Abstract
Rodent and clinical studies have documented that myeloid cell infiltration of tumors is associated with neutrophilia, lymphocytopenia and poor patient outcomes. This contrasts with lymphocyte infiltration of tumors, which is associated with improved outcomes. Lifestyle parameters such as high fat diets and omega (ω)-6 polyunsaturated fatty acids (PUFA) intake may influence these inflammatory parameters including extramedullary myelopoiesis that can contribute to a metastatic “niche”. While, tumor secretion of growth factors (GFs) and chemokines regulate tumor-immune-cell crosstalk, in this chapter, we also emphasize how lifestyle choices, including, obesity, high-fat and high ω-6 PUFA dietary content, contribute to inflammation and myeloid cell infiltration of tumors. A relationship between obesity and high-fat diets (notably the saturated fats in Western diets) and tumor incidence, metastasis, and poor outcomes is generally accepted. However, the mechanisms of dietary promotion of inflammatory microenvironments and targeted drugs to inhibit the clinical sequel remain an unmet challenge. One approach, modification of dietary intake may have a preventative or therapeutic approach to regulate tumor-associated inflammation and remains an attractive, but little studied intervention.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
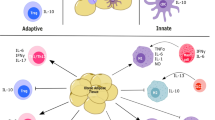
Leukocyte infiltration of tumors can have either a pro-tumorigenic or tumor-inhibitory functions. As an example tumor-associated macrophages (TAMs) have tumoricidal activity and can induce antitumor T-cells; but, can also suppress cytotoxic T-cell responses capable of inhibiting tumor growth (Fig. 10.1). Myeloid cell infiltration of tumors is associated, in part, with tumor-derived cytokines, GFs, chemokines, and expression of immune checkpoint molecules that regulate the expansion of myeloid progenitors within the marrow and at extramedullary sites and to an extent within the tumor (Fig. 10.2). Numerous studies have demonstrated that activated macrophages can kill tumor cells in vitro. However, macrophage infiltration of tumors is predominately, a pro-tumorigenic/tumor-progressive phenotype [1]; although, some human studies have been equivocal [2]. Indeed, most studies have found no relationship between immunogenicity, metastatic propensity and infiltrating TAM frequency [3,4,5]. Despite this lack of an immune correlation, TAM infiltration is associated with a poor prognosis [6] and rapid tumor progression [7, 8]. Myeloid-derived suppressor cells (MDSCs) have also been identified in the circulation of tumor bearing (TB) hosts and to infiltrate tumors [9,10,11,12,13]. The immunosuppressive activity of MDSCs (both murine and human) occurs through multiple mechanisms including the upregulation of reactive oxygen species (ROS), nitric oxide (NO) production and arginase levels, as well as the secretion of immunosuppressive cytokines [14]. Preclinical studies have shown that MDSCs can control tumor growth [3, 15], while immune augmenting type-1 macrophages (M1) and/or dendritic cells (DC1) cells contribute to the induction of an antitumor T-cell response, although their presence is not sufficient for tumor rejection [16]. M1 macrophage depletion or an increase in infiltrating M2 macrophages, DC2s, and MDSCs are associated with a poor prognosis and increased tumor relapse post resection.
The leukocytes infiltrating tumors regulates their growth and progression. Tumor regression is associated with infiltration by mature dendritic cells (DCs), cytotoxic T cells (CTL) and type 1T-helper cells (Th1). Contrasting with this, tumor growth is facilitated by immune mediated immunosuppression and neoangiogenesis by immature DCs, myeloid-derived suppressor cells, (MDSCs) plasmacytoid DCs, (pDCs) M2 macrophages, as well as T regulatory (T-reg) cells and a low frequency of CD4 and CD8 effector T cells. The expansion of myeloid cell proliferation, including immunosuppressive populations, is regulated by colony stimulating factors (CSFs), chemokines and dietary w-6 PUFA
Tumors secrete growth factors that expand and mobilize committed myeloid progenitors (CMP) and hematopoietic progenitor cells (HPC) from the marrow to extramedullary sites of myelopoiesis including the spleen, liver, lungs and primary and metastatic tumor lesions. Diets with increased levels of ω6 polyunsaturated fatty acids (PUFA) can increase myeloplasia largely as an extramedullary process. These CMPs can mature into dendritic cells (DCs), myeloid derived suppressor cells (MDSCs); both monocytic (M) and granulocytic (G), monocytes, endothelial progenitor cells and macrophages including tumor-associated macrophages (TAMs), as well as become activated, or “paralyzed”, within the tumor environment. DC1 and DC2 are dendritic cell subsets that are immune augmenting and suppressive respectively. Dependent upon the infiltrating subset and extent of maturation and activation, these cells are critical components and regulators of immune suppression, angiogenesis, vasculogenesis, and tumor regression or growth
Lymphocytes also infiltrate tumors (Fig. 10.1) and the associated adaptive immune response has a positive prognosis. However, the infiltrating lymphocytes can also be T-cell suppressive. Thus, while T-cells have the potential to kill tumor cells, frequently they are of low frequency and avidity [17], and cannot control tumor growth [18]. Nonetheless, increased T-cell infiltration of tumors is associated with an improved outcome [19,20,21,22,23,24,25,26], and an increased understanding of infiltrating T-cell phenotypes and their functions has resulted in an improved understanding of their prognostic potential. However, some tumor cells express checkpoint molecules that downregulate immune responses. Myeloid cells, including macrophages, PMNs and MDSCs, can also express immunosuppressive checkpoint mediators, such as PD-L1 [27], providing another mechanism to down regulate T-cell proliferation and function. Consequently, although anti-tumor T cells are present in the tumor microenvironment their anti-tumor activity may be limited. However, antibodies that inhibit immune checkpoints are demonstrating efficacy in reactivating anti-tumor T cell responses [28].
