Abstract
Renal and perinephric abscess can result in significant morbidity and mortality with complications including sepsis, renal failure, and fistula formation.
Image-guided percutaneous abscess drainage is the treatment of choice in large-sized renal and perirenal abscess because it offers a relatively simple, minimally invasive option with the goal of averting the development of sepsis, reducing duration of hospital stay, and reducing the cost of treatment.
Ultrasound (US) and computed tomography (CT) are the most commonly used imaging modalities to guide percutaneous abscess drainage, and fluoroscopy is often also used to guide serial dilatation and catheter placement following successful needle access.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
An abscess is a localized collection of purulent fluid [1]. Perinephric and renal abscesses are uncommon but potentially lethal complications which may lead to sepsis from hematogenous spread of infection [2].
A renal abscess is confined to the renal parenchyma; a perinephric abscess is a pocket of pus in the perinephric space between the renal capsule and Gerota’s fascia; perirenal abscesses may also develop from extension of inflammatory disease outside the Gerota’s fascia [3].
The most common causes are either ascending infections of the lower urinary tract or hematogenous seeding from primary infectious sites [4].
Perinephric abscess may result from rupture of a renal abscess into the perirenal space but most often develops directly from hematogenous spread of infection. Alternative mechanisms include extension from extrarenal inflammatory processes such as diverticulitis and pyelosinus extravasation of infected urine [5].
Despite their rarity, also abscesses remain an important complication of renal transplantation [6].
Moreover, abscesses can develop secondary to spontaneous or iatrogenic infection after recent surgery [7]. They commonly manifest in the first postoperative month but can arise at any time.
Perinephric abscesses can result from infection of the surgical site, spontaneous or iatrogenic infection of a previously sterile fluid collection, or complicated pyelonephritis [8].
Common predisposing conditions are systemic diseases such as diabetes mellitus and renal or urologic diseases such as malignancy or renal stones [9].
Renal or perinephric hematoma, spontaneous, traumatic, or iatrogenic, can become infected [10].
Bacterial pyelonephritis is most common due to Gram-negative organisms such as Escherichia coli [11]. The following represents a description of indications, techniques, complications, and management of percutaneous drainage in patients with renal collections [12].
2 Indications
In most cases, small-sized renal abscesses <3 cm are successfully treated with intravenous antibiotics alone; small fluid collections can be sampled or aspirated for the assessment of optimal antibiotic coverage or for fluid characterization. If material appears infected, a drainage catheter may then be placed [13].
For instance, although fever, leukocytosis, malaise, anorexia, or other systemic symptoms point to an infection, these signs and symptoms may be absent in elderly, very ill, or immunocompromised patients [14].
Large (>5 cm) or rapidly enlarging collections and obstructing and infected collecting systems are readily amenable to percutaneous drainage [9].
3 Contraindications
Significant coagulopathy and severe compromised cardiopulmonary function or hemodynamic instability are common contraindications for all types of percutaneous procedures [15].
These contraindications should be addressed and corrected or controlled before the procedure whenever is possible. Percutaneous drainage is contraindicated in calcified masses. Septation and multiloculation are not absolute contraindications for percutaneous drainage because these conditions can be resolved by inserting several catheters or by septal perforation.
Pre-procedural planning may be the most important step of the procedure to avoid potential complications. Lack of a safe pathway to the abscess or fluid collection is a contraindication.
Inability of the patient to cooperate with, or to be positioned correctly for, the procedure may prevent the success of treatment [16].
4 Antibiotics Prophylaxis
The authors of the Society of Interventional Radiology (SIR) standards of practice guidelines for adult antibiotic prophylaxis consider percutaneous abscess drainage a dirty procedure, and, as such, routine pre-procedural prophylactic antibiotic administration is recommended [17].
5 Procedure
5.1 Approach
Pre-procedural planning represents the most important step in order to avoid complications, especially major vessel injuries and the formation of a pseudoaneurysm and/or bleeding.
First of all, aseptic technique is mandatory to prevent the spread of pathogens and the development of sepsis and septic shock.
