Abstract
This chapter provides an overview of ECG lead systems and commercial devices for atrial fibrillation monitoring, with special emphasis on devices using few leads. The rapid development of electronics has given rise to various approaches, ranging from invasive devices to short-term screening recorders, as well as to non-ECG mobile devices. Contemporary devices offer patient comfort and can therefore be used for extended time periods, for example, to detect silent, previously undocumented atrial fibrillation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
2.1 Introduction
The surface ECG is the most widely used clinical tool for detecting atrial fibrillation (AF), with the standard 12-lead ECG, recorded at rest, as the preferred lead system [1]. However, fewer leads are sufficient to confirm AF, being particularly advantageous when the patient is monitored for an extended period of time, for example, to detect silent, previously undocumented AF. Since extended ECG monitoring may last for several days, even a three-lead system, requiring five electrodes, may become inconvenient for the patient [2]. For this reason, various types of single-lead devices have been developed to ensure patient comfort in exchange for diagnostic reliability provided by more leads. While reduced lead systems are well-suited for detecting AF, they only provide limited information on the underlying mechanisms. On the other hand, comprehensive characterization of AF, such as identification of wavefront propagation patterns in the atria [3], benefits from using a large number of electrodes.
Commercial devices, especially those employing a small number of leads, tend to produce false positives due to electromyographic noise, motion artifacts, ectopic beats, or pronounced sinus arrhythmia [4], calling for manual review of computer-detected arrhythmic episodes. This shortcoming is particularly pronounced when it is of interest to analyze brief AF episodes. Since manual review of long-term continuous ECG recordings is time-consuming, and at times unreliable [5], it is essential to improve AF detection performance.
2.2 Lead Systems
Historically, the electrode placement of most lead systems has been focused on ventricular activity, and, consequently, they are suboptimal for atrial activity—an observation which applies especially to atrial activity during AF. Since the amplitudes of atrial waves, i.e., P and f waves, are much smaller than those of ventricular waves, better discrimination of atrial tachyarrhythmias, e.g., atrial tachycardia, atrial flutter, and AF, can be achieved in leads with larger atrial amplitude [6, 7]. Moreover, large-amplitude leads facilitate the characterization of f waves, especially when the f waves have been separated from ventricular activity (Chap. 5). For example, the atrial fibrillatory rate (AFR), a parameter useful for selection of treatment strategy [8], is more reliably estimated.
When the standard 12-lead ECG system is employed, limb lead II normally produces the largest P wave amplitude. On the other hand, since the atrial activity is disorganized during AF, the precordial leads V\(_{1}\) and V\(_{2}\) usually have the largest f wave amplitude due to their proximity to the atria. In general, the f waves in V\(_{1}\) to V\(_{6}\) have decreasing amplitude as the distance to the atria increases. Moreover, the precordial electrodes are placed close to each other, thus causing the f waves to be correlated.
For sake of clarity, it should be noted that the term “lead” is used for defining the voltage difference between two electrodes. For example, lead I is the voltage between the left and right arm electrodes, whereas lead V\(_{1}\) is the voltage between the electrode placed at C\(_{1}\), i.e., chest position #1, and Wilson’s central terminal which is the average of the three limb leads.
2.2.1 Body Surface Potential Mapping
While the standard 12-lead ECG serves as the reference system for AF diagnosis, it only provides limited information on the wavefront propagation patterns of atrial activity. A larger number of electrodes, distributed over the body surface, better reflect spatial differences. Indeed, reconstruction of body surface potentials from the f waves of the 12-lead ECG is associated with a 53% reconstruction error, indicating that additional electrodes are needed to provide a more accurate representation of the f waves [9]. For this reason, body surface potential mapping (BSPM) is an important tool for reconstructing f waves [10]. The number of electrodes used in BSPM systems has ranged from 56 [3] to 252 [11], involving anterior, posterior, and sometimes lateral sites. Electrodes can either be distributed nonuniformly [9] or arranged as a uniform grid around V\(_{1}\) [3, 12].
In one of the very first BSPM studies on AF, wavefront propagation patterns were identified, representing either a single wavefront, single wavefront with breakages, or multiple wavefronts [3]. Signals were recorded using a custom-made vest with 56 (40 anterior and 16 posterior) electrodes arranged around V\(_{1}\) in a uniform grid with a distance of 2.2 cm between the electrodes. Despite the low quality of the f waves, the constructed maps of wavefront propagation were consistent with those observed using invasive or optical mapping. When comparing f waves in V\(_{1}\) to those recorded using BSPM, f waves in V\(_{1}\) were representative only when a single wavefront propagated across the whole atria; in such cases, f wave amplitude and AFR were similar, regardless of the site where the signal was recorded. However, for a single wavefront breaking or multiple simultaneous wavefronts coexisting, the f wave pattern differed and depended on the site.
Obviously, more electrodes result in higher spatial resolution. However, many electrodes are impractical in clinical routine, and, therefore, the following questions should be answered:
-
What is the optimal number of electrodes?
-
Which electrode placement provides the best atrial information?
To answer these questions, BSPM with 64 nonuniformly distributed electrodes (48 anterior and 16 posterior) was employed [9]. The criterion for selecting the optimal leads was given by the total root mean square (RMS) error of the reconstructed signals of the remaining leads not included in the lead system under consideration. Since the ventricular activity of the whole body surface can be reconstructed from the standard 12-lead ECG with an error of 25% [9], the optimal number of electrodes needed to record the atrial activity was defined by the same error percentage. The results showed that 23 electrodes were needed to achieve this reconstruction error, using the placement shown in Fig. 2.1. To reduce the error to 10%, at least 45 electrodes were required for reconstructing the atrial activity, while only 22 electrodes for the ventricular activity.
Assuming that the type of wavefront propagation can be successfully identified using BSPM, this technique has the potential to characterize the degree of atrial organization. The application of BSPM is limited by the time-consuming preparation procedure; however, this problem may be mitigated by integrating the electrodes into smart textiles, which would reduce the time required for electrode placement considerably.
2.2.2 Modifications of the Standard 12-Lead ECG
A lead system tailored to the analysis of AF is more likely to be clinically accepted if it can be viewed as a modified standard 12-lead ECG. Accordingly, several such modifications have been proposed [9, 13,14,15]. The design of a modified lead system is restricted to the 10 electrodes defining the standard 12-lead ECG. To retain Wilson’s central terminal, used as the reference potential of the precordial leads, the positions of the extremity electrodes V\(_{\text {R}}\), V\(_{\text {L}}\), and V\(_{\text {F}}\) should be retained. Moreover, it is desirable to place the electrodes in relation to conventional sites to simplify electrode placement, especially since incorrect placement is a well-known problem when acquiring the standard 12-lead ECG [16]. The electrode sites are determined heuristically, either by placing the electrodes close to the atria or using some optimization criterion.
A heuristic approach to determining ECG leads with increased f wave amplitude is to place V\(_{3}\) to V\(_{6}\) in the vicinity of V\(_{1}\) and V\(_{2}\). For example, the precordial electrodes can be rearranged to form a \(2\times 3\) grid on the right side of the chest, with V\(_{1}\) and V\(_{2}\) unmodified and V\(_{3}\) to V\(_{6}\) replaced by the new electrodes V\(_{\text {LS}}\), V\(_{\text {S}}\), V\(_{\text {RS}}\), and V\(_{\text {R}}\) [13]. The electrode V\(_{\text {LS}}\) (left superior, LS) is placed one intercostal space above V\(_{2}\). The electrode V\(_{\text {S}}\) (superior, S) is placed one intercostal space above V\(_{1}\). The electrode V\(_{\text {RS}}\) (right superior, RS) is moved to the right of V\(_{\text {S}}\), whereas the electrode V\(_{\text {R}}\) is moved to the right of V\(_{1}\) and aligned vertically with V\(_{\text {RS}}\), see Fig. 2.2a. Preliminary results based on simulated f wave signals, using a biophysical model of the human atria and thorax, showed that the resulting lead system, coined as the electroatriogram, provides more information on atrial activity than the standard 12-lead ECG [13, 14].
