Abstract
Anthocyanins are a unique group of flavonoids from plants. They have promising health-promoting activities in medicine as well as great applications in food processing, cosmetic production, and other areas. At present, extraction from plant materials is the main source of commercially available anthocyanins. Plant extraction, however, has a number of limitations including heavy dependence on seasonal climate, fluctuating availability, and variable anthocyanin content from different sources. To provide an alternative to the current production method, new technologies are under development including plant cell suspension cultures and microbial production. In this chapter, we focus on metabolic engineering of anthocyanin biosynthesis in bacterial strains. The specific engineering strategies followed for strain optimization such as enzyme selection, enzyme engineering, and regulation of UDP-glucose supply are discussed. In addition, a two-step bioconversion strategy, in which cell growth is favored at the first stage while the production of anthocyanins occurs at the second phase, is also described.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Anthocyanins and Their Industrial Applications
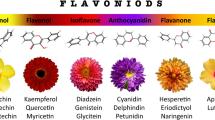
Anthocyanins are ubiquitous pigments in many plants. Macroscopically , they endow flowers, leaves and fruits with diverse colors, which are important traits pursued by floriculture and horticulture. Microscopically , these chemicals protect plants from irradiation damage, oxidative stress, and pathogens [1,2,3,4]. As a group of colorful compounds with similar structures that belong to the flavonoid group of polyphenols (Fig. 4.1), anthocyanins have found their uses extended beyond their physiological roles in plants. With an increasing preference for natural food colorants, there is an ever-growing demand for anthocyanins as dietary supplements, colorants, and cosmetic additives [5,6,7]. Great effort has been invested towards improving the production of natural anthocyanins and developing new technologies for highly efficient and stable anthocyanin production [8].
The basic structure of natural anthocyanins. Different decorations including glycosylation, methylation and acylation can occur at C3’ (R1), C4’ (R2), C5’ (R3), C3 (R4), C5 (R5), and C7 (R6), and over 600 anthocyanins with such a basic core structure have been identified. Most anthocyanins are distinguished into six anthocyanidins shown in the table. Me: a methyl group
1.1 Pharmaceutical Applications
Anthocyanins are promising drug candidates in preventing and treating diseases in animal models and in humans [9,10,11,12]. With the specific mechanisms remaining elusive, there have been many studies probing the cellular and global response to anthocyanins, and in vivo anthocyanin metabolism [13]. Among other things, it has been shown that anthocyanins may inhibit body fat accumulation and obesity-induced inflammation in animal models, presumably by suppressing fat synthesis in the liver and white adipose tissue [10, 14], and by increasing glutathione peroxidase 3 expression while reducing the expression of inflammatory genes [15]. In treating diabetes in mice, cyanidin 3-O-glucoside was shown to downregulate retinol-binding protein 4 (RBP4) [16], while crude bilberry extract was shown to activate AMP-activated protein kinase (AMPK) [10], both ameliorating hyperglycemia symptoms. Anthocyanin extracts also help mitigate osteoclast-induced postmenopausal bone loss [17], lower blood pressure [18], and improve visual functions [10, 19]. In addition, anthocyanins block interleukin-1β, tumor necrosis factor-α, and nuclear factor (NF)-κβ in animal models, and therefore help with the suppression of neuroinflammation, neurodegradation, and brain aging [10]. These health benefits, though, are mostly observed with animal models and have yet to be verified in human clinical trials.
1.2 Food Colorants
Colorants are important additives to enhance the attractiveness of processed foods to consumers. Artificial colorants such as azo dyes dominated the market until a few decades ago, when consumers became more concerned about safety issues. The social tendency of “going natural” has stimulated the rapid increase in the use of pigments from natural sources as colorants owing to their specific characteristics, such as color variation at different pHs, pharmaceutical activities, biosafety, etc. [6].
Among natural pigments, anthocyanins, with potential health attributes and relatively low toxicity in animals and humans at high doses [20], are leading the market (together with carotenoids) [21,22,23]. In the US, four anthocyanin-based colorants are exempt from FDA certification [24]. In the European Union, anthocyanin-containing colorants are treated as natural colorants [25]. Besides anthocyanins, their acylated products are also widely used for improved color stability [26]. Nowadays, most anthocyanins are derived from grape pomace in winemaking processes [5], and grape extracts are widely used in coloring ice creams, dairy products, and sweets [21].
1.3 Cosmetic Industry
Besides the nutraceutical and food industries, anthocyanins also have potential applications in the cosmetic industry [27]. As effective antioxidants against reactive oxygen species, anthocyanins strongly absorb visible and ultraviolet (UV) light owing to the specific polyphenol structure [28], and protect skin from aging and UV-induced damage [29], such as inflammation and oxidative damage in the epidermis, dermis, and adnexal organs [27]. The underlying mechanisms have been demonstrated in several in vitro cellular and animal models, although detailed in vivo investigations are to be established [30, 31]. In general, anthocyanins reduce the UV-induced elevation of cyclooxygenase-2 and prostaglandin E2 through the NF-κβ-dependent pathways . Moreover, anthocyanins decrease apoptotic cell death by inhibiting caspase-3 activation and reduce the proapoptotic Bax protein levels [30, 32]. So far, no cosmetic products containing pure anthocyanins have been approved; however, there have been trials on the development of anthocyanin-colored lipsticks [33]. The incorporation of anthocyanins into cosmetics and skin care products may facilitate the alleviation of skin problems caused by direct contact with certain chemicals in these products, and may help to rejuvenate skin by reducing wrinkles, dark spots, redness, and other problems resulting from aging and skin damage [34, 35]. At present, a few companies have been trying to incorporate anthocyanins into cosmetics. Among them, Nutrasorb, LLC. developed engineered lettuce, whose extract is used both as a food supplement and a cosmetic additive. With more investigations into the skin protecting functions of anthocyanins and their decreased production costs, anthocyanin-based cosmetics may find their way into the market in the near future.
