodium hydroxide for 1 h at room temperature. After that, flax fibers were washed for several times in distilled water and dried in an oven. It was observed that after alkalization, the surface roughness of the flax fibers was considerably increased improving the flax fiber/polypropylene interfacial shear strength values. Furthermore, surface energy measurements reported that after alkali treatment the polar component of surface energy of flax fibers decreased suggesting that alkali fibers became more hydrophobic. As a consequence, alkali treatment could improve the wettability with hydrophobic polymer matrices. In order to study the influence of fiber modification on the mechanical properties of composites, flax/PP composites were compounded with 30 wt% fiber content. It was observed that the addition of untreated flax fiber bundle decreased the tensile strength of neat PP, indicating lack of stress transfer from the matrix to the fiber. Even though, alkali treatment improved significantly the wettability and the interfacial shear strength determined by pull-out test, tensile, and flexural strength values were not changed after the addition of alkali treated flax fiber. It must be mentioned that the methods based on the single fiber tests to determine fiber/polymer adhesion could deviate significantly from the real composite performance.
Fernandes et al. (2013) treated sisal fibers in NaOH solution (5% w/v) for 2 h at room temperature and they observed by FTIR analysis and TGA the removal of noncellulosic compounds. In addition, X-ray diffraction (XRD) results showed that after removal lignin, hemicelluloses and other surface impurities the crystallinity index of alkali treated sisal fibers increased and consequently a small increase in density values was observed. They also observed a decrease of mechanical properties of alkali treated sisal fibers. They reinforced high-density polyethylene (HDPE)/cork blends with 10 wt% of untreated and alkali treated sisal fibers and they observed that composites based on the alkali treated fibers showed higher tensile strength and Young modulus values than untreated ones. They suggested that this fact could be related to the increase of fiber surface roughness after alkali treatment improving fiber/HDPE adhesion.
4.1.2 Silane Treatment
Silanes are considered versatile coupling agents that could improve the interface between lignocellulosic fiber and polymer matrix (Orue et al. 2016b; Le Moigne et al. 2014). Silane is a multifunctional molecule which can form a chemical link with the fiber surface through a siloxane bridge. A typical general structure of silane coupling agent is shown in Fig. 8.
During the treatment process of the fiber, silane forms silanols in the presence of water and by a condensation process, one end of silanol could react with the cellulose hydroxyl groups. Finally, when silane modified fibers are blended with the polymeric matrix, the other functional groups of silane react with the matrix functional group (Kabir et al. 2012; Zhang et al. 2005). Therefore, it is important to choose a silane agent which is suitable to react with functional groups of fiber and the polymeric matrix. On the contrary, the stress would not transfer efficiently from polymer matrix to lignocellulosic fibers and consequently, the mechanical properties of composites would not improve. The possible reaction scheme between silane agent and lignocellulosic fiber is shown in Fig. 9.
Silane chemical agent concentration, the medium used (water, organic solvent, or mixtures), the soaking time, the drying temperature, and time are all important factors to carry out silane treatment (Arbelaiz et al. 2016).
In previous works (Orue et al. 2015, 2016b) the effect of silane treatment on sisal fiber properties as well as on composite mechanical performance was studied. Sisal fibers were soaked in silane aqueous solution (2% w/v) under continuous stirring for 3 h. The pH of the solution was adjusted to 3–4 with glacial acetic acid and silane/fiber weight ratio used was 2:1 (w/w). 3-(2 aminoethylamino) propyltrimethoxysilane chemical agent was used as silane chemical agent and it was thought that the silane concentration employed could produce a multilayer polysiloxane network on the sisal fibers bundles (Abdelmouleh et al. 2002).
FTIR spectra of untreated and silane treated sisal fiber are shown in Fig. 10. It can be confirmed that silane treated fibers showed a new absorption band around 1560 cm−1 related to NH2 bending vibration of organosilane agent. Moreover, it was observed in the thermogravimetric analysis that fibers treated with silane chemical agent showed a higher percentage of char which could be related to the presence of grafted silane (Rachini et al. 2012).
After treating sisal fibers with silane chemical agent, the contact angle with a drop of water increased from 68.2° to 88.2° suggesting that the silane treated fibers were more hydrophobic than untreated ones (Orue et al. 2015, 2016a). Figure 11 showed that after silane treatment, the tensile strength values of sisal fibers decreased slightly. This reduction could be related to the acid medium used for silane treatment that might decrease cellulose molecular weight resulting in lower mechanical properties (Orue et al. 2016b; Klemm et al. 1998).
As observed in Table 3, tensile strength values of composites based on silane treated sisal fibers increased slightly as fiber bundle content increased indicating that the adhesion between the polymer and fiber was improved (Orue et al. 2016b).
The improvement of adhesion between silane treated sisal fiber and PLA matrix was corroborated by pull-out test, being the interfacial shear strength value of silane treated fibers slightly higher than untreated fibers ones. This improvement was attributed to the chemical bond between PLA matrix and amino groups of silane agent linked to the fiber surface (Orue et al. 2015). In SEM, micrographs of composites based on the silane treated fibers, sisal fibers cannot be clearly distinguished suggesting that the fiber bundle adhesion was improved. However, a few amount of pulled-out fibers and holes were observed which meant that the adhesion was not very strong (Orue et al. 2016b).
Zou et al. (2012) studied the effect of silane treatment on the properties of short sisal fiber/PLA biocomposites. For this purpose, sisal fibers were soaked in 5% w/v γ-amine propyl triethoxysilane ethanol solution at room temperature for 2 h. The pH of the solution was adjusted to 4–5 with acetic acid and fibers were washed with ethanol and dried under vacuum at 70 °C before the preparation of the biocomposites. Composites with 10 wt% untreated and treated sisal fibers were compounded by compression molding technique and they observed that silane treatment improved the mechanical properties. They suggested that silane with ethoxy groups can be hydrolyzed in water and produced silanols which reacted with the hydroxyl groups of sisal fibers forming covalent bonds on the fiber surface. Furthermore, amine groups from the silane may further react with terminal groups of PLA matrix. The bridge formed at the interface led to the improvement of sisal fiber/PLA matrix adhesion.
In previous works, (Arbelaiz et al. 2005a, b) flax fibers were treated with vinyltrimethoxysilane (VTMO) chemical agent. It was observed that the polar component of surface energy was decreased considerably indicating that silane treated flax fibers could be better wetted out by nonpolar polymer matrices. However, when silane treated flax fiber/PP composites were compounded, the tensile and flexural strength were not improved suggesting that stress transfer from the matrix to the fiber occurred with a disappointing lack of efficiency. This fact could indicate that the vinyl group was not able to react with PP chains and consequently fiber/polymer matrix adhesion was not improved.
4.1.3 Esterification
4.1.3.1 Esterification with Acetyl Groups
Acetylation treatment of lignocellulosic fibers is an esterification reaction and it was reported that the treatment improved the lignocellulosic fiber/matrix adhesion (Li et al. 2007; Kabir et al. 2012; Kumar et al. 2011). Usually, the procedure included an alkali treatment initially, followed by acetylation treatment (Li et al. 2007; Kumar et al. 2011; Efanov and Ovchinnikov 2012). Among other reactive, acetic anhydride and acetic acid can be used (Paul et al. 2010b; De Rosa et al. 2011; Hill et al. 1998). The hydrolysis of acetic anhydride to acetic acid is a moderately highly exothermic fast reaction (Hirota et al. 2010) and the reaction with acetic anhydride involves the generation of acetic acid as by-product which must be removed from the lignocellulosic fibers (Hill et al. 1998). The hydroxyl groups of the lignocellulosic fibers react with the acetyl reducing the hydrophilic character of the fiber. The possible reaction between the hydroxyl groups of lignocellulosic fiber and carboxylic groups of acid is shown in Fig. 12.
The use of catalyst increases the rate of acetylation and the most common catalyst that can be used are pyridine, sulfuric acid, potassium, and sodium acetate. Nevertheless, strong acid catalyst conditions could damage the fiber structure (Kabir 2012). Therefore, selection of catalyst conditions is an important factor for the acetylation treatment.
De Rosa et al. (2011) studied the effect of acetic acid treatment on the mechanical properties of okra (Abelmoschus esculentus) fibers. They treated lignocellulosic fibers with 10% acetic acid and they observed that after the treatment the tensile strength and Young modulus values of okra fibers decreased considerably. The fracture surface of untreated and acetylated fiber was observed by SEM and they observed that the acetylation treatment tended to expose the lumen of lignocellulosic fibers and the acetylated fibers appear to be “cleaner” than raw materials. Untreated and acetylated fibers exhibited adsorption bands in the regions 1730–1745 and 1235–1240 cm−1 attributed to the C=O stretching of carbonyl in the ester and C–O stretching of acetyl groups, respectively. However, the low intensity of absorbance bands in the analyzed regions indicated that the fibers have a low degree of acetylation. Comparison of relative weight loss, defined as (W o − W f)/W o in which W o is the weight of the sample at time 0 and W f is the weight at the end of the test after drying at 70 °C for 2 h, confirmed that acetylation treatment reduced the hydrophilic character suggesting a better wettability with hydrophobic polymer resins could be achieved.
Zou et al. (2012) studied the effect of esterification treatment on the properties of short sisal fiber/PLA biocomposites. Chopped sisal fiber was soaked in acetic anhydride solution at room temperature for 2 h. The fibers were then filtered off and washed with distilled water until free from acid and dried under vacuum at 70 °C. After that, composites with 10 wt% treated sisal fibers and PLA polymer matrix were compounded by compression molding technique. They observed that the acetylation treatment improved the mechanical properties of composites suggesting that the anhydride groups covalently link with sisal fiber enhanced the interfacial adhesion.
4.1.3.2 Esterification with Fatty Acids
Fatty acids, such as stearic acid, or fatty acid salts are used to esterify the hydroxyl groups of lignocellulosic fibers (Saha et al. 2016). The hydroxyl groups of lignocellulosic fibers react with the carboxyl groups reducing the hydrophilic character of the fiber and improving the wettability between lignocellulosic fibers and hydrophobic polymer matrices. Therefore, the adhesion between lignocellulosic fibers and polymer matrix could be improved and better mechanical performance would achieve. The possible reaction between hydroxyl groups of lignocellulosic fibers and stearic acid is given in Fig. 13.