Two other, heuristically derived modifications are intended for either anterior or posterior monitoring [15]. Similarly to the lead system in [13], V\(_{1}\) and V\(_{2}\) remain unmodified, whereas the other four leads are rearranged counterclockwise around V\(_{1}\) and V\(_{2}\), see Fig. 2.2b and c. The only difference compared to the placement in [13] is that the lead on the right upper side of the chest, denoted V\(_{\text {RS}}\), is placed one intercostal space below V\(_{1}\), denoted V\(_{\text {B}}\). The posterior electrodes V\(_{\text {1P}}\), V\(_{\text {1PS}}\), V\(_{\text {2P}}\), and V\(_{\text {2PS}}\) (posterior superior, PS) are rearranged in a similar fashion. Two electrodes are placed opposite to V\(_{1}\) and V\(_{2}\), whereas the remaining two are placed one intercostal space below V\(_{1}\) and V\(_{2}\). Following cancellation of the ventricular activity, the usefulness of the proposed electrode placement was investigated in terms of interlead dispersion of the AFR. The proximity of V\(_{1}\) to V\(_{2}\) gave rise to nearly the same AFR, whereas the dispersion was larger among the anterior leads. Although the posterior electrode placement was associated with 35% lower frequency dispersion than that of the anterior, the combination of both approaches can be used to extract additional information. It was shown that anterior leads mostly reflect the AFR of the right atrium [17], whereas posterior leads mostly reflect the AFR of the left atrium [18]. Therefore, inclusion of both anterior and posterior leads should be applied to identify frequency gradients, and the driving atrium.
The above-mentioned heuristic approaches are useful for finding electrode placement which offers larger f wave amplitude, but not for finding electrode placement which increases the atrial information. A quantitative approach to optimal placement of precordial electrodes is to maximize the ratio of the smallest to the largest singular value of the f wave signal obtained at different sites on the body surface [14]. Similar to the heuristic approaches, four of the precordial electrodes are rearranged, while two remain unmodified. The placement of V\(_{1}\) is unmodified due to its proximity to the atria, as is the placement of V\(_{4}\) because its f waves are the ones which are the least correlated to those in V\(_{1}\). In [14], using a biophysical model to simulate f waves, the search for optimal placement of the four precordial electrodes resulted in four distinct areas on the thorax where the electrodes should be placed to ensure additional atrial information, see Fig. 2.3a. The electrode V\(_{\text {S}}\) is placed one intercostal space above V\(_{1}\). The electrode V\(_{\text {RS}}\) is positioned to the right of V\(_{\text {S}}\) at the same intercostal space. The electrode V\(_{\text {LC}}\) is placed slightly below the left clavicle (LC), whereas the electrode V\(_{\text {P}}\) is placed on the back behind the atria at the same level as V\(_{1}\). Interestingly, two of the four new electrode sites, namely V\(_{\text {S}}\) and V\(_{\text {RS}}\), were the same as those derived heuristically in [13]. In addition, the placement of V\(_{\text {S}}\) was the same as that used for lead S in the EASI lead system, defined by the E, A, and I electrode positions of the Frank lead system, plus an electrode S positioned over the upper end of the sternum [19].
Results obtained from simulated f wave signals showed that more information can be extracted using the optimized as well as the heuristically-derived lead systems than with the systems using conventional electrode placement. Nevertheless, the difference between the proposed lead systems in terms of gained atrial information was not large. Considering that the electrodes of the heuristically-derived placement are closer to the atria, thus producing larger f wave amplitude, the use of optimized placement, involving electrodes with smaller f waves (V\(_{4}\) and V\(_{\text {LC}}\)), is questionable.
Two other modified 12-lead ECG systems for improving AF analysis have been derived using an iterative lead selection principle. Only those leads were chosen which increase the information contained in each selected lead set [20]. Similarly to the previously described lead systems, only four precordial electrodes were repositioned based on the criteria for selecting leads with additional atrial information [9]. Depending on the constraint that either V\(_{1}\) and V\(_{2}\) or V\(_{1}\) and V\(_{4}\) should be kept at their conventional sites, two electrode placements were derived of which one had the two additional electrodes V\(_\text {RI}\) (right inferior) and V\(_\text {W}\) (waist), see Fig. 2.3b. Both lead systems were associated with similar f wave reconstruction error, about 10% lower than that obtained with the standard 12-lead ECG, although the electrodes were placed on different parts of the body. A relatively small improvement in reconstruction error implies that modifications of the 12-lead ECG do not result in markedly increased atrial information content. Considering the increased complexity of the electrode placement, it is doubtful whether such modifications will be adopted clinically.
2.2.3 Reduced Lead Systems
So far, no specialized lead system is used in clinical routine when ambulatory monitoring is prescribed in patients with AF. Therefore, five-electrode, standard ambulatory monitoring is typically applied, capable of recording six limb leads, i.e., I, II, III, aVR, aVL, aVF, in combination with a single precordial lead, e.g., V\(_{1}\) [21]. It is well-known that standard ambulatory monitors lead to reduced quality of life and have lower patient compliance [2, 22]. For this reason, single-lead monitors are considered as a promising alternative for long-term ambulatory monitoring of AF [22]. To facilitate AF detection in reduced-lead ECGs, it is desirable to employ electrode placement optimized for f wave analysis.
A reduced lead system for atrial activity enhancement was proposed already in the very first book on electrocardiography, authored by Sir Thomas Lewis and published in 1913 [23]. The Lewis lead system consists of five leads, where two, L\(_{1}\) and L\(_{2}\), are derived for the purpose of enhancing the f waves, see Fig. 2.4a. The bipolar chest lead L\(_{1}\) is obtained by placing electrode 1 over the upper end of the sternum, and electrode 2 on the right side of the sternum at the second intercostal space. Lead L\(_{2}\) is the voltage between electrodes 2 and 3, placed on the right side of the sternum at the fourth intercostal space.
The Lewis lead system has two out of six electrodes placed directly on the pectoral muscle, where artifacts due to arm movement are likely to occur. In order to avoid leads on the chest muscles, a modified Lewis lead was proposed which is more immune to noise and with good projection of the f waves [24]. The modified Lewis lead L\(_{\text {M}}\) is obtained by removing electrode 2 of the Lewis lead system, and moving electrode 3 one intercostal space downwards, i.e., from the fourth to the fifth, to improve the immunity to arm movement artifacts, see Fig. 2.4b. A comparative study showed that L\(_{1}\) and L\(_{2}\) exhibit a high atrial-to-ventricular amplitude ratio [24]. However, f wave enhancement is achieved at the expense of a much reduced ventricular amplitude, rather than increased f wave amplitude. Despite the fact that L\(_{1}\) and L\(_{2}\) are proximal, L\(_{1}\) was found to be twice as susceptible to electromyographic noise as L\(_{2}\), and, therefore, L\(_{2}\) is considered the preferred lead. For long-term monitoring, where high noise levels are often encountered, L\(_{\text {M}}\) may be more advantageous since it has larger f wave amplitude than L\(_{2}\). Hence, L\(_{2}\) offers better immunity to electromyographic noise [24].
Recent results have shown that the largest P wave amplitude is obtained when the distance between electrodes is 12–18 cm [25]. Depending on torso size, the distance between the electrodes of the modified Lewis lead L\(_{\text {M}}\) is 14–20 cm, whereas the distance for L\(_{1}\) and L\(_{2}\) is less than half. Since L\(_{1}\), L\(_{2}\), and L\(_{\text {M}}\) are roughly along the same axis with respect to the heart’s electrical vector, the longer distance between electrodes is probably the primary reason for a larger f wave amplitude in L\(_{\text {M}}\) than in L\(_{1}\) and L\(_{2}\).