1.4 Other Fields
Beyond the applications in food, drugs and cosmetics that come into direct contact with humans and animals, anthocyanins have been exploited in dye sensitive solar cells (DSSCs) for the conversion of visible light to electricity. The key component in a DSSC is the sensitizer, which should have strong absorption in a wide spectrum and good adherence to the TiO2 surface [36]. Traditionally, a transition metal coordination complex is used, making the synthesis expensive and complicated [37]. Natural dyes such as anthocyanins, however, are vastly available at much lower cost. Anthocyanins interact with TiO2 through hydroxyl and carbonyl groups, allowing for electron transfer to the conducting band of TiO2 films [38, 39]. The energy conversion efficiency is influenced by many factors such as the source of anthocyanins and the extraction method [37, 40], and efficiencies of up to 2% have been achieved with anthocyanins extracted from different plants [37, 41, 42]. Although the efficiency of anthocyanin-based DSSCs is substantially lower compared to those using synthetic sensitizers (~10% efficiency), it can be further enhanced by chemical modifications [43, 44]. Research is continuing in this field to improve the efficiency of DSSCs using anthocyanins alone or in combination with other natural dyes as photosensitizers.
2 Plant-Based Anthocyanin Production
Anthocyanins are synthesized via the general flavonoid pathway in plants, whereby three molecules of malonyl-CoA and one molecule of 4-coumaroyl-CoA derived from the general phenylpropanoid pathway are condensed to form naringenin chalcone by chalcone synthase (CHS) (Fig. 4.2) . In the subsequent step, naringenin chalcone is converted to its isomer naringenin by chalcone isomerase (CHI). Next, naringenin is hydroxylated by enzymes such as flavanone 3-hydroxylase (F3H) , flavonoid 3′-hydroxylase (F3’H) and flavonoid 3′, 5′-hydroxylase (F3’5’H) , forming different dihydroflavonols. The dihydroflavonols are then reduced to the corresponding leucoanthocyanidins by dihydroflavonol 4-reductase (DFR) , followed by the oxidation from anthocyanidin synthase (ANS ) to generate the unstable precursor anthocyanidins. Anthocyanidins are flavylium cations that undergo glycosylation at C3 or other positions by flavonoid glucosyltransferases (FGTs) , giving rise to anthocyanins (see also Chap. 9 of this book). The most common saccharide unit incorporated in this step is glucose, whereas galactose, xylose, and other sugar units are also found in natural anthocyanins [45]. Beyond glycosylation, other modifications, such as acylation, and methylation of the hydroxyl groups on B ring, have also been reported [46]. These modifications are performed in plants for improved stability or for specific physiological functions.
The biosynthetic pathway of anthocyanins in plants. The general precursor phenylalanine, obtained from the shikimate pathway, enters the phenylpropanoid pathway to provide the intermediate coumaroyl-CoA for the production of flavonoids. Coumaroyl-CoA undergoes condensation with malonyl-CoA to form naringenin chalcone, which experiences various modifications to form diverse anthocyanin compounds. R1-R5 are functional groups involved in the modification of different carbons in anthocyanin molecules, such as glycosyl, acyl, methyl, and hydroxyl groups. Abbreviations of enzymes: CHS chalcone synthase, CHI chalcone isomerase, F3H flavanone 3-hydroxylase, F3’H flavonoid 3′-hydroxylase, F3’5’H flavonoid 3′, 5′-hydroxylase, DFR dihydroflavonol reductase, ANS anthocyanidin synthase, FGT flavonoid-glucosyltransferase, OMT O-methyltransferase, ACT acyltransferase
Anthocyanins in plants show different colors according to the pH in vacuoles [5, 45], which also affects anthocyanin stability. These compounds are quite labile at neutral and basic pH values. Structural modifications, lowered pH and co-pigmentation in vacuoles are all means adopted by plants to stabilize anthocyanins. The complexity of anthocyanin biosynthesis and their instability make their production in controlled systems a great challenge.
2.1 Extraction from Plants
So far the prevailing way of industrial anthocyanin production is by extraction from plants. For example, the anthocyanin supply for food colorants mainly comes from the waste products of the winemaking industry. The extraction is typically performed in solvents, of which the most commonly used is ethanol, because of its environmental friendliness, safety, and little interference with anthocyanin recovery [47]. Other extraction methods, such as pressurized liquid extraction, and novel extraction tools/agents, including ultrasound, subcritical water, and polymeric absorber resins, have also been reported to be effective in obtaining anthocyanins from crops and fruits [48,49,50,51]. These methods have different applications and should be selected with care. For example, water-extracted anthocyanins from plant flowers can be directly used for making DSSCs, whereas ethanol assisted extraction leads to photocatalytic decomposition of the extracted anthocyanins by TiO2 in the solar cell, and hence lead to low efficiency of energy conversion [37].
