Abstract
Patients with cancer frequently overexpress inflammatory cytokines with an associated neutrophilia both of which may be downregulated by diets with high omega-3 polyunsaturated fatty acids (ω-3 PUFA). The anti-inflammatory activity of dietary ω-3 PUFA has been suggested to have anticancer properties and to improve survival of cancer patients. Currently, the majority of dietary research efforts do not differentiate between obesity and dietary fatty acid consumption as mediators of inflammatory cell expansion and tumor microenvironmental infiltration, initiation, and progression. In this chapter, we discuss the relationships between dietary lipids, inflammation, neoplasia and strategies to regulate these relationships. We posit that dietary composition, notably the ratio of ω-3 vs. ω-6 PUFA, regulates tumor initiation and progression and the frequency and sites of metastasis that, together, impact overall survival (OS). We focus on three broad topics: first, the role of dietary lipids in chronic inflammation and tumor initiation, progression, and regression; second, lipid mediators linking inflammation and cancer; and third, dietary lipid regulation of murine and human tumor initiation, progression, and metastasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Omega 3 polyunsaturated fatty acids
- Inflammation
- Cancer
- Tumor progression
- Metastasis
- Diet
- Prostaglandins
- Lipoxygenases
- Myeloid-derived suppressor cells
- Neutrophils
- Myeloplasia
8.1 Introduction
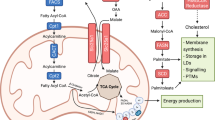
Bioactive lipids include a wide variety of metabolites that regulate essential cellular functions including membrane fluidity, cellular energy storage, lipid signaling, inflammation, and immunity. These mediators have pleiotropic and opposing activities secondary to their metabolism. The biosynthesis and metabolism of fatty acids are dependent on dietary intake and composition of polyunsaturated fatty acids (PUFA). Recent studies into bioactive fatty acids and their precursors in the tumor microenvironment have emphasized the importance of improving our understanding of the cellular lipidome as a regulator of tumor initiation, progression and metastasis, inflammatory and immune responses, and their tumor therapeutic potential as targets.
Chronic inflammation has multiple roles in carcinogenesis, tumor progression, and metastasis. Evidence from preclinical and clinical studies supports a role for chronic inflammation in the initiation and progression of cancer. Further, multiple mechanisms contribute to tumor initiation including the induction of genomic instability, alterations in epigenetic events and subsequent inappropriate expression of genes, enhancing the proliferation of tumor initiated cells, resistance to apoptosis, tumor neovascularization, and tumor invasion and metastasis. Inflammation-associated reactive oxygen and nitrogen species can also result in damage to cellular components including DNA, proteins, and lipids, which may directly or indirectly contribute to malignant transformation. Overexpression, elevated secretion, or abnormal activation of proinflammatory mediators, including cytokines, growth factors (GFs), chemokines, cyclooxygenase-2 (COX-2), prostaglandins (PGs), arginase, lipoxygenases (LOX), pattern recognition receptors (toll and notch), inducible nitric oxide synthase (iNOS) and nitric oxide (NO), and a distinct network of intracellular signaling molecules including upstream kinases and transcription factors, can all contribute to tumor progression. While inflammation supports tumor development, the tumor microenvironment, including tumor and stromal cells, as well as, inflammatory/immune cells, both activate in situ, or mobilized into the tumor microenvironment, can result in an inflammatory state by aberrant expression of proinflammatory mediators. Many of the proinflammatory mediators, especially cytokines, GFs, chemokines, PGs, and leukotrienes (LTs), upregulate angiogenic switches inducing inflammatory angiogenesis and tumor-stroma-cell communication, resulting in tumor angiogenesis, local and systemic immune suppression, and tumor invasion and metastasis.
Rodent and clinical studies have shown that myeloid cell infiltration of the tumor microenvironment is associated with poor clinical outcomes, as well as, neutrophilia and lymphocytopenia. In contrast, an increased lymphocytic infiltration of tumors is associated with improved clinical outcomes. Lifestyle parameters, including obesity and diets with high amounts of saturated fat and/or omega (ω)-6 PUFAs, influence tumor leukocytic infiltration, as well as an increase in extramedullary myelopoiesis (EMM). Tumor secretion of GFs and chemokines can regulate tumor-immune-cell crosstalk; dietary lifestyle choices can also contribute to inflammation, tumor induction and progression, and tumor leukocyte infiltration. A relationship between obesity and high-fat diets (notably saturated fats in Western diets) and the regulation of inflammation, tumor induction, metastasis, and poor clinical outcomes is accepted. Further, mechanisms of dietary promotion of an inflammatory microenvironment are little studied, and few targeted drugs to inhibit the clinical sequelae have neither been identified nor studied. Similarly, adipose tissue, within the tumor microenvironment and its regulation by diet, needs additional study. Thus, modifications of obesity and dietary lipids may inform preventative or therapeutic approaches in the control of tumor-associated inflammation and neoplastic progression.