A short distance between the bipolar electrodes is desirable since the electrodes can then be integrated into a single recording device, and, consequently, increase patient compliance [22, 27]. However, a reduced distance between the electrodes will also reduce P and f wave amplitudes, which in turn may reduce the performance of AF detectors exploring the presence of f waves [28, 29]. Thus, another approach to finding the best lead for ambulatory monitoring of f waves is to account for both signal amplitude and distance between the electrodes. Based on the analysis of 120 combinations of bipolar leads, obtained from 16 electrodes placed on the precordial area, the best leads for monitoring of P and f waves were identified [25]. A lead for P wave monitoring is obtained by placing one electrode on the right side of the sternum at the second intercostal space, and another electrode on the sternum in line with the fourth intercostal space. A lead for f wave monitoring (referred to as “f-lead”) is obtained by placing one electrode on the sternum at the level of the second intercostal space, and another electrode on the right side of the sternum at the fourth intercostal space, see Fig. 2.4c. Although a distance of 8 cm was found to be optimal for both P and f waves, these leads involve different electrodes, and, thus, a lead can only be optimal for either P or f wave monitoring. Considering that one electrode is placed on the sternum, while the other on the right side of the chest, both leads are susceptible to motion artifacts. Hence, the signal quality during daily activities should be investigated before an AF monitor is designed for these particular leads.
However, this approach is not necessarily optimal with respect to maximized atrial amplitude. Therefore, a bipolar lead (referred to as “P-lead”) for maximized P wave amplitude was derived based on the analysis of 117-lead BSPM, recorded from more than 200 healthy individuals [26]. Since only healthy individuals were included, the best lead coincided, not surprisingly, with the electrical axis of the heart, see Fig. 2.4d. To obtain the P-lead, one electrode has to be placed on the right sternal clavicular junction and the other on the midpoint of the left costal margin in line with the seventh intercostal space. The study showed that the P-lead has nearly three times larger P wave amplitude than L\(_{2}\), and 35% larger amplitude than L\(_{\text {M}}\). While the P-lead is attractive for AF detectors involving P wave analysis, it will not necessarily produce larger f wave amplitude.
Although the EASI lead system was not specifically developed for the analysis of atrial activity, lead ES provides relatively large P wave amplitude [24]. This lead system uses four electrodes placed on the torso, where the electrodes E, A, and I are placed at the same sites as in the Frank lead system [19]. The electrode S is placed over the upper end of the sternum (the manubrium) and the electrode E at the bottom of the sternum at the level of the fifth intercostal space.
The aforementioned reduced lead systems, except EASI, were developed for the purpose of enhancing atrial activity, and, therefore, less suitable for evaluating ventricular beat morphology, except for fundamental information such as the occurrence times of the QRS complexes. Since RR interval irregularity, together with P wave absence and f wave presence, represent the landmark properties of AF, a single-lead system may even provide sufficient information for AF detection. Considering that the electrodes of the reduced lead systems are closely positioned to the right atrium, the spectral content of the f waves is largely related to the right atrium. However, it remains to be shown, for example, how well AFR, determined from the surface ECG, agrees with that measured by an intra-atrial recording. Moreover, there is a lack of studies examining noise immunity of different ECG lead systems.
2.3 Recording Devices
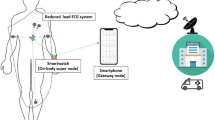
For many years, the resting ECG and 24-h ambulatory monitoring were the only available techniques for analyzing AF. However, the rapid development of electronics and communication technologies has given rise to novel approaches to AF monitoring and screening, ranging from invasive devices, providing a convenient way to continuously monitor arrhythmias for months and years, to short-term screening recorders, see Fig. 2.5 [30]. Contemporary mobile ECG devices (smartphones, smartwatches, smart wristbands, tablets) can immediately transfer data to the physician via wireless communication (Bluetooth, Wi-Fi, GSM networks) and Internet. Together with the rapidly growing cloud-based software, new opportunities are created to collect and analyze large amounts of data. Since most mobile devices can simultaneously acquire other types of information than the ECG, e.g., physical activity, body position, and respiration, such information can be used to provide a more complete picture of the factors initiating and maintaining AF.
Given that there are many devices on the market capable of recording the ECG, this section only provides an overview of the most representative technologies applied to AF detection.
2.3.1 Standard Resting ECG
Since the standard 12-lead ECG is globally recognized, cost-effective, straightforward to record, and easy to interpret for a trained physician/technician, the majority of patients with AF are identified using the 12-lead ECG. Its main disadvantage is the short recording time, normally lasting only 10–30 s. Thus, persistent and permanent AF can be detected with the 12-lead ECG, whereas paroxysmal AF, especially in its early stages, is likely to go undetected.
2.3.2 Standard Ambulatory Monitors
Since the ambulatory monitor was introduced by Norman Holter in 1961 [31], it has become widely accepted in clinical applications. The monitor is a portable device capable of recording the ECG continuously for one or two days during normal daily activities, usually with a three-lead configuration. Occasionally, seven-day and even one-month ambulatory monitoring may be prescribed. The ambulatory monitor is a valuable tool not only for arrhythmia detection, including AF, but also to evaluate drug effects on AF recurrence. Once monitoring is finished, the ECG is analyzed offline using commercial software. Although the software for AF detection has improved over the years, the results from automated AF detection still need to be manually reviewed by a physician/technician to ensure that AF episodes are correctly detected.
The major drawbacks of ambulatory monitoring are the adhesive electrodes and the connecting wires, which can be uncomfortable for certain patients and sometimes lead to premature termination of monitoring [32]. Moreover, certain patients are allergic to adhesive electrodes, and therefore other techniques need to be considered.
2.3.3 Cardiac Event Recorders
External cardiac event recorders are portable devices similar to the standard ambulatory monitor, but smaller and lighter since a single-lead ECG is usually recorded. Most cardiac event recorders are not operating continuously, but record when activated by the patient when symptoms occur, or started automatically when rhythm abnormalities are detected by an embedded algorithm. Two main types of cardiac event recorders can be distinguished : continuous loop recorders and symptom event recorders.
Continuous loop recorders are continuously refreshing, i.e., recording and erasing the data. Data refreshing is terminated when the device is triggered by the patient or an algorithm. In such a way, the ECG signal of the entire event, as well as a few minutes before and after, are stored in the memory. Due to limited storage capacity, only the onset and the end of the episode are saved if the arrhythmia lasts for a longer period of time. Similar to the standard ambulatory monitor, the continuous loop recorder is connected to adhesive electrodes.
Symptom event recorders are, in contrast to continuous loop recorders, not required to be worn at all times, but can be temporarily attached to the body by the patient when arrhythmia symptoms are experienced. However, this device is neither suitable for capturing the very onset of an arrhythmia, nor for detecting nocturnal and asymptomatic events. Moreover, when symptoms are severe, e.g., fainting, it is difficult for the patient to correctly attach the device to the body.
Various studies have demonstrated that cardiac event recorders are prone to false alarms due to ectopic beats, since runs of such beats may resemble AF. For example, a study of the external loop recorder Vitaphone 3100 BT (Vitaphone GmbH, Mannheim, Germany) showed that each patient in sinus rhythm, in average, had more than five false positive ECGs during the 24-h recording period, caused by ventricular and supraventricular premature beats and sinus arrhythmia [33]. Similar performance was reported in another study where nearly 3,000 events were collected, and roughly 1,200 were classified as AF by a proprietary algorithm [34]. However, only 5% were confirmed as AF after manual revision.
2.3.4 Biopatches
The biopatch technology provides a comfortable and safe way to monitor health status, by employing a leadless, wearable, single-use device which is designed to record the long-term continuous ECG [35]. In addition to the ECG, this type of device can acquire other physiological parameters, e.g., skin temperature, accelerometer data, and respiration. Due to its minimalistic design, the device can be placed on body areas associated with less motion artifacts, and thus record signals with better quality.
The Zio-Patch device (iRhythm Technologies, San Francisco, CA, USA) is a noninvasive, small size (\(123 \,\times \,53 \,\times \,10.7\) mm), lightweight (34 g), single-use ECG monitor, capable of recording the ECG up to 14 days [36]. Unlike standard ambulatory monitors, the Zio-Patch can remain attached during showering to ensure continuous monitoring. The device is attached over the left pectoral muscle with skin adhesive. Besides continuous monitoring, symptomatic arrhythmia events can be captured when the patient presses a button on the device. Once monitoring is completed, the device is mailed back to the manufacturer, where the data are analyzed. The detection of AF is performed in a two-step procedure: Arrhythmia episodes are first identified using an algorithm relying on heart rate, rhythm irregularity, and ECG morphology, and then the detected episodes are reviewed by a technician to eliminate false positives.