2.2 Anthocyanin Production from Suspension Cell Culture
Plant suspension cell culture is a technology that introduces the bioreactor concept to cultivate plant cells for anthocyanin production with tight control over the fermentation processes [52, 53]. This technology involves development of suitable cell lines, optimization of operating conditions of the bioreactors, and scaling up of fermentation (see also Chap. 8 of this book). For several decades, plants have been used to develop anthocyanin-producing cell lines, such as grapes, Cleome rose, sweet potatoes, aspen, wild carrots, etc. [54, 55]. However, currently there are no systems that are commercially feasible.
At present, the total anthocyanins obtained from plant suspension cell culture can reach up to 10% of the total dry weight of the producing cells, whereas the yields of specific types of anthocyanins are usually low [5, 56]. A major difficulty in suspension cell culture is the instability of cell lines, with anthocyanin production decreasing drastically over time [5]. Although the underlying mechanisms are not fully understood, one possible cause is the inadequate cell differentiation, since fast-growing, undifferentiated cells cannot produce anthocyanins. Besides the low anthocyanin yield in unstable and inefficient cell lines, other hurdles restricting the application of plant suspension cell culture in anthocyanin production include high production cost, low consumer acceptance, and strict biosafety regulations on cell line-derived compounds, especially those obtained from genetically modified cell lines [57, 58]. These limitations directly affect the economic competitiveness and attractiveness to investment and commercialization of anthocyanins. Subsequent metabolic engineering of cell lines and cell line selection are necessary for enhanced metabolic flux towards the anthocyanin pathway and improved accumulation of specific anthocyanins. In the future, the emerging global genome editing technologies, such as the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) system, zinc finger nucleases (ZFNs), and transcription activator-like effector nucleases (TALENs), can be applied in developing engineered cell lines to support stable and efficient anthocyanin production [59]. Moreover, detailed investigations on fermentation design, in terms of medium composition, culture conditions, elicitation, and precursor feeding, are required to release the maximal potential of plant cell culture.
3 Anthocyanin Production in Microorganisms
As the most commonly used workhorse in metabolic engineering , E. coli has been engineered for the production of many flavonoids including naringenin, kaempferol, and catechin [46, 60,61,62]. Anthocyanins have also attracted much attention. In 2005, the genes of F3H and ANS from Malus domestica, DFR from Anthurium andraeanum, and flavonoid 3-glucosyltransferase (F3GT) from Petunia hybrida were successfully expressed in E. coli [63], and the recombinant strain could produce 6.0 μg/L of cyanidin 3-O-glucoside and 5.6 μg/L of pelargonidin 3-O-glucoside (see also Chap. 9 of this book) using naringenin and eriodictyol as the respective feeding precursors. Subsequent optimization of the enzyme source and the UDP-glucose pool, regulation of precursor uptake, and optimization of the production process greatly increased final product titers [64,65,66]. The highest production of cyanidin 3-O-glucoside and pelargonidin 3-O-glucoside was 350 mg/L and 113 mg/L using catechin and afzelechin as the respective precursors. These approaches have also been extended to the microbial biosynthesis of methylated anthocyanins. For example, the production of peonidin 3-O-glucoside (an O-methylated anthocyanin) from catechin was achieved in E. coli with the introduction of P. hybrida ANS, Arabidopsis thaliana F3GT, and Vitis vinifera anthocyanin O-methyltransferase (AOMT), and a final titer of 56 mg/L was reported upon pathway optimization [67]. To date, the reported microbial hosts of anthocyanin biosynthesis are still limited to E. coli, although the heterologous production of other flavonoids has been extended to Saccharomyces cerevisiae and Streptomyces venezuelae [46, 63], and the production of stilbenes and flavanones has been established in the amino acid-producing strain Corynebacterium glutamicum [68,69,70]. It will remain to be seen if other microbial hosts can be engineered for the production of anthocyanins.
3.1 Engineering of Pathway Enzymes
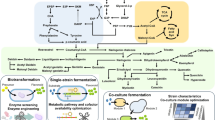
Engineering of the anthocyanin pathway involves coexpression of enzymes from plants. Heterologous expression of plant genes in prokaryotes is generally challenging, and typically, the genes/enzymes need to be modified prior to their functional expression (Fig. 4.3). For example, to achieve functional expression of a plant P450 F3’5’H from Catharanthus roseus, the four codons at the 5′-end of the gene were removed, and the fifth codon was replaced with ATG as the new start codon, while the sixth codon was changed from leucine to alanine [71]. The resulting new F3’5’H was fused to a shortened P450-reductase from C. roseus to form a chimeric protein, which catalyzed the formation of the flavonol quercetin by feeding coumaric acid.