Tumor-associated adipocyte-derived elements can promote tumor growth, as well as, dedifferentiation into fibroblast-like cells. Free-fatty acids (FFAs) released by adipocytes are used by cancer cells to support proliferation. In addition, these FFAs activate and modulate monocytes, macrophages, and vascular endothelial cells so as to favor a protumorigenic microenvironment [1, 2]. Obesity- and tumor-associated adipose tissues regulate tumor development in multiple ways, by providing energy via FFAs or through adipokines, cytokines, and miRNAs. Tumor-associated adipocytes can recruit macrophages to the tumor microenvironment and stimulate their polarization to the alternative M2 functionality via CCL2, interleukin (IL)-1β, and CXCL12 [3]. In addition, adipocytes produce inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-6, IL-1β, and CCL2 [3, 4], which results in inflammatory cell recruitment, infiltration, and accumulation, resulting in foci of low-grade chronic inflammation [5, 6]. Visceral adipose tissues from obese individuals are also frequently associated with increased systemic levels of CCL2, TNF-α, IL-1, IL-6, and iNOS [7]. Moreover, the level of CCL2 produced is increased due to recognition of cell-free DNA (cfDNA) from degraded adipocytes. Obese mice also have increased cfDNA release resulting in a higher accumulation of macrophages, which aggravates inflammation [8], increases angiogenesis, and supports tumor growth and progression [3].
8.2 Role of Dietary Lipids in Chronic Inflammation and Tumor Initiation, Progression, and Regression
Dietary fats can increase inflammatory responses, particularly Western diets, predominantly due to the inclusion of fatty acids (FAs) from animal sources, which are mainly saturated fatty acids (SFAs), and FAs from plants that are predominantly ω-6 PUFAs. All are proinflammatory. In contrast, FAs derived from some plant-based oils, and fatty fish, which consist mainly of ω-3 PUFA, inhibit inflammation. Rodent and clinical studies have shown that subjects given diets rich in ω-6 PUFAs have an increased risk of inflammatory mediators and diseases, including asthma, rheumatoid arthritis, and inflammatory bowel disease [9]. In contrast, diets with high levels of long chain (LC)-ω-3 PUFAs are anti-inflammatory, such that consuming individuals have a decreased risk of inflammatory diseases [9]. These clinically relevant activities are associated with the oxidization of PUFAs to either proinflammatory or pro-resolving lipid mediators (Fig. 8.1), both of which can regulate inflammation and immunity [10]. Proinflammatory mediators, notably PGs and LTs, are secreted in response to “foreign” substances and cleared by pro-resolving lipid mediators, restoring cellular and tissue homeostasis [11]. Diets with high levels of the ω-3 PUFAs, shorter chain α-linolenic acid (ALA), and more critically, LC eicosapentaenoic (EPA) and docosahexaenoic (DHA) are associated with a decrease in inflammation [12]. The beneficial effects of dietary LC-ω-3 PUFAs include their metabolism into anti-inflammatory metabolites including LTs, thromboxanes (TX), resolvins and a decrease in inflammatory cytokines. The ω-3 PUFAs differ from the ω-6 PUFAs based on the position of their double bonds in the acyl chain, such as linoleic acid (LA) as compared to the ω-6 PUFA arachidonic acid (AA) (Fig. 8.1). However, the inflammatory activities of PUFAs are not clearly separated, based on the number and placement of double bonds, counting from the methyl end of the FA (i.e., ω-3 vs. ω-6). The dietary addition of the short-chain ω-3 PUFA, ALA, an essential FA, and the main precursor of LC-ω-3 PUFAs, is proinflammatory and can result in enhanced secretion of superoxides from macrophages and neutrophils [13], and adhesion of inflammatory cells to endothelial cells [14]. Further, ALA can limit the proliferation of rodent and human lymphocytes [15,16,17], supporting immunosuppressive functions. Consistent with these in vitro activities, rodents provided a high-fat diet, rich in ALA, have a decreased mitogen-stimulated lymphocyte proliferation and natural killer (NK) cell activity [18].
