Abstract
Percutaneous drainage of abdomino-pelvic abscess or fluid collection has become in this last 30 years the standard of care in the treatment of abdominal abscesses. It can allow an effective drainage with minimal trauma to the tissues and lower morbidity and mortality rates.
Imaging guidance for drainage is most commonly performed with ultrasonography (US), computed tomography, or US and fluoroscopy combined.
Catheter insertion procedures include the trocar and Seldinger techniques.
Abscesses in locations that are difficult to access, such as those located deep in the pelvis, subphrenic regions, or epigastric region, can be drained by using the appropriate approach (transrectal, transgluteal, intercostal, or transhepatic).
Success rate of the percutaneous drainage in the treatment of abdominal abscess are high, and with adequate expertise in imaging-guided drainage techniques it is possible to successfully drain most abdominal infected collection and avoid surgery.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
21.1 Pathophysiology
Intra-abdominal abscesses are localized collections of pus confined in the peritoneal cavity or in the retroperitoneal space by an inflammatory wall. This barrier may include the omentum, inflammatory adhesions, or contiguous viscera. The abscesses usually contain a mixture of aerobic and anaerobic bacteria, coming from the gastrointestinal tract.
Bacteria in the peritoneal cavity, in particular those arising from the large intestine, stimulate the arriving of acute inflammatory cells. The omentum and viscera tend to localize the site of infection, producing a phlegmon. The resulting hypoxia in the area facilitates the growth of anaerobes and impairs the bactericidal activity of granulocytes. The phagocytic activity of these cells degrades cellular and bacterial debris, creating a hypertonic milieu that expands and enlarges the abscess cavity in response to osmotic forces.
If untreated, the process continues until bacteremia develops, which then progresses to generalized sepsis with shock.
21.2 Etiology
Although multiple causes of intra-abdominal abscesses exist, the following are the most common:
-
Perforation of bowels, which includes peptic ulcer perforation
-
Perforated appendicitis and diverticulitis
-
Gangrenous cholecystitis
-
Pancreatitis or pancreatic necrosis progressing to pancreatic abscess
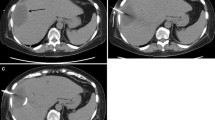
Other causes include untreated penetrating trauma to the abdominal viscera with missing HVI and postoperative complications, such as anastomotic leakage [1, 2] or missed gallstones during laparoscopic cholecystectomy (Fig. 21.1).
21.3 Percutaneous Treatment
The first studies on the percutaneous abscess drainage have been published around 30 years ago as an alternative to the more invasive surgical treatments [3–5] and have since then become the standard of care.
The drainage of abdominal fluid collection can be performed with a diagnostic or therapeutic purpose.
Diagnostic
-
Characterization of the abdominal fluid collection (pus, bile, blood, urine, lymph, pancreatic juice)
-
Differentiate infected from noninfected collections and characterization of the responsible microorganism
Therapeutic
-
To treat abscesses that can be fully drained
-
Adjuvant measure in the cases of multiple incompletely drainable abscesses or in critical patients
21.4 Indications
The main indications of the percutaneous drain placement are the presence of an abdominal infected collection. The abscess size is usually bigger than 4 cm. For the management of smaller (<3 cm) collections, most authors advocate a trial of antibiotics alone with consideration given to needle aspiration to hone antibiotic coverage for persistent cases [6, 7].
Symptoms such as pain or pressure from a large noninfected fluid collection, or obstruction of the bowel or ureter, are also indications for drainage.
The only absolute contraindications to perform a percutaneous drainage are the lack of a safe access or when the access can be impaired by the interposition of organs of vascular structures.
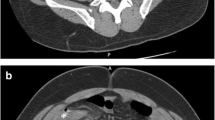
However, in most cases the absence of a safe way to reach the collection can be resolved by changing decubitus or by using curved needles or choosing alternative approaches: transgluteal (Fig. 21.2), transvaginal, or transperineal paths are used for pelvic collections and transhepatic to reach the epiploon and peripancreatic cavities.