Paul et al. (2010b) studied the influence of esterification treatment on the mechanical properties of composites based on PP matrix and short banana fibers. Alkali pretreated banana fibers were soaked in 1% stearic acid in alcohol for 1 h and dried in an air oven at 60 °C for 1 h. They observed that the carboxyl groups of the stearic acid reacted with hydroxyl groups of the fiber through an esterification reaction, and hence, the treatment reduced the number of hydroxyl groups available for bonding with water molecules. As a consequence, they reported that the stearic acid treatment lowered the acidity and polarity values of banana fiber improving the wettability with hydrophobic polymer matrices. SEM micrographs of the stearic acid treated fiber surface revealed more fibrillation and roughness than untreated banana fibers. Therefore, the mechanical adhesion between banana fiber and PP could be improved. They characterized PP/banana fiber composites mechanical properties with 50% fiber loading and they observed that stearic acid treated fiber composites showed a slight enhancement of tensile and flexural properties compared to the composites based on the untreated fibers.
4.1.3.3 Esterification with Maleate Groups
Maleate coupling agents could provide an efficient link between the fiber surface and matrix. During grafting, maleic anhydride functional groups react with the hydroxyl groups of lignocellulosic fibers (Kabir et al. 2012). Maleic anhydride is not only used to modify fiber surface but also the polymer matrix, such as PP and PE, to achieve better interfacial bonding and mechanical properties in composites (Gassan and Bledzki 1997; Joseph et al. 2003; Lu and Chung 2000). For example, the PP and PE chains allow maleic anhydride to be cohesive and produce maleic anhydride grafted polypropylene (MAPP) and polyethylene (MAPE) copolymers, respectively. After that, the treatment of cellulose fibers with hot MAPP and MAPE copolymers provides covalent bonds across the interface (Bledzki and Gassan 1999; Li et al. 2007). The mechanism of reaction between maleate copolymer and lignocellulosic fiber consisted on the activation of the copolymer by heating and the subsequent esterification of cellulose fiber (Bledzki et al. 1996). After the treatment, the surface energy of lignocellulosic fiber is increased to a level much closer to the surface energy of the matrix improving the wettability of fiber and esterification provides higher interfacial adhesion between fiber and polymer matrix (Li et al. 2007).
In previous works (Arbelaiz et al. 2005a, b) flax fibers were treated with maleic anhydride (MA) and with maleic anhydride polypropylene copolymer (MAPP). It was observed that untreated fiber showed a tensile strength and modulus of 802 MPa and 46.9 GPa, respectively. After MA treatment, tensile strength, and modulus decreased around 15 and 30%, respectively. However, MAPP treated flax fibers showed slightly higher tensile strength values whereas the Young modulus of MAPP treated fibers were similar to untreated flax fibers. It was suggested that the increments could be explained by the deposition of MAPP copolymer on the surface of the unit cells becoming the surface uniform and smooth. Contact angle measurements showed that after treating flax fibers, they became more hydrophobic being the polar component of surface energy similar to that for neat PP. Moreover, it was observed that after the treatment with MAPP, the interfacial shear strength (IFSS) value was improved around 15%. Flexural and tensile properties of flax/PP composites with 30 wt% fiber content showed that MAPP treatment increased flexural and tensile strength indicating an improvement of stress transfer from the matrix to flax fiber bundle. However, it was reported that MA treatment seemed not to act as stress transfer bridge between PP and flax fiber bundle. Even though the wettability was improved after MA treatment, composite tensile, and flexural results were not improved due to lack of adhesion efficiency.
Cisneros-Lopez et al. (2016) studied the effect of fiber surface treatment with maleic anhydride grafted polyethylene copolymer (MAPE) on the mechanical properties of PE/agave fiber composites. In this case, 1 wt% of MAPE was dissolved in 1,2,4-trichlorobenzene (TCB) at 90 °C. Then, alkali pretreated agave fibers were left for 30 min in the solution at the same temperature under high-intensity mixing. At least, the MAPE treated fibers were dried for 5 days in an oven at 80 °C for complete TCB removal. They observed that the MAPE treated fiber FTIR spectrum showed two strong bands at 2918 and 2852 cm−1 which are the characteristic bands of the vibration C–H groups of alkane associated with the polyethylene chains of MAPE that were grafted on the fiber surface (Benitez et al. 2013; Mohanty and Nayak 2006). SEM micrographs of MAPE treated fibers showed a smoother surface than untreated fibers with a more uniform texture confirming that MAPE was effectively grafted on the agave sisal surface. Moreover, they said that the significant decrease in fiber surface porosity observed as a result of MAPE treatment was also a sign that MAPE was able to penetrate this porosity and grafted with the alkali pre-treated fibers. Both phenomena will have a positive effect on fiber/matrix interactions in terms of adhesion, wettability, and dispersion (Jandas et al. 2013). After that, they compounded PE/agave fiber composites with a fiber content of 15 wt%. Composited based on the MAPE treated fibers showed significant improvements in tensile strength by up to 38% (from 13 to 18 MPa) compared with composites based on untreated fibers and 13% with respect to neat polymer matrix. Same behavior was observed in flexural and impact properties. They suggested that the improvements were related to better affinity, compatibility, and adhesion between agave fiber and PE polymer matrix.
4.1.4 Reaction with Free Radicals
4.1.4.1 Peroxide Treatment
Peroxide is a chemical compound with the specific functional group RO–OR containing the divalent ion bond O–O (Zhou et al. 2016). Lignocellulosic fiber surface can be modified with organic peroxides such as benzoyl peroxide, (C6H5CO)2O2, and dicumyl peroxide, (C6H5C(CH3)2O)2 (Zhou et al. 2016; Li et al. 2007). Fibers were soaked with an organic peroxide in an acetone solution for about 30 min after alkali pretreatment (Paul et al. 2010b; Sreekala et al. 2000). Organic peroxides are highly reactive and decompose to create free radicals \( \left( {{\text{RO}} \bullet } \right) \) which can be grafted onto cellulose and polymer matrix chain by reacting with the hydrogen groups of lignocellulosic fiber and polymer matrix (Zhou et al. 2016; Li et al. 2007). The free radical reaction initiated by peroxide compound between the polymer matrix and cellulose fibers is shown in Fig. 14.
Goriparthi et al. (2012) soaked jute fibers in 6% benzoyl peroxide acetone solution for about 30 min after alkali pretreatment and jute/PLA composites were compounded containing 50 wt% jute fibers. They observed that after the peroxide treatment, the tensile strength and moduli values of the composites were increased considerably. They suggested that the peroxide treatment produced many structural and chemical modifications on jute fiber surface improving the interfacial bonding mechanism between the fiber and the matrix. The same behavior was observed in flexural properties of peroxide treated composites. Izod impact strength of peroxide treated composites was slightly lower than the untreated composites. Debonding, pull-out, and fiber fractures are the mechanisms of energy absorption during impact and they mentioned that fiber pull-out requires higher energy than that of the fracture of fiber and debonding (Goriparthi et al. 2012). They suggested that high impact strength of untreated composites may be attributed to more fiber pull-outs due to weak interface between the fiber and the matrix. However, in the case of peroxide treated composites, fiber fracture occurred, being the reason for lower impact strength of peroxide treated composites compared to untreated composites.
4.1.4.2 Acrylonitrile Grafting
Grafting acrylonitrile groups on fiber surface is an effective method of surface modification. Acrylonitrile initiates free radicals to react with the cellulose molecules by dehydrogenation and oxidation. Then, the activated free radical sites interact with the matrix. The resulting bond between the fiber and matrix enhances the interlocking efficiency at the interface (Kabir et al. 2012; Kalia et al. 2009).
Khan et al. (2015) grafted bleached jute fibers by acrylonitrile monomer (50 wt% of fiber) in the presence of K2S2O8 as initiator (1 wt% of fiber), and FeSO4 as a catalyst for 90 min at 70 °C in a water bath. Untreated and treated fibers were used for compounding jute/PLA composites by compression molding technique. In order to study the effect of acrylonitrile grafting, bleached jute composites were taken as reference material for comparison. They observed that the tensile, flexural, and impact strengths were increased by 14, 8, and 10%, respectively, in comparison of composites based on bleached fibers suggesting that the compatibility between the polymer matrix and jute fibers was improved after modification. SEM micrographs of the fractured surface of composites based on bleached fibers showed interfacial failure and fiber pull-out suggesting that the fiber/PLA adhesion was not very strong. In the case of acrylonitrile modified composites, the failure surface showed fibers completely covered with the matrix with no fiber pull-out. Therefore, they concluded that the increase of tensile and flexural strength of the composite was due to the improvement of the fiber/matrix interface.
4.1.4.3 Acrylate Grafting
Acrylate groups can be grafted on fiber surface by (i) reaction with free radicals (Li et al. 2007; Kalia et al. 2009) or (ii) an esterification reaction (Kabir et al. 2012). Depending on the polymer matrix used, the most interesting via should be used. In the first via, the acrylate groups can be grafted to cellulose molecule radicals. Cellulose can be treated with high-energy radiation to generate radicals together with chain scission (Li et al. 2007). In the second via, the acrylic acid can react with the cellulosic hydroxyl groups of the fiber and provide more access of reactive cellulose macro radicals to the polymerization medium. The carboxylic acids from coupling agents create ester linkages with the cellulose hydroxyl groups reducing the hydrophilic character of fiber and improving moisture resistance properties (Kabir et al. 2012). The reaction between the hydroxyl groups of lignocellulosic fibers and acrylic acid is given in Fig. 15. Afterward, the grafting of treated lignocellulosic fiber onto the matrix can be initiated by peroxides.
Zhang et al. (2013) immersed alkali pretreated wood fiber in a 0.3 M acrylic acid and 0.01 M benzoyl peroxide solution for 1 h at 85 °C. The volume ratio of water to benzene was 9:1 and benzoyl peroxide was used as an initiator. The acrylic acid treated fibers were soaked in a 6 M sodium chloride and 1 M sodium hydroxide solution or 15 min at room temperature. Then, wood fiber was removed and washed with an abundant amount of distilled water until the unreacted acrylic acid was eliminated. Finally, fibers were dried in an oven at 90 °C for 12 h. After that, the treated wood fiber was mixed with unsaturated polyester resin and composites were compounded. They observed that the flexural and tensile strength values increased as wood fiber content was increased suggesting that the chemical treatments could remove the impurities on the fiber surface and enhance the roughness improving the fiber/polymer adhesion. SEM micrographs of the impact-fractured surface of composites based on the untreated fiber evidenced a poor adhesion between the wood fiber and unsaturated polyesters. Nevertheless, they observed that after treating wood fibers with acrylic acid, SEM micrograph of the impact-fractured surfaces of composites suggested that fiber/polymer interfacial adhesion was improved.
