Abstract
Hybrid PET/MR combines two advanced imaging techniques, offering the potential to improve image quality, reduce radiation dose and combine functional information from MR (dynamic contrast-enhanced sequences, diffusion-weighted imaging and magnetic resonance spectroscopy) with PET in the management of lymphoma. The reduction in radiation dose afforded by PET/MR in Hodgkin lymphoma, which is the commonest cancer in teenagers and young adults, is particularly appealing. PET/MR reduces radiation dose by approximately 60–77% compared with PET combined with full-dose diagnostic CT and 20–27% compared with PET combined with lower-dose CT.
To date, studies directly comparing PET/MR with PET/CT suggest they are likely equivalent for staging, although data regarding extranodal involvement are more limited than nodal lymphoma. Whole-body diffusion-weighted imaging (DWI) in isolation has been reported to provide similar staging information to half-body PET/CT without incurring any radiation, but DWI misses a substantial number of lesions and introduces some ‘false positive’ lesions when compared to PET/CT depending on the DWI criteria used to establish involvement, which are not currently standardised. This is a significant limitation when assessing sites of involvement on repeat imaging for response assessment. DWI does not appear to add value in addition to anatomical sequences in PET/MR at the current time.
PET/MR probably underestimates FDG uptake compared to PET/CT and MR attenuation in comparative studies, and correction algorithms will need to be optimised before PET/MR can replace PET/CT in most cases for response assessment using recommended international criteria with the five-point ‘Deauville’ scale.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
20.1 Introduction
Imaging has played a critical role in the management of patients with lymphoma for decades. Although not used to screen asymptomatic individuals, imaging has proven useful at virtually all other stages of the disease including for diagnosis in suspected cases, initial staging, treatment response assessment and recurrence detection and surveillance in high-risk individuals [1]. Imaging provides guidance for biopsies [2] and is used to determine the extent of disease based on identification of lymph node enlargement and extranodal disease [3].
PET/CT imaging with 18F-fluorodeoxyglucose (FDG) offered a significant advancement in the ways in which imaging could be used to manage patients with lymphoma. The metabolic signature generated by FDG not only increases sensitivity for lesion detection compared to CT [4] but carries significant prognostic value and has been proven to monitor treatment response and detect recurrent disease with higher performance compared to conventional imaging [1]. For these reasons, FDG PET/CT is a clinical standard for evaluation of patients with most types of lymphoma.
PET/MRI is an exciting new technology that has the potential to improve the value of imaging in patients with lymphoma even more. Advantages include lower radiation exposure (particularly beneficial for children and young adults) and potentially improved image quality with the use of MR-based motion correction [5]. Simultaneous scanners offer improved registration of PET data and anatomical datasets [6], thus facilitating lesion characterisation and potentially helping direct tissue biopsies. Finally, the biological information derived from advanced MR techniques including dynamic contrast-enhanced MRI (DCE-MRI), diffusion-weighted imaging (DWI) [7] and MR spectroscopy (MRS) [8] is an active area of study that holds promise to potentially improve the ability of imaging to detect viable tumour, better assess treatment response and perhaps one day guide selection of specific treatment regimens. PET/MRI scanners offer the two most advanced imaging technologies combined in one scanner which makes it an excellent research tool.
This chapter will provide the reader with an overview of lymphoma biology, management and treatment, review conventional imaging and then provide a detailed discussion of how PET, PET/CT and MR alone have been used in the management of patients with lymphoma. Following this background, the existing literature studying the use of combined PET and MR data (typically acquired on PET/MRI scanners although studies combining separately acquired PET and MR datasets (‘PET + MR’) will be covered as well) will be comprehensively reviewed. Case examples highlighting the concepts discussed in the emerging PET/MRI literature will be presented. The reader will learn about new PET radiopharmaceuticals that may be relevant to future PET/MRI research, and pitfalls in the PET/MR imaging of patients with lymphoma will be discussed.
20.2 Overview of Lymphoma Biology, Staging and Existing and Emerging Treatments
Lymphomas are the commonest lymphoproliferative disorder worldwide. They are divided into Hodgkin and non-Hodgkin lymphomas.
20.3 Biology
Hodgkin lymphoma (HL) has an annual incidence of 8500 cases in the USA (https://seer.cancer.gov/statfacts/html/hodg.html) and 2100 cases in the UK (http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/hodgkin-lymphoma). HL is the commonest cancer in the adolescent age group, with a further peak in people aged over 45. It originates from mature B cells with the hallmark of classical HL being the Reed-Sternberg cell, which expresses CD30, but which accounts for less than 1% of nodal masses [9]. Tumour cells are surrounded by many benign inflammatory cells including T cells, macrophages, B cells and eosinophils that produce cytokines which promote tumour growth and help the lymphoma to avoid host mechanisms. The abundance of inflammatory cells has been suggested as a reason why HL is so well imaged with FDG and why FDG changes rapidly in response to treatment [10].
Classical HL (cHL) is divided into nodular sclerosing (around 80% of cHL), mixed cellularity, lymphocyte depleted and lymphocyte rich. Nodular lymphocyte predominant HL (NLPHL) is rare, usually negative for CD30 with lymphocyte predominant cells that are similar to germinal centre B cells [9].
The non-Hodgkin lymphomas have an annual incidence of 72,600 cases in the USA (https://seer.cancer.gov/statfacts/html/nhl.html) and 13,600 cases in the UK. Half of the patients are over 70 years (http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/non-hodgkin-lymphoma). NHLs can be divided into B- and T-cell malignancies.
The aggressive B-cell NHLs are a heterogeneous group that arise at different stages of B-cell differentiation. They more often involve extranodal sites than HL. The commonest subtype is diffuse large B-cell lymphoma (DLBCL) which nowadays accounts for nearly 50% of NHL in western countries (http://www.cancerresearchuk.org/health-professional/cancer-statistics). The International Prognostic Index (IPI) is used to predict pretreatment prognosis, and more recently the National Comprehensive Cancer Network-IPI has been shown to have better prognostic value [11]. Gene expression profiling has identified genetic alterations which mean DLBCL can be separated by cell of origin into the germinal centre B-cell (GCB) subtype and non-GCB, usually activated B-cell (ABC) subtype, with worse prognosis for the ABC subtype [12]. Subtypes that include translocation of the MYC gene (MYC+) and/or the BCL2 or BCL6 are especially resistant to treatment. Where both MYC and BCL translocations occur, this is referred to as ‘double-hit’ lymphoma.
The most common type of indolent NHL is follicular lymphoma which accounts for about 20% of NHL (http://www.cancerresearchuk.org/health-professional/cancer-statistics) and is derived from germinal B cells [13]. Taken together HL, DLBCL and FL account for 70% of cases of lymphoma, and most data about PET imaging relates to these subtypes [14].
20.4 Staging
The same staging applies to HL and NHL with a recent modification of the Ann Arbor staging suggested in the Lugano classification (Cheson 2014) as follows:
Stage I—a single lymph node region (I) or a single extralymphatic site (IE)
Stage II—two or more lymph node regions on the same side of diaphragm (II) or stage I or II with contiguous involvement of an extralymphatic site (IIE)
Stage III—nodes on both sides of diaphragm (III) which may include the spleen (sometimes referred to as IIIS)
Stage IV—disseminated extranodal involvement
In Hodgkin lymphoma, the suffix ‘A’ or ‘B’ refers to the absence or presence of systemic symptoms, respectively. Where bulky disease is present, the Lugano classification suggests to record the largest tumour diameter.
20.5 Treatment
Early stage good-risk HL is treated with two to four cycles of adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy and radiotherapy, the length of which is determined by clinical factors and prognostic scores such as the German Hodgkin Study Group and European Organisation for Research and Treatment of Cancer criteria (Europe) and the Eastern Cooperative Oncology Group (USA) [15].
Advanced-stage disease or early stage with poor risk is treated with longer courses of ABVD or bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisolone (BEACOPP) chemotherapy. Radiotherapy is sometimes used to treat patients with initial bulky or residual disease. Progression-free survival (PFS) with ABVD is around 65–75% [16]. PFS with BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisolone) is around 85–90% [17] but is associated with more treatment-related side-effects including haematological toxicity, increased risk of infertility and second malignancies. Which chemotherapy to use in advanced-stage disease is debated. Some argue the more effective BEACOPP chemotherapy regimens should be used, whilst others argue that many patients are cured with ABVD which is less toxic than BEACOPP, which should be reserved for a subset of patients [16, 18]. Recent clinical trials have focused on how to assess which patients would benefit most from ABVD and/or BEACOPP, some of which include a PET response-adapted design.