10.2 Immune Cell Infiltration of Tumors
The hypothesis that hematological markers of systemic inflammation, in particular the neutrophil–lymphocyte ratio (NLR), can predict survival in tumor bearing patients has recently received much interest. Many groups have investigated the prognostic value of the NLR in a variety of tumors and at different disease stages. To date, over 60 studies (>37,000 patients) have examined the clinical utility of the NLR to predict outcomes [29]. There is also an emerging relationship between proinflammatory cytokines in the plasma of patients with elevated NLR (>5) and the tumor microenvironment. A number of studies have measured circulating cytokines together with the NLR [30, 31] providing insight into the mechanisms underlying the NLR, including one study that documented an elevated NLR associated with an increased peritumoral infiltration of macrophages [30]. Together, these observations suggest that the NLR reflects, at least in part, the up-regulation of innate immunity providing easily measurable biomarkers that can predict OS and PFS in cancer patients.
The interactions between tumor infiltrating immune effector cells takes place primarily around the tumor. Thus, while the NLR may have prognostic significance, specific subsets of infiltrating cells, as discussed above, may prove more informative. Specifically, cytotoxic CD8+ lymphocytes, as a component of tumor-specific adaptive immunity, may constitute a critical mediator. Further, the T-cell suppressive nature of myeloid cells, including MDSCs, M2 macrophages, and DC2s suggests the potential sensitivity and criticality of the myeloid cell-to-CD8+ lymphocyte ratio in tumor tissue. A few studies have undertaken such analyses observing, for example, that CD66+ myeloid cells provide an independent prognostic factor for poor disease free survival (DFS) and overall survival (OS) [32]. This observation has been extended by the analysis of infiltrating NLR (iNLR) as a CD66b:CD8 cell ratio with the observation of a relationship with a cumulative incidence of relapse, OS and tumor stage [33]. As discussed below, a patient’s lifestyle, both preceding and following diagnosis, can contribute to not only cancer initiation and progressions but also outcome. Thus, hosts eating a high-fat diet, or one with a high level of saturated fat or ω-6 PUFAs generally have an inflammatory phenotype with neutrophilia, which may contribute to cancer development and poor outcomes. Conversely, and with little data to date, diets with a high ω-3 PUFA content have been associated with decreased inflammation and extramedullary myelopoiesis, and potentially improved clinical outcomes. We posit, herein, that dietary ω3 PUFA may also increase infiltrating T-cells thereby contributing to improved clinical outcomes.
10.3 PUFA Regulation of Inflammatory Cells in Rodents
Several lines of evidence suggest that the dietary PUFA composite can influence inflammatory or anti-inflammatory cellular responses. Fatty acids from animal sources, mainly contain saturated fatty acids (SFAs) or ω6 PUFA. In contrast, fatty acids derived from some plant-based oils, and certain types of fatty fish consist mainly of ω3 PUFA. Recent studies have suggested that diets rich in ω6 PUFAs increase the risk of inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, and asthma [34]. In contrast, diets rich in ω3 PUFAs have anti-inflammatory effects as supported by a decreased risk and control of these diseases [34]. PUFAs can be oxidized to generate either pro-inflammatory or pro-resolving lipid mediators (Fig. 10.3). These mediators have potent immune modulatory capacities and are generated rapidly during an inflammatory response [35]. Pro-inflammatory mediators, including prostaglandin (PG)s and leukotrienes (LTs), are induced in response to “foreign” materials and when they are cleared, pro-resolving lipid mediators restore normal tissue homeostasis [36]. Diets rich in ω3-PUFAs such as α linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are associated with a decreased incidence and severity of inflammatory diseases [37]. The beneficial effects of these dietary FAs include anti-inflammatory metabolites such as a subset of PGs, LTs, thromboxanes, resolvins and lowered levels of inflammatory cytokines. However, the activities of ω3-PUFA contrast with other FAs that differ mainly in the position of their double bonds in the acyl chain, such as linoleic acid (LA) and arachidonic acid (AA) found with ω6-PUFA containing diets and their corresponding metabolites (Fig. 10.3).