Prasad et al. (2016) soaked alkali pretreated banana fibers in a 1% acrylic acid solution maintaining fiber/solution ratio of 1:15 (w/v) for 20 min. Then, they washed and dried the banana fibers in an air oven at 70 °C for 48 h to obtain acrylic acid treated fibers. By FTIR analysis they observed that the absorbance bands at about 2919 and 2854 cm−1 appeared to be slightly stronger for the acrylic acid treated fiber than untreated fiber, corresponding to the introduction of –CH– and –CH2–groups by the acrylic acid treatment. Furthermore, they observed that the band near 1632 cm−1 for acrylic acid treated fibers was found to be broader, suggesting that some ester groups were grafted on banana fibers. Finally, they observed that the alkali treatment removed noncellulosic compounds. The absorbance band at about 1733 cm−1 related to the carbonyl of hemicelluloses, pectin and wax was not observed in the spectrum of acrylic acid treated fibers. In the same way, the change in the band near 1252 cm−1 was due to the C–O stretching vibration of acetyl groups of lignin which was related to the removal of lignin. The surface of untreated and treated banana fiber was studied by SEM. They observed that untreated banana fibers surface showed the presence of impurities, globular particles, wax, and fatty substances. However, the acrylic acid treated banana fiber surface appeared to be rough with the slight disintegration of the fiber along with fibrillation, which was attributed to the removal of noncellulosic compounds. After that, they compounded LDPE/banana fiber composite with 25 wt% fiber loading. After the acrylic acid treatment, the tensile strength of composites increased around 10% whereas the modulus of composites did not change compared to composites based on the untreated fibers. The increment of tensile strength was related to an improvement in the fiber wetting and bonding with LDPE matrix. In order to study the fractured surface of composites, SEM analysis was carried out. In SEM micrograph of untreated fiber composite, fibers seemed to be detached from the LDPE matrix and pulled-out fibers and voids were observed indicating poor interfacial bonding between fiber and matrix. Nevertheless, after acrylic acid treatment, no pull-out fibers and holes were observed, indicating better interlocking between banana fibers and the polymer matrix.
4.1.5 Etherification Reactions
Modification of cellulosic fibers by etherification reaction can change fiber properties and make it more useful and acceptable for some applications (Kalia et al. 2009; Mansour et al. 1994). Sodium hydroxide plays an important role in forming a charged intermediate species with the fiber, which allows the faster nucleophilic addition of epoxides, alkyl halides, and benzyl chloride (Kalia et al. 2009; Matsuda 1996).
Chen et al. (2012) added 10 g kenaf fiber and 100 ml of 40 wt% NaOH aqueous solution into a three neck round bottom flask with strong mechanically stirring and refluxing with water. The temperature was raised to 110 °C and 50 ml of benzyl chloride was added into the mixture and the reaction was allowed to proceed for different periods of time (90, 180 and 300 min). Then, benzylated kenaf fibers were washed with ethanol and water and dried in the vacuum oven at 60 °C. They observed that the chemical structure of kenaf fibers after the introduction of the benzyl group varied according to the reaction time. All benzylated kenaf fibers spectrum showed that the hydroxyl vibration absorption at about 3500 cm−1 was decreased after benzylation, while the absorptions at 1800–1950, 1600, 736, and 695 cm−1 increased, indicating the formation of mono-substituted benzene rings. Moreover, the band of benzylated kenaf at 1206–1207 cm−1 was assigned to the asymmetric and symmetric axial deformation of the C–O–C bonds of alkyl-aryl ether, which were not observed for the untreated kenaf fiber. In addition, they observed that the benzylation decreased considerably the hydrophilic character of the kenaf fibers because higher contact angle values with a drop of water were observed.
Sun et al. (2016) mixed 10 g of alkali pretreated bamboo fibers with 20 ml of benzyl chloride at 35 °C in a double glass reaction kettle equipped with a reflux condenser and a stirring bar. Then, the solution was heated to 120 °C and the reaction was carried out for 1 h. Finally, benzylated bamboo fibers were washed and dried in a vacuum oven at 73 °C at a pressure of 0.08 MPa for 12 h. Finally, bamboo fiber/PE composites panels containing 10, 20, and 30 wt% fiber loading were prepared using a hot press molding technique. After the benzylation treatment, they observed that the flexural and tensile strength values of composites increased considerably. The tensile and flexural strength of composites with a 30:70 fiber/PE mass ratio increased up to 24.2 and 26.7%, respectively, compared to the values obtained for the untreated bamboo fiber/PE composites. They suggested that the improvement of the mechanical properties of the benzyl treated fiber composites was mainly attributed to the enhancement of the interface compatibility.
4.1.6 Benzoylation Treatment
Benzoylation treatment, an important transformation in organic synthesis, is another treatment used to decrease the hydrophilic character of lignocellulosic fiber and improve fiber/matrix interfacial adhesion (Zhou et al. 2016; Kalaprasad et al. 2004). Prior to react lignocellulosic fiber with benzoyl groups, the lignocellulosic fiber should be initially pretreated with NaOH aqueous solution in order to activate and expose the hydroxyl groups on the fiber surface (Zhou et al. 2016; Kabir et al. 2012). Afterward, the lignocellulosic fiber was treated with benzoyl chloride.
Sampathkumar et al. (2014) activated the hydroxyl groups of areca natural fibers using 6 wt% NaOH solution. For this purpose, areca fibers were soaked in 6 wt% NaOH solution and agitated with benzoyl chloride for 15 min. Then, the treated areca fibers were soaked in ethanol solution for 1 h to remove benzoyl chloride and fibers were washed thoroughly using distilled water and dried in air. They observed that FTIR spectra of benzoylated areca fibers showed an absorption band in the range of 1400–1600 cm−1 due to the C=C stretching of aromatic rings in the treated fibers. The alkali pretreatment resulted in the absence of 1727 cm−1 band suggesting that the alkali pretreatment removed the noncellulosic compounds of areca fibers. Nevertheless, after benzoyl chloride treatment, they observed a carbonyl group absorption in the range of 1700 cm−1 suggesting the ester linkage in the benzoyl chloride treated areca fiber. The SEM micrograph of untreated areca fiber showed a network structure in which the fibrils were bound together by hemicelluloses and lignin. On the other hand, they observed that benzoyl chloride treated fibers showed a large number of pits and much more rough surface due to the removal of noncellulosic compounds. Finally, they reported that after treating with benzoyl chloride, the areca fibers reduced its hydrophilic character and became more compatible with the polymer matrix.
Paul et al. (2010b) soaked chopped banana fiber in 2 wt% NaOH solution for 90 min and agitated with benzoyl chloride for 30 min. Banana fibers were washed with water and dried in air oven at 70 °C. After that, they compounded PP/banana fiber composites with untreated and treated fiber with 50 wt% fiber loading and the mechanical properties of composites were studied. They observed that the tensile and flexural properties of benzoylated fiber composite were found to be higher than those untreated fiber composites. The tensile strength of benzoylated fiber composites improved around 13% whereas the flexural strength improved 6%. On the other hand, they observed that the Young modulus increased from 1521 to 1595 MPa while the flexural modulus improved from 1400 to 1520 MPa. The improvement in mechanical properties of composites based on the benzoylated treated fibers was attributed to the reduction in the hydrophilicity of fibers (Nair et al. 1996; Joseph et al. 2002). In another work, Paul et al. (2010a) studied the fractured surface of PP/banana composites by SEM analysis and they observed that tensile fractured surface of composites based on the benzoylated treated fibers showed a fiber breakage rather than fiber debonding due to better banana fiber/PP adhesion. In SEM micrograph of untreated banana/PP composites can be observed that the tensile rupture was accompanied by the debonding of the banana fibers leaving holes, which indicated a poor adhesion between PP matrix and untreated banana fibers.
4.1.7 Isocyanate Treatment
Isocyanate is reported to work as coupling agent in fiber reinforced composites (Paul et al. 1997; Joseph and Thomas 1996, Sreekala and Thomas 2003). The isocyanate functional group (–N=C=O) reacts with the hydroxyl groups of cellulose and lignins of fiber and a urethane linkage is formed (Li et al. 2007; Kabir et al. 2012). Isocyanate also reacts with the moisture present on the fiber surface and forms urea which can further react with the hydroxyl groups of the celluloses (George et al 2001). This secondary reaction results in higher moisture resistance properties of the fiber and provides better bonding with the matrix to enhance composite properties (Kalia et al. 2009; Kabir et al. 2012). The reaction between the fiber and isocyanate coupling agent is shown in Fig. 16, where R could be different chemical groups such as alkyl which react with the polymer matrix.
Datta and Kopczynska (2015) studied the effect of kenaf modification on morphology and mechanical properties of thermoplastic polyurethane materials. Among other treatments, modification of a blocked isocyanate was carried out. In order to obtain blocked isocyanate, the molten 4,4′-diphenylmethane diisocyanate (MDI) was mixed with methanol in a molar ratio of 1:1 for 30 min to obtain a 20 wt% solution of MDI and methanol in acetone. Then, fibers were soaked in this solution for one week and washed repeatedly with acetone and dried at 100 °C for 2 h. Spectra of blocked isocyanate treatment showed a band at 1595 cm−1 which was associated to the benzene rings of MDI structure and a band at 1541 cm−1 was related to the amide symmetric stretching vibration. SEM micrographs of untreated fibers showed a relatively smooth surface, and small particles of impurities attached to the surface were also observed. In order to study the effect of isocyanate treatment on kenaf/thermoplastic polyurethane composites, they compounded composites with 10 and 30 wt% fiber contents. They observed that isocyanate treated fibers improved the tensile strength values of composites with fiber loading of 10 wt%. However, with 30 wt% content of fibers, they observed that composite tensile strength was not improved. They reported that this fact was due to fiber agglomerations and poor adhesion between matrix and kenaf fibers.