Patients with refractory or relapsed disease are treated with salvage chemotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT), if fit, and this approach cures approximately 50% of patients [19]. Patients unable to have ASCT may be offered consolidation with radiotherapy and/or palliative chemotherapy.
Recently new agents have been developed with good responses in relapsed and refractory HL. Brentuximab vedotin (BV) is an antibody-drug conjugate targeted against CD30 which was reported to have an overall response rate of 75% and a complete response rate of 34% in patients who progressed after ASCT [20]. Overall PFS was short, but 16/34 patients with complete response remained in remission at a median follow-up of 53 months. BV with AVD has recently been evaluated in a clinical trial ‘ECHELON’ in first-line treatment (https://clinicaltrials.gov/ct2/show/NCT01712490), and results are awaited.
Another promising development in treatment is the immune checkpoint inhibitors. The programmed cell death 1 (PD1) receptor protein and its ligands (PDL1 and PDL2) interfere with T-cell function and enable tumours to evade T-cell attack. Checkpoint inhibitors such as nivolumab and pembrolizumab have demonstrated good responses in patients with Hodgkin lymphoma, most of whom had relapsed after ASCT [21, 22]. Other targeted agents including histone deacetylase (HDAC) inhibitors, e.g. panobinostat; mTOR inhibitors, e.g. everolimus; and phosphatidyl-3-kinase inhibitors, e.g. idelalisib; have shown promising results [23].
DLBCL is most commonly treated with 6–8 cycles of rituximab and cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) with or without consolidation RT which cures about 60–70% of patients [12]. Early stage non-bulky disease may be treated with 3–4 cycles of R-CHOP and involved field radiotherapy (IFRT). The use of rituximab has been associated with significant improvements in outcome for first-line treatment, but patients treated with R-CHOP chemotherapy who relapse have very poor outcomes. The hope is that newer targeted agents will enable therapy aimed at specific molecular subtypes with better patient outcomes.
Recent studies adding the proteasome inhibitor bortezomib in the treatment of non-ABC subtypes to R-CHOP have so far not been shown to improve PFS [24]. Other trials investigating combinations of R-CHOP with the immunomodulatory agents lenalidomide and idelalisib are underway [12].
Second-line treatment options in DLBCL are salvage chemotherapy followed by high-dose chemotherapy and ASCT, but response rates are low. For patients who are not suitable for ASCT, various palliative treatments are available, some of which induce durable remissions [12].
Follicular lymphoma is not curable unless localised and usually follows a relapsing and remitting course over several years. The disease may transform into an aggressive lymphoma. Treatment is aimed at prolonging and maximising the quality of life. Treatment consists of radiotherapy if localised, immunochemotherapy or observation in selected cases until treatment is required. Maintenance treatment is often given for 2 years after immunochemotherapy with the monoclonal antibody, rituximab, which improves disease-free survival but not overall survival [25].
20.6 Overview of Conventional Imaging in Lymphoma
Prior to the advent of FDG PET, PET/CT and advanced MR techniques, x-ray computed tomography (CT) was the standard imaging test for evaluation of patients with lymphoma. To this day, anatomical assessment of disease burden within the lymph nodes, spleen and visceral organs remains critically important and is a key indicator of disease stage and marker of treatment response [26]. Definition of the extent of disease anatomically remains important, given that not all lymphomas are highly FDG-avid, and thus PET imaging with FDG is predominantly used in classical Hodgkin lymphoma and diffuse large B-cell lymphomas. Other histological subtypes including marginal zone lymphoma, small lymphocytic lymphoma and primary cutaneous T-cell lymphomas are less FDG-avid and may be routinely staged by CT alone [14]. The most recent lymphoma staging criteria (Lugano classification) incorporate information from PET but still heavily rely on anatomical disease assessment by CT with short- and long-axis measurements being used to calculate a ‘sum of the product diameters’ as a means of treatment response, for less FDG-avid histologies [26].
Standard x-rays and ultrasound (US) play a much more limited role in the evaluation of patients with lymphoma. US-guided biopsy techniques may be useful for needle biopsies of enlarged lymph nodes or when lymphoma involvement of the liver is suspected. Typically, fine needle aspiration (FNA) can be used as a screening test, but surgical lymph node excision is required to obtain enough tissue to allow full histological, immunologic and molecular biological characterisations of lymphoma [27]. MR-guided or CT-guided biopsies may be reserved for lesions presenting in challenging anatomical locations [28]. Bone marrow staging is typically done by blind biopsy; however, focal lesions identified on CT can prompt more advanced biopsy techniques for characterisation of focal destructive lesions [29].
20.7 PET in Lymphoma
20.7.1 Adult
PET/CT has become the main imaging modality for assessment of aggressive lymphomas. PET/CT, using 18F-fluorodeoxyglucose (FDG), is recommended for staging and response assessment of FDG-avid lymphomas, replacing CT for these lymphoma subtypes in the most recent international guidelines [14, 26]. Most subtypes of lymphoma are FDG-avid, but exceptions that do not consistently take up FDG include marginal zone lymphomas, small lymphocytic lymphoma and some cutaneous lymphomas [30]. Most published data about PET, however, relate to the most common disease subtypes of Hodgkin lymphoma (HL), diffuse large B-cell lymphoma and follicular lymphoma.
Prognostic indices are used to risk stratify patients at diagnosis, but as most include stage, imaging stage is important. PET/CT using FDG is the most accurate staging technique in HL and non-Hodgkin lymphoma (NHL) with increased sensitivity over CT alone, particularly for extranodal disease. A number of publications report changes in stage, using PET/CT with upstaging occurring more often than downstaging and management alterations in a proportion of patients [14]. Most often PET/CT is performed as a low-/intermediate-dose examination without contrast for the purposes of localisation and attenuation correction rather than as a full-dose contrast-enhanced scan. In many subtypes this suffices with evidence suggesting using low-dose PET/CT has little, if any, impact on management [31,32,33,34,35]. There are however situations where contrast-enhanced CT offers superior assessment of disease, such as the assessment of bowel involvement in mantle cell lymphomas [36]. Baseline PET/CT also improves the accuracy of subsequent response assessment [37, 38].
PET/CT is sensitive for bone marrow involvement in lymphomas that have predominantly focal involvement of the marrow, including Hodgkin lymphoma [35, 39] and DLBCL [40,41,42]. In these subtypes, PET/CT is more sensitive than bone marrow biopsy for detecting bone marrow disease. This means that the bone marrow biopsy is no longer considered to be a routine requirement for staging in HL [43]. In DLBCL, reports also suggest that routine bone marrow biopsy does not add value in the majority of patients [44]. PET may however miss small cells in the marrow [45, 46]. When patients have a mix of more indolent disease in the marrow and aggressive large cells in the lymph nodes, this is referred to as ‘discordant disease’. For this reason, omitting biopsy in patients with DLBCL is more controversial [47] even though discordant disease does not confer a worse prognosis and there is no evidence that patients with discordant disease have better outcomes if treatment or follow-up is altered [44]. Similarly, PET may not be sufficiently sensitive to detect low-volume disease comprising 10–20% of the marrow, although again this does not affect prognosis [48]. PET/CT is less sensitive in subtypes with diffuse often low-volume marrow disease, and in follicular and other indolent lymphomas, PET is unable to reliably exclude bone marrow involvement [49].
FDG uptake is higher in aggressive than indolent lymphomas, and PET/CT may be used to target sites for biopsy where there is clinical concern regarding suspected transformation [36, 50, 51].
PET/CT is a reliable tool for assessing remission from disease in aggressive lymphomas [14]. Patients with lymphomas often have residual nodal masses at the end of treatment. A ‘negative’ PET/CT scan excludes the presence of viable tumour cells within masses with a high degree of certainty and has led to the abandonment of the previous response category of complete response unconfirmed which was used to refer to masses on CT thought likely to contain fibrous tissue [52]. The positive predictive value (PPV) is lower than the negative predictive value of course, because FDG is not specific for lymphoma and is taken up in processes with enhanced glycolysis such as infection and inflammation, often treatment related. The PPV is dependent on the subtype and disease prognosis [14]. Residual FDG uptake at the end of treatment may require biopsy in the case of poor prognosis disease when salvage therapy is being contemplated or at the least an interval scan in the case of good prognosis disease where time allows.
Scans performed during treatment are commonly referred to as ‘interim’ scans. In HL the ability of PET to discriminate chemosensitive from chemoresistant disease after 2–3 courses of ABVD treatment [53, 54] led to testing of response-adapted approaches in international trials. These trials are beginning to report results.