Few recommendations can help to minimize the risk of complications: first of all, it is important to use the safest and most direct percutaneous route, as to minimize the length of the internal catheter; another concern is to avoid organs or vital anatomical structures—often that part can be achieved easier with an angled approach, which helps the needle to maintain a smooth coiling and an easier advancement of the wire. Finally, it can be helpful to place the drainage catheter in the most dependent portion of the cavity in order to facilitate the evacuation of the collection [2].
5.2 Imaging Diagnosis and Guidance
US, CT, and magnetic resonance imaging (MRI) are accurate modalities for diagnosis of renal and retroperitoneal abscess.
The US appearance of renal abscess is variable. It can appear as either a hyper- or hypoechoic focal mass or complex cystic structure. On CT renal or perirenal abscess appears as a low attenuation mass that may enhance after contrast administration, although not to the extent of a solid renal tumor.
CT and MRI reveal a heterogeneously enhancing, complex, cystic lesion with enhancing internal septa and a variable degree of infiltration of the perinephric space [12].
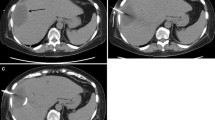
However, ultrasound is a useful real-time guidance for percutaneous catheter drainage (Fig. 21.1).
(a) Unenhanced CT axial image shows a collection in the left perirenal space. (b) After administration of contrast media, an enhanced rim was shown, adjacent to renal parenchyma. (c) The drainage was deployed under US guidance. (d) One week later, contrast-enhanced CT showed partial resolution of the collection
The combination of sonographic and fluoroscopic guidance is the most dynamic method because it provides multiplanar real-time visualization of needle advancement and direct visualization of dilator and catheter placement [11].
Conventional fluoroscopy fails to provide internal body detail, limiting its use to the drainage of large superficial fluid cavities or intraorgan cavities containing a sufficient amount of air that can be used for targeting and as an adjunctive modality to US and CT. A combination of initial US or CT guidance for the placement of the access needle and guidewire followed by fluoroscopic guidance for the wire and catheter manipulations and completion of the procedure can be useful for difficult drainages such as small or relatively deep cavities [2].
Intracavitary air may prevent optimal visualization of an abscess using US guidance.
CT may be used for air-containing cavities, for small or deep cavities, and for those with a potentially intervening hollow viscus or solid organ along the path of the access needle [18].
CT fluoroscopy using the “quick-check” technique has been shown to decrease total procedure time and patient radiation dose when compared to CT guidance without fluoroscopy [19].
5.3 Techniques
Two methods may be applied for the percutaneous approach to the collection and a safe deployment of a drainage catheter.
The Seldinger technique uses an 18-gauge sharp hollow needle (trocar) to puncture the rim of the fluid collection. Once punctured, the stylet is withdrawn, and the fluid is aspirated through the trocar needle to confirm intracavitary location. A 0.035-in. floppy-tipped guidewire is advanced through the lumen of the trocar, and the needle is then withdrawn, leaving the distal tip of the wire coiled in the collection. Imaging at this point in the procedure is useful to document appropriate placement of the wire prior to track dilation. Fascial dilators are then advanced over the wire with a stepwise increase in diameter to dilate the intended track of the catheter. Once the track is dilated, the drainage catheter, assembled with stiffener but without the trocar, is advanced along the wire to the previously marked depth of the collection. Once the track has been dilated, the drainage catheter, assembled with the stiffener but without the trocar, is advanced along the wire to the collection. Once in the exact point, the catheter is released from the metal cannula and the pigtail is formed. To secure the catheter in place, a string locking mechanism is used to fix the pigtail in the coiled position. The string is then cut and fixed to the stopcock. Catheters should be secured at the skin, preferably with an adhesive-backed locking device.
The trocar technique, the alternative to the Seldinger method, is performed using a direct puncture approach using the catheter with stylet in place. After access to the collection is obtained, the catheter is advanced and fed off the stiffener and stylet and is retained in place with the pigtail locking device. The trocar technique is faster than the Seldinger technique, obviates the need for an assistant, and is well suited for large or superficial fluid collections [20].