The strategies applied in microbial production of anthocyanins . The whole strategies include the engineering of anthocyanin-producing strains and the optimization of the biocatalytic process. Strain modification focuses on screening and engineering of enzymes in the metabolic pathway, the transportation of the substrate and the product, and the supply of UDP-glucose. The biocatalysis is separated into two phases to maximize anthocyanin accumulation while maintaining normal cell growth. The content shown here is the example of cyanidin 3-O-glucoside production from catechin
Apart from modification of individual enzymes in the metabolic pathway, translational fusion of multiple enzymes in successive steps is another effective method of improving anthocyanin production (Fig. 4.3). Such fusions can maximize the local concentrations of substrates for each enzyme in the fusion system while minimizing the degradation of unstable intermediates, allowing multiple reactions to occur efficiently [72]. Using this strategy, it has been shown in E. coli that the translational fusion of F3GT from Arabidopsis to the N-terminus of ANS from Petunia could better convert catechin to cyanidin 3-O-glucoside compared with the tandem expression of ANS and F3GT [64]. In this case, the fused protein complex could catalyze the successive biochemical reactions 16.9% more efficiently than the uncoupled enzymes due to the faster conversion of the unstable intermediate anthocyanidin.
Beyond direct enzyme engineering , selection of enzymes from diverse species is another way of improving the production of anthocyanins and other flavonoids (Fig. 4.3) [73]. The orthologous enzymes from different species that catalyze the same reactions usually exhibit diverse kinetic and thermodynamic properties, resulting in varied metabolic behaviors and different levels of production. In a study, the in vivo activities of ANS from four plants were compared, and the enzyme from P. hybrida produced 0.19- to 5.4-fold and 0.47- to 4.9-fold higher cyanidin and cyanidin 3-O-glucoside, respectively, in E. coli than the enzymes from Antirrhinum majus, Gerbera hybrida, and M. domestica [64]. In the production of peonidin 3-O-glucoside , five sources of AOMTs were compared and the one from V. vinifera led to the best substrate conversion with the lowest byproduct production [67]. Similarly, selection of DFR was conducted based on in vitro characterization of DFR orthologs from different plant sources during the de novo production of anthocyanins from flavonols [63, 74]. In another study, different combinations of three 4-coumaroyl CoA ligases, two CHSs and two CHIs, each enzyme having a distinct plant origin, resulted in a 3-fold increase in naringenin production in E. coli [75]. In an effort to synthesize resveratrol in recombinant E. coli, the in vitro kinetics of stilbene synthases from four plants were analyzed, and the data correlated well with the in vivo production [76] (see also Chap. 3 of this book).
3.2 Supply of Cofactors and Cosubstrates
Besides pathway enzymes, the biosynthesis of anthocyanins is also dependent on cofactors and cosubstrates that are involved in electron transfer, and enzyme activation or stabilization. For example, the enzyme ANS uses ferrous ions and sodium ascorbate as cofactors, and 2-oxoglutarate as a cosubstrate to conduct a two-electron oxidation of its substrates [64, 77]. The glycosylation of cyanidin at the C3 position requires an equimolar amount of UDP-glucose. Therefore, sufficient supply of cofactors and cosubstrates is a prerequisite for efficient, high-yield production of anthocyanins.
UDP-glucose is required for glycosylation in some anthocyanins (see also Chap. 9 of this book). As a valuable chemical that takes part in many cellular functions, from the generation of metabolic intermediates to the biosynthesis of cellular structural components, UDP-glucose must undergo global and elaborate regulation to reach a suitable level. In general, the regulation lies in altered expression of genes involved in UDP-glucose biosynthesis and/or its consumption. In an E. coli strain that produced cyanidin 3-O-glucoside, the abundance of UDP-glucose was increased by overexpressing one or more genes responsible for its biosynthesis from orotic acid (pyrE, pyrR, cmk, ndk, pgm, and galU) while blocking the competitive UDP-glucose consumption pathways. The resulting production of cyanidin 3-O-glucoside increased by 20-fold [64, 65]. Interestingly, even the overexpression of pgm and galU alone, under the control of independent T7 promoters on the same plasmid, led to a 57.8% increase in cyanidin 3-O-glucoside production [64]. These studies demonstrate that the supply of UDP-glucose is an important limiting factor for the overproduction of glycosylated anthocyanins. Considering the high cost of UDP-glucose and its precursor orotic acid, engineered intracellular biosynthesis of UDP-glucose from cheap nutrients would be useful for its supplementation.
Sodium ascorbate is another necessary ingredient to support the overproduction of anthocyanins. The addition of sodium ascorbate was found to significantly increase the consumption of the substrate catechin and the production of anthocyanin 3-O-glucoside in E. coli, whereas extra addition of the cosubstrate 2-oxoglutarate was unnecessary, probably because 2-oxoglutarate is the intermediate compound in the Krebs cycle and its supply is commonly abundant [64].
S-Adenosyl-L-methionine (SAM) , a cosubstrate commonly involved in the transfer of methyl groups by methyltransferases, is generally required for the production of methylated anthocyanins. SAM supply can be increased by supplementing methionine and/or upregulating genes associated with SAM production. However, the generation of SAM undergoes feedback repression by methionine biosynthesis regulator MetJ based on the intracellular concentration of SAM [78], thus limiting the high-level accumulation of SAM and the rate of methylation of the target compounds. Recently, this difficulty was overcome by CRISPRi-mediated deregulation of the methionine and SAM biosynthetic pathways through the silencing of MetJ in the production of peonidin 3-O-glucoside from catechin in E. coli, and a twofold increase in the production titer was achieved with such an approach [67].