In early stage HL, two European studies examined whether radiotherapy could be omitted in patients with complete metabolic response (CMR) on interim PET [55, 56]. PFS was superior with combined modality treatment compared to ABVD alone by approximately 6% at 3 years; however, patients treated with chemotherapy, but without radiotherapy, still had good prognosis with 3y-PFS of around 90%. Longer follow-up will determine if omitting radiotherapy may improve overall survival for some patient subgroups despite inferior PFS, by ameliorating late effects, e.g. cardiopulmonary disease and second malignancies. Omission of radiotherapy is now considered to be an option for some patients with early stage disease treated with ABVD and CMR on interim PET [57].
In advanced HL, an international study reported that bleomycin could be safely omitted from further treatment in cycles 3–6 after an interim scan showing CMR with ABVD treatment with fewer side-effects [58]. 3y-PFS rates were however 85% even in PET-negative patients, which is lower than reported for BEACOPP chemotherapy [17]. Trials are also in progress investigating response-adapted approaches according to interim PET after BEACOPP chemotherapy for advanced-stage disease (https://clinicaltrials.gov/ct2/show/NCT00515554) and using BEACOPP and ABVD sequentially in intermediate-stage disease (https://clinicaltrials.gov/ct2/show/NCT01356680).
For patients who do not achieve CMR or PET ‘negative’ status on interim scans, escalation from ABVD to BEACOPP in early and advanced-stage disease appears to be beneficial, improving PFS [58]. A recent Italian study also reported good outcomes for patients with advanced HL escalated to high-dose chemotherapy and transplant on the basis of a ‘positive’ interim PET scan [59].
At the end of treatment with 6–8 cycles of BEACOPP chemotherapy, a large German Hodgkin Study Group trial reported that consolidation radiotherapy was not required for patients with advanced disease achieving CMR at the end of chemotherapy [17].
In DLBCL, recent reports suggest that CMR on interim PET confers a very good prognosis [60,61,62]. Failure to achieve CMR at interim is associated with a worse prognosis, but even so most patients have PFS rates of around 50%, and unlike HL, in DLBCL treatment options are more limited. So far, most response-adapted treatments based on an interim PET scan showing inadequate response have failed to improve patient outcomes [63,64,65].
The place of interim PET scans in HL is generally accepted. In DLBCL the role of interim PET is more controversial [47], but if interim scanning is performed, then PET/CT is more reliable than CT [66].
In follicular lymphoma, PET/CT performed at the end of chemotherapy and rituximab treatment is predictive of relapse [67], but so far, response-adapted treatments have not been tested.
PET/CT is used in the pre-transplant setting to predict prognosis in both HL [68] and DLBCL [69]. Patients who achieve a complete metabolic response on PET have longer disease-free survival than patients with persistent FDG uptake after high-dose chemotherapy. Patients with a PET-positive scan have been the focus of trials testing alternative regimens or consolidation [70].
The recommended method of assessing response in lymphoma is a five-point scale that compares uptake, if present, with sites of initial disease on a baseline scan using the normal mediastinum and liver as reference regions. The scale is commonly referred to as the ‘Deauville criteria’ after the place where the first international workshop on PET in lymphoma was held, where the method was adopted and later validated in HL, DLCBL and FL [14, 67, 71]. Scores 1, 2 and 3 on the scale are regarded as showing complete metabolic response with standard treatment, although in some clinical trials, scores 1 and 2 have been used to define CMR to avoid the risk of under-treatment when de-escalating therapy [14].
Deauville criteria score the most intense uptake in a site of initial disease, if present, as:
1. No uptake |
2. Uptake ≤ mediastinum |
3. Uptake > mediastinum but ≤ liver |
4. Uptake moderately higher than liver |
5. Uptake markedly higher than liver and/or new lesions |
X new areas of uptake unlikely to be related to lymphoma
20.8 Paediatric
PET has high sensitivity and specificity for staging in paediatric patients [72] and for the detection of bone marrow involvement. Similar to adult practice, high negative predictive values are reported in children for interim and end of treatment PET and PET/CT, although the positive predictive value is more variable [72,73,75]. A low positive predictive value is observed in HL at the end of treatment [73], likely related to the good prognosis of the disease. Radiotherapy is used in intermediate- and advanced-stage HL, and PET/CT is advocated for planning purposes [76]. In the first international study for classical HL in children, patients with early stage disease did not receive radiotherapy after treatment with OEPA, if the early response assessment PET scan was regarded as showing adequate response [77]. In the second international study, this approach has been extended to the intermediate- and advanced-stage groups (https://clinicaltrials.gov/ct2/show/NCT02684708). In this study a less stringent definition of adequate response is being used similar to adults with Deauville scores of 1, 2 and 3 being regarded as CMR. The trial employs a quantitative modification of the Deauville criteria with standardised regions of interest for the residual most intense uptake and the liver [78] referred to as ‘qPET’.
Pitfalls that may make interpretation of scans more challenging in children include the occurrence of thymic hyperplasia/rebound with treatment and the more frequent physiological uptake of FDG in brown fat.
20.9 MR in Lymphoma
MR imaging offers the potential of a radiation-free method to obtain high-quality anatomical images in patients with lymphoma. The high tissue contrast associated with this modality makes it an ideal tool for imaging of the brain and spinal regions. Outside of the central nervous system, the performance of anatomical MR has been more limited. Studies of lymphoma focusing on the bone marrow demonstrate high sensitivity [79] but low specificity resulting from false positives related to regenerating marrow or bone marrow inflammation [80]. MR imaging of the lungs has not yet reached the performance of CT [81], and no anatomical MR technique has offered performance that would replace the information obtained from FDG PET. In contrast to limited results in anatomical MR, developments in the arena of functional MR imaging with whole-body diffusion-weighted imaging (DWI) have yielded interesting results.
20.10 Whole-Body Diffusion-Weighted Imaging
Historically, lymphoma has been staged and restaged using CT, and with the advent of FDG PET and PET/CT, molecular imaging has become the standard of care to detect, stage, restage and monitor treatment response in lymphoma. MRI has typically been reserved for evaluation of more unusual scenarios including primary CNS lymphoma or lymphoma with suspected CNS involvement. Visceral organ infiltration may be evaluated by MRI when conventional imaging is equivocal. More recently, technical advances have yielded whole-body techniques that have been compared to CT and PET/CT. The lack of radiation associated with MR has made this an attractive modality for study, particularly for paediatric patients and young adults.
Whole-body diffusion imaging has been studied at numerous centres as a possible replacement for FDG PET/CT. In a recent meta-analysis of six studies, Regacini et al. reported that whole-body MRI with diffusion imaging agreed with findings on FDG PET/CT (with respect to staging) in 91% of all cases. In some cases, MRI detected additional lesions, but the authors could not fully address the potential for these lesions to represent false positives, citing that imaging artefacts in the chest and normal lymph nodes in the inguinal regions can be difficult to assess [82]. With respect to detection of focal bone marrow involvement, preliminary data suggests good agreement between FDG PET/CT and whole-body MRI with diffusion imaging [83].
With respect to treatment response assessment, preliminary data suggests potential utility of diffusion imaging. Lin et al. reported a mean increase of ADC values from 0.658 to 1.501 in residual enlarged lymph nodes after four cycles of chemotherapy and similar changes in areas of organ involvement [84]. Other authors have reported similar increases in ADC in the setting of treatment response, but just how these findings compare to the performance of FDG PET/CT requires further studies before definitive recommendations for or against DWI can be made in the setting of response assessment. One author has demonstrated significant changes in nodal ADC as early as 4.5 days after starting a first cycle of chemotherapy [85]. Response assessment is more challenging in bone due to changes in red marrow and fatty marrow elements in response to chemotherapy and marrow stimulation, but it has been suggested that an overall increase in ADC is usually associated with a good treatment response [86].
20.11 CNS Lymphoma (Primary and Secondary)
MR imaging has played a more central role in the imaging of known or suspected CNS lymphoma involving the brain parenchyma, meninges, eyes or spinal cord. Most cases of CNS lymphoma present without evidence of disease outside of the CNS, although FDG PET literature has suggested that up to 15% of patients may harbour disease elsewhere [87]. MRI has higher sensitivity than CT and can identify enhancing tumour on the surface of the brain and spinal cord, within the ventricles or in the region of the spinal nerve roots [88, 89]. It is not possible to differentiate between primary and secondary lymphomas of the brain based on MR features alone [90], and therefore body scanning is indicated in patients presenting with CNS lesions.