6 Success Rate
Curative drainage, defined as complete resolution of infection requiring no further operative intervention (Fig. 21.2), may be achieved in more than 80% of patients. Partial success is defined as either adequate drainage of the abscess with surgery subsequently performed to repair an underlying problem or as temporizing drainage performed to stabilize the patient’s condition before surgery. Partial success occurs in 5–10% of patients. Failure occurs in 5–10% and recurrence in 5–10%. These results are similar for both abdominal and chest drainage procedures [16].
7 Complications
All complications are recorded and classified as minor and major.
Major complications were defined as complications that, if untreated, might threaten the patient’s life, lead to substantial morbidity and disability, result in hospital admission, or substantially lengthen hospital stay.
Minor complications include conditions (like pain or mild hematuria) that do not lead to consequences, and require only symptomatic therapy and observation [16].
Complications after percutaneous drainage of renal and perirenal abscess are unusual: a transient febrile episode without sequelae in the first 12 h after placement of the catheter is the most common complication related to percutaneous abscess drainage [21], occurring in less than 10% of the patients [22].
The erosion or the inadvertent placement of the catheter into the gastrointestinal tract, the inadvertent dislodgment of the drainage catheter, and the renal vascular or ureteral injury [23] are less frequent, but they can also occur as complications after percutaneous drainage [24]. Hemorrhagic event represents a possible event, rarely requiring transfusion [16].
Another rare complication described in the literature is pyopneumothorax, resulted from an inappropriately placed drainage catheter that violated the pleural space [25].
8 Management
Daily catheter care with irrigation of the catheter, preferably every 8 h with at least 10 mL of sterile saline, is recommended. The decision to remove the catheter is multifactorial and includes normalization of temperature and white blood cell count as well as reduction of drainage volume to less than 10 mL/day [20].
9 Fluid Collections in the Transplanted Kidney
Perinephric fluid collections after renal transplantation are common and are associated with a number of serious complications, one of these is a perirenal abscess, which account for 2–30% of all aspirated fluid collections in the peritransplant period. Classically, these patients present with fever alone or with perigraft pain plus tenderness in a period ranging from the first 2–3 days to weeks after transplantation [6].
9.1 Lymphocele
Postoperative lymphoceles are caused by lymphatic leakage from the allograft bed or from the allograft itself and are the most common perirenal fluid collection, usually occurring weeks to months after transplantation [26].
Renal transplant patients are predisposed to prolonged lymphatic leakage as a result of graft rejection, the use of steroids or diuretics, or retransplantation [27].
Most lymphoceles are small and asymptomatic, and intervention is not necessary. However, some lymphoceles compress adjacent structures and may cause hydronephrosis, edema, or deep venous thrombosis in the ipsilateral lower extremity, and percutaneous aspiration of the fluid becomes indicated [28] (Fig. 21.3).
The most effective therapy is the combination of indwelling catheter drainage and sclerotherapy with a reported success rate of 68–100% [26].
Various sclerosing agents can be used with multiple treatments required in most cases, with the catheter left in place for anywhere from 4 to 35 days [29].
If an uninfected lymphocele recurs, it is usually treated by un-roofing into the peritoneal cavity by either open or laparoscopic technique [6].
9.2 Abscess
An abscess may arise from an infected wound or from a secondarily infected lymphocele, hematoma, or urinoma after attempts at aspiration or as a consequence of graft pyelonephritis [6] (Fig. 21.4).
Any perigraft fluid collection can become infected; usually, the affected patient presents with fever or local pain. US or CT findings usually are nonspecific, but air within the perirenal fluid collection strongly suggests a perirenal abscess. Also, in the clinical setting of fever and leukocytosis in a transplant patient, the detection of a perinephric fluid collection is presumptive evidence that the fluid is infected. In these situations, ultrasound- or CT-guided needle aspiration may confirm the diagnosis and permit the planning of a percutaneous drainage [30].
Prompt surgical or percutaneous drainage combined with systemic antibiotics is mandatory because of the immunosuppressed state of transplant patients. Percutaneous drainage under US or CT guidance is associated with a high rate of success and a low complication rate [28], with the modalities previously described in this chapter [28].
If the fluid is purulent, microscopic examination of the fluid for pus cells and organisms is done, and antibiotic treatment is initiated. Open surgical drainage becomes necessary when the percutaneous drainage of the infected fluid collections is ineffective completely or partially [6].