In a large study by the Zio-Patch, including more than 26,700 patients, the mean wear time was found to be \(7.6\,\pm \,3.6\) days, thus being about half the expected monitoring time [22]. Although only 16% reached the maximum monitoring duration of about 13 days, 96% of the patients exceeded the ambulatory monitoring time of 48 h. Given that nearly 25% of all AF cases were detected after 48 h, this finding justifies the Zio-Patch for detection of AF episodes which otherwise would have been missed with standard ambulatory monitoring. In 87% of all patients, the device produced analyzable signal quality during at least 22 h per day.
The NUVANT Mobile Cardiac Telemetry system (Corventis, San Jose, CA, USA) is another biopatch device that offers prolonged monitoring of arrhythmias [37, 38]. In contrast to the Zio-Patch, the NUVANT system analyzes the ECG in real-time. The system consists of a wearable sensor, designed for single-lead recordings, and a portable transmitter which transmits the data to a proprietary monitoring center via a cloud-based application. The device incorporates activation functionality allowing the patient to trigger the device on-demand when symptoms are experienced. The sensor is activated automatically and starts recording the ECG immediately after being attached to the body. The data are transmitted whenever rhythm abnormalities are detected, followed by review of certified technicians. Clinical reports on rhythm trends and AF burden are then prepared. The sensor lasts up to 7.5 days, however, multiple sensors can be employed in sequence to extend the monitoring period up to 30 days. Preliminary studies performed by the Corventis team themselves demonstrated an AF prevalence of 20% among those who used the NUVANT system [37, 38]. However, larger independent clinical studies are needed to establish the clinical usefulness of this device relative to other monitoring technologies.
Compactness, absence of wires, and water resistance of biopatches contribute to better patient compliance and signal quality, which in turn lead to that more cases with AF are detected than with standard ambulatory monitoring. Therefore, further shrinking of device size and extended monitoring duration will likely promote biopatch-based AF monitoring as an alternative to implanted loop recorders. Nevertheless, the clinical implications and the cost effectiveness of biopatches have to be further investigated.
2.3.5 Handheld Recorders
Handheld recorders rely on the single-lead ECG, acquired by placing the hands (thumbs, fingers, palms) on two electrodes during a period from 10 s to several minutes. Handheld recorders have been proposed as an alternative screening tool to pulse palpation. Compared to other ECG modalities, handheld recorders offer certain advantages such as low cost, ease of use, and the absence of adhesive electrodes and connecting wires.
Among the handheld AF screening devices, thumb-ECG recorders are gaining recognition around the world. The Zenicor thumb-ECG recorder (Zenicor Medical Systems AB, Stockholm, Sweden) is used by several hundred clinics in Scandinavia. The device records a 30-s single-lead ECG several times a day, at predetermined times as well as when the patient has AF-related symptoms. The signal is transmitted to a web server via a mobile connection. In such a way, ECGs are stored on the internet, automatically categorized into clinically useful groups, and, if needed, made available to a trained technician for evaluation. The Zenicor device was evaluated on a large population of 7,173 individuals of 75–76 years of age who underwent intermittent screening during two weeks. The results showed that four times more cases with AF were detected than with 24-h standard ambulatory monitoring [39, 40]. Based on information from the thumb-ECG, indicating new onset AF, anticoagulant treatment was initiated in 93% of all patients.
The AfibAlert AF monitor (Lohman Technologies, Sussex, WI., USA) is another commercially available thumb-ECG recorder. The AfibAlert acquires the ECG in two different ways: either by pressing thumbs on the electrodes or using wrist electrodes. The signal is recorded for 45 s, and then analyzed for AF. The preliminary decision is immediately reported by an LED indicator. If AF is suspected, the patient has to transmit the ECG to a physician for confirmation of the preliminary decision by uploading the data through a USB connection. The company website declares an AF detection accuracy of 94%, however, no clinical study has been published which supports this figure.
The MyDiagnostick recorder (Applied Biomedical Systems BV, Maastricht, The Netherlands) is designed to record a palm ECG. The device has the form of a stick with metallic handles at both ends, serving as electrodes. In order to acquire data for arrhythmia detection, the user has to hold the metallic handles for one minute. To reduce the number of false positives, the procedure is repeated twice. The recorded ECG is then analyzed using an embedded, proprietary AF detection algorithm. The patient is informed about the outcome of the analysis via an LED indicator. The MyDiagnostick device was tested on 181 patients, where the majority had confirmed AF. Thus, a highly exaggerated AF prevalence of 53% was reported when the ECGs were acquired [41]. Sensitivity of 94% and specificity of 93% resulted when the recommended protocol of three subsequent measurements was followed (see Sect. 4.5 for the definition of different detection performance measures). Since most patients had AF, the influence of non-AF ECGs with ectopic beats on the false positive rate remains to be established.
In summary, handheld devices offer a simple and fast means for detecting AF, since measurements can be performed whenever arrhythmia symptoms are experienced. Moreover, such devices may be used by a physician/technician to check whether the patient needs a standard 12-lead ECG for confirmation of AF. On the other hand, the huge amount of data to be manually reviewed is a significant problem for some of the handheld ECG recorders. In addition, poor signal quality due to large electrode–skin impedance and motion artifacts, rapid changes in the ECG signal due to lost electrode contact, and low f wave amplitude are obstacles which make the analysis particularly challenging [42].
2.3.6 Smartphone-Based Devices
Smartphone-based devices are emerging tools for screening of general health status [43, 44]. In 2016, there were 2.3 billion smartphone users around the world, and more than 259,000 mobile health applications available on app stores for personal use. It is highly likely that smartphones incorporating healthcare technologies will occupy a large part of the medical screening devices in the future. Considering that smartphones are well-suited for data acquisition, storage, and processing, as well as for display and transmission of analysis results, they represent an inexpensive means for mass screening of AF [45, 46].
Smartphones are used to acquire the ECG between the fingers of the left and right hands, placed on the electrodes at the back of the smartphone case. AliveCor Heart Monitor (AliveCor, San Francisco, CA, USA) and CardiacDesigns ECG Check (CardiacDesigns, Park City, UT, USA) have cases with integrated dry electrodes for acquiring a single-lead ECG. Before a recording is made, special instructions on arm relaxation are provided with the aim of reducing the noise level and the amount of artifacts. The ECG is recorded for about one minute and transmitted to the microphone of the iPhone, using a modulated ultrasound signal. The ECG is sent to a cardiologist for review when an abnormal rhythm has been identified by a proprietary algorithm.
Although studies are underway to assess the suitability of this technology for mass screening [47], its performance remains unclear. For example, the original study reported excellent performance of the AliveCor Heart Monitor with sensitivity of 98% and specificity of 97% [48]. However, a subsequent study on two different groups, cardiac patients and geriatric patients, revealed much lower sensitivities of 55% and 79%, respectively [49]. This dramatic reduction in sensitivity was explained by errors in the software and the decision taken by the company to favor specificity over sensitivity [50]. The motivation behind this decision was to minimize the number of false positives, since the device is sold to patients who will not necessarily seek ECG revision by certified technicians.
2.3.7 Implantable Devices
Implantable loop recorders are invasive leadless devices used exclusively for diagnostic purposes. Such recorders have proved to be useful for diagnosing recurrent syncope events when the patient temporarily has lost consciousness and then recovers spontaneously [51]. In case of AF, the implantable loop recorder plays a special role in certain situations, e.g., when evaluating the success of AF treatment procedures such as radiofrequency or cryoablation, assessing the efficacy of rate control therapy, or detecting asymptomatic paroxysmal AF episodes after cryptogenic ischemic stroke.
Several implantable loop recorders with embedded AF detection are available for clinical use, e.g., Reveal XT (Medronic, Minneapolis, MN, USA), SJM Confirm (St, Jude Medical, St. Paul, MN, USA), and Sleuth (Transoma Medical, St. Paul, MN, USA). These devices include two built-in electrodes suitable for recording the bipolar electrogram, have a thickness of several millimeters, weigh less than 20 g, and are inserted subcutaneously. Similarly to the external, continuous loop recorder earlier mentioned, the implantable equivalent involves looping memory, and can operate either in automated self-activation mode or patient-activated mode using a handheld control device when symptoms are experienced.