Primary CNS lymphoma (PCNSL) usually presents as a supratentorial intracranial mass, frequently involving the periventricular white matter and may cross the midline. Smaller deep brain structures can be involved, and a more rare subtype of PCNSL is limited to the dura, usually presenting as a low-grade marginal zone lymphoma [91]. Primary leptomeningeal lymphoma can also occur. Ocular lymphoma may represent direct extension from adjacent structures or in rare cases can originate within the eye [92]. Due to high cellularity, PCNSL typically presents with low to intermediate signal on T2 imaging and relatively low ADC values on diffusion-weighted sequences. Lesions usually enhance on DCE-MRI either homogeneously or peripherally in necrotic lesions [93].
Advanced MR imaging techniques have been employed in the management of PCNSL. Diffusion-weighted imaging research has demonstrated the ADC values are typically lower in PCNSL compared to brain tumours, cellular metastases or toxoplasmosis, but significant overlap remains [94]. ADC has been shown to have prognostic value and can serve as a marker of treatment response [95].
Perfusion imaging based on arterial spin-labelling techniques has demonstrated relatively higher blood flow in gliomas compared to lymphoma and relatively lower values in toxoplasmosis [96, 97]. Vessel permeability measured by DCE-MRI is higher in gliomas than lymphomas [98]. Also, MR spectroscopy has been studied in PCNSL, but overlapping high levels of lipid and macromolecule resonance between PCNSL and toxoplasmosis has limited applications [99]. Susceptibility-weighted imaging is also under study [100]. Unfortunately, none of these techniques are specific enough to obviate tissue biopsy.
20.12 PET/MRI in Lymphoma
Several studies have reported results from hybrid PET/CT and PET/MRI scans performed on the same day in lymphoma patients to assess whether the examinations are equivalent (Table 20.1). It is inevitable with these types of studies that there will be limitations. All include a mix of lymphoma subtypes and patients scanned at different time points—staging, response assessment and sometimes surveillance. Many patients had no disease at the time of the scan, and very few patients in the studies had extranodal disease, certainly lower than what is usually encountered in lymphoma patient populations. PET/MRI was mostly done following PET/CT to avoid the chance that the patient might not complete the diagnostic or ‘standard’ examination.
The gold standard, as with all lymphoma studies, for the presence or absence of lymphomatous lesions is imperfect, because it is not ethical to biopsy lesions for these purposes. Some studies simply used PET/CT as the reference standard; others used a combination of biopsy (where available because clinically indicated), clinical and imaging follow-ups. Nonetheless, despite limitations, some important conclusions can be drawn.
20.13 Diagnostic Performance of PET/MRI Compared with PET/CT
The diagnostic performance of PET/MRI appears to be similar to PET/CT with respect to the detection of nodal disease in common lymphoma subtypes (Figs. 20.1, 20.2, and 20.3). Fewer cases with extranodal disease have been assessed, but most report that extranodal lesions were seen using both modalities. One study which evaluated PET/MRI but did not directly compare with PET/CT reported high sensitivity, comparable to PET/CT using MR for AC only [101]. Studies using anatomical sequences reported equal sensitivity for PET/CT and PET/MRI.
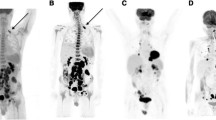
85-year-old male with newly diagnosed diffuse large B-cell lymphoma. FDG PET/MRI demonstrates large mediastinal mass in the posterior mediastinum. Intense FDG uptake (upper middle frame) is seen corresponding to hyperintense foci on diffusion-weighted images (lower left frame) with corresponding relatively low ADC values (lower middle frame; see dark regions anterior to spine and aorta)
77-year-old female with Hodgkin lymphoma. FDG PET/MRI demonstrates intense uptake in left cervical lymphadenopathy. Lymph node margins are well defined on axial HASTE MR (lower left frame), corresponding DW imaging demonstrates high signal (upper right frame) in left cervical adenopathy with corresponding low signal (dark) on ADC images (lower right frame)
Comparative studies also reported equivalent staging using PET/CT and PET/MRI. Staging was identical in all cases performed at baseline in 18 patients with mostly aggressive lymphomas [102]. Herrmann et al. [103] reported that all 188 lesions, which were considered to be ‘positive’ for lymphoma using their reference standard, were detected on both PET/CT and PET/MRI (29 scans). Eighteen of 188 lesions were extranodal. Lesions assessed included patients with HL [28], DLBCL [26] and intermediate- and low-grade lymphomas [28]. Fourteen out of eighty two of these patient scans were performed for primary staging.
Some studies have reported additional lesions on PET/MRI carried out after PET/CT, possibly related to delayed acquisition with increased uptake occurring in lymphomatous lesions over time [102, 104, 105].
In the study by Afaq et al., nodal lesions in the mesentery and the retroperitoneum in one patient and nodal lesions in the axilla in another patient were seen only on PET/MRI, but PET/MRI scans were carried out on average more than 2 h later than PET/CT [102]. For the first patient, this resulted in an assessment of residual metabolic disease on PET/MRI but complete metabolic response on PET/CT. The second patient had additional lesions such that the ‘missed’ axillary lesions did not affect disease status which was the same on PET/MRI and PET/CT.
Regarding extranodal disease, Heacock et al. reported discrepancy in bone marrow involvement in the right femoral neck in a patient with follicular lymphoma [105]. Bone marrow involvement was reported by readers on PET/MRI and DWI but not when reading PET/CT. The lesion was more FDG-avid on the PET component of the PET/MRI which was carried out after PET/CT, but there was also a more conspicuous bone lesion seen on the MR sequences including DWI than on the CT component of the PET/CT [105]. Advantages in the assessment of the bone marrow using PET/MRI have not been demonstrated on other studies, but so far these have included only three patients with BMI [102, 103], and more data are needed.
In one patient a probable adrenal lesion was reported as disease on PET/MRI but not PET/CT. The adrenal lesion reduced in size and activity on follow-up imaging and was deemed to be involved by lymphoma. The PET/MRI scans were carried out on average more than 2 h later than PET/CT [102].
In the studies to date, few patients with lung involvement have been reported [102]. This is an area where theoretically PET/CT might have an advantage, but the sizes of lung lesions in lymphoma are often larger than with solid tumours and were all resolved using PET/MRI. A recent study which included patients with solid cancers and lymphoma reported that the vast majority (97%) of small lung nodules that did not take up FDG and were missed on PET/MRI were likely to be benign, as they resolved or remained stable on follow-up [106].
Interobserver agreement between readers for evaluation of the presence or absence of disease was reported as perfect for nodal sites on PET/CT and almost perfect for nodal sites on PET/MRI in an evaluation of 95 nodal sites by 2 observers [102]. Both readers detected the same eight extranodal sites on PET/CT and nine extranodal sites on PET/MRI. Interobserver agreement for assessment of disease status was perfect.
20.14 Diffusion-Weighted Imaging Compared with PET/CT and PET/MRI Diagnostic Sequences
Authors have concluded that DWI either has no additional value or is inferior to PET/CT and PET/MRI.
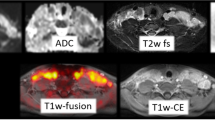
Afaq et al. reported that no additional sites were found on DWI compared to PET/CT and PET/MRI and that staging was identical, concluding that DWI had no additional value in lymphoma [102]. Other studies reported that DWI was inferior to PET/CT and PET/MRI. Herrmann et al. reported that DWI missed 33% of nodal lesions and 44% of extranodal lesions in their study which included 82 patient scans [103]. Lesions were defined as high signal on high b-value DWI using the optimal cut-off derived from their data and low signal on the corresponding ADC map. No threshold for lesion size was used. This resulted in a large number of false positive lesions on DWI as well as poor sensitivity. The authors commented that the lack of standardised criteria, especially with respect to extranodal involvement, contributed to the high false positive rate. The spleen was a particularly difficult area to assess on DWI. Three cases of splenic involvement were missed using DWI, and three other cases showed restricted diffusion in spleens with normal activity on PET/CT [103] (Fig. 20.4). PET/CT and DWI were concordant for imaging stage in only 18/82 scans. DWI upstaged 60 scans including 45 scans where there was no assessable disease according to Deauville criteria on PET/CT.
Demonstrates a patient scanned with PET/MRI (a) and PET/CT (b) after a single administration of FDG on the same day. Note that the spleen has normal activity on FDG PET/MRI (c) but restricted diffusion on DWI (d) (courtesy of Dr. Andrew Mallia, PET Imaging Centre at St Thomas’, King’s College London)
Heacock et al. reported 19/51 (37%) of nodal lesions were missed on DWI with 5 false positive lesions [105]. There was disagreement in stage in 10/28 patients compared to PET/CT, one of which was bone marrow involvement correctly assigned by DWI but also on PET/MRI (as above). Atkinson et al. reported 17% of lesions were missed on DWI in a smaller series of 10 patients with increased FDG uptake on PET/CT and PET/MRI [107]. Four out of eleven of the missed lesions on DWI were in the chest which they attributed to signal loss due to respiratory motion artefact.