4.1.8 Oxidation Reactions
4.1.8.1 Permanganate Treatment
Permanganate treatment on natural fibers is conducted by potassium permanganate (KMnO4) in acetone solution (Kabir et al. 2012). In permanganate treatment, highly reactive permanganate ions can react with cellulose hydroxyl groups. This treatment could enhance the chemical interlocking at the interface and also provide better adhesion with the matrix (Rahman et al. 2007). Furthermore, permanganate ions could also react with the hydroxyl groups located in lignin constituents reducing the hydrophilic character of the fiber (Kabir et al. 2012; Paul et al. 1997). Higher concentrations than 1% of KMnO4 could degrade the fiber properties (Li et al. 2007; Paul et al. 1997).
Paul et al. (2010b) soaked alkali pretreated banana fiber in 0.5% KMnO4 in acetone for 90 min. By SEM analysis, they found that permanganate could etch the fiber surface and made it physically rougher than untreated ones. In addition, they observed that the contact area between the fiber and the matrix was also increased after treatment as a result of the fiber roughness increment. In consequence, interfacial properties can be improved by mechanical interlocking. Empirical polarity parameters calculated in terms of Kamlet–Taft solvent polarity scale revealed that permanganate treatment lowered the acidity as well as the polarity of the banana fibers improving the wettability of permanganate treated fiber and polymer matrices. They said that the fiber/matrix interactions were dependent on the polarity parameters of the modified banana fiber surface and they suggested that lower polarity of the banana fibers led to better compatibility with PP matrix. Thus, they observed that the tensile and flexural properties were slightly increased for permanganate treated banana fiber/polypropylene (PP) composites.
Zou et al. (2012) studied the effect of permanganate treatment on the properties of short sisal fiber/PLA biocomposites. The chopped sisal fibers were soaked in 0.1% KMnO4/acetone solution for 2 h. Fibers were then taken out and washed many times with distilled water. Finally, sisal fibers were dried in vacuum at 70 °C. Scanning electron microscopy (SEM) micrographs showed that the permanganate treatment roughened the sisal surface improving the interfacial bonding between permanganate treated sisal fiber and the polymer matrix. Composites with PLA matrix and 10 wt% of untreated and permanganate treated sisal fibers were prepared. They observed that permanganate treated sisal fiber reinforced composites showed an improvement in the tensile and impact properties compared to the composites based on untreated sisal fibers. Moreover, fractured surfaces of composites showed that the sisal fibers were tightly connected with PLA matrix suggesting that the adhesion between sisal fiber and PLA was improved after permanganate treatment.
4.1.8.2 Furfuryl Alcohol Modification
This modification is based on the selective oxidation by chlorine dioxide of guaiacyl and syringyl phenols of the lignin, generating ortho- and para- quinones able to react Diels-Alder reaction with furfuryl alcohol (Trindade et al. 2004). The latter were reacted with furfuryl alcohol, creating a coating around the fiber. This modification favored the fiber-matrix interaction at the interface but caused some fiber degradation that affected the mechanical properties (Trindade et al. 2005). The fiber modified with furfuryl alcohol exhibited degradation of hemicelluloses, but cellulose maintained most of its crystallinity (Trindade et al. 2004).
Saw et al. (2011) oxidized coir fiber with an aqueous chlorine dioxide solution, which was prepared by reactions between sodium chlorite and acetic acid in aqueous medium. After oxidation reaction, the yellow-red colored fibers were washed with distilled water until neutrality. Then, the oxidized coir fibers were impregnated with furfuryl alcohol and heated at 100 °C for 4 h in presence of N2 flow. The excess of furfuryl alcohol was removed by soxhlet extraction using ethanol for 15 h. Finally, fibers were dried at 50 °C for 24 h. They observed that a broad adsorption band at 3700–3300 cm−1 region characteristic of the polymeric association of the hydroxyl groups and bonded –OH stretching vibration present in cellulose, hemicellulose, and lignins, decreased after the chemical treatment. They explained that the results reflected the decrease of phenolic/aliphatic hydroxyl groups in the fiber lignins after the oxidation reaction. Furthermore, they observed that after the modification of fibers, a decrease in intensity was observed for an aromatic band of lignin unit at 1606, 1512, and 1424 cm−1. Scanning electron micrographs of untreated coir fibers showed the presence of a large number of regularly placed holes or pits and layer over layers of sheet substances like wax of fatty substances on the surface of unmodified fibers. After oxidation, the surfaces of the fibers became rougher due to the removal of the inter-cellulose binding materials and the amorphous waxy cuticle layer. Nevertheless, the SEM image of furfuryl alcohol grafted fibers showed lower surface roughness compared to oxidized treated fibers. Furthermore, to determine the surface roughness of the untreated and treated fiber, atomic force microscope was used. They observed that the untreated and oxidized treated coir fibers exhibited a root mean square roughness values of 71 and 135 nm, respectively, whereas the furfuryl alcohol grafted fiber exhibited a surface roughness of 85 nm proving that the surface of the fiber was covered with furfuryl alcohol. This results suggested that the mechanical adhesion between modified coir fibers and polymer matrix could be improved. They also observed that the oxidation and furfuryl alcohol modifications increased the water contact angle of coir fiber but, decrease the contact angle with other low-polar liquids like glycerol or ethylene glycol indicating the reduction of hydrophilic character of the modified fiber. This meant that the wettability between the modified coir fibers and hydrophobic polymer matrix could be improved.
In another work, Saw et al. (2013) studied the effect surface treatments on luffa fiber reinforced epoxy composites. Luffa fibers were firstly oxidized and then furfuryl alcohol was grafted on oxidized luffa fiber as detailed previously (Saw et al. 2011). After that, untreated and treated luffa fibers mats were separately impregnated with epoxy resin for fabricating the composites. In each composite, the weight ratio of matrix to fiber was maintained at 70:30. They observed that the chemical treatment of the fiber improved chemical bonding and helped it to withstand high tensile load by the composites made of modified luffa fibers. They observed that for furfuryl alcohol grafted fiber composites, the tensile strength and modulus values were increased by 100 and 123%, respectively, due to the wettability and the mechanical adhesion between modified luffa fiber and epoxy polymer matrix were improved. Finally, they found by SEM images that furfuryl alcohol grafted luffa fibers were well embedded in the epoxy matrix and many fewer fiber pull-out and holes were observed compared to that of composites based on untreated fibers suggesting that the furfurylation is an adequate surface treatment to improve the mechanical adhesion between lignocellulosic fiber and the epoxy polymer matrix.
4.2 Physical Treatments
Physical treatments do not change considerably the chemical composition of the lignocellulosic fibers but structural and surface properties of the fiber change considerably. Usually, physical treatments are used as a preparation stage for chemical treatments (Bataille et al. 1994). By physical treatments, fiber bundles can be separated into individual filaments or fiber surface can be modified (Mukhopadhyay and Fangueiro 2009). Methods like a steam explosion is used, when a separation of the bundles into individual filaments is required. However, plasma treatments, dielectric-barrier discharge technique or corona discharge treatments are employed when modification of the fiber surface is desired.
4.2.1 Steam Explosion Treatment
Steam explosion process separates the lignocellulosic fiber into its main components, namely cellulose, lignins, and hemicelluloses (Josefsson et al. 2002). This process involves heating lignocellulosic fibers at high temperature and pressures, followed by mechanical disruption of the pretreated materials by violent discharge into a collecting tank (Kestur et al. 2009). Steam explosion process can include catalysts at different concentrations and different conditions of temperatures, pressures, and times are used. The conditions used in steam explosion process depend on lignocellulosic fiber type (Kestur et al. 2009).
Hou et al. (2014) prepared lightweight composites based on PP and cotton stalk fibers treated with steam flash explosion (SFE) process. Treated cotton stalk fibers were carded with the flip cotton machine for two times and then blended with PP matrix to prepare composites using compression molding technique. They noticed that after treating SFE fibers with an alkali solution (SFE-AT), composite tensile strength and Young modulus values were 47.5 and 27.0% higher than the values obtained for composites reinforced with SFE treated fibers. The fractured surface of composites was studied by SEM and they observed that in composites reinforced with SFE treated fibers, cotton stalks fibers were pulled-out and fibers were hardly coated by PP matrix, which revealed a poor fiber-matrix adhesion. In contrast, in composites reinforced with SFE-AT fibers, cotton stalks fibers were coated by the PP matrix. Moreover, most of the fibers were fractured suggesting that the cotton stalk fibers were strained up to their breaking point. Therefore, they suggested that the interfacial adhesion was improved due to the removal of noncellulosic compounds and lower moisture regain of alkali treated fibers. SEM micrographs showed that SFE-AT cotton stalks fiber had a smaller diameter and more grooves on the surface compared with the non-alkali treated SFE fibers. Besides, SEM micrographs suggested that alkali treatment removed noncellulosic compounds from fiber surface (Wang et al. 2009) and steam explosion could dissolve some pectin and hemicelluloses (Ibrahim et al. 2010). On the other hand, PP composites reinforced SFE-AT fibers exhibited the smallest thickness swelling due to water absorption. They suggested that the water absorption of composites was mainly due to the diffusion of water molecules into the interface between cotton stalk fibers and PP. A better adhesion between PP and SFE-AT fibers was obtained and consequently, there was less space in the interfacial region where water molecules can diffuse.
4.2.2 Plasma and Corona Treatments
The plasma discharge can be generated by either corona treatment or cold plasma treatment. Both methods are considered plasma treatment when ionized gas has an equivalent number of positive and negative charged molecules that react with the surface of the present material (Young 1992). The difference between the corona treatments and cold plasma treatments is the frequency of the electric discharge. At high-frequency, cold plasma can be produced by microwave energy, while a lower frequency alternating current discharge at atmospheric pressure produces a corona plasma (Young 1992). Depending on the nature and composition of feed gases, a wide range of surface modifications can be achieved. One advantage of plasma treatment, among others, is the short time required for surface treatment.
Bozaci et al. (2013) studied the effect of plasma treatment on flax fiber surface properties and flax fiber/high-density polyethylene (HDPE) adhesion. Flax fibers were treated during 2 min by air and argon gas at different plasma powers; 100, 200, and 300 W, respectively. They observed that argon plasma treatment was slightly more harmful than air plasma treatment because worst tensile strength values were obtained. In fact, after air and argon plasma treatment of flax fibers at 300 W, tensile strength decreased at about 9 and 12%, respectively, respect to untreated fibers ones. The fiber surface observed by SEM showed a smooth surface for untreated flax fiber whereas plasma treated fiber surface exhibited tiny grains and roughness, increasing surface roughness when plasma power was increased. They observed that argon plasma treated fibers had more grooves and grains than air plasma treated ones due to the higher etching tendency of the argon plasma. Consequently, air plasma treated flax fibers seemed to be smoother than that argon plasma treated flax fiber ones. Pull-out test results showed that after plasma treatment, flax fiber/HDPE interfacial shear strength (IFSS) values became higher than that of untreated flax fiber. They suggested that IFSS value improved due to the increase of roughness and contact area between the flax fiber and the HDPE polymer matrix.