The Reveal XT implantable loop recorder identifies atrial tachycardia and AF on the basis of Poincaré plot analysis [52], see also Sect. 4.2.2. In addition, the device can be programmed to detect arrhythmia episodes with a user-defined minimum duration. In a study with 247 patients using a minimum AF episode duration of two minutes, the sensitivity and the positive predictive value were found to be 88.2% and 73.5%, respectively. However, these figures increased to 92.1 and 79.6% when the minimum duration was increased to as much as six minutes [53]. As a result, six minutes has usually been preferred in clinical studies. Manual review of simultaneously recorded electrograms showed that false detection due to ectopic beats represented the most common problem [53, 54]. A more detailed review of the causes showed that 35% of the false positives were due to activity of the pectoral muscle, 15% due to atrial and ventricular premature beats, 4% due to false QRS detection, and 1.5% due to T wave oversensing [55].
Many clinical studies have demonstrated that continuous AF monitoring using implantable loop recorders is superior to noninvasive techniques. Hence, implantable cardiac monitors are gaining in popularity, although the false positive rate is usually high. However, mass implantation of invasive devices is unrealistic due to the high cost associated with the device and the required surgical procedure, as well as the potential risk of infection. Another notable drawback is that the device has to be replaced after 2–3 years of usage, although emerging, energy-effective hardware and software solutions may, at least in theory, extend the operation time of the device up to 10 years [56].
Implantable devices, such as the pacemaker, the cardioverter–defibrillator, and the biventricular pacemaker for cardiac resynchronization therapy are used for therapeutic purposes. However, they can also be programmed to detect arrhythmias such as AF. This type of device can record the intra-atrial electrogram directly in the heart via an atrial lead. In contrast to the surface ECG, the intra-atrial electrogram mostly reflects atrial activations, whereas the ventricular activity usually has lower amplitude (Fig. 2.6). Thus, a device with an atrial electrode not only makes it possible to detect episodes of rapid atrial rate, but also to characterize individual episodes with respect to atrial rate.
Since the dual-chamber cardioverter–defibrillator has an electrode placed in the right ventricle, both the intra-atrial and intra-ventricular signals are involved in the detection of atrial tachyarrhythmias. A small study showed that 98% of 132 AF episodes were correctly detected by a dual-chamber cardioverter–defibrillator Medtronic Jewel AF (Medtronic, Minneapolis, MN, USA) [57]. All false positives were due to over-sensing of far-field ventricular activity. It should be noted that all falsely detected episodes were shorter than five minutes, with a mean duration of \(2.6\pm 2.0\) min for atrial tachycardia and \(3.2 \pm 1.6\) min for AF. This result suggests that the false positive rate increases for arrhythmic events of shorter duration. Although AF may be discriminated from atrial tachycardia or atrial flutter using information on AFR and irregularity of atrial events, some AF episodes may be incorrectly classified as atrial tachycardia.
As mentioned above, incorrect AF detection in a dual-chamber cardioverter–defibrillator may be due to far-field ventricular activity in the intra-atrial electrogram. In some patients, especially when the atrial electrode is positioned outside the right atrial appendage, the amplitude of the ventricular activity is very large. Therefore, to avoid over-sensing of the far-field ventricular activity, the detection sensitivity of atrial waves has either to be reduced or the post-ventricular atrial blanking prolonged [58]. For this reason, in many clinical studies, an episode is flagged as atrial tachyarrhythmia when the atrial rate exceeds 190 beats per minute for at least six minutes. Moreover, a rapid atrial rate may be due to other supraventricular tachycardias, such as atrial flutter or atrial tachycardia, or even to bursts of atrial premature beats. Thus, manual review of intra-atrial signals may not be enough to distinguish between AF and other arrhythmias.
2.3.8 Non-ECG Devices
Emerging technologies for signal acquisition provide interesting means for recording physiological signals in a less obtrusive way, without the need for disposable electrodes. Several photoplethysmographic approaches to AF detection have been proposed, including the built-in camera of an iPhone [59], a web camera [60], an earlobe sensor [61], and a smart wristband [62] to acquire a pulsatile signal, see Fig. 2.7. Since AF detection based on the RR interval series of the ECG has been found useful (Sect. 4.2), it can be anticipated that AF detection based on the PP interval series of the photoplethysmogram (PPG) should be equally useful, although the PP intervals do not always match the RR intervals [63, 64]. In fact, the RR-based approach dominates in PPG-based AF detection, just as it does in ECG-based AF detection, because information on pulse morphology is more vulnerable to noise and artifacts.
Since a camera is available in any smartphone, the least expensive alternative to mass AF screening is based on the smartphone. The main idea is to produce a pulsatile PPG signal from the video obtained by placing the fingertip directly on the camera lens, with the LED flash illuminating the fingertip [46]. The resulting PPG signal is then processed with respect to AF detection. An iPhone-based prototype was validated in 76 patients with persistent AF undergoing electrical cardioversion, based on the pulsatile signal acquired before and after cardioversion [65]. Detection performance was expressed in terms of sensitivity and specificity, found to be 96% and 97%, respectively. The Cardiio Rhythm smartphone application (Cardiio, Cambridge, MA, USA) exhibited similar performance, with sensitivity and specificity of 93% and 98%, respectively [45]. In that study, the signal was acquired under the instruction of a trained observer. However, when acquired without supervision, the signal quality will most likely deteriorate, leading to reduced performance.
The commercially available smart wristbands facilitate unobtrusive AF monitoring, since the PPG can be acquired intermittently or even continuously for several days. Using the Empatica E4 smart wristband (Empatica, Milan, Italy), the PPG signal was acquired at rest for 10 min in 31 patients with persistent AF, 29 with sinus rhythm, and 9 with non-AF arrhythmias [62]. The sensitivity and specificity of AF detection were found to be 75% and 96%, respectively.
Given that the oscillometric principle of self-screening blood pressure monitors involves the analysis of a pulsatile signal, which in turn represents pressure oscillations in the sphygmomanometer cuff, the same signal can be employed for evaluation of pulse rhythm irregularity. Such an approach is especially attractive for mass screening, since home blood pressure monitors are widespread among hypertensive patients at high risk for developing AF. The Microlife BP A200 (Microlife AG, Widnau, Switzerland) and the Omron M6 (Omron Healthcare, Kyoto, Japan) are widely used blood pressure monitors with an integrated function for AF detection. In both monitors, detection is performed during cuff deflation by calculating the mean and standard deviation of 10 consecutive pulse intervals. Then, the irregularity index is computed by dividing the standard deviation by the mean, i.e., the coefficient of variation. Since these measures are especially sensitive to the presence of ectopic beats, intervals 25% shorter and 25% longer than the mean are removed from the series before computation of the irregularity index. The only notable difference between these two blood pressure monitors lies in their respective recommendations: three consecutive measurements should be performed using the Microlife BP A200, whereas only one for the Omron M6.
Several studies have investigated the feasibility of the Microlife BP A200 blood pressure monitor to detect AF. The performance differed slightly between the studies, depending primarily on the number of consecutive measurements taken for decision-making. Rather high sensitivity of 92–100% and specificity of 89–97% were obtained when three consecutive measurements were performed [66,67,68,69]. However, such monitors are prone to false alarms due to the presence of ectopic beats or highly variable pulse rates. For example, respiratory sinus arrhythmia is very common in the younger population, thus it is not surprising that 18% of the measurements were false positives for individuals of 13–18 years of age [70]. Hence, it is essential to evaluate the usefulness of this technique for AF detection, requiring large-scale studies where the ECG is simultaneously recorded.
Although the idea to detect AF using the PPG is promising, artifacts tend to play an important role when recordings are made at home, without the supervision of trained staff. Therefore, reliable artifact detection is necessary to ensure that the workload, as well as the expenses, generated by many false detections can be held to a minimum. Another problem arises in situations when the patient has impaired blood flow in the fingers—a problem commonly encountered in patients suffering from diabetes. So far, no guidelines exist on how to interpret the PPG signal, and, therefore, the ECG still needs to be recorded to confirm the presence of AF. As a result, PPG-based devices are suitable for AF screening, but not for diagnostic purposes.