The difference between the number of positive lymphoma lesions on DWI compared to PET/CT and PET/MRI did not reach statistical significance, when response and surveillance scans were considered in the study by Herrmann et al. [103]. There were however fewer lesions during and after treatment, and DWI still missed ≥50% of positive lymphoma lesions according to the follow-up criteria.
Fewer lesions were also missed in patients with low-grade disease compared to patients with aggressive lymphoma using DWI, but DWI was statistically inferior for the detection of lymphoma even in this group (p = 0.03). It seems reasonable to conclude that tumour cellularity is inferior to glucose metabolism in assessing lymphomatous disease.
It has been suggested there may be a role for DWI in subtypes that are not routinely FDG-avid such as MALT lymphoma. Giraudo et al. reported higher sensitivity in non-FDG-avid lymphomas when DWI was added to PET/MRI because of low FDG-avidity in six cases of mucosa-associated lymphoid tissue (MALT) lymphoma, one case of mantle cell lymphoma and one case of marginal zone lymphoma [108]. Size criteria were used for non-FDG-avid lesions on CT and MR and for DWI lesions with restricted diffusion. In these less common subtypes, however, international guidelines already recommend that if not FDG-avid, contrast-enhanced CT and/or MR is a better investigation. DWI may possibly have added value. In another small study by the same group looking at response assessment in 15 patients with MALT lymphoma, the change in SUV on interim PET at 3 cycles of treatment was better at predicting CT response at the end of treatment than change in ADC. Changes in ADC in patients with end of treatment complete response on CT showed much larger standard deviations than changes in SUV which the authors attributed to inhomogeneities in the magnetic field and artefacts [109].
20.15 Quantitation
High correlation has been observed for measurement of the maximum standardised uptake value (SUVmax) on PET/CT and PET/MRI in studies involving 158 patients [102, 104, 105, 107]. Grueneisen et al. however found that SUVmax was significantly higher for PET/MRI with a mean difference in SUVmax between PET/CT and PET/MRI of −2.5 (95% CI 3.1 to −7.9) which they attributed to the delay in acquisition of PET/MRI with increasing uptake in lymphoma lesions over time [104].
Conversely other studies have reported that SUVmax was higher with PET/CT even when PET/MRI was performed later, suggesting that PET/MRI may underestimate uptake. In the study by Afaq et al. [102], the mean difference in SUVmax was only 0.32 (95% CI −0.12 to 0.75) when PET/MR imaging was performed over 2 h later than PET/CT (68 patient scans). Heacock et al. [105] reported the mean difference was 1.7 (95% CI −5.8 to 9.2) when PET/MR imaging was performed an average of 1 h after PET/CT.
The mean SUV (SUVmean) was also reported to be significantly higher for PET/CT compared to PET/MRI with SUVmean measured at 20.1 ± 2.1 (PET/CT) and 13.7 ± 1.4 (PET/MRI) [107] where the mean delay between PET/CT and PET/MRI was 151 min. This also suggests that PET/MRI may underestimate intensity of uptake compared to PET/CT. Underestimation or overestimation of SUV measurements with PET/MRI could potentially have implications when measuring response using Deauville criteria.
Afaq et al. reported a moderate inverse correlation between SUVmax and ADC mean in 27 scans where the most FDG-avid lesion corresponded to a measurable lesion on the ADC map [102]. Others have not demonstrated any relationship between SUVmax and SUVmean with ADC values [105, 107].
20.16 Radiation Dose
Radiation dose is reduced in hybrid PET scanning if MR is used instead of CT for anatomical localisation and attenuation correction. The reduction is marked if full-dose ‘diagnostic’ CT is used but more modest if low-dose CT is used which is usually sufficient for staging and response assessment in aggressive lymphomas [31,32,33, 35]. Dose reduction with full-dose CT has been reported to be around 60–77% and with low-dose CT around 20–27% [104, 107]. Dose savings may therefore be considerable but may become less with further advances in CT iterative reconstruction.
20.17 Other Factors
Set-up costs are higher for PET/MRI and duration of scans is generally longer than PET/CT. This translates into higher scan costs, and PET/MRI remains a limited resource in most countries [102]. The scan duration depends of course on the sequences chosen for MR imaging. Using a ‘fast’ protocol that included a coronal 3D volume interpolated breath-hold examination (VIBE) for attenuation correction, DWI, an echo-planar imaging (EPI) sequence, a transverse 2D half Fourier acquisition single-shot turbo spin echo (HASTE) sequence and transverse post-contrast 3D fat-saturated VIBE sequence, Gruniesen reported imaging times of 27.8 ± 3.7 m for PET/MRI versus 17.3 ± 1.9 m for PET/CT using a 4 min per bed acquisition for PET and 4–5 bed positions [104]. Other studies reported imaging times of up to 120 min for anatomical sequences and DWI [108] which is unlikely to be tolerated by elderly or very unwell patients with lymphoma.
Using MR for attenuation correction only, the scan duration for PET/MRI was reported as 23–25 min in another study of which the PET duration was 20–22 min [101]. Using MR just for attenuation correction is however suboptimal as discussed above and results in inferior sensitivity to PET/CT or PET/MRI with anatomical sequences.
20.18 Paediatric PET/MRI Experience
Ponisio et al. performed 9 patient scans in children aged 12–17 years at the time of response assessment. Assessment of response category was the same using PET/CT and PET/MRI, but there were some discordant findings [110]. PET/MRI missed one focus of uptake in a mediastinal mass compared to two foci seen on PET/CT. Conversely PET/CT missed a focus of uptake in the neck compared to two foci on PET/MRI. In one case a renal lesion was not reported on PET/CT because it was obscured by physiological urinary activity but was reported on DWI as it had restricted diffusion. Artefacts were reported on the MR attenuation correction map from dental braces [2], a port catheter [1] and the lungs due to motion artefact [1]; however, there was high correlation in measurements of SUVmax; the average difference was only 1.6% between PET/CT and PET/MRI.
Sher et al. performed 40 patient scans in children with a mean age of 14.6 ± 3.9 years [111]. Sensitivity was similar: 95% with PET/CT and 92% with PET/MRI. Specificity was also similar: 56% with PET/CT and 61% with PET/MRI for individual lesions. Staging was correct in 35 of 40 patients with both modalities, but of these, 29 children had no evidence of lymphoma at the time of scanning. Six lesions were misclassified compared to the reference standard. Two were due to interobserver variation rather than the imaging modality. Three were misclassified on PET/MRI. One reported as mesenteric nodal disease on PET/MRI was reported as physiological uptake in bowel on PET/CT. One dismissed as muscle uptake on PET/MRI was reported as axillary nodal disease on PET/CT. One patient had a left hilar node with lower uptake on PET/MRI (SUVmax 1.6) compared to PET/CT (SUVmax 3.3) which was overlooked on PET/MRI. Dedicated anatomical MR sequences were omitted in this study to reduce scan duration, and this likely accounted for the discrepancies, but scan duration is an issue when imaging children. One lesion was misclassified on PET/CT. A prevascular node was reported correctly on PET/MRI but as physiological right atrial uptake on PET/CT. In one patient a lytic bone lesion that was not FDG-avid was overlooked on both modalities.
This was the only study in those undertaken to date, to perform any PET/MRI scans prior to PET/CT [111]. The authors observed no difference in SUV values when PET/CT was done first but a significant difference when PET/MRI was done first in SUVmax and SUVmean for both benign and malignant lesions identified. This again supports the premise that PET/MRI may underestimate SUV values, as reported in adult studies [102, 105, 107]. In malignant lesions the SUVmax was 6.3 ± 2.8 on PET/MRI and 10.1 ± 4.9 on PET/CT when the PET/MRI was performed first (p < 0.001) but 6.2 ± 3.1 on PET/MRI and 5.9 ± 3.2 on PET/CT when the PET/CT was done first (p < 0.001) [111]. This suggests that differences may be due to attenuation correction being less reliable in PET/MRI. At these levels, the differences could conceivably have an impact on response assessment, although as with other studies, there was no significant difference in accuracy between the techniques compared to the reference standard. Compared with low-dose PET/CT, there was a 45 ± 10% reduction in effective dose with PET/MRI in the children in this study [111].
20.19 Novel Tracers for PET/MRI in Lymphoma
FDG PET has been a mainstay of lymphoma lesion detection, tumour grading, treatment response monitoring and recurrence detection. This agent is not without flaws, as false positives occur due to inflammation and tumour grading by SUVmax is limited by significant quantitative overlap between low- and high-grade histologies. There is a need to develop better tracers to address these limitations.