Sever et al. (2011) studied the mechanical properties of jute/HDPE composites. Jute fibers were modified by oxygen plasma with different frequencies and discharge powers for 15 min. Jute/HDPE composites were prepared using two plies of jute fiber mats and three plies of HDPE sheets. Then, the composite laminate was heated beyond its melting temperature without applying any pressure. In the second step, the composite laminate was pressed at 195 °C at a pressure of 10 MPa for 15 min. For jute fiber/HDPE composites, they observed that after oxygen plasma treatment, the interlaminar shear strength (ILSS) value of composites was increased. In low frequency, ILSS value of oxygen plasma treated jute/HDPE composite increased up to 7.7 and 8.5 MPa, respectively, when 30 and 60 W of plasma power was applied. On the other hand, in radio frequency, the ILSS values of composites were enhanced when the plasma power rose up to 90 W increasing ILSS values of the composites by 65% for 30 W, 84% for 60 W, and 189% for 90 W in comparison with composites based on the untreated fiber ones. They suggested that the increment was related to the improved interfacial adhesion between the fiber surface and the HDPE matrix. The flexural and tensile strength values of jute fiber/HDPE composites improved after plasma treatment. In low frequency, when jute fibers were treated with oxygen plasma power at 60 W, the tensile, and flexural strength values of jute fiber/HDPE composites were increased around 31 and 45%, respectively. On the other hand, in radio frequency, they observed that the tensile and flexural strength values improved with increasing the plasma power. Even though jute fibers were treated with greater radio frequency plasma power, the tensile, and flexural strength of composites did not decrease suggesting that the interfacial adhesion between fiber surface and HDPE polymer matrix was improved. SEM micrographs of the fractured surface of untreated jute fiber/HDPE showed that jute fibers were pulled-out from the thermoplastic matrix and the fibers had a smooth and clean surface with no matrix adhered suggesting that interfacial adhesion between untreated jute fibers and HDPE matrix was very poor. In SEM micrographs of composites reinforced with oxygen plasma treated fibers, fiber surface was covered with large quantities of the matrix and a great number of fibers were appeared to be embedded in HDPE matrix, indicating a better fiber/matrix adhesion.
Sinha and Panigrahi (2009) studied the effect of plasma treatment on structure and wettability of jute fibers as well as on the flexural strength of jute/unsaturated polyester composites. Jute fibers were treated by argon at a plasma power of 20 W varying the exposing time from 5 to 15 min. They compounded unsaturated polymer resin composites with 15 wt% of untreated and plasma treated jute fibers. They observed that the surface of untreated jute fiber was smooth while plasma treated fiber presented a rough surface. Moreover, they found that plasma treatment increased the contact angle of jute fiber with water, therefore decreasing its polarity. The decrease of the polar component was more pronounced when higher was the exposure time of plasma treatment. Thus, the wettability of jute fibers and polymer matrix may be increased. The increase of hydrophobicity was corroborated by FTIR analysis, since they observed that after plasma treatment the signal of characteristic bands of hydrophilic groups, such as carboxylic and hydroxyl groups were decreased. For these reasons, composites reinforced with plasma treated fibers during 10 min showed the highest flexural strength values. The flexural strength value improved about 14% compared to composites reinforced with untreated jute fibers. SEM micrograph of the fractured surface of composite reinforced with 15 min plasma treated fiber showed improved fiber/polymer adhesion. Nevertheless, the mechanical properties of composites were decreased respect to composites based on the 10 min treated fibers. This fact suggested that after 15 min plasma treatment jute fibers were degraded lowering the strength of the composite. In fact, SEM micrographs of 15 min plasma treated fiber showed the formation of pits on the fiber surface, corroborating that 15 min exposing time could damage the fiber.
4.2.3 Dielectric-Barrier Discharge Treatment
In dielectric-barrier discharge (DBD) treatment, glass, or ceramic barrier is placed between two electrodes connected to a very high-voltage alternating current (AC) generator. The advantage of this method is that the electrodes are not in close contact with the plasma (Mukhopadhyay and Fangueiro 2009). High-voltage alternating current produces random arc in a matter of milliseconds in the discharge gap which ionizes the gaseous molecules present within the gap and hence produce plasma. The conventional setting is a coaxial glass tube connected to the continuous alternating power source. The produced plasma depends on the gas pressure in the tube and hence the chance of collision between the molecules.
Li et al. (2014) studied the effect of helium plasma treatment on ramie fiber/polybutylene succinate (PBS) adhesion. Fibers were washed successively with acetone and distilled water followed by drying in an oven. Then, fibers were soaked in ethanol for 10 min with a mass gain of 5.0% before plasma treatment. The DBD was generated between two parallel copper electrodes. A power supply of 15.98 kHz frequency was applied to generate the plasma between the electrodes within a gap of 10 mm. In the process, helium was used as the treatment gas and treatment time was set up on 30 s. In addition, four voltages of 1.5, 3, 6, and 9 kV were chosen to treat ramie fibers. Surface morphologies of the untreated and treated ramie fibers were observed by SEM. Untreated ramie fibers had a relatively smooth yet naturally streaked surface while the treated fibers showed the different extent of plasma etching. Moreover, they observed that the fiber roughness increased with the increase of plasma treatment voltage. Many visible spots, small notched and large area of protuberances were observed especially for fibers treated with 9 kV. This fact suggested that treatment conditions could damage ramie fiber properties. The effect of different plasma treatment voltage on surface wettability of ramie fibers was investigated using dynamic contact angle analysis. They found that the advancing contact angle of untreated ramie fiber was 55.9° and plasma treated fiber with 1.5 kV voltage showed similar contact angle. Nevertheless, the contact angles of fibers treated with 3, 6, and 9 kV notably increased probably due to the plasma treatment induced grafting of ethyl groups onto the fiber surface. Additionally, they corroborated this suggestion by X-ray photoelectron spectroscopy (XPS) technique. Therefore, it was thought the fiber/matrix adhesion would be improved since the wettability of ramie fibers and the fiber surface roughness was increased. Thus, the IFSS values of ramie/PBS composites were determined using microbond pull-out test. Compared with untreated ramie fiber/PBS, the IFSS value for fiber treated with the lowest voltage, 1.5 kV, did not show significant differences with respect to untreated fiber/PBS system. Meanwhile, the IFSS value for fibers treated with 3, 6, and 9 kV raised about 29.6, 45.5, and 19.4%, respectively, compared to untreated fiber ones due to the combination of the better chemical compatibility and improved mechanical interlocking between the fiber and polymeric matrix.
Ragoubi et al. (2012) treated miscanthus fibers by corona discharge treatment (CDT). Fibers were treated in CDT device on a dielectric barrier technique. The treatment was assured by a low-frequency high-voltage generator (typically 15 kV, 50 Hz) and the samples were disposed between the electrodes and treated for several minutes. After the CDT, obtained XPS results evidenced that in treated samples there was an increase in oxygen content and a decrease in carbon content one. The increase of the O/C ratio was attributed to the surface oxidation generated by the corona treatment. On the other hand, SEM micrographs showed that after treating fiber for 15 min, some little cracks appeared on the surface of miscanthus fiber. For longer treatment time, the fibrils were pulled-off of the fibers resulting in the formation of some cavities that could be observed after 45 min. They observed that CDT produced many activated sites that could react with oxygen to give etching effect. In order to confirm this suggestion, they compounded composites based on miscanthus fiber using PP and PLA as matrices. For PP/treated miscanthus system, an increase in Young modulus, stress, and strength at yield values were observed compared with those values obtained for composites based on untreated fibers. They suggested that the wettability of miscanthus fibers was increased by CDT. Also, they suggested that the improvement of interfacial properties could be attributed mainly to a mechanical anchorage. The fractured surface of composites was observed by SEM and they reported that in PP composites reinforced with untreated miscanthus, the fiber surface appeared smooth and clean. At higher magnification, the pull-out fiber was observed indicating poor fiber/PP interfacial adhesion. On the other hand, fractured surfaced of composites based on the treated fibers showed that miscanthus fibers were covered with matrix and there were no signs of pull-out fiber which means that the interfacial adhesion between PP and miscanthus fiber was improved by corona treatment. Similar behavior was reported on the mechanical properties of PLA/miscanthus fibers after treating fibers by CDT.
4.3 Biological Treatments
Biological treatments are a promising technology due to its several advantages like low energy requirements and mild environmental conditions which make them eco-friendly and economically viable strategy (Kumar et al. 2009). Furthermore, biological modifications can selectively remove noncellulosic materials (George et al. 2014). Biological treatments with microorganisms, such as fungi, bacteria, and enzymes have been used for lignocellulosic fibers treatments (Buschle-Diller et al. 1999).
4.3.1 Fungal Treatment
Fungal treatments produce extracellular enzymes that degrade lignins, as well as extensive range of other noncellulosic materials (Li et al. 2009). It has been reported that fungal treatments produce an extensive system of hyphae which can make fine holes on the lignocellulosic fiber surface and may roughen the surface of the lignocellulosic fiber (Li et al. 2009; Daniel et al. 2004).