2.3.9 Monitoring Strategies
At an early stage of arrhythmia progression, AF detection is particularly challenging because the episodes may be asymptomatic, brief, and infrequent. Therefore, AF is usually identified during planned examinations of health status or by pronounced symptoms. Today, pulse palpation followed by a 12-lead ECG or 24-h ambulatory monitoring is the standard procedure for AF screening in individuals over 65 years [1]. However, ambulatory monitoring, let alone the 12-lead ECG, is usually insufficient for detecting paroxysmal AF. Hence, there is an ongoing debate on the monitoring strategies which are better suited for specific tasks, such as, monitoring of AF recurrence after catheter ablation, cryptogenic ischemic stroke, and coronary artery bypass grafting [71, 72]. Moreover, when selecting the most appropriate strategy for AF detection, factors such as cost effectiveness and patient compliance should be considered as well.
A comprehensive study was conducted in which various strategies of intermittent AF monitoring (24-h, 7-, 14-, and 30-day standard ambulatory monitoring) were investigated in terms of the likelihood to detect at least one AF episode during a one-year period [72]. Since the study excluded AF episodes shorter than five minutes, the chance to detect even a single AF episode during the monitoring period increases if a high-performing algorithm for brief AF detection is applied. The study was based on mathematical simulations using data from invasive, continuous monitoring of 647 patients. The simulation results showed that in order to identify paroxysmal AF in half of the monitored patients, four random tests of 24-h ambulatory monitoring should, in average, be prescribed. To reach a sensitivity of 80%, at least three random tests of 30-day ambulatory monitoring, five tests of 14 days, or seven tests of 7 days are needed.
An essential point to be made regarding the temporal occurrence pattern of AF episodes is that it is considerably more challenging to detect AF with intermittent monitoring when AF episodes are highly aggregated in time, see Fig. 2.8 [72]. In such cases 24-h ambulatory monitoring can turn out to be completely ineffective, requiring extended monitoring to improve the detection rate.
A number of studies have been conducted which compare the standard monitoring strategy, i.e., the 12-lead ECG or 24-h ambulatory monitoring, to potentially more advantageous AF detection strategies [72]. For example, the above-mentioned large scale population study, involving individuals of 75–76 years of age, showed that short-term intermittent screening, using the handheld Zenicor ECG recorder for at least twice a day over two weeks, detected new onset AF in 7.4% of all patients [73].
Another problem is the selection of effective strategy for AF detection after cryptogenic ischemic stroke; both intermittent screening and continuous monitoring are considered. For example, patients having suffered from a stroke or a transient ischemic attack were screened for one month, using a patient-activated event recorder [74]. Only patients with a negative outcome of the initial 24-h ambulatory monitoring were prescribed with screening (one ECG recorded per day of about 30-s duration). Surprisingly, as many as 9.2% of all cases with new onset AF were identified using such a simplistic screening approach. It was emphasized in [74] that the cost of transtelephonic ECG monitoring was almost one third lower than that of 24-h ambulatory monitoring.
Since stroke patients are predisposed to have brief AF episodes, intermittent screening may result in lower than actual AF detection rates. Hence, continuous monitoring, using either invasive or noninvasive technologies, has been applied in several studies. For example, one-month monitoring after ischemic stroke, using noninvasive event-triggered loop recorder, improved the detection rate of paroxysmal AF by more than five times compared to 24-h ambulatory monitoring [75]. In another study, patients having suffered from cryptogenic ischemic stroke were prescribed with continuous AF monitoring using an insertable loop recorder, where half a year of monitoring yielded up to a sixfold higher AF detection rate compared to 24-h ambulatory monitoring [76].
These findings clearly demonstrate that 24-h ambulatory monitoring is not particularly efficient for detecting AF, despite the fact that it represents the standard procedure in most countries. However, a unified agreement on how each different situation (opportunistic AF screening, evaluation of cardioversion/catheter ablation success, AF detection after cryptogenic stroke, monitoring of drug effect) should be handled remains to be established in order to achieve the highest efficiency of AF detection.
References
P. Kirchhof, S. Benussi, D. Kotecha, A. Ahlsson, D. Atar, B. Casadei, M. Castella, H.C. Diener, H. Heidbuchel, J. Hendriks, G. Hindricks, A.S. Manolis, J. Oldgren, B.A. Popescu, U. Schotten, B. Van Putte, P. Vardas, S. Agewall, J. Camm, G. Baron Esquivias, W. Budts, S. Carerj, F. Casselman, A. Coca, R. De Caterina, S. Deftereos, D. Dobrev, J.M. Ferro, G. Filippatos, D. Fitzsimons, B. Gorenek, M. Guenoun, S.H. Hohnloser, P. Kolh, G.Y. Lip, A. Manolis, J. McMurray, P. Ponikowski, R. Rosenhek, F. Ruschitzka, I. Savelieva, S. Sharma, P. Suwalski, J.L. Tamargo, C.J. Taylor, I.C. Van Gelder, A.A. Voors, S. Windecker, J.L. Zamorano, K. Zeppenfeld, 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37, 2893–2962 (2016)
L. Roten, M. Schilling, A. Häberlin, J. Seiler, N.G. Schwick, J. Fuhrer, E. Delacrétaz, H. Tanner, Is 7-day event triggered ECG recording equivalent to 7-day Holter ECG recording for atrial fibrillation screening? Heart 98, 645–649 (2012)
M.S. Guillem, A.M. Climent, F. Castells, D. Husser, J. Millet, A. Arya, C. Piorkowski, A. Bollmann, Noninvasive mapping of human atrial fibrillation. J. Cardiovasc. Electrophysiol. 20, 507–513 (2009)
K. Harris, D. Edwards, J. Mant, How can we best detect atrial fibrillation? J. R. Coll. Physicians. Edinb. 42, 5–22 (2012)
J. Mant, D.A. Fitzmaurice, F.D.R. Hobbs, S. Jowett, E.T. Murray, R. Holder, M. Davies, G.Y.H. Lip, Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (safe) trial. Br. Med. J. 335, 1–6 (2007)
A.L. Bakker, G. Nijkerk, B.E. Groenemeijer, R.A. Waalewijn, E.M. Koomen, R.L. Braam, H.J. Wellens, The Lewis lead: making recognition of P waves easy during wide QRS complex tachycardia. Circulation 119, e592–e593 (2009)
A. Mizuno, K. Masuda, K. Niwa, Usefulness of Lewis lead for visualizing P-wave. Circ. J. 78, 2774–2775 (2014)
P.G. Platonov, V.D.A. Corino, M. Seifert, F. Holmqvist, L. Sörnmo, Atrial fibrillatory rate in the clinical context: natural course and prediction of intervention outcome. Europace 16, iv110–iv119 (2014)
M.S. Guillem, A. Bollmann, A.M. Climent, D. Husser, J. Millet, F. Castells, How many leads are necessary for a reliable reconstruction of surface potentials during atrial fibrillation? IEEE Trans. Inf. Technol. Biomed. 13, 330–340 (2009)