18F-Fluorodeoxythymidine (FLT) is a synthetic amino acid tracer that has been developed to indirectly measure cellular proliferation in vivo and is of interest for use in patients with lymphoma. Preliminary studies suggest sensitivity that is very similar to FDG, with some suggestion of potentially improved tumour grading compared to FDG [112]. More recent work has demonstrated potential utility in assessing treatment response and determining prognosis during mid-treatment imaging [113]. A question for PET/MRI researchers is to determine if the performance of FLT can be synergistic with MR parameters in a manner that offers advantages over FDG.
Fludarabine is a drug used in the treatment of low-grade lymphomas, often as a part of combination regimens with other drugs. Given the presence of a fluorine atom on this compound, investigators have substituted the fluorine with 18F-fluorine and thus produced a PET-tracer version of this drug. Preliminary murine studies demonstrated rapid uptake in lymphoma cells that was more intense than FDG and minimal background organ activity [114]. Subsequent mouse studies have demonstrated persistent uptake in viable tumour cells following immunotherapy with rituximab [115] and good specificity with lower uptake in inflammatory lesions compared to FDG [116]. Further studies with PET/MRI to correlate the intratumoural distribution of this new agent and relate it to findings on diffusion or DCE-MR imaging sequences may be of interest to further improve lesion detection and monitoring of treatment responses.
Conclusions
-
1.
PET/MRI is likely equivalent to PET/CT in staging for nodal disease and extranodal disease (but there are limited data regarding extranodal disease to date).
-
2.
PET/MRI may underestimate FDG uptake compared to PET/CT, which could have implications for response assessment, and more data are needed. This may improve as attenuation correction algorithms are optimised.
-
3.
DWI will not replace PET/CT or PET/MRI for evaluation of FDG-avid lymphomas and can probably be omitted in PET/MR imaging for lymphoma.
-
4.
PET/MRI reduces radiation dose, although dose savings may be less as CT dose reduces with iterative reconstruction techniques and needs to be weighed against longer scan duration.
References
Wright CL, Maly JJ, Zhang J, et al. Advancing precision nuclear medicine and molecular imaging for lymphoma. PET Clin. 2017;12(1):63–82. S1556-8598 (16)30090-6
Povoski SP, Hall NC, Murrey DA Jr, et al. Feasibility of a multimodal (18)F-FDG-directed lymph node surgical excisional biopsy approach for appropriate diagnostic tissue sampling in patients with suspected lymphoma. BMC Cancer. 2015;15:378. https://doi.org/10.1186/s12885-015-1381-z.
Sin KM, Ho SK, Wong BY, et al. Beyond the lymph nodes: FDG-PET/CT in primary extranodal lymphoma. Clin Imaging. 2016;42:25–33. S0899-7071 (16)30180-2 [pii]
la Fougere C, Hundt W, Brockel N, et al. Value of PET/CT versus PET and CT performed as separate investigations in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2006;33(12):1417–25. https://doi.org/10.1007/s00259-006-0171-x.
Catalano OA, Masch WR, Catana C, et al. An overview of PET/MRI, focused on clinical applications. Abdom Radiol. 2016;42(2):631–44. https://doi.org/10.1007/s00261-016-0894-5.
Rakheja R, DeMello L, Chandarana H, et al. Comparison of the accuracy of PET/CT and PET/MRI spatial registration of multiple metastatic lesions. Am J Roentgenol. 2013;201(5):1120–3. https://doi.org/10.2214/AJR.13.11305.
Sun B, Song L, Wang X, et al. Lymphoma and inflammation in the orbit: diagnostic performance with diffusion-weighted imaging and dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2016;45(5):1438–45. https://doi.org/10.1002/jmri.25480.
de la Pena MD J, Vicente LG, Alonso RC, et al. The multiple faces of nervous system lymphoma. Atypical magnetic resonance imaging features and contribution of the advanced imaging. Curr Probl Diagn Radiol. 2016;46(2):136–45. S0363-0188(16)30007-X [pii]
Mathas S, Hartmann S, Kuppers R. Hodgkin lymphoma: pathology and biology. Semin Hematol. 2016;53(3):139–47. https://doi.org/10.1053/j.seminhematol.2016.05.007.
Meignan M, Itti E, Gallamini A, et al. FDG PET/CT imaging as a biomarker in lymphoma. Eur J Nucl Med Mol Imaging. 2015;42(4):623–33. https://doi.org/10.1007/s00259-014-2973-6.
Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–42. https://doi.org/10.1182/blood-2013-09-524108.
Caimi PF, Hill BT, Hsi ED, et al. Clinical approach to diffuse large B cell lymphoma. Blood Rev. 2016;30(6):477–91. S0268-960X(16)30033-9 [pii]
Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055–63. https://doi.org/10.1182/blood-2015-11-624288.
Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32(27):3048–58. JCO.2013.53.5229 [pii]
Engert A, Raemaekers J. Treatment of early-stage Hodgkin lymphoma. Semin Hematol. 2016;53(3):165–70. https://doi.org/10.1053/j.seminhematol.2016.05.004.
Vassilakopoulos TP, Johnson PW. Treatment of advanced-stage Hodgkin lymphoma. Semin Hematol. 2016;53(3):171–9. https://doi.org/10.1053/j.seminhematol.2016.05.006.
Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–9. https://doi.org/10.1016/S0140-6736(11)61940-5.
Engert A. XVII. Treatment of advanced-stage Hodgkin lymphoma. Hematol Oncol. 2015;33(Suppl 1):87–9. https://doi.org/10.1002/hon.2225.
Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–62. https://doi.org/10.1016/S0140-6736(15)60165-9.
Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9. https://doi.org/10.1200/JCO.2011.38.0410.
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. https://doi.org/10.1056/NEJMoa1411087.
Moskowitz CH, Ribrag V, Michot J, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after Brentuximab Vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). Blood. 2014;124(21):290.
Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(4):434–42. https://doi.org/10.1002/ajh.24272.
Davies AJ, Caddy J, Maishman T, et al. A prospective randomised trial of targeted therapy for diffuse large B-cell lymphoma (DLBCL) based upon real-time gene expression profiling: the Remodl-B study of the UK NCRI and SAKK lymphoma groups. Blood. 2015;126(23):812.
Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. https://doi.org/10.1016/S0140-6736(10)62175-7.
Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. JCO.2013.54.8800 [pii]
Gascoyne RD. Establishing the diagnosis of lymphoma: from initial biopsy to clinical staging. Oncology (Williston Park). 1998;12(10 Suppl 8):11–6.
Zangos S, Eichler K, Wetter A, et al. MR-guided biopsies of lesions in the retroperitoneal space: technique and results. Eur Radiol. 2006;16(2):307–12. https://doi.org/10.1007/s00330-005-2870-2.
Agid R, Sklair-Levy M, Bloom AI, et al. CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: experience of 267 biopsies. Clin Radiol. 2003;58(2):143–7. S0009926002910615 [pii]
Weiler-Sagie M, Bushelev O, Epelbaum R, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51(1):25–30. https://doi.org/10.2967/jnumed.109.067892.
Chalaye J, Luciani A, Enache C, et al. Clinical impact of contrast-enhanced computed tomography combined with low-dose (18)F-fluorodeoxyglucose positron emission tomography/computed tomography on routine lymphoma patient management. Leuk Lymphoma. 2014;55(12):2887–92. https://doi.org/10.3109/10428194.2014.900761.
Elstrom RL, Leonard JP, Coleman M, et al. Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol. 2008;19(10):1770–3. https://doi.org/10.1093/annonc/mdn282.
Pinilla I, Gomez-Leon N, Del Campo-Del Val L, et al. Diagnostic value of CT, PET and combined PET/CT performed with low-dose unenhanced CT and full-dose enhanced CT in the initial staging of lymphoma. Q J Nucl Med Mol Imaging. 2011;55(5):567–75. R39102188 [pii]
Raanani P, Shasha Y, Perry C, et al. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol. 2006;17(1):117–22. mdj024 [pii]
Barrington SF, Kirkwood AA, Franceschetto A, et al. PET-CT for staging and early response: results from the response-adapted therapy in advanced Hodgkin lymphoma study. Blood. 2016;127(12):1531–8. https://doi.org/10.1182/blood-2015-11-679407.
Bodet-Milin C, Touzeau C, Leux C, et al. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from the GOELAMS group. Eur J Nucl Med Mol Imaging. 2010;37(9):1633–42. https://doi.org/10.1007/s00259-010-1469-2.