Outline of the eicosanoid and resolvin related mediator synthesis pathways from arachidonic acid (AA) and alpha linolenic acid and their inflammatory and anti-inflammatory activities. COX cyclooxygenase, CYT p450 cytochrome p450, CXC CXC chemokines, HETE hydroxyeicosatetraenoic acid, HDHA hydroxyldocosahexaenoic acid, HPETE hydroperoxyeicosatetraenoic acid, HPDHA, hydroperoxydocosahexaenoic acid, HPEPE hydroperoxyeicosapentaenoic acid, IL interleukin, IFN interferon, LOX lipoxygenase, LT leukotriene, LX lipoxin, PG prostaglandin, PMN polymorphonuclear leukocytes, ROS reactive oxygen synthetase, TNF tumor necrosis factor, TX thromboxane
Omega-3 PUFAs are anti-inflammatory in part by modulating the metabolism of inflammatory eicosanoids, cytokines, ROS and the expression of adhesion molecules [38]. EPA and DHA dietary supplementation has proven effective in decreasing intestinal damage and improving gut histology in inflammatory bowel disease [39], as well as, decreased joint pain, number of tender and swollen joints, and duration of morning stiffness in patients with arthritis [40]. Due to these responses, the effects on the immune response in various organs has been the subject of recent review articles [41].
10.4 PUFA and Immune Function
Studies using both ω6 and ω3 PUFA in rodent dietary studies, have documented different effects depending on the type of study (in vitro or in vivo), and the response measured. In vitro studies with ALA have shown an enhanced secretion of superoxides from neutrophils and macrophages [42], resulting in neutrophil adhesion to endothelial cells [43] promoting pro-inflammatory effects. However, ALA has also been shown to inhibit the proliferation of rodent and human lymphocytes following mitogen stimulation [44] suggesting that ALA may also be immunosuppressive. Studies where rodents were fed a high-fat diet, rich in ALA resulted in decreased mitogen-stimulated lymphocyte proliferation and NK cell activity [45].
In vitro studies using the ω6 PUFA; AA, have documented inflammatory properties including enhanced superoxide release [42], neutrophil adhesion to endothelial cells [43], and IL-1β production by macrophages [46]. Feeding mice a diet with high levels of ω-6 PUFA has been shown, in a dose dependant manner, to result in increased levels of LTE4 and PGE2 following in vivo stimulation with zymosan [47]. In a recent study, diets high in AA were shown to increase angiotensinogen, IL6 and MCP-1 levels in response to the proinflammatory transcription factor; nuclear factor κβ (NFκβ) stimulation [48].
A number of studies have shown that the ω3 PUFA, ALA inhibits the proliferation of rodent and human lymphocytes in vitro [44, 49, 50]. Studies where rats were fed an oil with a high ALA composition (linseed oil, 100 g/kg diet) for 8 weeks, a decrease in superoxide production by peritoneal macrophages in response to phorbol esters, was observed [51]. However, rodents fed linseed oil also had an increase in TNF secretion by resident macrophages, but no effect on TNF production by inflammatory macrophages [52]. Thus, the precise effect of the w-3 PUFA, ALA on lymphocyte functions appears to depend on the levels of ALA and the total PUFA content of the diet [53].
Because dietary fish oil leads to decreased PGE2 production, it has been suggested that ω3 PUFAs should have anti-inflammatory activities, enhance the production of Th1-type cytokines, increase MHC II expression, lymphocyte proliferation and NK cell activity, as well as, decrease IgE production. Culture of human neutrophils with EPA or DHA has been shown to inhibit superoxide production and phagocytosis [54]. Similarly, the incubation of murine peritoneal macrophages with EPA or DHA inhibits expression of MHC II [55]. In a study, in which human monocytes were incubated with either EPA or DHA, both were shown to decrease the proportion of HLA-DR or -DP positive monocytes in response to IFN-γ [56] resulting in a reduced ability to present antigen [57]. The addition of fish oil to rodent diets can also decrease superoxide and hydrogen peroxide production by macrophages [58]. As compared to safflower oil, the addition of fish oil to murine diets results in lower peak plasma levels of TNF-α, IL-1β, and IL-6 following endotoxin injection [59]. Furthermore, parenteral nutrition that includes fish oil can decrease serum TNF-α, IL-6, and IL-8 levels in rats with burns compared with animal given ω6 PUFA–rich parenteral nutrition [54]. However, the majority of rodent studies with dietary fish oil use a diet in which EPA plus DHA comprise up to 30% of dietary fatty acids and up to 12% of dietary energy. The conclusions from these studies have been refined by studies in rats and mice that have indicated that relatively low levels of EPA or DHA at a level of 4.4% of total fatty acids or 1.7% of dietary energy are sufficient to provide anti-inflammatory activities [60].