Conclusions
Nowadays, the procedures described became the first choice in the treatment of abscess collections. They have resulted in reduced morbidity and mortality and have helped to reduce length of hospital stay and hospital costs. In conclusion, three fundamental steps can be identified: patient selection, performing the procedure, and correct management of the patient. In all three steps, interventional radiologist, supported by clinicians, has the most important role.
References
Hung CH, Liou JD, Yan MY, Chang CC (2007) Immediate percutaneous drainage compared with surgical drainage of renal abscess. Int Urol Nephrol 39:51–55. https://doi.org/10.1007/s11255-006-9033-5
Charles HW (2012) Abscess drainage. Semin Interv Radiol 29:325–336. https://doi.org/10.1055/s-0032-1330068
Krishna GS, Vijayalakshmidevi B, Lakshmi AY, Mutheswaraiah B, Sivakumar V (2012) Perinephric abscess with extension into mediastinum and epidural space. Indian J Nephrol 22:224–225. https://doi.org/10.4103/0971-4065.98770
Dielubanza EJ, Mazur DJ, Schaeffer AJ (2014) Management of non-catheter-associated complicated urinary tract infection. Infect Dis Clin N Am 28:121–134. https://doi.org/10.1016/j.idc.2013.10.005
Coelho RF, Schneider-Monteiro ED, Mesquita JLB, Mazzucchi E, Marmo Lucon A, Srougi M (2007) Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg 31:431–436. https://doi.org/10.1007/s00268-006-0162-x
Ahmadnia H, Yarmohamadi A (2003) Percutaneous drainage of perirenal abscess after kidney transplantation: a 4-year experience. Transplant Proc 35:2670–2671. https://doi.org/10.1016/j.transproceed.2003.08.074
Meng MV, Mario LA, McAninch JW (2002) Current treatment and outcomes of perinephric abscesses. J Urol 168:1337–1340. https://doi.org/10.1097/01.ju.0000027904.39606.32
Nixon JN, Biyyam DR, Stanescu L, Phillips GS, Finn LS, Parisi MT (2013) Imaging of pediatric renal transplants and their complications: a pictorial review. Radiographics 33:1227–1251. https://doi.org/10.1148/rg.335125150
Lee SH, Jung HJ, Mah SY, Chung BH (2010) Renal abscesses measuring 5 cm or less: outcome of medical treatment without therapeutic drainage. Yonsei Med J 51:569–573. https://doi.org/10.3349/ymj.2010.51.4.569
Dietrich CF, Lorentzen T, Appelbaum L, Buscarini E, Cantisani V, Correas JM, Cui XW, D’Onofrio M, Gilja OH, Hocke M, Ignee A, Jenssen C, Kabaalioğlu A, Leen E, Nicolau C, Nolsoe CP, Radzina M, Serra C, Sidhu PS, Sparchez Z, Piscaglia F (2016) EFSUMB guidelines on interventional ultrasound (INVUS), part III - abdominal treatment procedures (short version). Ultraschall Med 37:27–45. https://doi.org/10.1055/s-0035-1553965
Heller MT, Haarer KA, Thomas E, Thaete FL (2012) Neoplastic and proliferative disorders of the perinephric space. Clin Radiol 67:e31–e41
Demertzis J, Menias CO (2007) State of the art: imaging of renal infections. Emerg Radiol 14:13–22. https://doi.org/10.1007/s10140-007-0591-3
Lorenz JM, Al-Refaie WB, Cash BD, Gaba RC, Gervais DA, Gipson MG, Kolbeck KJ, Kouri BE, Marshalleck FE, Nair AV, Ray CE, Hohenwalter EJ (2015) ACR appropriateness criteria radiologic Management of Infected Fluid Collections. J Am Coll Radiol 12:791–799. https://doi.org/10.1016/j.jacr.2015.04.025
Siegel JF, Smith A, Moldwin R (1996) Minimally invasive treatment of renal abscess. J Urol 155:52–55
Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WE, Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement, Standards of Practice Committee of the Society of Interventional Radiology (2013) Addendum of newer anticoagulants to the SIR consensus guideline. J Vasc Interv Radiol 24:641–645. https://doi.org/10.1016/j.jvir.2012.12.007