M.S. Guillem, A.V. Sahakian, S. Swiryn, Derivation of orthogonal leads from the 12-lead electrocardiogram. Performance of an atrial-based transform for the derivation of P loops. J. Electrocardiol. 41, 19–25 (2008)
M. Haissaguerre, M. Hocini, A.J. Shah, N. Derval, F. Sacher, P. Jaïs, R. Dubois, Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J. Cardiovasc. Electrophysiol. 24, 711–717 (2013)
M.S. Guillem, A.M. Climent, J. Millet, Á. Arenal, F. Fernández-Avilés, J. Jalife, F. Atienza, O. Berenfeld, Noninvasive localization of maximal frequency sites of atrial fibrillation by body surface potential mapping. Circ. Arrhythm. Electrophysiol. 6, 294–301 (2013)
Z. Ihara, V. Jacquemet, J.M. Vesin, A. van Oosterom, Adaption of the standard 12-lead ECG system focusing on atrial electrical activity, in Proceedings of Computers in Cardiology vol. 32, 203–205 (2005)
Z. Ihara, A. van Oosterom, V. Jacquemet, R. Hoekema, Adaptation of the 12-lead electrocardiogram system dedicated to the analysis of atrial fibrillation. J. Electrocardiol. 40, 68.e1–68.e8 (2007)
D. Husser, M. Stridh, L. Sörnmo, I. Toepffer, H.U. Klein, S.B. Olsson, A. Bollmann, Electroatriography–time-frequency analysis of atrial fibrillation from modified 12-lead ECG configurations for improved diagnosis and therapy. Med. Hypotheses 68, 568–573 (2007)
W. Wenger, P. Kligfield, Variability of precordial electrode placement during routine electrocardiography. J. Electrocardiol. 29, 179–184 (1996)
A. Bollmann, N. Kanuru, K. McTeague, P. Walter, D.B. DeLurgio, J. Langberg, Frequency analysis of human atrial fibrillation using the surface electrocardiogram and its response to ibutilide. Am. J. Cardiol. 81, 1439–1445 (1998)
S. Petrutiu, A.V. Sahakian, W.B. Fisher, S. Swiryn, Manifestation of left atrial events in the surface electrocardiogram, in Proceedings of Computers in Cardiology vol. 33, 1–4 (2006)
G.E. Dower, A. Yakush, S.B. Nazzal, R.V. Jutzy, C.E. Ruiz, Deriving the 12-lead electrocardiogram from four (EASI) electrodes. J. Electrocardiol. 21(Suppl.), S182–187 (1988)
R.L. Lux, C.R. Smith, R.F. Wyatt, J.A. Abildskov, Limited lead selection for estimation of body surface potential maps in electrocardiography. IEEE Trans. Biomed. Eng. 25, 270–276 (1978)
B.J. Drew, R.M. Califf, M. Funk, E.S. Kaufman, M.W. Krucoff, M.M. Laks, P.W. Macfarlane, C. Sommargren, S. Swiryn, G.F. Van Hare, Practice standards for electrocardiographic monitoring in hospital settings. Circulation 110, 2721–2746 (2004)
M.P. Turakhia, D.D. Hoang, P. Zimetbaum, J.D. Miller, V.F. Froelicher, U.N. Kumar, X. Xu, F. Yang, P.A. Heidenreich, Diagnostic utility of a novel leadless arrhythmia monitoring device. Am. J. Cardiol. 112, 520–524 (2013)
T. Lewis, Clinical Electrocardiography (Shaw & Sons, New York, 1913)
A. Petrėnas, V. Marozas, G. Jaruševičius, L. Sörnmo, A modified Lewis ECG lead system for ambulatory monitoring of atrial arrhythmias. J. Electrocardiol. 48, 157–163 (2015)
S. Nedios, I. Romero, J.-H. Gerds-Li, E. Fleck, C. Kriatselis, Precordial electrode placement for optimal ECG monitoring: implications for ambulatory monitor devices and event recorders. J. Electrocardiol. 47, 669–676 (2014)
A. Kennedy, D.D. Finlay, D. Guldenring, R.R. Bond, J. McLaughlin, Detecting the elusive P-wave: a new ECG lead to improve the recording of atrial activity. IEEE Trans. Biomed. Eng. 63, 243–249 (2016)
M.A. Rosenberg, M. Samuel, A. Thosani, P.J. Zimetbaum, Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin. Electrophysiol. 36, 328–333 (2013)
S. Babaeizadeh, R.E. Gregg, E.D. Helfenbein, J.M. Lindauer, S.H. Zhou, Improvements in atrial fibrillation detection for real-time monitoring. J. Electrocardiol. 42, 522–526 (2009)
S. Asgari, A. Mehrnia, M. Moussavi, Automatic detection of atrial fibrillation using stationary wavelet transform and support vector machine. Comput. Biol. Med. 60, 132–142 (2015)
J.S. Steinberg, N. Varma, I. Cygankiewicz, P. Aziz, P. Balsam, A. Baranchuk, D.J. Cantillon, P. Dilaveris, S.J. Dubner, N. El-Sherif, J. Krol, M. Kurpesa, M.T. La Rovere, S.S. Lobodzinski, E.T. Locati, S. Mittal, B. Olshansky, E. Piotrowicz, L. Saxon, P.H. Stone, L. Tereshchenko, G. Turitto, N.J. Wimmer, R.L. Verrier, W. Zareba, R. Piotrowicz, 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiacmonitoring/telemetry. Heart Rhythm 17, e55–e96 (2017)
N.J. Holter, New method for heart studies: continuous electrocardiography of active subjects over long period is now practical. Science 134, 1214 (1961)
H.T. Tu, S. Spence, J.M. Kalman, S.M. Davis, Twenty-eight day Holter monitoring is poorly tolerated and insensitive for paroxysmal atrial fibrillation detection in cryptogenic stroke. Int. Med. J. 44, 505–508 (2014)
A. Müller, W. Scharner, T. Borchardt, W. Och, H. Korb, Reliability of an external loop recorder for automatic recognition and transtelephonic ECG transmission of atrial fibrillation. J. Telemed. Telecare 15, 391–396 (2009)
B.O. Velthuis, J. Bos, K. Kraaier, J. Stevenhagen, J.M. van Opstal, J. van der Palen, M.F. Scholten, Performance of an external transtelephonic loop recorder for automated detection of paroxysmal atrial fibrillation. Ann. Noninvasive Electrocardiol. 18, 564–570 (2013)
S.S. Lobodzinski, ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog. Cardiovasc. Dis. 56, 224–229 (2013)
P.M. Barrett, R. Komatireddy, S. Haaser, S. Topol, J. Sheard, J. Encinas, A.J. Fought, E.J. Topol, Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 127, 95.e11–95.e17 (2014)
J. M. Engel, V. Mehta, R. Fogoros, A. Chavan, Study of arrhythmia prevalence in NUVANT mobile cardiac telemetry system patients, in Proceedings Conference of the IEEE Engineering in Medicine and Biology Society (2012), pp. 2440–2443
J.M. Engel, N. Chakravarthy, G. Nosbush, M. Merkert, M.D.R. Fogoros, A. Chavan, Comparison of arrhythmia prevalence in NUVANT Mobile Cardiac Telemetry System patients in the US and India, in Proceedings Conference of the IEEE Engineering in Medicine and Biology Society (2014), pp. 2730–2733
L. Friberg, J. Engdahl, V. Frykman, E. Svennberg, L.-Å. Levin, M. Rosenqvist, Population screening of 75- and 76-year-old men and women for silent atrial fibrillation (STROKESTOP). Europace 15, 135–140 (2012)
E. Svennberg, J. Engdahl, F. Al-Khalili, L. Friberg, V. Frykman, M. Rosenqvist, Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 131, 2176–2184 (2015)
B. Vaes, S. Stalpaert, K. Tavernier, B. Thaels, D. Lapeire, W. Mullens, J. Degryse, The diagnostic accuracy of the MyDiagnostick to detect atrial fibrillation in primary care. BMC Fam. Pract. 15, 113 (2014)
M. Stridh, M. Rosenqvist, Automatic screening of atrial fibrillation in thumb-ECG recordings, in Proceedings of Computing in Cardiology vol. 39, 193–196 (2012)
E. Agu, P. Pedersen, D. Strong, B. Tulu, Q. He, L. Wang, Y. Li, The smartphone as a medical device: assessing enablers, benefits and challenges, in 10th Annual IEEE Communications Society Conference on Sensor, Mesh and Ad Hoc Communications and Networks (SECON) (2013), pp. 76–80
A.R.J. Mitchell, P. Le Page, Living with the handheld ECG. BMJ Innov. 2 (2015)