10.5 Clinical Anti-Inflammatory Activity of ω3 PUFA
There have been a number of clinical trials assessing the benefits of dietary supplementation with fish oil for the treatment of inflammatory diseases in humans, including rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, lupus, and multiple sclerosis [61]. Many of the placebo-controlled, double-blind trials of fish oil in chronic inflammatory diseases have shown significant benefits, including decreased disease activity and a lowered use of anti-inflammatory drugs. The evidence for a beneficial effect of fish oil is strongest in rheumatoid arthritis, where ω3 PUFA has been found to cause a concentration-dependent decrease in enzymes that degrade cartilage, expression of COX-2, but not COX-1, and TNF-α and IL-1β expression in cultured articular cartilage chondrocytes [62]. The mechanisms by which ω3 PUFAs have a beneficial effect in patients with arthritis has been postulated to be a competition with the canonical ω6 substrate AA resulting in eicosanoids that are less potent at inducing inflammation [63]. Recent observations have shown that ω3 PUFAs can be enzymatically converted to novel bioactive lipid mediators, termed resolvins, protectins and maresins, which promote the resolution of inflammation and that are log- orders more potent than their lipid precursors [64]. These observations have generated a paradigm shift documenting that the resolving phase of inflammation is not a passive process, but is actively ‘switched-off’ via endogenous anti-inflammatory mediators [65]. This contrasts with ω-6 PUFA associated metabolites, PGD2, LTC4, LTD4, and LTE4, which mediate pulmonary inflammation in asthma and are major mediators of asthmatic bronchoconstriction. AA is a precursor to LTs, which promote allergic inflammation, PGE2 also regulates macrophage and lymphocyte function. Thus, it has been suggested that increased dietary intake of the w-6 PUFA LA, as the precursor of AA, is causally linked to allergic diseases and suggests a potential treatment focus for ω3 fatty acids [66].
10.6 PUFA Modulated Inflammation and Neoplasia in Rodent Tumor Models
As discussed above, clinically there have been varying associations between PUFA consumption/composition and inflammation; but there are many confounding factors including genetic susceptibility, tissue microenvironments, stress, obesity, age and duration. Murine models have identified a number of mechanisms in the association of dietary PUFA and tumor initiation and progression focused on systemic and tissue inflammation. Inflammation at tumor initiation can be regulated by risk factors, including hormones, obesity and age. However, following tumor initiation, inflammation is modulated by tumor growth in addition to existing risk factors. Thus, inflammatory microenvironments are created by cross talk between tumor-secreted GFs and host immunity.
Using mammary tumors as an example, the cellular microenvironment of mammary glands incorporate hormonal responsive epithelial cells, stromal cells, as well as, immune cells, in association with adipose tissue, that can result in an endocrine as well as an inflammatory organ [67]. The role of inflammation in tumorigenesis is supported by the evidence of a progressive increase in infiltrating inflammatory cells, which include activated macrophages and granulocytes, during the progression from normal tissue to dysplastic cells, which are believed to support tumor initiation [68].
The effect of dietary PUFA in tumor progression and metastasis has been studied in animal, and xenograft models of mammary cancer. In a xenograft model using MDA-MB-435 injected athymic nude mice given diets of either LA, EPA or DHA, significant retardation of tumor growth and metastasis was observed in the mice given EPA or DHA including a reduction in AA levels in tumor membrane phospholipids [69]. Further when EPA and DHA were given as a neoadjuvant therapy, prior to tumor excision, pulmonary metastases were significantly suppressed compared to mice maintained on a LA diet [70]. Similar immune-augmenting and therapeutic activities were observed in R3230RC and MCF-7 mammary adenocarcinoma models [71, 72]. These anti-inflammatory activities may also include the regulation of MDSCs that can inhibit both non-antigen specific and antigen-specific CD4+ and CD8+ T-cell responses. The mechanisms of MDSC immunosuppression are diverse, including up-regulation of ROS, NO, and L-arginine metabolism, as well as immunosuppressive cytokines. In one tumor survival study, mice were switched from an 8% corn oil (1% ALA) diet to an 8% canola oil (10% ALA) diet, when the mice had an average primary tumor volume of 60 mm3. In these studies tumor growth was significantly lower in mice fed the ω-3 based canola oil diet compared to the ω-6 based, corn oil cohort [73].