Quarles van Ufford HM, van Tinteren H, Stroobants SG, et al. Added value of baseline 18F-FDG uptake in serial 18F-FDG PET for evaluation of response of solid extracerebral tumors to systemic cytotoxic neoadjuvant treatment: a meta-analysis. J Nucl Med. 2010;51(10):1507–16. https://doi.org/10.2967/jnumed.110.075457.
Barrington SF, Mackewn JE, Schleyer P, et al. Establishment of a UK-wide network to facilitate the acquisition of quality assured FDG-PET data for clinical trials in lymphoma. Ann Oncol. 2011;22(3):739–45. https://doi.org/10.1093/annonc/mdq428.
El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30(36):4508–14. https://doi.org/10.1200/JCO.2012.42.4036.
Khan AB, Barrington SF, Mikhaeel NG, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122(1):61–7. https://doi.org/10.1182/blood-2012-12-473389.
Berthet L, Cochet A, Kanoun S, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54(8):1244–50. https://doi.org/10.2967/jnumed.112.114710.
Cerci JJ, Gyorke T, Fanti S, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med. 2014;55(10):1591–7. https://doi.org/10.2967/jnumed.113.134486.
Cheson BD. Hodgkin lymphoma: protecting the victims of our success. J Clin Oncol. 2012;30(36):4456–7. https://doi.org/10.1200/JCO.2012.45.5402.
Alzahrani M, El-Galaly TC, Hutchings M, et al. The value of routine bone marrow biopsy in patients with diffuse large B-cell lymphoma staged with PET/CT: a Danish-Canadian study. Ann Oncol. 2016;27(6):1095–9. https://doi.org/10.1093/annonc/mdw137.
Paone G, Itti E, Haioun C, et al. Bone marrow involvement in diffuse large B-cell lymphoma: correlation between FDG-PET uptake and type of cellular infiltrate. Eur J Nucl Med Mol Imaging. 2009;36(5):745–50. https://doi.org/10.1007/s00259-008-1021-9.
Pelosi E, Penna D, Douroukas A, et al. Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: results from a large multicentre study. Q J Nucl Med Mol Imaging. 2011;55(4):469–75. R39102239 [pii]
Moskowitz CH, Schoder H. Current status of the role of PET imaging in diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):138–42. https://doi.org/10.1053/j.seminhematol.2015.01.004.
Campbell J, Seymour JF, Matthews J, et al. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol. 2006;76(6):473–80. EJH644 [pii]
Luminari S, Biasoli I, Arcaini L, et al. The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol. 2013;24(8):2108–12. https://doi.org/10.1093/annonc/mdt137.
Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(21):4643–51. JCO.2005.12.072 [pii]
Watanabe R, Tomita N, Takeuchi K, et al. SUVmax in FDG-PET at the biopsy site correlates with the proliferation potential of tumor cells in non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51(2):279–83. https://doi.org/10.3109/10428190903440953.
Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86. JCO.2006.09.2403 [pii]
Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–9. 2005-06-2252 [pii]
Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–52. JCO.2007.11.6525 [pii]
Raemaekers JM, Andre MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–94. https://doi.org/10.1200/JCO.2013.51.9298.
Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598–607. https://doi.org/10.1056/NEJMoa1408648.
Follows GA, Ardeshna KM, Barrington SF, et al. Guidelines for the first line management of classical Hodgkin lymphoma. Br J Haematol. 2014;166(1):34–49. https://doi.org/10.1111/bjh.12878.
Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419–29. https://doi.org/10.1056/NEJMoa1510093.
Zinzani PL, Broccoli A, Gioia DM, et al. Interim positron emission tomography response–adapted therapy in advanced-stage Hodgkin lymphoma: final results of the phase II part of the HD0801 study. JCO. 2016;34(12):1376–85. https://doi.org/10.1200/JCO.2015.63.0699.
Mamot C, Klingbiel D, Hitz F, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015;33(23):2523–9. https://doi.org/10.1200/JCO.2014.58.9846.
Huntington SF, Nasta SD, Schuster SJ, et al. Utility of interim and end-of-treatment [(18)F]-fluorodeoxyglucose positron emission tomography-computed tomography in frontline therapy of patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56(9):2579–84. https://doi.org/10.3109/10428194.2015.1007506.
Carr R, Fanti S, Paez D, et al. Prospective international cohort study demonstrates inability of interim PET to predict treatment failure in diffuse large B-cell lymphoma. J Nucl Med. 2014;55(12):1936–44. https://doi.org/10.2967/jnumed.114.145326.
Zijlstra JM, Burggraaff CN, Kersten MJ, et al. FDG-PET as a biomarker for early response in diffuse large B-cell lymphoma as well as in Hodgkin lymphoma? Ready for implementation in clinical practice? Haematologica. 2016;101(11):1279–83. haematol.2016.142752 [pii]
Stewart DA, Kloiber R, Owen C, et al. Results of a prospective phase II trial evaluating interim positron emission tomography-guided high dose therapy for poor prognosis diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55(9):2064–70. https://doi.org/10.3109/10428194.2013.862242.
Pardal E, Coronado M, Martin A, et al. Intensification treatment based on early FDG-PET in patients with high-risk diffuse large B-cell lymphoma: a phase II GELTAMO trial. Br J Haematol. 2014;167(3):327–36. https://doi.org/10.1111/bjh.13036.
Barrington SF, Mikhaeel NG. PET scans for staging and restaging in diffuse large B-cell and follicular lymphomas. Curr Hematol Malig Rep. 2016;11(3):185–95. https://doi.org/10.1007/s11899-016-0318-1.
Trotman J, Luminari S, Boussetta S, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1(1):e17–27. https://doi.org/10.1016/S2352-3026(14)70008-0.
Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–70. https://doi.org/10.1182/blood-2011-10-388058.
Sauter CS, Matasar MJ, Meikle J, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125(16):2579–81. https://doi.org/10.1182/blood-2014-10-606939.
Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosfamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–92. https://doi.org/10.1016/S1470-2045(15)70013-6.
Meignan M, Itti E, Bardet S, et al. Development and application of a real-time on-line blinded independent central review of interim PET scans to determine treatment allocation in lymphoma trials. J Clin Oncol. 2009;27(16):2739–41. https://doi.org/10.1200/JCO.2009.22.4089.
Uslu L, Doing J, Link M, Rosenberg J, Quon A, Daldrup-Link HE. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. 2015;56(2):274–86.
Furth C, Steffen IG, Amthauer H, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol. 2009;27(26):4385–91. https://doi.org/10.1200/JCO.2008.19.7814.
Riad R, Omar W, Kotb M, et al. Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging. 2010;37(2):319–29. https://doi.org/10.1007/s00259-009-1276-9.
Bakhshi S, Radhakrishnan V, Sharma P, et al. Pediatric nonlymphoblastic non-Hodgkin lymphoma: baseline, interim, and posttreatment PET/CT versus contrast-enhanced CT for evaluation--a prospective study. Radiology. 2012;262(3):956–68. https://doi.org/10.1148/radiol.11110936.
Paulino AC, Margolin J, Dreyer Z, et al. Impact of PET-CT on involved field radiotherapy design for pediatric Hodgkin lymphoma. Pediatr Blood Cancer. 2012;58(6):860–4. https://doi.org/10.1002/pbc.23273.
Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680–6. https://doi.org/10.1200/JCO.2009.26.9381.
Hasenclever D, Kurch L, Mauz-Korholz C, et al. qPET – a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(7):1301–8. https://doi.org/10.1007/s00259-014-2715-9.
Kwee TC, Kwee RM, Verdonck LF, et al. Magnetic resonance imaging for the detection of bone marrow involvement in malignant lymphoma. Br J Haematol. 2008;141(1):60–8. https://doi.org/10.1111/j.1365-2141.2008.07020.x.
Daldrup-Link HE, Henning T, Link TM. MR imaging of therapy-induced changes of bone marrow. Eur Radiol. 2007;17(3):743–61. https://doi.org/10.1007/s00330-006-0404-1.
Cieszanowski A, Lisowska A, Dabrowska M, et al. MR imaging of pulmonary nodules: detection rate and accuracy of size estimation in comparison to computed tomography. PLoS One. 2016;11(6):e0156272. https://doi.org/10.1371/journal.pone.0156272.
Regacini R, Puchnick A, Shigueoka DC, et al. Whole-body diffusion-weighted magnetic resonance imaging versus FDG-PET/CT for initial lymphoma staging: systematic review on diagnostic test accuracy studies. Sao Paulo Med J. 2015;133(2):141–50. https://doi.org/10.1590/1516-3180.2014.8312810.
Albano D, Patti C, Lagalla R, et al. Whole-body MRI, FDG-PET/CT, and bone marrow biopsy, for the assessment of bone marrow involvement in patients with newly diagnosed lymphoma. J Magn Reson Imaging. 2016;45(4):1082–9. https://doi.org/10.1002/jmri.25439.