Wallace MJ, Chin KW, Fletcher TB, Bakal CW, Cardella JF, Grassi CJ, Grizzard JD, Kaye AD, Kushner DC, Larson PA, Liebscher LA, Luers PR, Mauro MA, Kundu S (2010) Quality improvement guidelines for percutaneous drainage/aspiration of abscess and fluid collections. J Vasc Interv Radiol 21:431–435. https://doi.org/10.1016/j.jvir.2009.12.398
Venkatesan AM, Kundu S, Sacks D, Wallace MJ, Wojak JC, Rose SC, Clark TWI, D’Othee BJ, Itkin M, Jones RS, Miller DL, Owens CA, Rajan DK, Stokes LS, Swan TL, Towbin RB, Cardella JF (2010) Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J Vasc Interv Radiol 21:1611–1630. https://doi.org/10.1016/j.jvir.2010.07.018
Carlson SK, Bender CE, Classic KL, Zink FE, Quam JP, Ward EM, Oberg a L (2001) Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology 219:515–520. https://doi.org/10.1148/radiology.219.2.r01ma41515
Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT (2001) CT fluoroscopy--guided interventional procedures: techniques and radiation dose to radiologists. Radiology 220:161–167. https://doi.org/10.1148/radiology.220.1.r01jl29161
Jaffe TA, Nelson RC (2016) Image-guided percutaneous drainage: a review. Abdom Radiol 41:629–636
Deyoe LA, Cronan JJ, Lambiase RE, Dorfman GS (1990) Percutaneous and perirenal drainage of renal abscesses: results in 30 patients. AJR Am. J. Roentgenol. 155:81–83
Rubilotta E, Balzarro M, Lacola V, Sarti A, Porcaro AB, Artibani W (2014) Current clinical management of renal and perinephric abscesses: a literature review. Urologia 81:144–147. https://doi.org/10.5301/urologia.5000044
Mueller PR, Ferrucci JT, Butch RJ, Simeone JF, Wittenberg J (1985) Inadvertent percutaneous catheter gastroenterostomy during abscess drainage: significance and management. Am J Roentgenol 145:387–391. https://doi.org/10.2214/ajr.145.2.387
vanSonnenberg E, Mueller PR, Ferrucci JT (1984) Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part I: results, failures, and complications. Radiology 151:337–341. https://doi.org/10.1148/radiology.151.2.6709901
Lang EK (1990) Renal, perirenal, and pararenal abscesses: percutaneous drainage. Radiology 174:109–113. https://doi.org/10.1148/radiology.174.1.2294535
Johnson SP, Berry RS (2001) Interventional radiological Management of the Complications of renal transplantation. Semin Interv Radiol 18:047–058. https://doi.org/10.1055/s-2001-12838
Khauli RB, Stoff JS, Lovewell T, Ghavamian R, Baker S (1993) Post-transplant lymphoceles: a critical look into the risk factors, pathophysiology and management. J Urol 150:22–26
Kobayashi K, Censullo ML, Rossman LL, Kyriakides PN, Kahan BD, Cohen AM (2007) Interventional radiologic management of renal transplant dysfunction: indications, limitations, and technical considerations. Radiographics 27:1109–1130. https://doi.org/10.1148/rg.274065135
Pollak R, Veremis SA, Maddux MS, Mozes MF (1988) The natural history of and therapy for perirenal fluid collections following renal transplantation. J Urol 140:716–720
Bouali K, Magotteaux P, Jadot A, Saive C, Lombard R, Weerts J, Dallemagne B, Jehaes C, Delforge M, Fontaine F (1993) Percutaneous catheter drainage of abdominal abscess after abdominal surgery. Results in 121 cases. J Belg Radiol 76:11–14
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ierardi, A.M. et al. (2018). Interventional Radiology in the Treatment of Abscess Collections. In: Tonolini, M. (eds) Imaging and Intervention in Urinary Tract Infections and Urosepsis. Springer, Cham. https://doi.org/10.1007/978-3-319-68276-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-68276-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68275-4
Online ISBN: 978-3-319-68276-1
eBook Packages: MedicineMedicine (R0)