Pickering et al. (2007) modified hemp fibers using fungal treatments. Hemp fibers were sterilized in an autoclave for 15 min at 121 °C before fungal treatment. The fungi:hemp ratio was approximately 10:12 (mg/g) and incubation were carried out at 27 °C for 2 weeks. After fungal treatment, fibers were sterilized and washed for 10 min before being dried in an oven at 80 °C. The fungi used for hemp fiber treatment were Phanerochaete sordida, Pycnoporus species, Schizophyllum commune, Absidia, and Ophiostomn floccosun. The first three belong to white rot fungi group, whereas the fourth and the fifth belong to zygomycetes and ascomycetes groups, respectively. They observed that the untreated fiber surface appeared smooth and glossy, whereas after fungal treatment with white rot fungi, the surface of fibers led to less glossy and striations becoming more visible along the fiber. They observed by XRD analysis that after all fungal treatments, crystallinity values of fungal treated fibers increased slightly respect to untreated fibers supporting the removal of noncellulosic compounds. In addition, the removal of noncellulosic compound was corroborated by TGA analysis since after the fungal treatment hemp fiber were thermally more stable than untreated ones. However, they observed that after the fungal treatment, the tensile strength values of treated fibers were reduced respect to untreated fibers, especially, when hemp fibers were treated with Schizophyllum commune fungal. They observed that tensile strength reduced about 50% suggesting that the fiber strength reduction could be related to the cellulose degradation during fungal treatment. After the characterization of untreated and fungal treated fibers, they compounded composites with 40 wt% hemp fibers and PP matrix and they added 3 wt% MAPP coupling agent. Despite the tensile strength values of all fungal treated fibers were reduced respect to untreated fibers, composites reinforced with fungal treated fiber showed higher tensile strength values than composites reinforced with untreated hemp fibers. Thus, the highest tensile strength value was observed in composites based on fibers treated with Schizophyllum commune. Hemp fiber treated with Schizophyllum commune showed the lowest fiber tensile strength value, therefore, composites tensile strength data suggested that the interfacial bonding between PP and hemp fibers were greatly improved due to the removal of noncellulosic compounds and the increment of fiber surface roughness.
Li et al. (2009) carried out an analysis of hemp/PP composites using white rot fungal treatments. For fungal treatments, dried hemp fibers were sterilized using gamma radiation. Irradiated fibers were inoculated with white rot fungi for 2 weeks at 27 °C and water was added for all fungal treatments to give a moisture content of 60 wt%. The fungi used for hemp fiber treatment were Phanerochaete sordid, Pycnoporus species, and Schizophyllum commune, respectively. They observed that all fungal treatments reduced the amount of wax, pectin, hemicelluloses, and lignin in the hemp fiber. FTIR spectra of fungal treated fibers showed that the band around 1736 cm−1 attributed to the presence of the carboxylic ester in pectins showed a significant intensity reduction supporting the removal of pectins. In addition, the band around 1268 cm−1, related to the COO stretching in lignins was disappeared in white rot fungi treated fibers. The surface morphology of untreated and treated fibers was characterized by SEM. Untreated hemp fiber showed a large amount of debris adhering to the surface of the fiber bundles, because they were coated with noncellulosic material. After fungal treatment, relatively cleaner surface was observed which supported the removal of noncellulosic compound. Nevertheless, they mentioned that the resolution of the available SEM was inadequate to detect fine holes caused by fungal hyphae attack on fungal treated fiber surfaces. The removal of noncellulosic compound was also corroborated by XRD analysis since the crystallinity values were improved after the white rot fungal treatment. The strength values of white rot fungi treated fibers were lower than untreated fibers. They observed that the tensile strength values of fungal treated hemp fiber decreased at least 23% and the highest decrease, around 38%, was observed for Schizophyllum commune treated fiber. They suggested that the decrease of tensile strength values was related to the creation of fine holes in the fiber surface. After that, they compounded PP/hemp composites with 40 wt% fiber content using 3 wt% MAPP coupling agent. Despite the fungal treatments reduced hemp fiber tensile strength value, the tensile strength values of composites reinforced with white rot fungi treated fibers were higher than composites based on the untreated ones. This fact suggested that the interfacial bonding adhesion between fiber and matrix seemed to be improved due to the removal of noncellulosic compounds and the increase of surface roughness.
4.3.2 Enzyme Treatment
Enzymes are biocatalysts that accelerate biomechanical reactions acting on a specific reactant called “substrate”. All substrates have their own enzymes such as cellulase for cellulose, xylanase for hemicellulose, laccase for lignins, etc. (Karaduman et al. 2012). Enzyme treatments are environmentally friendly and require mild operating conditions (George et al. 2016).
George et al. (2014) studied the surface morphology and thermal properties of lignocellulosic fibers treated with enzymes. Hemp and flax fibers were treated with different enzymes such as xylanase, xylanase + 10% cellulase, polygalacturonase, laccase, and pectinmethylesterase, respectively. The liquid to fiber ratio was maintained at 50:1 (v/w) to facilitate complete wetting of fiber. Enzymatic treatments were conducted for 90 min under constant agitation of 80 rpm at optimum pH and temperature conditions (see Table 4) and enzymes were deactivated by heating at 90 °C for 10 min.
They observed that after treating lignocellulosic fibers with xylanase + 10% cellulase enzymes, hemp, and flax fibers showed a reduction in the cellulosic content whereas lignocellulosic fibers treated with pectinases enzymes (polygalacturonase and pectinmethylesterase) showed a decrease in the hemicelluloses content. In addition, laccase treated flax fibers showed a significant reduction in the lignin content. It was observed from SEM micrographs that after enzyme treatment the elementary fibers became more visible compared to the surface of untreated fibers. On the other hand, they observed that a combination of xylanase and cellulase, and polygalacturonase treated samples exhibited an increase in surface roughness. The onset degradation temperature of enzyme treated hemp and flax fibers were improved due to the removal of pectin and hemicellulose materials. This improvement was achieved when lignocellulosic fibers were treated with xylanase and pectinases enzyme. However, no change in the onset degradation temperature was observed when lignocellulosic fibers were treated with laccase enzyme. On the other hand, they reported that the enzyme treatment resulted in higher surface hydrophilicity because of the removal of lignin and hemicellulose, thereby exposing the cellulosic backbone. In summary, enzyme treatment of lignocellulosic fibers can be used to selectively degrade components that limit the thermal stability and to modify the fiber surface characteristic.
George et al. (2016) studied the mechanical and moisture absorption of enzyme treated fibers/PP composites with 20 wt% of fiber content. They observed that after the enzyme treatment there was no change in mechanical properties. By TGA analysis, they observed that enzymatic treatment improved the thermal properties of the resulting composites. Furthermore, composites based on the enzyme treated fiber showed lower moisture absorption values than untreated fiber composites. Xylanase + cellulase treatments provided the greatest reduction in moisture both for hemp and flax fiber composites. After 4 weeks of immersion in water at room temperature, they observed that the xylanase + cellulase treatment decreased the average moisture absorption of composites based on the hemp fiber around 250% whereas the average moisture absorption of flax fibers reduced around 200%. Finally, in order to study the fiber/matrix adhesion, the fractured surface of the composite was observed by SEM. Composites based on the untreated fibers showed pulled-out fibers due to the poor interfacial adhesion. However, composites based on the enzyme treated samples revealed a better distribution of fibers indicating a better fiber dispersion and fiber/matrix adhesion.
Hanana et al. (2015) treated alfa fibers with several enzymes. The enzyme treatments were carried out after alfa fiber were treated with 0.5 M NaOH solution in autoclave for 20 min at 120 °C with a pressure of 1 bar. The optimal pH and temperature conditions of enzyme treatment are listed in Table 5. For all experiments, the liquid to fiber ratio was maintained at 150:1 (v/w) to facilitate complete wetting of fiber and different enzyme reaction times were used, 2, 4, and 8 h, respectively.
In enzyme treated fibers, the cellulose micro-fibrils were visible indicating that the lignin and other noncellulosic materials were greatly removed from the fiber surface becoming fiber surface rougher. They reported that pectinase enzyme treatment was the most effective for separating alfa fiber into smaller and single fiber due to the removal of materials from the fiber surface and the degradation of polygalacturonan. In consequence, they observed that pectinase enzyme treatment improved the mechanical properties of composite based on the alfa fibers. The tensile strength values of composites based on the pectinase treated fiber improved around 30% compared to alkali treated composites. However, the tensile strength values of composites based on the laccase and xylanase treated fibers did not observe important changes.
5 Conclusion
The use of natural fibers as reinforcement in polymer composites is attracting a lot of interest due to its mechanical properties, processing advantages, and environmental benefits. However, the hydrophilic character of the fibers lowers the compatibility with the hydrophobic matrix which results in poor mechanical properties of the composites. Therefore, it is necessary to modify the fiber surface morphology in order to reduce the hydrophilic character of the fibers and improve the fiber/matrix adhesion. The fiber treatments could modify tensile properties of treated fibers, being often lower than untreated fibers. Nevertheless, composites based on the treated fibers could show higher tensile strength values than composites based on untreated fibers due to the adhesion improvement between the lignocellulosic fiber and polymer matrix after the surface treatments. The adhesion improvement for each fiber/polymer system depends on the fiber treatment type chosen as well as the chemical composition and morphological features of the composite components. In composites based on the lignocellulosic fibers, usually, the chemical treatments are used for fiber surface modification, such as alkali, silane, and acetylation among others. Inadequate treatment conditions could damage fiber structure, decreasing considerably fiber mechanical properties and consequently, the optimization of surface treatment conditions is a key factor to prepare composites with superior mechanical performance. Chemical treatments are the most used to modify fiber surface, however, physical and especially biological treatments, could be promising alternatives to improve lignocellulosic fiber/matrix adhesion because they are more environmentally friendly treatments than chemical ones. In most cases, physical and biological treatments are combined with chemical treatments in order to make more effective treatments.