Interventions using ω-3 PUFA in chemically induced mammary tumor models support the results from xenograft tumor models. In a 7, 12-dimethylbenz (α) anthracene (DMBA) induced mammary tumor model, a fish oil diet significantly reduced tumor incidence, growth and metastasis [74, 75]. The effect of an ω-3 diet on tumor induction and growth correlated with reduced AA serum levels, protection against DNA single strand breaks, suppressed tumor cell proliferation; c-Myc and HER-2/neu expression and an increase in the apoptosis markers Bcl-2 and Bax [75,76,77]. Similarly, in a model of N-methyl-N-nitrosourea (MNU)-induced rat mammary tumors, the activity of dietary fat compositions including, saturated fatty acid (SFA), monounsaturated fat (MUFA), ω−6 PUFA alone or different ratios of ω−6:ω−3 PUFA were studied. It was found that a 1:1 ratio of ω−6:ω−3 PUFA was more effective in the prevention of mammary tumor development as compared to the other dietary cohorts, by decreasing mRNA expressions of fatty acid synthase, cyclooxygenase-2 (COX-2), and 5-lipoxygenase (5-LOX) in mammary tissues and decreasing peroxisome proliferator-activated receptor gamma (PPAR-γ) levels [78]. Together, these studies directly support a role for ω-3 PUFA in modulating an inflammatory tumor microenvironment by the up regulation of PPAR-γ [77, 78]. When the ω-3 PUFA content was significantly increased to a ω−6:ω3 ratio of 1:14.6 compared to 1:0.7, a 60% reduction in tumor growth was observed. This was associated with decreased cyclin-D1 and phospho-retinoblastoma protein expression and increased levels of cyclin-dependent kinase inhibitors, CIP1 (p21) and KIP1 (p27), an increased apoptotic index, reduced inflammation and mammalian target of rapamycin (mTOR) activity [79]. In an orthotopic 4 T1 mammary tumor model, 5% fish oil was used as therapy beginning when hosts had primary tumors that were 8–10 mm3 and documented a significant reduced tumor growth and metastasis, which was correlated with inhibition of cancer cell proliferation [80].
The ability of ω3 PUFA to downregulate inflammatory mediators and increase apoptotic proteins emphasizes the importance of exogenous regulation of the tumor microenvironment. However the mechanism of regulation is not clear. In-vitro studies, have focused on cellular phenotypes and the effect of ω3 PUFA on inflammatory cells in both LPS and tumor induced inflammation. The majority of these studies have focused on inflammatory pathway factors. Although ω3 PUFA has anti-inflammatory effects in inflammatory diseases including cancer, its regulation of MDSCs, which is a critical regulator of the tumor microenvironments is understudied. Further, the majority of murine models, involve diets that are isocaloric but fully equivalent, raising the question of obesity verses dietary constituents. Since obesity itself is an inflammatory disorder, ruling out the effects of obesity associated inflammation as a confounding factor, is crucial to determine the actual effects of dietary components such as fatty acids in tumor initiation, progression and metastasis.
10.7 PUFA Regulation of Immune Cells: Consequences for Clinical Outcomes in Cancer
Epidemiological studies of the incidence and progression of breast cancer in populations of women of Japanese descent in the USA compared to women in Japan, have indicated a significantly higher incidence in the USA compared to Japan [81]. This observation was supported by the finding that offspring of Japanese immigrants to the United States, but not the immigrants themselves, had breast cancer rates similar to the general American population [82]. In the 1990s, dietary components that were implicated in these different incidences were identified [83]. These relatively weak and sometimes contradictory correlative epidemiologic data were considered plausible, given experiments demonstrating ω3 PUFAs had the potential to reduce pro-inflammatory cytokines, inflammation and development of cancer [84]. Similarly, there are indications that high fat diets increase breast cancer risk and are associated with an increased incidence of aggressive prostate cancer [85].
In an epidemiological study of 56,007 French women over 8 years, it was noted that breast cancer risk was not related to dietary PUFA overall, but a significant risk was associated with ω6 vs. ω3 PUFAs that was inversely related to ω3 PUFAs in women with the highest intake of ω6 PUFAs indicating interactions between PUFA consumption [86]. The decreased risk of breast cancer with ω3 PUFA intake (from fish) was confirmed in a case controlled study [87]. A population based study showed all-cause mortality was reduced 16–34% in women with a high intake of ω3 PUFAs [88]. Overall, during the past 20 years, data has accumulated to indicate that a high ω6 PUFA intake is pro-inflammatory, likely involving COX-2 and NFκβ activation leading to increased breast cancer incidence and all-cause mortality whereas high ω3 PUFA intake is protective, against high ω6 PUFA consumption downregulating NFκβ and decreasing breast cancer incidence and all-cause mortality.