If the way of access is covered by a small bowel loop or the stomach, the drainage can be done relatively safely through those organs via a needle. However, it is not recommended to pass a needle through the colon.
Coagulopathy, transpleurical pass, echinococcal cyst, and asymptomatic sterile fluid collections are relative contraindication.
The presence of coagulation disorders have to be corrected before the procedure by the administration of fresh frozen plasma or platelets.
Transpleural passage should be avoided as much as possible because it is painful and can cause a pleuric effusion or a secondary infection of the pleural space.
The drainage of echinococcal cysts is not therapeutic and can be complicated by anaphylactic shock and spreading of the disease. Percutaneous treatment of such cysts can be, however, achieved using the so-called PAIR technique (puncture, aspiration, alcohol injection, re-aspiration) [8] with low rate of recurrence and complications.
The drainage of an asymptomatic sterile fluid collection is not recommended, and if it is done, the catheter should be left in place for the shortest possible time, to avoid possible secondary infections.
21.5 Imaging Guidance
Ultrasound and CT are the modalities used most of the times to guide the drainage procedures. Fluoroscopy is sometimes needed in addition to the sonography to monitor the drainage and the eventual presence of fistulas by iodinated contrast injection.
Ultrasound is the most useful way to visualize intra-abdominal abscesses. The main advantages of this technique are:
-
The possibility to follow the needle pathway in real time, thus allowing the rapid execution of the drainage
-
The possibility to perform this technique in the same patient’s room (e.g., in cases of patients in intensive care unit)
-
The easy availability of the equipment
Limitations of ultrasound are deep-located collections (retroperitoneum, pelvis, peripancreatic region) or when there is an overlapping structure (e.g., a bowel loop) impairing the ultrasounds guide.
CT is the preferred choice when a reasonably safe pathway cannot be found using ultrasonography. The main advantage of CT is the perfect visualization of all the anatomic structures and thus the possibility to track particularly difficult and/or deep paths. Another benefit of CT is that, through the administration of EV contrast media, it is possible to further improve the visualization of the fluid collection (Figs. 21.2 and 21.3).
MIP oblique reconstruction of the control scan after positioning of drainage catheter in the collection: 30 ml of pus were aspired. During the multistep procedure of the needle insertion, a sample of the free fluid in the Douglas space was aspirated and cultured and it resulted negative for infection
The drawbacks of CT are the length of the procedure, the limited access to the equipment, the lack of real-time needle visualization during the procedure, the difficulty in following nonorthogonal paths, and the exposition to x-rays.
21.6 Patient Preparation
-
Coagulative tests: PT < 15 s; INR < 1,5; platelet > 75,000
-
Test of the kidneys functionality if an intravenous contrast is foreseen.
-
Fasting
-
Venous access
-
Informed consent
-
Monitoring
21.7 Punction Technique and Other Technical Issues
The first common steps of the procedure are the study of the preliminary imaging to determine the optimal approach, the disinfection and preparation of the skin, a sterile work field large enough to place the guides and catheter and an adequate local anesthesia.
Seldinger Technique
-
Choose the material. Chiba needles are preferable, having a good echogenicity and being relatively atraumatic.
-
Position the needle in the collection (Fig. 21.4a). This phase can be done in one step if US-guided, or in more steps when using CT guidance. Using US we have a real time vision of the needle path while with CT, after each step, it will be necessary to monitor the correct needle direction through repeated scans limited to the specific area of interest.
-
Once the collection is reached, a sample of fluid will be collected and submitted for culture test and eventually laboratory analyses.
-
A guide will be passed through the needle cannula and the cannula is withdrawn (Fig. 21.4c).
-
The drain will be inserted over the wire to reach the correct position (Fig. 21.4d).
If the access is difficult, it is possible to use a fine needle (20–22G) through which can be inserted a 0.018 guide (Fig. 21.4b). Further to this, with specific coaxial kits it will be possible to place a larger guide (0.035–0.038), allowing the insertion of dilators and ultimately the drainage (usually 8–12f).