Abbreviations
- AC:
-
Alternating current
- CDT:
-
Corona discharge treatment
- DBD:
-
Dielectric-barrier discharge
- FTIR:
-
Fourier Infrared Spectroscopy
- HDPE:
-
High-density polyethylene
- IFSS:
-
Interfacial shear strength
- ILSS:
-
Interlaminar shear strength
- MA:
-
Maleic anhydride
- MAPE:
-
Maleic anhydride grafted polyethylene
- MAPP:
-
Maleic anhydride grafted polypropylene
- MDI:
-
4,4′-diphenylmethane diisocyanate
- PBS:
-
Polybutylene succinate
- PE:
-
Polyethylene
- PLA:
-
Poly(lactic acid)
- PP:
-
Polypropylene
- SEM:
-
Scanning electron microscopy
- SFE:
-
Steam flash explosion
- SFE-AT:
-
Steam flash explosion with alkali solution
- TAPPI:
-
Technical Association of Pulp and Paper Industry
- TCB:
-
1,2,4-trichlorobenzene
- TGA:
-
Thermogravimetric analysis
- VTMO:
-
Vinyltrimethoxysilane
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray diffraction
References
Abdelmouleh M, Boufi S, Salah A, Belgacem MN, Gandini A (2002) Interaction of silane coupling agents with cellulose. Langmuir 18:3203–3208
Allaby M (1998) A dictionary of plant sciences, 2nd edn. Oxford University Press, Oxford
Arbelaiz A, Cantero G, Fernández B, Mondragon I, Gañán P, Kenny JM (2005a) Flax fiber surface modifications: effects on fiber physico mechanical and flax/ polypropylene interface properties. Polym Compos 26:324–332
Arbelaiz A, Fernandez B, Cantero G, Llano-Ponte R, Valea A, Mondragon I (2005b) Mechanical properties of flax fiber/polypropylene composites. Influence of fiber/matrix modification and glass fiber hybridization. Composites, Part A 36(2):637–644
Arbelaiz A, Fernandez B, Ramos JA, Retegi A, Llano-Ponte R, Mondragon I (2005c) Mechanical properties of short flax fiber bundle/polypropylene composites: influence of matrix/fiber modification, fiber content, water uptake and recycling. Compos Sci Technol 65(10):1582–1592
Arbelaiz A, Trifol J, Peña-Rodriguez C, Labidi J, Eceiza A (2016) Modification of Poly(lactic acid) matrix by chemically modified flax fiber bundles and Poly(ethylene glycol) plasticizer. In: Visakh PM, Sigrid L (eds) Polyethylene-based biocomposites and bionanocomposites. ISBN: 978-1-119-03845-0
Bataille P, Dufourd M, Sapieha S (1994) Copolymerization of styrene on to cellulose activated by corona. Polym Int 34(4):387–391
Benitez AN, Monzón MD, Angulo I, Ortega Z, Hernandez PM, Marrero MD (2013) Treatment of banana fiber for use in the reinforcement of polymeric matrices. Measurement 46(3):1065–1073
Bledzki AK, Gassan J (1999) Composites reinforced with cellulose based fibres. Prog Polym Sci 24:221–274
Bledzki AK, Reihmane S, Gassan J (1996) Properties and modification methods for vegetable fibers for natural fiber composites. J Appl Polym Sci 59:1329–1336
Bozaci E, Sever K, Sarikanat M, Seki Y, Demir A, Ozdogan E (2013) Effects of the atmospheric plasma treatments on surface and mechanical properties of flax fiber and adhesion between fiber—matrix for composite materials. Compos B 45(1):565–572
Buschle-Diller G, Fanter C, Loth F (1999) Structural changes in hemp fibers as a result of enzymatic hydrolysis with mixed enzyme systems. Text Res J 69(4):244–251
Chen C, Cho M, Kim B, Nam J, Lee Y (2012) Thermo plasticization and characterization of kenaf fiber by benzylation. J Ind Eng Chem 18(3):1107–1111
Cisneros-Lopez EO, Perez-Fonseca AA, Fuentes-Talavera FJ, Anzaldo J (2016) Rotomolded polyethylene-agave fiber composites: effect of fiber surface treatment on the mechanical properties. Polym Eng Sci 56(8):856–865
Daintith J (2000) A dictionary of chemistry, 4th edn. Oxford University Press, Oxford
Daniel G, Volc J, Niku-Paavola ML (2004) Cryo-FE-SEM & TEM immune-techniques reveal new details for understanding white-rot decay of lignocellulose. C R Biol 327(9–10):861–871
Datta J, Kopczynska P (2015) Effect of kenaf fibre modification on morphology and mechanical properties of thermoplastic polyurethane materials. Ind Crops Prod 74:566–576
De Rosa IM, Kenny JM, Puglia D, Santulli C, Sarasini F (2010) Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites. Compos Sci Technol 70:116–122
De Rosa IM, Kenny JM, Maniruzzaman M, Moniruzzaman Md, Monti M, Puglia D, Santulli C, Sarasini F (2011) Effect of chemical treatments on the mechanical and thermal behaviour of okra (Abelmoschus esculentus) fibres. Compos Sci Technol 71:246–254
Efanov MV, Ovchinnikov PV (2012) Acetylation of alkali treated peat. Solid Fuel Chem 46(2):138–141
Egües I (2013) Lignocellulosic materials as new renewable resources: extraction, purification and potential use of hemicelluloses. Ph.D. thesis, Faculty of Engineering, Gipuzkoa, Spain
Fernandes EM, Mano JF, Reis RL (2013) Hybrid cork-polymer composites containing sisal fibre: morphology, effect of the fibre treatment on the mechanical properties and tensile failure prediction. Compos Struct 105:153–162
Focher B (1992) Physical characteristics of flax fibre. In: Sharma HSS, Van Sumere CF (eds) The biology and processing of flax. M. Publications, Belfast, UK, pp 11–32
Gassan J, Bledzki AK (1997) The influence of fiber-surface treatment on the mechanical properties of jute-polypropylene composites. Compos A 28(12):1001–1005
Gassan J, Bledzki AK (1999) Alkali treatment of jute fibers: relationship between structure and mechanical properties. J Appl Polym Sci 71(4):623–629
George J, Sreekala MS, Thomas S, George J, Sreekala MS, Thomas S (2001) A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym Eng Sci 41(9):1471–1485
George M, Mussone PG, Bressler DC (2014) Surface and thermal characterization of natural fibres treated with enzymes. Ind Crops Prod 53:365–373
George M, Mussone PG, Alemaskin K, Chae M, Wolodko J, Bressler DC (2016) Enzymatically treated natural fibres as reinforcing agents for biocomposite material: mechanical, thermal, and moisture absorption characterization. J Mater Sci 51(5):2677–2686
Goriparthi BK, Suman KNS, Mohan N (2012) Effect of fiber surface treatments on mechanical and abrasive wear performance of polylactide/jute composites. Compos A 43(10):1800–1808
Haiping Y, Rong Y, Hanping C, Dong HL, Chuguang Z (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Hanana S, Elloumi A, Placet V, Tounsi H (2015) An efficient enzymatic-based process for the extraction of high-mechanical properties alfa fibres. Ind Crops Prod 70:190–200
Hill CAS, Abdul Khalil HPSA, Hale MD (1998) A study of the potential of acetylation to improve the properties of plant fibres. Ind Crops Prod 8:53–63
Hirota WH, Rodrigues RB, Sayer C, Giudici R (2010) Hydrolysis of acetic anhydride: non-adiabatic calorimetric determination of kinetics and heat exchange. Chem Eng Sci 65:3849–3858
Hou X, Sun F, Yan D, Xu H, Dong Z, Li Q (2014) Preparation of lightweight polypropylene composites reinforced by cotton stalk fibers from combined steam fl ash-explosion and alkaline treatment. J Cleaner Prod 83:454–462
Ibrahim MM, Agblevor FA, El-Zawawy WK (2010) Isolation and characterization of cellulose and lignin from steam-exploded lignocellulosic biomass. BioResources 5(1):397–418
Jandas PJ, Mohanty S, Nayak SK (2013) Surface treated banana fiber reinforced poly (lactic acid) nanocomposites for disposable applications. J Cleaner Prod 52:392–401
Jiang A, Xi J, Wu H (2012) Effect of surface treatment on the morphology of sisal fibers in sisal/polylactic acid composites. J Reinf Plast Compos 31:621–630
Josefsson T, Lennholm H, Gellerstedt G (2002) Steam explosion of aspen wood. Characterisation of reaction products. Holzforschung 56(3):289–297
Joseph K, Thomas S (1996) Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites. Polymer 37(23):5139–5149
Joseph PV, Joseph K, Thomas S (2002) Short sisal fiber reinforced polypropylene composites: the role of interface modification on ultimate properties. Compos Interfaces 9:171–205
Joseph PV, Joseph K, Thomas S, Pillai CKS, Prasad VS, Groeninckx G, Sarkissova M (2003) The thermal and crystallization studies of short sisal fiber reinforced polypropylene composites. Compos A 34(3):253–266
Kabir MM (2012) Effects of chemical treatments on hem fibre reinforced polyester composites. Ph.D. thesis, Faculty of Engineering and Surveying, University of Southern Queensland, Toowoomba, Australia
Kabir MM, Wang H, Lau KT, Cardona F (2012) Chemical treatments on plant-based natural fiber reinforced polymer composites: an overview. Compos B 43(7):2883–2892
Kalaprasad G, Francis B, Thomas S, Kumar CR, Pavithran C, Groeninckx G, Thomas S (2004) Effect of fibre length and chemical modifications on the tensile properties of intimately mixed short sisal/glass hybrid fibre reinforced low density polyethylene composites. Polym Int 53(11):1624–1638
Kalia S, Kaith BS, Kaur I (2009) Pretreatments of natural fibers and their application as reinforcing material in polymer composites—a review. Polym Eng Sci 49(7):1253–1272
Karaduman Y, Gokcan D, Onal L (2012) Effect of enzymatic pretreatment on the mechanical properties of jute fiber-reinforced polyester composites. J Compos Mater 47(10):1293–1302
Kestur SG, Ramos LP, Wypych F (2009) Comparative study of Brazilian natural fibers and their composites with other. In Thomas S, Pothan LA (eds) Natural fibre reinforced polymer composites: from Macro to Nanoscale. Old City Publishing, Inc. Philadelphia, USA
Khan GMA, Shaikh H, Alam MS, Gafur MA (2015) Effect of chemical treatments on the physical properties of non-woven Jute/PLA. BioResources 10(4):7386–7404
Kim JK, Pal K (2010) Surface modifications in WPC with pre-treatment methods. In: Kim JK, Pal K (eds) Recent advances in the processing of wood-plastic composites. Springer, Berlin, pp 23–57
Klemm D, Philipp B, Heinze T, Heinze U, Wagenknecht W (1998) Comprehensive cellulose chemistry: fundamentals and analytical methods, vol 1. Wiley-VCH, Weinheim
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729
Kumar R, Obrai S, Sharma A (2011) Chemical modifications of natural fiber for composite material. Chem Sin 2(4):219–228