Recent studies have shed additional light on the mechanisms involved in these clinical effects, as well as their relationship to the previously discussed innate and adaptive immune cells in the tumor microenvironment. The regulation by ω3 PUFA of macrophage function, has been documented with the use of antagonists to GPR120 (free fatty acid receptor 4 (FFA4R)) which is expressed by some myeloid cell populations [89]. It is noted that ω3 PUFAs mediate anti-inflammatory effects via this receptor. However, the nuclear receptor PPAR-γ is also a receptor for PUFAs and the regulatory mechanisms of ω3 and ω6 PUFA on obesity [90], postmenopausal breast mammary cancer [91] and microenvironmental inflammation [41] require additional study. Changes in the lipid content of cell membranes associated with ω3 and ω6 PUFA intake have effects on oncogenic signalling through modulation of lipid raft profiles and a reduction in cytokine production [92]. In addition, PUFAs contribute to the regulation of hematopoiesis in the BM, at extramedullary sites such as the spleen [93, 94] and have been suggested to induce the expansion of myeloid derived suppressor cells [95].
In summary, dietary intake of PUFAs have shown significant effects on clinical outcomes in cancer patients. In general ω6 PUFAs are associated with increased risk due to both direct effects on the mammary gland and promotion of a pro-inflammatory tumor microenvironment. In contrast, ω3 PUFAs have protective effects and counter tumor and ω6 PUFA associated inflammation. A general recommendation can be made that individuals should decrease dietary ω−6 PUFA intake and increase their ω3 PUFA consumption such that a dietary ratio of no more than 1–3 to 1 is consumed to support cancer prevention. PPAR-γ and GPR120 agonists also have potential use as neoplastic chemopreventive drugs; although both these drugs and dietary PUFA regulation have yet to definitively document anti-cancer activity. In contrast, long-term use of anti-inflammatory drugs has a clearly documented cancer preventive activity associated with inflammatory cell infiltration of tumors [96]. However, these benefits need to be weighed against the risks associated with the long-term use of anti-inflammatory drugs, which highlights the potential for dietary PUFA regulation of inflammation.
References
Eccles SA, Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974;250:667–9.
Gauci GL, Alexander P. The macrophage content of some human tumors. Cancer Lett. 1975;1:29.
Key M, Talmadge JE, Fidler IJ. Lack of correlation between the progressive growth of spontaneous metastases and their content of infiltrating macrophages. J Reticuloendothel Soc. 1982;32:387–96.
Evans R, Lawler EM. Macrophage content and immunogenicity of C57BL/6J and BALB/cByJ methylcholanthrene-induced sarcomas. Int J Cancer. 1980;26:831–5.
Steele RJ, Eremin O, Brown M, Hawkins RA. A high macrophage content in human breast cancer is not associated with favourable prognostic factors. Br J Surg. 1984;71:456–8.
Sun XF, Zhang H. Clinicopathological significance of stromal variables: angiogenesis, lymphangiogenesis, inflammatory infiltration. MMP and PINCH in colorectal carcinomas. Mol Cancer. 2006;5:43.
Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64.
Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42.
Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Alexander P, Eccles SA, Gauci CL. The significance of macrophages in human and experimental tumors. Ann N Y Acad Sci. 1976;276:124–33.
Sinha P. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–51.
Karanikas V, Zamanakou M, Soukou F, Kerenidi T, Gourgoulianis KI, Germenis AE. Naturally occurring tumor-specific CD8+ T-cell precursors in individuals with and without cancer. Immunol Cell Biol. 2010;88:575–85.
Gervois N, Guilloux Y, Diez E, Jotereau F. Suboptimal activation of melanoma infiltrating lymphocytes (TIL) due to low avidity of TCR/MHC-tumor peptide interactions. J Exp Med. 1996;183:2403–7.
Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, Kudoh S, Ochiai A. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–95.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942.
Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501.
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4.
Piersma SJ, Jordanova ES, van Poelgeest MIE, Kwappenberg KMC, van der Hulst JM, Drijfhout JW, Melief CJM, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–61.
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–6.
Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009;29:1093–102.
Gorgun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, White RE, Singh A, Ohguchi H, Suzuki R, Kikuchi S, Harada T, Hideshima T, Tai YT, Laubach JP, Raje N, Magrangeas F, Minvielle S, Avet-Loiseau H, Munshi NC, Dorfman DM, Richardson PG, Anderson KC: Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21(20):4607–4618.
Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33:23–35.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30.
Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, Soejima Y, Maehara Y. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64.
Kantola T, Klintrup K, Vayrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Napankangas J, Makela J, Karttunen TJ, Tuomisto A, Makinen MJ. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729–36.
Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G, Cheng Y. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12:7.
Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coelle C, Mouroux J, Hofman P. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–37.
Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–9.
Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37.
Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61.
Mocellin MC, Camargo CQ, Nunes EA, Fiates GM, Trindade EB. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr. 2016;35(2):359–69.
Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505.