P.-H. Chan, C.-K. Wong, Y. C. Poh, L. Pun, W.W.-C. Leung, Y.-F. Wong, M.M.-Y. Wong, M.-Z. Poh, D. W.-S. Chu, C.-W. Siu, Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J. Am. Heart Assoc. 5 (2016)
B. Freedman, Screening for atrial fibrillation using a smartphone: Is there an app for that? J. Am. Heart Assoc. 5 (2016)
N. Lowres, S.B. Freedman, J. Redfern, A. McLachlan, I. Krass, A. Bennett, T. Briffa, A. Bauman, L. Neubeck, Screening education and recognition in community pharmacies of atrial fibrillation to prevent stroke in an ambulant population aged \(\ge \)65 years (SEARCH-AF stroke prevention study): a cross-sectional study protocol. Br. Med. J. Open 2 (2012)
J.K. Lau, N. Lowres, L. Neubeck, D.B. Brieger, R.W. Sy, C.D. Galloway, D.E. Albert, S.B. Freedman, iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int. J. Cardiol. 165, 193–194 (2013)
L. Desteghe, Z. Raymaekers, M. Lutin, J. Vijgen, D. Dilling-Boer, P. Koopman, J. Schurmans, P. Vanduynhoven, P. Dendale, H. Heidbuchel, Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 19, 29–39 (2017)
D.E. Albert, Letter on Desteghe et al., Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace, euw218 (2016)
K. Kanjwal, V.M. Figueredo, B. Karabin, B. Grubb, The implantable loop recorder: current uses, future directions. J. Innov. Cardiac Rhythm Manag. 2, 215–222 (2011)
S. Sarkar, D. Ritscher, R. Mehra, A detector for a chronic implantable atrial tachyarrhythmia monitor. IEEE Trans. Biomed. Eng. 55, 1219–1224 (2008)
G. Hindricks, E. Pokushalov, L. Urban, M. Taborsky, K.-H. Kuck, D. Lebedev, G. Rieger, H. Pürerfellner, and on behalf of the XPECT Trial Investigators, Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation results of the XPECT trial. Circ. Arrhythm. Electrophysiol. 3, 141–147 (2010)
A.S. Montenero, A. Quayyum, P. Franciosa, D. Mangiameli, A. Antonelli, L. Barbieri, N. Bruno, F. Zumbo, M. Vimercati, Implantable loop recorders: a novel method to judge patient perception of atrial fibrillation. Preliminary results from a pilot study. J. Interv. Card. Electrophysiol. 10, 211–220 (2004)
C. Eitel, D. Husser, G. Hindricks, M. Frühauf, S. Hilbert, A. Arya, T. Gaspar, U. Wetzel, A. Bollmann, C. Piorkowski, Performance of an implantable automatic atrial fibrillation detection device: impact of software adjustments and relevance of manual episode analysis. Europace 13, 480–485 (2011)
O. Andersson, K.H. Chon, L. Sörnmo, J.N. Rodrigues, A 290 mV sub-V\(_\text{ T }\) ASIC for real-time atrial fibrillation detection. IEEE Trans. Biomed. Circuits Syst. 9, 377–386 (2015)
C.D. Swerdlow, W. Schsls, B. Dijkman, W. Jung, N.V. Sheth, W.H. Olson, B.D. Gunderson, Detection of atrial fibrillation and flutter by a dual-chamber implantable cardioverter-defibrillator. For the Worldwide Jewel AF Investigators. Circulation 101, 878–885 (2000)
J.W. Fung, J. Sperzel, C.M. Yu, J.Y. Chan, R.N. Gelder, M.X. Yang, R. Rooke, P. Boileau, G. Fröhlig, Multicenter clinical experience with an atrial lead designed to minimize far-field R-wave sensing. Europace 11, 618–624 (2009)
J. Lee, B.A. Reyes, D.D. McManus, O. Mathias, K.H. Chon, Atrial fibrillation detection using an iPhone 4S. IEEE Trans. Biomed. Eng. 60, 203–206 (2013)
J.P. Couderc, S. Kyal, L. Mestha, B. Xu, D. Peterson, X. Xia, B. Hall, Detection of atrial fibrillation using contactless facial video monitoring. Heart Rhythm 12, 195–201 (2015)
T. Conroy, J.H. Guzman, B. Hall, G. Tsouri, J.-P. Couderc, Detection of atrial fibrillation using an earlobe photoplethysmographic sensor. Physiol. Meas. 38, 1906–1918 (2017)
V.D.A. Corino, R. Laureanti, L. Ferranti, G. Scarpini, F. Lombardi, L.T. Mainardi, Detection of atrial fibrillation episodes using a wristband device. Physiol. Meas. 38, 787–799 (2017)
G. Lu, F. Yang, J.A. Taylor, J.F. Stein, A comparison of photoplethysmography and ECG recording to analyse heart rate variability in healthy subjects. J. Med. Eng. Technol. 33, 634–641 (2009)
E. Gil, M. Orini, R. Bailón, J.M. Vergara, L. Mainardi, P. Laguna, Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas. 31, 1271 (2010)
D.D. McManus, J. Lee, O. Maitas, N. Esa, R. Pidikiti, A. Carlucci, J. Harrington, E. Mick, K.H. Chon, A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 10, 315–319 (2013)
J. Wiesel, L. Fitzig, Y. Herschman, F.C. Messineo, Detection of atrial fibrillation using a modified Microlife blood pressure monitor. Am. J. Hypertens. 22, 848–852 (2009)
G.S. Stergiou, N. Karpettas, A. Protogerou, E.G. Nasothimiou, M. Kyriakidis, Diagnostic accuracy of a home blood pressure monitor to detect atrial fibrillation. J. Hum. Hypertens. 23, 654–658 (2009)
G. Marazzi, F. Iellamo, M. Volterrani, M. Lombardo, F. Pelliccia, D. Righi, F. Grieco, L. Cacciotti, L. Iaia, G. Caminiti, G. Rosano, Comparison of Microlife BP A200 Plus and Omron M6 blood pressure monitors to detect atrial fibrillation in hypertensive patients. Adv. Ther. 29, 64–70 (2012)
J. Wiesel, B. Arbesfeld, D. Schechter, Comparison of the Microlife blood pressure monitor with the Omron blood pressure monitor for detecting atrial fibrillation. Am. J. Cardiol. 114, 1046–1048 (2014)
A.J. Cheung, B.M.Y. Cheung, False detection of atrial fibrillation in children by a blood pressure monitor with atrial fibrillation detection function. Br. Med. J. Case Rep. 1–2 (2015)
G. Hindricks, C. Piorkowski, Atrial fibrillation monitoring: mathematics meets real life. Circulation 126, 791–792 (2012)
E.I. Charitos, U. Stierle, P.D. Ziegler, M. Baldewig, D.R. Robinson, H. Sievers, T. Hanke, A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 126, 806–814 (2012)
J. Engdahl, L. Andersson, M. Mirskaya, M. Rosenqvist, Stepwise screening of atrial fibrillation in a 75-year-old population: Implications for stroke prevention. Circulation 127, 930–937 (2013)
N. Gaillard, S. Deltour, B. Vilotijevic, A. Hornych, S. Crozier, A. Leger, R. Frank, Y. Samson, Detection of paroxysmal atrial fibrillation with transtelephonic EKG in TIA or stroke patients. Neurology 74, 1666–1670 (2010)
D.J. Gladstone, M. Spring, P. Dorian, V. Panzov, K.E. Thorpe, J. Hall, H. Vaid, M. O’Donnell, A. Laupacis, R. Côté, M. Sharma, J.A. Blakely, A. Shuaib, V. Hachinski, S.B. Coutts, D.J. Sahlas, P. Teal, S. Yip, J.D. Spence, B. Buck, S. Verreault, L.K. Casaubon, A. Penn, D. Selchen, A. Jin, D. Howse, M. Mehdiratta, K. Boyle, R. Aviv, M.K. Kapral, M. Mamdani, Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 370, 2467–2477 (2014)
T. Sanna, H.-C. Diener, R.S. Passman, V. Di Lazzaro, R.A. Bernstein, C.A. Morillo, M.M. Rymer, V. Thijs, T. Rogers, F. Beckers, K. Lindborg, J. Brachmann, Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 370, 2478–2486 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Petrėnas, A., Marozas, V., Sörnmo, L. (2018). Lead Systems and Recording Devices. In: Sörnmo, L. (eds) Atrial Fibrillation from an Engineering Perspective. Series in BioEngineering. Springer, Cham. https://doi.org/10.1007/978-3-319-68515-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-68515-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68513-7
Online ISBN: 978-3-319-68515-1
eBook Packages: EngineeringEngineering (R0)