3.3 Engineering Anthocyanin Secretion
Metabolic engineering has resulted in accomplishing the production of many compounds, natural or unnatural, in microorganisms. However, some of these compounds are toxic to cells by either directly reducing cell viability or indirectly interfering with cellular functions and metabolism, thus limiting their high-yield production. A feasible scheme is to pump out the products continuously during their biosynthesis to extracellular media, where their toxic effects are attenuated. To achieve this, identification of specific transporters is critical. In addition, the incorporation of transporters for enhanced substrate uptake also facilitates the production of the target chemicals. In an E. coli strain that converted catechin to cyanidin 3-O-glucoside, the overexpression of the product-associated efflux pump YadH increased the production by 15%, and the deletion of another efflux pump TolC, which was probably responsible for the secretion of catechin, enhanced production by 55%. The combined effect was a 63% promotion in cyanidin 3-O-glucoside production [66].
Anthocyanins in their natural plant hosts are transported to vacuoles after their synthesis, and this process requires both cytoplasmic transporters and transmembrane transporters. The most commonly studied plant-based transporters are glutathione S-transferase and ATP-binding cassette (ABC) transporters (see also Chap. 9 of this book). Since both plant tonoplasts (membranes surrounding the vacuoles) and microbial cell membranes comprise lipid bilayers, it may be useful to investigate the performance of engineered plant-based transporters in microorganisms for anthocyanin delivery across the cytoplasmic membrane and the outer membrane.
3.4 Optimization of the Production Process
The instability of anthocyanins is a major problem for accomplishing their efficient production in microorganisms. In plants, the naturally synthesized anthocyanins are stabilized in vacuoles through pH adjustment and co-pigmentation [45, 79]. However, in bacterial cells that are engineered as artificial producing hosts, there is a shortage of protection mechanisms for produced anthocyanins. The microbially synthesized anthocyanins are quite unstable inside or outside cells, considering that the intracellular and extracellular pH is around 7 for commonly used bacteria under their normal growth conditions. To solve this issue, a two-step biocatalysis strategy has been proposed [64]. During the first phase, cells are cultured in a medium at pH 7 to support normal growth and enzyme expression. In the second step, cells at a particular growth stage are transferred to fresh medium at pH 5.0 to facilitate anthocyanin production and accumulation (Fig. 4.3). Protective agents such as glutamate can be added to minimize acid-induced cell lysis. With such an approach, the production of cyanidin 3-O-glucoside in E. coli was ~15-fold higher than that from the traditional single-step production [64].
Concentration of dissolved oxygen is another parameter that shows great impact on anthocyanin biosynthesis and stability. Oxygen is critical for the maintenance of ANS functionality and the synthesis of anthocyanins; however, an excessive amount of dissolved oxygen may oxidize anthocyanins. Although the specific roles of oxygen in microbial production of anthocyanins are poorly understood, it is clear that an optimal supply of oxygen is important. In a study of catechin production from eriodictyol, increased concentration of dissolved oxygen led to enhanced production of catechin, which might be related to increased NADPH supply [73]. However, no such investigations have been reported for anthocyanin production.
Besides pH and oxygen, temperature and induction point also have remarkable effect on anthocyanin production [66, 75]. Temperature generally imposes direct impact on cell viability and protein expression or folding, and hence influences anthocyanin bioconversion indirectly. Induction time-points are correlated with growth stages and conditions of the producing cells, and differential enzyme expression at diverse growth phases can result in significantly different production efficiencies.
Pathway balancing should also be considered for efficient anthocyanin production. The aim is to reduce metabolic burden exerted on host cells during the overproduction of anthocyanins, and meanwhile, to maintain normal cell growth and metabolism to the most extent [80]. Many tools have been established to balance the metabolic pathways, such as the ePathBrick vectors, the ePathOptimize platform , and biosensor-based dynamic regulation in flavonoid biosynthesis [81,82,83,84,85]. Recently, a dCas9-based toolbox has been developed to orchestrate the expression of multiple genes simultaneously in E. coli [86, 87]. This strategy can be exploited for the identification of the potential regulation points relevant for anthocyanin production.
4 Conclusions and Future Perspectives
Anthocyanins are very useful flavonoids with applications as dietary supplements, food colorants, and cosmetic additives. The current supply is largely dependent on extraction from plant materials, while emerging technologies delve into sustainable production either in engineered plant cells or in recombinant microbial cells. In this chapter, we focused on metabolic engineering of anthocyanin production in microorganisms, especially in E. coli. We presented the strategies that have been applied in optimizing the biosynthetic pathways, the host strains, and the bioreaction processes. However, many issues still remain to be addressed, such as poor expression of anthocyanin biosynthetic genes, imbalance of genes in the pathway, and stabilization of the final product. With the elucidation of anthocyanin biosynthesis in plants, sophisticated redesign of related enzymes, and regulation of the constructed pathways based on metabolic models, it is expected that engineered microorganisms will become an important source of providing anthocyanins.