Le Moigne N, Longerey M, Taulemesse J, Bénézet J, Bergeret A (2014) Study of the interface in natural fibres reinforced poly (lactic acid) biocomposites modified by optimized organosilane treatments. Ind Crops Prod 52:481–494
Li X, Tabil LG, Panigrahi S (2007) Chemical treatments of natural fiber for use in natural fiber-reinforced composites: a review. J Polym Environ 15:25–33
Li Y, Pickering KL, Farrell RL (2009) Analysis of green hemp fibre reinforced composites using bag retting and white rot fungal treatments. Ind Crops Prod 29(2–3):420–426
Li Y, Zhang J, Cheng P, Shi J, Yao L, Qiu Y (2014) Helium plasma treatment voltage effect on adhesion of ramie fibers to polybutylene succinate. Ind Crops Prod 61:16–22
Lu B, Chung TC (2000) Synthesis of maleic anhydride grafted polyethylene and polypropylene, with controlled molecular structures. J Polym Sci, Part A-1: Polym Chem 38:1337–1343
Lu T, Liu S, Jiang M, Xu X, Wang Y, Wang Z, Gou J, Hui D, Zhou Z (2014) Effects of modifications of bamboo cellulose fibers on the improved mechanical properties of cellulose reinforced poly (lactic acid) composites. Compos B 62:191–197
Mansour OY, Nagaty A, El-Zawawy WK (1994) Variables affecting the methylation reactions of cellulose. J Appl Polym Sci 54(5):519–524
Matsuda H (1996) Chemical modification of solid wood. In: Hon DNS (ed) Chemical modification of lignocellulosic materials. Marcel Dekker Inc, New York, pp 159–184
Merkel K, Rydarowski H, Kazimierczak J, Bloda A (2014) Processing and characterization of reinforced polyethylene composites made with lignocellulosic fibres isolated from waster plant biomass such as hemp. Compos B 67:138–144
Mohanty S, Nayak SK (2006) Mechanical and rheological characterization of treated jute-HDPE composites with different morphology. J Reinf Plas Compos 25(13):1419–1439
Mohanty AK, Misra M, Drzal LT (2001) Surface modifications of natural fibers and performance of the resulting biocomposites: an overview. Compos Interfaces 8(5):313–343
Mondragon G, Fernandes S, Retegi A, Peña C, Algar I, Eceiza A, Arbelaiz A (2014) A common strategy to extracting cellulose nanoentities from different plants. Ind Crops Prod 55:140–148
Mukhopadhyay S, Fangueiro R (2009) Physical modification of natural fibers and thermoplastic films for composites—a review. J Thermoplas Compos Mater 22(2):135–162
Mwaikambo LY, Ansell MP (2002) Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J Appl Polym Sci 84:2222–2234
Nair KCM, Diwan SM, Thomas S (1996) Tensile properties of short sisal fiber reinforced polystyrene composites. J Appl Polym Sci 60:1483–1497
Orue A, Jauregi A, Peña-Rodriguez C, Labidi J, Eceiza A, Arbelaiz A (2015) The effect of surface modifications on sisal fiber properties and sisal/poly (lactic acid) interface adhesion. Compos B 73:132–138
Orue A, Eceiza A, Peña-Rodriguez C, Arbelaiz A (2016a) Water uptake behavior and Young modulus prediction of composites based on treated sisal fibers and poly (lactic acid). Materials 9(5):400–414. https://doi.org/10.3390/ma9050400
Orue A, Jauregi A, Unsuain U, Labidi J, Eceiza A, Arbelaiz A (2016b) The effect of alkaline and silane treatments on mechanical properties and breakage of sisal fibers and poly (lactic acid)/sisal fiber composites. Compos A 84:186–195
Paul A, Joseph K, Thomas S (1997) Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforced with short sisal fibers. Compos Sci Technol 57(1):67–79
Paul SA, Sinturel C, Joseph K, Mathew GDG, Pothen LA, Thomas S (2010a) Dynamic mechanical analysis of novel composites from commingled polypropylene fiber and banana fiber. Polym Eng Sci 50(2):384–395
Paul SA, Joseph K, Mathew GDG, Pothen LA, Thomas S (2010b) Influence of polarity parameters on the mechanical properties of composites from polypropylene fiber and short banana fiber. Compos A 41(10):1380–1387
Pickering KL, Li Y, Farrell RL, Lay M (2007) Interfacial modification of hemp fiber reinforced composites using fungal and alkali treatment interfacial modification of hemp fiber reinforced composites using fungal and alkali treatment. J Biobased Mater Bioenergy 1:109–117
Prasad N, Kumar V, Shishir A (2016) Banana fiber reinforced low—density polyethylene composites: effect of chemical treatment and compatibilizer addition. Iran Polym J 25(3):229–241
Praveen KM, Thomas S, Grohens Y, Mozetic M, Junkar I, Primc G, Gorjanc M (2016) Applied surface science investigations of plasma induced effects on the surface properties of lignocellulosic natural coir fibres. Appl Surf Sci 368:146–156
Rachini A, Le Troedec M, Peyratout C, Smith A (2012) Chemical modification of hemp fibers by silane coupling agents. J Appl Polym Sci 123(1):601–610
Ragoubi M, George B, Molina S, Bienaimé D, Merlin A, Hiver J, Dahoun A (2012) Effect of corona discharge treatment on mechanical and thermal properties of composites based on miscanthus fibres and polylactic acid or polypropylene matrix. Compos A 43(4):675–685
Rahman MM, Mallik AK, Khan MA (2007) Influences of various surface pretreatments on the mechanical and degradable properties of photografted oil palm fibers. J Appl Polym Sci 105(5):3077–3086
Raj G, Balnois E, Baley C, Grohens Y (2011) Role of polysaccharides on mechanical and adhesion properties of flax fibres in flax/PLA biocomposite. Int J Polym Sci 2011:1–11
Ranganathan N, Oksman K, Nayak SK, Sain M (2016) Structure property relation of hybrid biocomposites based on jute, viscose and polypropylene: The effect of the fibre content and the length on the fracture toughness and the fatigue properties. Compos A 83:169–175
Saba N, Tahir P, Jawaid M (2014) A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 6:2247–2273
Saha P, Chowdhury S, Roy D, Adhikari B, Kuk J (2016) A brief review on the chemical modifications of lignocellulosic fibers for durable engineering composites. Polym Bull 73(2):587–620
Sampathkumar D, Punyamurthy R, Bennehalli B, Ranganagowda RP, Venkateshappa SC (2014) Natural areca fiber: surface modification and spectral studies. J Adv Chem 10(10):3263–3273
Saritha M, Arora A, Lata (2012) Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol 52(2):122–130
Saw SK, Sarkhel G, Choudhury A (2011) Applied surface science surface modification of coir fibre involving oxidation of lignins followed by reaction with furfuryl alcohol: characterization and stability. Appl Surf Sci 257(8):3763–3769
Saw SK, Purwar R, Nandy S, Ghose J, Sarkhel G (2013) Fabrication, characterization, and evaluation of luffa cylindrica fiber reinforced epoxy composites. BioResources 8(4):4805–4826
Sever K, Erden S, Gülec HA, Seki Y, Sarikanat M (2011) Oxygen plasma treatments of jute fibers in improving the mechanical properties of jute/HDPE composites. Mater Chem Phys 129(1–2):275–280
Sinha E, Panigrahi S (2009) Effect of plasma treatment on structure, wettability of jute fiber and flexural strength of its composite. J Compos Mater 43(17):1791–1802
Sreekala MS, Thomas S (2003) Effect of fibre surface modification on water-sorption characteristics of oil palm fibres. Compos Sci Technol 63(6):861–869
Sreekala MS, Kumaran MG, Joseph S, Jacob M, Thomas S (2000) Oil palm fibre reinforced phenol formaldehyde composites: influence of fibre surface modifications on the mechanical performance. Appl Compos Mater 7(5):295–329
Sun E, Sun F, Dong Y (2016) Interface morphology and thermoplasticization behavior of bamboo fibers benzylated with benzyl chloride. Surf Interface Anal 48:64–72
Trindade WG, Hoareau W, Razera IAT, Ruggiero R, Frollini E, Castellan A (2004) Phenolic thermoset matrix reinforced with sugar cane bagasse fibers: attempt to develop a new fiber surface chemical modification involving formation of quinones followed by reaction with furfuryl alcohol. Macromol Mater Eng 289:728–736
Trindade WG, Hoareau W, Megiatto JD, Razera IAT, Castellan A, Frollini E (2005) Thermoset phenolic matrices reinforced with unmodified and surface-grafted furfuryl alcohol sugar cane bagasse and curaua fibers: properties of fibers and composites. Biomacromol 6(5):2485–2496
Wang B, Panigrahi S, Tabil L, Crerar W (2007) Pre-treatment of flax fibers for use in rotationally molded biocomposites. J Reinf Plast Compos 26(5):447–463
Wang K, Jiang JX, Xu F, Sun RC (2009) Influence of steaming pressure on steam explosion pretreatment of Lespedeza stalks (Lespedeza crytobotrya): Part 1. Characteristics of degraded cellulose. Polym Degrad Stab 94(9):1379–1388
Xie Y, Hill CAS, Xiao Z, Militz H, Mai C (2010) Silane coupling agents used for natural fiber/polymer composites: a review. Compos A 41(7):806–819
Young R (1992) Activation and characterization of fiber surfaces for composites. In: Rowell R, Shulz TP, Narayan R (eds) Emerging technologies for materials and chemicals from biomass. American Chemical Society, Washington D.C., pp 115–135
Yu Y, Huang X, Yu W (2014) A novel process to improve yield and mechanical performance of bamboo fiber reinforced composite via mechanical treatments. Compos B 56:48–53
Zhang SM, Liu J, Zhou W, Cheng L, Duo XD (2005) Interfacial fabrication and property of hydroxyapatite/polylactide resorbable bone fixation composites. Curr Appl Phys 5:516–518
Zhang H, Cui Y, Zhang Z (2013) Chemical treatment of wood fiber and its reinforced unsaturated polyester composites. J Vinyl Addit Technol 19(1):18–24
Zhou Y, Fan M, Chen L (2016) Interface and bonding mechanisms of plant fibre composites: an overview. Compos B 101:31–45
Zou H, Wang L, Gan H, Yi C (2012) Effect of fiber surface treatments on the properties of short sisal fiber/poly(lactic acid) biocomposites. Polym Compos 33:1659–1666
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Orue, A., Eceiza, A., Arbelaiz, A. (2018). Pretreatments of Natural Fibers for Polymer Composite Materials. In: Kalia, S. (eds) Lignocellulosic Composite Materials. Springer Series on Polymer and Composite Materials. Springer, Cham. https://doi.org/10.1007/978-3-319-68696-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-68696-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68695-0
Online ISBN: 978-3-319-68696-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)