If the access is easier, it is possible to reach the collection area with an 18G needle that can allow to pass a larger guide and thus avoid the need of using a coaxial kit.
After the removal of the needle, with the guide only in place, a small cutaneous cut with the scalpel tip is performed to allow an easier insertion of dilators and catheter. At this stage, as the guide could be displaced or looped if it is not firmly kept in tension, caution should be taken.
Trocar Technique
When collections are superficial and large, Trocar catheters could be used, a faster and more simple procedure. Trocars are made up of a metallic sharp obturator, a cannula, and a seal. After skin cut with the scalpel tip, the trocar is advanced in one-step maneuver, under US real-time guide or CT guide, until it reaches the collection.
After drainage catheter positioning and guidewire removal, if the procedure is performed in a US-radiological suite, a small injection of iodine contrast media allows to study the abscess morphology and eventually the presence of fistulas (Fig. 21.5).
Same patient as in Fig. 21.1. Iodinated contrast subministration via the catheter: a fistula with the sigma was depicted
Then drainage catheter is secured to the patient skin and covered with sterile gauzes taking care to avoid kinking and connected to collection pouch.
Once the catheter is in place, the cannula/catheter is attached to a drainage bag through a three-way stopcock. The cavity should be aspirated until there is no more return or the aspirate becomes blood stained. The cavity should then be irrigated with small volumes of saline solution (10–20 ml) and then re-aspirated until all particulate material is removed.
21.8 Complications
Complication rate is ranging between 2.5 and 10.4 % [9, 10]; major and minor complications can be distinguished.
Hemorrhage, septicemia, shock, enteric fistula, peritonitis, and hemopneumothorax account for major complications.
Accidental injury of an artery may lead to severe hemorrhage requiring blood transfusion or surgical repair. It is therefore mandatory to monitor vital signs after the procedure to identify an occult bleeding.
Particularly with US guide, the passage of the catheter through a bowel can be neglected in the beginning but can lead to enteric fistula, peritonitis, and occlusion. A CT scan with injection through the drainage catheter of diluted iodine contrast media is mandatory when bowel accidental puncture is suspected. Surgical repair is often required in such an event, particularly if a colon lesion is seen due to contaminated content, while gastric lesions usually are self-healing.
Minor complications include local pain and tenderness, cutaneous inflammation, self-limiting bleeding, catheter displacement, and pericatheter leak.
Pericatheter leak, if resulted by catheter malfunctioning, for instance kinking or obstruction, requires catheter unblock or replacement.
21.9 Success Rates
Drainage catheter should be kept in place until complete draining of the cavity and until vital signs are gone back to normal. Premature removal could lead to abscess recurrence.
When the output of the collected fluid is below 20 ml/die, usually the catheter could be removed.
If no clinical improvements occur, or an abrupt decrease or not satisfactory volume of fluid collected are seen, an imaging study is recommended.
If the drainage collects more than 50 ml of fluids after 4–5 days, a fistula should be suspected; in such an event, a prolonged period of drainage (4–6 weeks) or surgical repair is required.
The overall failure rate of the percutaneous drainage was 9 % in two studies [11, 12]. Simple unilocular abscesses are managed almost always by percutaneous drainage; more complicated abscesses, such as those with enteric fistulas (e.g., diverticular abscess) or pancreatic abscesses, have cure rates ranging from 65 to 90 % [13]. Percutaneous catheter change over a wire is the preferred intervention in cases of undrained abscess fluid despite technically adequate catheter placement, with 66 % of success [14].
Risk factors associated with failure of the drainage procedure are the complexity of the abscess cavity (septation, multiloculation, or clotted blood) and the presence of a fistula between the abscess and the bowel, biliary tree, or bladder.
Fibrinolysis of complex, multi-septated fluid collections refractory to PCD by intracavitary instillation of fibrinolytic agents has been reported to be safe and showed a reduction in hospital stay, drainage duration, and overall cost of treatment [15].