Abbreviations
- 4CL:
-
4-coumaroyl-CoA ligase
- ANS:
-
Anthocyanidin synthase
- AOMT:
-
Anthocyanin O-methyltransferase
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- DFR:
-
Dihydroflavonol 4-reductase
- DSSC:
-
Dye-sensitized solar cell
- F3’5’H:
-
Flavonoid 3′, 5′-hydroxylase
- F3’H:
-
Flavonoid 3′-hydroxylase
- F3GT:
-
Flavonoid 3-glucosyltransferase
- F3H:
-
Flavanone 3-hydroxylase
- FGT:
-
Flavonoid glucosyltransferase
- SAM:
-
S-adenosyl-L-methionine
- UV:
-
Ultraviolet
References
Gould KS, McKelvie J, Markham KR. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ. 2002;25(10):1261–9.
Winkel-Shirley B. Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126(2):485–93.
Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol. 2002;155(3):349–61.
Lorenc-Kukuła K, Jafra S, Oszmiański J, Szopa J. Ectopic expression of anthocyanin 5-O-glucosyltransferase in potato tuber causes increased resistance to bacteria. J Agric Food Chem. 2005;53(2):272–81.
Ananga A, Georgiev V, Ochieng J, Phills B, Tsolova V. Production of anthocyanins in grape cell cultures: a potential source of raw material for pharmaceutical, food, and cosmetic industries. In: Poljuha D, Sladonja B, editors. The Mediterranean genetic code – Grapevine and Olive. Rijeka: InTech; 2013.
Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J Dairy Food Sci. 2011;6(1):71–8.
Tanaka Y, Ohmiya A. Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol. 2008;19(2):190–7.
Wang J, Guleria S, Koffas MAG, Yan Y. Microbial production of value-added nutraceuticals. Curr Opin Biotechnol. 2016;37:97–104.
Lila MA, Burton-Freeman B, Grace M, Kalt W. Unraveling anthocyanin bioavailability for human health. Annu Rev Food Sci Technol. 2016;7:375–93.
Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2012;56(1):159–70.
de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11(4):1679–703.
Burton-Freeman B, Linares A, Hyson D, Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J Am Coll Nutr. 2010;29(1):46–54.
Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study. Am J Clin Nutr. 2013;97(5):995–1003.
Tsuda T. Regulation of adipocyte function by anthocyanins: possibility of preventing the metabolic syndrome. J Agric Food Chem. 2008;56(3):642–6.
DeFuria J, Bennett G, Strissel KJ, Perfield JW, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009;139(8):1510–6.
Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H, Horio F, Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74(11):1619–27.
Zheng X, Mun S, Lee SG, Vance TM, Hubert P, Koo SI, Lee S-K, Chun OK. Anthocyanin-rich blackcurrant extract attenuates ovariectomy-induced bone loss in mice. J Med Food. 2016;19(4):390–7.
Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87(2):323–31.
Zia Ul Haq M, Riaz M, Saad B. Anthocyanins and human health: biomolecular and therapeutic aspects. In: Book Anthocyanins and human health: biomolecular and therapeutic aspects. 1st ed. Cham: Springer; 2016.
Wallace TC, Giusti MM. Anthocyanins. Adv Nutr. 2015;6(5):620–2.
Mateus N, de Freitas V. Anthocyanins as food colorants. In: Winefield C, Davies K, Gould K, editors. Anthocyanins: biosynthesis, functions, and applications. New York: Springer; 2009.
Mora-Pale M, Sanchez-Rodriguez SP, Linhardt RJ, Dordick JS, Koffas MAG. Biochemical strategies for enhancing the in vivo production of natural products with pharmaceutical potential. Curr Opin Biotechnol. 2014;25:86–94.
Chin-Giaw L, Koffas MAG. Bioavailability and recent advances in the bioactivity of flavonoid and stilbene compounds. Curr Org Chem. 2010;14(16):1727–51.
Wrolstad RE. Anthocyanin pigments—bioactivity and coloring properties. J Food Sci. 2004;69(5):C419–25.
Socaciu C. Food colorants: chemical and functional properties. In: Food colorants: chemical and functional properties. Boca Raton: CRC Press; 2007.
Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochem Eng J. 2003;14(3):217–25.
Wallace TC, Giusti MM, Rojo LE, Roopchand DE, Graf B, Cheng DM, Ribnicky D, Fridlender B, Raskin I. Role of anthocyanins in skin aging and UV-induced skin damage. In: Anthocyanins in health and disease. Boca Raton: CRC Press; 2013.
Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Current protocols in food analytical chemistry. New York: Wiley; 2001.
Chan CF, Lien CY, Lai YC, Huang CL, Liao WC. Influence of purple sweet potato extracts on the UV absorption properties of a cosmetic cream. J Cosmet Sci. 2010;61(5):333–41.
Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Shim HJ, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. Protective effect of anthocyanins from black soybean seed coats on UVB-induced apoptotic cell death in vitro and in vivo. J Agric Food Chem. 2008;56(22):10600–5.
Tarozzi A, Marchesi A, Hrelia S, Angeloni C, Andrisano V, Fiori J, Cantelli-Forti G, Hrelia P. Protective effects of cyanidin-3-O-beta-glucopyranoside against UVA-induced oxidative stress in human keratinocytes. Photochem Photobiol. 2005;81(3):623–9.
Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-kappaB and phosphatidylinositol 3-kinase/Akt pathway. J Agric Food Chem. 2008;56(19):8969–74.
Westfall A. Evaluation of the efficacy of anthocyanins as biologically active ingredients in lipstick formulations. In: Food science and technology. Columbus: The Ohio State University; 2015.