Wild GE, Drozdowski L, Tartaglia C, Clandinin MT, Thomson AB. Nutritional modulation of the inflammatory response in inflammatory bowel disease—from the molecular to the integrative to the clinical. World J Gastroenterol. 2007;13:1–7.
James M, Proudman S, Cleland L. Fish oil and rheumatoid arthritis: past, present and future. Proc Nutr Soc. 2010;69:316–23.
Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84.
Badwey JA, Curnutte JT, Robinson JM, Berde CB, Karnovsky MJ, Karnovsky ML. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984;259:7870–7.
Bates EJ, Ferrante A, Smithers L, Poulos A, Robinson BS. Effect of fatty acid structure on neutrophil adhesion, degranulation and damage to endothelial cells. Atherosclerosis. 1995;116:247–59.
Soyland E, Nenseter MS, Braathen L, Drevon CA. Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Invest. 1993;23:112–21.
Calder PC. Dietary fatty acids and the immune system. Nutr Rev. 1998;56:S70–83.
Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–71.
German JB, Lokesh B, Kinsella JE. The effect of dietary fish oils on eicosanoid biosynthesis in peritoneal macrophages is influenced by both dietary N-6 polyunsaturated fats and total dietary fat. Prostaglandins Leukot Essent Fatty Acids. 1988;34:37–45.
Siriwardhana N, Kalupahana NS, Fletcher S, Xin W, Claycombe KJ, Quignard-Boulange A, Zhao L, Saxton AM, Moustaid-Moussa N. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-kappaB-dependent mechanisms. J Nutr Biochem. 2012;23:1661–7.
Santoli D, Phillips PD, Colt TL, Zurier RB. Suppression of interleukin 2-dependent human T cell growth in vitro by prostaglandin E (PGE) and their precursor fatty acids. Evidence for a PGE-independent mechanism of inhibition by the fatty acids. J Clin Invest. 1990;85:424–32.
Kelly JP, Parker CW. Effects of arachidonic acid and other unsaturated fatty acids on mitogenesis in human lymphocytes. J Immunol. 1979;122:1556–62.
Babu US, Bunning VK, Wiesenfeld P, Raybourne RB, O’Donnell M. Effect of dietary flaxseed on fatty acid composition, superoxide, nitric oxide generation and antilisterial activity of peritoneal macrophages from female Sprague-Dawley rats. Life Sci. 1997;60:545–54.
Turek JJ, Schoenlein IA, Bottoms GD. The effect of dietary n-3 and n-6 fatty acids on tumor necrosis factor-alpha production and leucine aminopeptidase levels in rat peritoneal macrophages. Prostaglandins Leukot Essent Fatty Acids. 1991;43:141–9.
Jeffery NM, Newsholme EA, Calder PC. Level of polyunsaturated fatty acids and the n-6 to n-3 polyunsaturated fatty acid ratio in the rat diet alter serum lipid levels and lymphocyte functions. Prostaglandins Leukot Essent Fatty Acids. 1997;57:149–60.
Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N, Suzuki N, Ito I. Effects of intravenous omega-3 and omega-6 fat emulsion on cytokine production and delayed type hypersensitivity in burned rats receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1998;22:363–7.
Khair-el-Din TA, Sicher SC, Vazquez MA, Wright WJ, Lu CY. Docosahexaenoic acid, a major constituent of fetal serum and fish oil diets, inhibits IFN gamma-induced Ia-expression by murine macrophages in vitro. J Immunol. 1995;154:1296–306.
Hughes DA, Southon S, Pinder AC. (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J Nutr. 1996;126:603–10.
Hughes DA, Pinder AC. N-3 polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes and inhibit antigen-presentation in vitro. Clin Exp Immunol. 1997;110:516–23.
Hubbard NE, Somers SD, Erickson KL. Effect of dietary fish oil on development and selected functions of murine inflammatory macrophages. J Leukoc Biol. 1991;49:592–8.
Sadeghi S, Wallace FA, Calder PC. Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology. 1999;96:404–10.
Peterson LD, Thies F, Sanderson P, Newsholme EA, Calder PC. Low levels of eicosapentaenoic and docosahexaenoic acids mimic the effects of fish oil upon rat lymphocytes. Life Sci. 1998;62:2209–17.
Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–97.
Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275:721–4.
Flower RJ, Perretti M. Controlling inflammation: a fat chance? J Exp Med. 2005;201:671–4.
Norling LV, Serhan CN. Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J Intern Med. 2010;268:15–24.
Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–16.
Rueter K, Haynes A, Prescott SL. Developing primary intervention strategies to prevent allergic disease. Curr Allergy Asthma Rep. 2015;15:537.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–7.
Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–23.
Rose DP, Connolly JM, Coleman M. Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res. 1996;2:1751–6.
Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–7.