21.10 Conclusions
As recommended from the Surgical Infection Society and the Infectious Disease Society of America, where feasible, percutaneous drainage of abscesses and other well-localized fluid collections is preferable to surgical drainage with a level of evidence (B-II) [16]. Open surgical techniques are likely required for poorly localized, loculated, complex, or diffuse fluid collections and necrotic tissue, high-density fluid, or percutaneously unaccessible collections (guidelines).
Acute peritonitis is better treated surgically rather than with percutaneous drainage.
References
Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: a case control study. Dis Colon Rectum. 2009;52(3):380–6.
Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11(16):2456–61.
Haaga JR, Alfidi RJ, Havrilla TR, et al. CT detection and aspiration of abdominal abscesses. AJR Am J Roentgenol. 1977;128:465–74.
Gerzof SG, Robbins AH, Birkett DH, Johnson WC, Pugatch RD, Vincent ME. Percutaneous catheter drainage of abdominal abscesses guided by ultrasound and computed tomography. AJR Am J Roentgenol. 1979;133:1–8.
Haaga JR, Weinstein AJ. CT-guided percutaneous aspiration and drainage of abscesses. AJR Am J Roentgenol. 1980;135:1187–94.
Kumar RR, Kim JT, Haukoos JS, et al. Factors affecting the successful management of intra-abdominal abscesses with antibiotics and the need for percutaneous drainage. Dis Colon Rectum. 2006;49(2):183–9.
Siewert B, Tye G, Kruskal J, et al. Impact of CT-guided drainage in the treatment of diverticular abscesses: size matters. AJR Am J Roentgenol. 2006;186(3):680–6.
Fìlice C, Brunetti E, Bruno R, Crippa FG. Percutaneous drainage of echinococcal cysts (PAIR—puncture, aspiration, injection, reaspiration): results of a worldwide survey for assessment of its safety and efficacy. Gut. 2000;47(1):156–7.
Shahnazi M, Khatami A, Jamzad A, Shohitavi S. Safety and efficacy of percutaneous CT-guided drainage in the management of abdominopelvic abscess. Iran J Radiol. 2014;11(3):e20876.
vanSonnenberg E, Wing VW, Casola G, et al. Temporizing effect of percutaneous drainage of complicated abscesses in critically ill patients. AJR Am J Roentgenol. 1984;142:821–6.
Lambiase RE, Deyoe L, Cronan JJ, Dorfman GS. Percutaneous drainage of 335 consecutive ab- scesses: results of primary drainage with 1-year follow-up. Radiology. 1992;184:167–79.
Akinci D, Akhan O, Ozmen MN, et al. Percutane- ous drainage of 300 intraperitoneal abscesses with long-term follow-up. Cardiovasc Intervent Radiol. 2005;28:744–50.
vanSonnenberg E, Wittich GR, Goodacre BW, Casola G, D’Agostino HB. Percutaneous abscess drainage: update. World J Surg. 2001;25:362–9.
Michael S, Gee MS, Kim JY, Gervais DA, Hahn PF, Mueller PR. Management of abdominal and pelvic abscesses that persist despite satisfactory percutaneous drainage catheter placement. AJR Am J Roentgenol. 2010;194:815–20.
Laborda A, De Gregorio MA, Miguelena JM, et al. Percutaneous treatment of intrabdominal abscess: urokinase versus saline serum in 100 cases using two surgical scoring systems in a randomized trial. Eur Radiol. 2009;19(7):1772–9.
Solomkin JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–64.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cinquantini, F., Piccinini, A., Montanari, N., Biscardi, A., Tugnoli, G., Di Saverio, S. (2017). Percutaneous Techniques for Management of Intra-abdominal Abscesses. In: Di Saverio, S., Catena, F., Ansaloni, L., Coccolini, F., Velmahos, G. (eds) Acute Care Surgery Handbook. Springer, Cham. https://doi.org/10.1007/978-3-319-15341-4_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-15341-4_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15340-7
Online ISBN: 978-3-319-15341-4
eBook Packages: MedicineMedicine (R0)