Lin C, Itti E, Luciani A, et al. Whole-body diffusion-weighted imaging with apparent diffusion coefficient mapping for treatment response assessment in patients with diffuse large B-cell lymphoma: pilot study. Investig Radiol. 2011;46(5):341–9. https://doi.org/10.1097/RLI.0b013e3182087b03.
Chen Y, Zhong J, Wu H, et al. The clinical application of whole-body diffusion-weighted imaging in the early assessment of chemotherapeutic effects in lymphoma: the initial experience. Magn Reson Imaging. 2012;30(2):165–70. https://doi.org/10.1016/j.mri.2011.09.019.
Toledano-Massiah S, Luciani A, Itti E, et al. Whole-body diffusion-weighted imaging in Hodgkin lymphoma and diffuse large B-cell lymphoma. Radiographics. 2015;35(3):747–64. https://doi.org/10.1148/rg.2015140145.
Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro-Oncology. 2008;10(2):223–8. https://doi.org/10.1215/15228517-2007-061.
Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32(6):984–92. https://doi.org/10.3174/ajnr.A2171.
DeAngelis LM, Boutros D. Leptomeningeal metastasis. Cancer Investig. 2005;23(2):145–54.
Senocak E, Oguz KK, Ozgen B, et al. Parenchymal lymphoma of the brain on initial MR imaging: a comparative study between primary and secondary brain lymphoma. Eur J Radiol. 2011;79(2):288–94. https://doi.org/10.1016/j.ejrad.2010.01.017.
Rottnek M, Strauchen J, Moore F, et al. Primary dural mucosa-associated lymphoid tissue-type lymphoma: case report and review of the literature. J Neuro-Oncol. 2004;68(1):19–23.
Kuker W, Herrlinger U, Gronewaller E, et al. Ocular manifestation of primary nervous system lymphoma: what can be expected from imaging? J Neurol. 2002;249(12):1713–6. https://doi.org/10.1007/s00415-002-0919-6.
Nabavizadeh SA, Vossough A, Hajmomenian M, et al. Neuroimaging in central nervous system lymphoma. Hematol Oncol Clin North Am. 2016;30(4):799–821. https://doi.org/10.1016/j.hoc.2016.03.005.
Schroeder PC, Post MJ, Oschatz E, et al. Analysis of the utility of diffusion-weighted MRI and apparent diffusion coefficient values in distinguishing central nervous system toxoplasmosis from lymphoma. Neuroradiology. 2006;48(10):715–20. https://doi.org/10.1007/s00234-006-0123-y.
Barajas RF Jr, Rubenstein JL, Chang JS, et al. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31(1):60–6. https://doi.org/10.3174/ajnr.A1750.
Yamashita K, Yoshiura T, Hiwatashi A, et al. Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and (1)(8)F-fluorodeoxyglucose positron emission tomography. Neuroradiology. 2013;55(2):135–43. https://doi.org/10.1007/s00234-012-1089-6.
Pollock JM, Tan H, Kraft RA, et al. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am. 2009;17(2):315–38. https://doi.org/10.1016/j.mric.2009.01.008.
Kickingereder P, Sahm F, Wiestler B, et al. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol. 2014;35(8):1503–8. https://doi.org/10.3174/ajnr.A3915.
Chinn RJ, Wilkinson ID, Hall-Craggs MA, et al. Toxoplasmosis and primary central nervous system lymphoma in HIV infection: diagnosis with MR spectroscopy. Radiology. 1995;197(3):649–54. https://doi.org/10.1148/radiology.197.3.7480733.
Kickingereder P, Wiestler B, Sahm F, et al. Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014;272(3):843–50. https://doi.org/10.1148/radiol.14132740.
Platzek I, Beuthien-Baumann B, Ordemann R, et al. FDG PET/MRI for the assessment of lymph node involvement in lymphoma: initial results and role of diffusion-weighted MR. Acad Radiol. 2014;21(10):1314–9. https://doi.org/10.1016/j.acra.2014.05.019.
Afaq A, Fraioli F, Sidhu H, et al. Comparison of PET/MRI with PET/CT in the evaluation of disease status in lymphoma. Clin Nucl Med. 2017;42(1):e1–7. https://doi.org/10.1097/RLU.0000000000001344.
Herrmann K, Queiroz M, Huellner MW, et al. Diagnostic performance of FDG-PET/MRI and WB-DW-MRI in the evaluation of lymphoma: a prospective comparison to standard FDG-PET/CT. BMC Cancer. 2015;15:1002. https://doi.org/10.1186/s12885-015-2009-z.
Grueneisen J, Sawicki LM, Schaarschmidt BM, et al. Evaluation of a fast protocol for staging lymphoma patients with integrated PET/MRI. PLoS One. 2016;11(6):e0157880. https://doi.org/10.1371/journal.pone.0157880.
Heacock L, Weissbrot J, Raad R, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. 2015;204(4):842–8. https://doi.org/10.2214/AJR.14.13181.
Raad RA, Friedman KP, Heacock L, et al. Outcome of small lung nodules missed on hybrid PET/MRI in patients with primary malignancy. J Magn Reson Imaging. 2016;43(2):504–11. https://doi.org/10.1002/jmri.25005.
Atkinson W, Catana C, Abramson JS, et al. Hybrid FDG-PET/MRI compared to FDG-PET/CT in adult lymphoma patients. Abdom Radiol (NY). 2016;41(7):1338–48. https://doi.org/10.1007/s00261-016-0638-6.
Giraudo C, Raderer M, Karanikas G, et al. 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance in lymphoma: comparison with 18F-fluorodeoxyglucose positron emission tomography/computed tomography and with the addition of magnetic resonance diffusion-weighted imaging. Investig Radiol. 2016;51(3):163–9. https://doi.org/10.1097/RLI.0000000000000218.
Mayerhoefer ME, Karanikas G, Kletter K, et al. Can interim 18F-FDG PET or diffusion-weighted MRI predict end-of-treatment outcome in FDG-avid MALT lymphoma after rituximab-based therapy?: a preliminary study in 15 patients. Clin Nucl Med. 2016;41(11):837–43. https://doi.org/10.1097/RLU.0000000000001395.
Ponisio MR, McConathy J, Laforest R, et al. Evaluation of diagnostic performance of whole-body simultaneous PET/MRI in pediatric lymphoma. Pediatr Radiol. 2016;46(9):1258–68. https://doi.org/10.1007/s00247-016-3601-3.
Sher AC, Seghers V, Paldino MJ, et al. Assessment of sequential PET/MRI in comparison with PET/CT of pediatric lymphoma: a prospective study. AJR Am J Roentgenol. 2016;206(3):623–31. https://doi.org/10.2214/AJR.15.15083.
Buck AK, Bommer M, Stilgenbauer S, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66(22):11055–61. 66/22/11055 [pii]
Mena E, Lindenberg ML, Turkbey BI, et al. A pilot study of the value of 18F-fluoro-deoxy-thymidine PET/CT in predicting viable lymphoma in residual 18F-FDG avid masses after completion of therapy. Clin Nucl Med. 2014;39(10):874–81. https://doi.org/10.1097/RLU.0000000000000539.
Dhilly M, Guillouet S, Patin D, et al. 2-[18F]fludarabine, a novel positron emission tomography (PET) tracer for imaging lymphoma: a micro-PET study in murine models. Mol Imaging Biol. 2014;16(1):118–26. https://doi.org/10.1007/s11307-013-0659-2.
Hovhannisyan N, Guillouet S, Fillesoye F, et al. Evaluation of the specificity of [(18)F]fludarabine PET/CT in a xenograft model of follicular lymphoma: comparison with [(18)F]FDG and impact of rituximab therapy. EJNMMI Res. 2015;5:23. eCollection 2015. https://doi.org/10.1186/s13550-015-0101-7.
Hovhannisyan N, Dhilly M, Guillouet S, et al. Comparative analysis between [(18)F]Fludarabine-PET and [(18)F]FDG-PET in a murine model of inflammation. Mol Pharm. 2016;13(6):2136–9. https://doi.org/10.1021/acs.molpharmaceut.6b00050.
Acknowledgements
King’s College London and UCL Comprehensive Cancer Imaging Centre. Funded by the CRUK and EPSRC in association with the MRC and DoH (England).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Barrington, S.F., Friedman, K. (2018). PET/MRI in Lymphoma. In: Iagaru, A., Hope, T., Veit-Haibach, P. (eds) PET/MRI in Oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-68517-5_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-68517-5_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68516-8
Online ISBN: 978-3-319-68517-5
eBook Packages: MedicineMedicine (R0)