Gonzalez MJ, Schemmel RA, Gray JI, Dugan L Jr, Sheffield LG, Welsch CW. Effect of dietary fat on growth of MCF-7 and MDA-MB231 human breast carcinomas in athymic nude mice: relationship between carcinoma growth and lipid peroxidation product levels. Carcinogenesis. 1991;12:1231–5.
Hardman WE. Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr Cancer. 2007;57:177–83.
Manna S, Janarthan M, Ghosh B, Rana B, Rana A, Chatterjee M. Fish oil regulates cell proliferation, protect DNA damages and decrease HER-2/neu and c-Myc protein expression in rat mammary carcinogenesis. Clin Nutr. 2010;29:531–7.
Noguchi M, Minami M, Yagasaki R, Kinoshita K, Earashi M, Kitagawa H, Taniya T, Miyazaki I. Chemoprevention of DMBA-induced mammary carcinogenesis in rats by low-dose EPA and DHA. Br J Cancer. 1997;75:348–53.
Manna S, Chakraborty T, Ghosh B, Chatterjee M, Panda A, Srivastava S, Rana A, Chatterjee M. Dietary fish oil associated with increased apoptosis and modulated expression of Bax and Bcl-2 during 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis in rats. Prostaglandins Leukot Essent Fatty Acids. 2008;79:5–14.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79.
Wei N, Wang B, Zhang QY, Mi MT, Zhu JD, Yu XP, Yuan JL, Chen K, Wang J, Chang H. Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr Cancer. 2008;60:810–25.
Jiang W, Zhu Z, McGinley JN, El Bayoumy K, Manni A, Thompson HJ. Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res. 2012;72:3795–806.
Xue M, Wang Q, Zhao J, Dong L, Ge Y, Hou L, Liu Y, Zheng Z. Docosahexaenoic acid inhibited the Wnt/beta-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J Nutr Biochem. 2014;25:104–10.
Berg JW. Can nutrition explain the pattern of international epidemiology of hormone-dependent cancers? Cancer Res. 1975;35:3345–50.
Tominaga S. Cancer incidence in Japanese in Japan, Hawaii, and western United States. Natl Cancer Inst Monogr. 1985;69:83–92.
Goodstine SL, Zheng T, Holford TR, Ward BA, Carter D, Owens PH, Mayne ST. Dietary (n-3)/(n-6) fatty acid ratio: possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women. J Nutr. 2003;133:1409–14.
Calder PC. N-3 polyunsaturated fatty acids and immune cell function. Adv Enzyme Regul. 1997;37:197–237.
Rose DP. Dietary fatty acids and cancer. Am J Clin Nutr. 1997;66:998S–1003S.
Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Benichou J, Clavel-Chapelon F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. 2009;124:924–31.
Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES. Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: a case-control study. BMC Cancer. 2009;9:216.
Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, Ahn J, Chen Y, Ahsan H, Terry MB, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: a population-based follow-up study on Long Island. New York: Cancer; 2015.
Im DS. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol. 2016;785:36–43.
Bjursell M, Xu X, Admyre T, Bottcher G, Lundin S, Nilsson R, Stone VM, Morgan NG, Lam YY, Storlien LH, Linden D, Smith DM, Bohlooly YM, Oscarsson J. The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS One. 2014;9:e114942.
Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ, Sears DD, Olefsky JM, Ellies LG. Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene. 2015;34:3504–13.
Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:43–7.
Xia S, Li XP, Cheng L, Han MT, Zhang MM, Shao QX, Xu HX, Qi L. Fish oil-rich diet promotes hematopoiesis and alters hematopoietic niche. Endocrinology. 2015;156:2821–30.
Schumann T, Adhikary T, Wortmann A, Finkernagel F, Lieber S, Schnitzer E, Legrand N, Schober Y, Nockher WA, Toth PM, Diederich WE, Nist A, Stiewe T, Wagner U, Reinartz S, Muller-Brusselbach S, Muller R. Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 2015;6:13416–33.
Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol. 2013;43:2943–55.
Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400.
Acknowledgements
James E Talmadge, Timothy R. McGuire and John Graham Sharp are members of the Fred and Pamela Buffet Cancer Center supported by 30CA036727. John Graham Sharp and Timothy R McGuire receive support via the Children’s Hospital/UNMC Pediatric Cancer Research Group, from the state of Nebraska and a Hyundai Foundation “Hope on Wheels” Grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Khadge, S., Sharp, J.G., McGuire, T.R., Thiele, G.M., Talmadge, J.E. (2017). Lipid Inflammatory Mediators in Cancer Progression and Therapy. In: Kalinski, P. (eds) Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy. Advances in Experimental Medicine and Biology, vol 1036. Springer, Cham. https://doi.org/10.1007/978-3-319-67577-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-67577-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67575-6
Online ISBN: 978-3-319-67577-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)