Ghimeray AK, Jung US, Lee HY, Kim YH, Ryu EK, Chang MS. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin Cosmet Invest Dermatol. 2015;8:389–96.
Mukherjee PK, Maity N, Nema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19(1):64–73.
Tennakone K, Kumara GRRA, Kumarasinghe AR, Sirimanne PM, Wijayantha KGU. Efficient photosensitization of nanocrystalline TiO2 films by tannins and related phenolic substances. J Photochem Photobiol A. 1996;94(2):217–20.
Wongcharee K, Meeyoo V, Chavadej S. Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol Energy Mater Sol Cells. 2007;91(7):566–71.
Hao S, Wu J, Huang Y, Lin J. Natural dyes as photosensitizers for dye-sensitized solar cell. Sol Energy. 2006;80(2):209–14.
Dai Q, Rabani J. Photosensitization of nanocrystalline TiO2 films by pomegranate pigments with unusually high efficiency in aqueous medium. Chem Commun. 2001;20:2142–3.
Calogero G, Marco GD. Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol Energy Mater Sol Cells. 2008;92(11):1341–6.
Calogero G, Yum J-H, Sinopoli A, Di Marco G, Grätzel M, Nazeeruddin MK. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol Energy. 2012;86(5):1563–75.
Ramamoorthy R, Radha N, Maheswari G, Anandan S, Manoharan S, Victor Williams R. Betalain and anthocyanin dye-sensitized solar cells. J Appl Electrochem. 2016;46(9):929–41.
Amao Y, Yamada Y, Aoki K. Preparation and properties of dye-sensitized solar cell using chlorophyll derivative immobilized TiO2 film electrode. J Photochem Photobiol A. 2004;164(1–3):47–51.
Calogero G, Sinopoli A, Citro I, Di Marco G, Petrov V, Diniz AM, Parola AJ, Pina F. Synthetic analogues of anthocyanins as sensitizers for dye-sensitized solar cells. Photochem Photobiol Sci. 2013;12(5):883–94.
Zhang Y, Butelli E, Martin C. Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol. 2014;19:81–90.
Pandey RP, Parajuli P, Koffas MAG, Sohng JK. Microbial production of natural and non-natural flavonoids: pathway engineering, directed evolution and systems/synthetic biology. Biotechnol Adv. 2016;34(5):634–62.
Delgado-Vargas F, Paredes-Lopez O. Natural colorants for food and nutraceutical uses. In: Natural colorants for food and nutraceutical uses. Boca Raton: CRC Press; 2002.
Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innovative Food Sci Emerg Technol. 2008;9(1):85–91.
Ju Z, Howard LR. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J Food Sci. 2005;70(4):S270–6.
Kammerer D, Gajdos Kljusuric J, Carle R, Schieber A. Recovery of anthocyanins from grape pomace extracts (Vitis vinifera L. cv. Cabernet Mitos) using a polymeric adsorber resin. Eur Food Res Technol. 2005;220(3):431–7.
ZY J, Howard LR. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J Agric Food Chem. 2003;51(18):5207–13.
Matkowski A. Plant in vitro culture for the production of antioxidants — a review. Biotechnol Adv. 2008;26(6):548–60.
Deroles S. Anthocyanin biosynthesis in plant cell cultures: a potential souce of natural colorants. In: Anthocyanins: Springer; 2008.
Simões C, Albarello N, de Castro TC, Mansur E. Production of anthocyanins by plant cell and tissue culture strategies. In: Biotechnological production of plant secondary metabolites. Dubai: Bentham Science Publishers; 2012.
Rao SR, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20(2):101–53.
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP. Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol. 2016;36(2):215–32.
Davies KM, Deroles SC. Prospects for the use of plant cell cultures in food biotechnology. Curr Opin Biotechnol. 2014;26:133–40.
Angelov A, Gotcheva V. Safety assessment and regulations for food ingredients derived from plant in vitro systems. In: Pavlov A, Bley T, editors. Bioprocessing of plant in vitro systems. Cham: Springer; 2017.
Bortesi L, Fischer R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol Adv. 2015;33(1):41–52.
Chemler JA, Leonard E, Koffas MAG. Flavonoid biotransformations in microorganisms. In: Winefield C, Davies K, Gould KS, editors. Anthocyanins: biosynthesis, funcitons, and applications: Springer; 2009.
Trantas EA, Koffas MA, Xu P, Ververidis F. When plants produce not enough or at all: metabolic engineering of flavonoids in microbial hosts. Front Plant Sci. 2015;6:7.
Chemler JA, Koffas MAG. Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin Biotechnol. 2008;19(6):597–605.
Yan Y, Chemler J, Huang L, Martens S, Koffas MAG. Metabolic engineering of anthocyanin biosynthesis in Escherichia coli. Appl Environ Microbiol. 2005;71(7):3617–23.
Yan Y, Li Z, Koffas MA. High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol Bioeng. 2008;100(1):126–40.
Leonard E, Yan Y, Fowler ZL, Li Z, Lim C-G, Lim K-H, Koffas MAG. Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm. 2008;5(2):257–65.
Lim CG, Wong L, Bhan N, Dvora H, Xu P, Venkiteswaran S, Koffas MAG. Development of a recombinant Escherichia coli strain for overproduction of plant pigment, anthocyanin. Appl Environ Microbiol. 2015;81(18):6276–84.
Cress BF, Leitz QD, Kim DC, Amore TD, Suzuki JY, Linhardt RJ, Koffas MA. CRISPRi-mediated metabolic engineering of E. coli for O-methylated anthocyanin production. Microb Cell Factories. 2017;16(1):10.
Kallscheuer N, Vogt M, Stenzel A, Gatgens J, Bott M, Marienhagen J. Construction of a Corynebacterium glutamicum platform strain for the production of stilbenes and (2S)-flavanones. Metab Eng. 2016;38:47–55.
Kallscheuer N, Vogt M, Marienhagen J. A novel synthetic pathway enables microbial production of polyphenols independent from the endogenous aromatic amino acid metabolism. ACS Synth Biol. 2017;6(3):410–5.
van Summeren-Wesenhagen PV, Marienhagen J. Putting bugs to the blush: metabolic engineering for phenylpropanoid-derived products in microorganisms. Bioengineered. 2013;4(6):355–62.
Leonard E, Yan Y, Koffas MAG. Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab Eng. 2006;8(2):172–81.
Springob K, Nakajima J, Yamazaki M, Saito K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat Prod Rep. 2003;20(3):288–303.
Zhao S, Jones JA, Lachance DM, Bhan N, Khalidi O, Venkataraman S, Wang Z, Koffas MAG. Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab Eng. 2015;28:43–53.
Leonard E, Yan Y, Chemler J, Matern U, Martens S, Koffas MAG. Characterization of dihydroflavonol 4-reductases for recombinant plant pigment biosynthesis applications. Biocatal Biotransfor. 2008;26(3):243–51.
Jones JA, Vernacchio VR, Sinkoe AL, Collins SM, Ibrahim MHA, Lachance DM, Hahn J, Koffas MAG. Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab Eng. 2016;35:55–63.
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MAG. High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol. 2011;77(10):3451–60.
Turnbull JJ, Nakajima J-i, Welford RWD, Yamazaki M, Saito K, Schofield CJ. Mechanistic studies on three 2-oxoglutarate-dependent oxygenases of flavonoid biosynthesis: anthocyanidin synthase, flavonol synthase, and flavanone 3β-hydroxylase. J Biol Chem. 2004;279(2):1206–16.
Martí-Arbona R, Teshima M, Anderson PS, Nowak-Lovato KL, Hong-Geller E, Unkefer CJ, Unkefer PJ. Identification of new ligands for the methionine biosynthesis transcriptional regulator (MetJ) by FAC-MS. J Mol Microbiol Biotechnol. 2012;22(4):205–14.
Passeri V, Koes R, Quattrocchio FM. New challenges for the design of high value plant products: stabilization of anthocyanins in plant vacuoles. Front Plant Sci. 2016;7:153.
Wu G, Yan Q, Jones JA, Tang YJ, Fong SS, Koffas MA. Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications. Trends Biotechnol. 2016;34(8):652–64.
Xu P, Vansiri A, Bhan N, Koffas MAG. ePathBrick: a synthetic biology platform for engineering metabolic pathways in E. coli. ACS Synth Biol. 2012;1(7):256–66.
Jones JA, Vernacchio VR, Lachance DM, Lebovich M, Fu L, Shirke AN, Schultz VL, Cress B, Linhardt RJ, Koffas MAG. ePathOptimize: a combinatorial approach for transcriptional balancing of metabolic pathways. Sci Rep. 2015;5:11301.
Xu P, Li L, Zhang F, Stephanopoulos G, Koffas M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci. 2014;111(31):11299–304.
Skjoedt ML, Snoek T, Kildegaard KR, Arsovska D, Eichenberger M, Goedecke TJ, Rajkumar AS, Zhang J, Kristensen M, Lehka BJ, Siedler S, Borodina I, Jensen MK, Keasling JD. Engineering prokaryotic transcriptional activators as metabolite biosensors in yeast. Nat Chem Biol. 2016;12(11):951–8.
Cress BF, Trantas EA, Ververidis F, Linhardt RJ, Koffas MAG. Sensitive cells: enabling tools for static and dynamic control of microbial metabolic pathways. Curr Opin Biotechnol. 2015;36:205–14.
Cress BF, Jones JA, Kim DC, Leitz QD, Englaender JA, Collins SM, Linhardt RJ, Koffas MAG. Rapid generation of CRISPR/dCas9-regulated, orthogonally repressible hybrid T7-lac promoters for modular, tuneable control of metabolic pathway fluxes in Escherichia coli. Nucleic Acids Res. 2016;44(9):4472–85.
Cress BF, Toparlak ÖD, Guleria S, Lebovich M, Stieglitz JT, Englaender JA, Jones JA, Linhardt RJ, Koffas MAG. CRISPathBrick: modular combinatorial assembly of type II-A CRISPR arrays for dCas9-mediated multiplex transcriptional repression in E. coli. ACS Synth Biol. 2015;4(9):987–1000.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Zha, J., Koffas, M.A.G. (2018). Anthocyanin Production in Engineered Microorganisms. In: Schwab, W., Lange, B., Wüst, M. (eds) Biotechnology of Natural Products. Springer, Cham. https://doi.org/10.1007/978-3-319-67903-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-67903-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67902-0
Online ISBN: 978-3-319-67903-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)