This is an outline of eicosanoid synthesis pathways from arachidonic acid (AA) and resolving-related mediators from α-linolenic acid (ALA) and their inflammatory and anti-inflammatory functions. The abbreviations include COX, cyclooxygenase; CYT p450 cytochrome, p450; CXC chemokine subtype, HETE, hydroxyeicosatetraenoic acid; HDHA, hydroxyldocosahexaenoic acid; HPETE, hydroperoxyeicosatetraenoic acid; HPDHA, hydroperoxydocosahexaenoic acid; HPEPE, hydroperoxyeicosapentaenoic acid; IL, interleukin, IFN, interferon; LOX, lipoxygenase; LT, leukotriene; LX, lipoxin; PG, prostaglandin; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen synthetase; TNF, tumor necrosis factor, TX, thromboxane
In vitro studies using the ω-6 PUFA, AA, have revealed an increase in inflammation, including enhanced superoxide release [13], neutrophil attachment to endothelial cells [14], and increased macrophage secretion of IL-1β [19]. Mice given diets with high ω-6 PUFA levels, in a dose-dependent manner, have an increased level of urinary leukotriene E4 (LTE-4) and prostaglandin E2 (PGE-2) following leukocyte stimulation in vivo [20]. Further, it has been reported that diets high in AA result in increased levels of angiotensinogen, IL-6, and monocyte chemoattractant protein (MCP)-1 and increased expression of the proinflammatory transcription factor, nuclear factor κβ (NFκβ) [21]. In studies with rats fed high AA diets for 8 weeks, a decrease in superoxide production by peritoneal macrophages was observed in response to phorbol esters [22] as well as an increase in TNF secretion by resident macrophages, although no effect on TNF production by inflammatory macrophages was observed [23]. Thus, the effects of the shorter ω-3 PUFA, ALA, on lymphocyte functions appear to be dependent on ALA levels and total PUFA diet content [24]. Studies with ALA and ω-6 PUFA contrast with the bioactivity of LC-ω-3 PUFA with 20 or more carbon atoms such as EPA and DHA, which are anti-inflammatory and immune augmenting [25]. The anti-inflammatory activity of diets incorporating LC-ω-3 PUFAs are due to a lower metabolism of ω-6 PUFA into inflammatory eicosanoids, cytokines, and the stimulation of reactive oxygen species (ROS) and nitric oxide synthase (NOS) mediators [26]. Clinically, dietary supplementation with EPA and DHA can decrease intestinal damage and improve gut histology in patients with inflammatory bowel disease [27], as well as decrease arthritic lesions including joint pain, number of tender and swollen joints, and duration of morning stiffness [28].
Tumor blood vessels are structurally and functionally abnormal, lacking a normal hierarchical structure composed of arterioles, capillaries, and venules [29]. Tumor endothelial cells are generally loosely connected and covered by fewer and more abnormal mural pericytes [29,30,31]. Clinically, a poor coverage of tumor blood vessels by pericytes is related to poor patient prognosis [32,33,34], and pericyte dysfunction has been associated with increased numbers of metastases [35]. Prostaglandin I2 (PGI2) is an important vascular prostanoid that provides an important balance in tumor angiogenesis [36, 37]. PGI2 and agonists of PGI2 have been suggested to reduce tumor metastasis by modifying tumor angiogenesis [38]. Thus, administration of PGI 2 analogs that affect endothelium-pericyte interaction has been shown to target angiogenesis tumor microenvironment and control neoplasia progression and growth [39]. The potential interaction between vascular prostacyclin and diet is supported by the finding of increased prostacyclin production by vessel walls in patients given diets with moderate levels of LC-ω-3 PUFAs [40]. Thus, LC-ω-3 PUFAs regulation of prostanoid synthesis by vascular endothelial cells within the tumor vascular provides another potential mechanism for LC-ω-3 PUFAs control of tumor growth.
Chronic inflammation contributes to the initiation and progression of malignancy [41]. The role of inflammation in carcinogenesis was first proposed in 1863 by Rudolf Virchow, when he noticed the presence of leukocytes in neoplastic tissues [42]. Since Virchow’s early observation linking inflammation and cancer, data supporting tumor initiation at sites of infection and chronic inflammation have been reported [43]. Indeed, approximately 25% of all cancers are associated with chronic infections and inflammation [44]. Although inflammation is an adaptive host defense against infection and is primarily a self-limiting process, inadequate resolution contributes to chronic pathologies including cancer [45, 46]. Evidence from laboratory- and population-based studies has suggested that organ-specific carcinogenesis is at least partly associated with inflammation [47,48,49,50]. Thus, the development of gastric, hepatic, gallbladder, prostate, and pancreatic tumors has been attributed to Helicobacter pylori-induced gastric inflammation, chronic hepatitis, cholecystitis, inflammatory atrophy of the prostate and chronic pancreatitis, respectively [46, 51, 52]. Patients suffering from inflammatory bowel disorders also have an increased risk of colorectal cancer [47, 53, 54], while management with anti-inflammatory drugs (COX-2 inhibitors) reduces this risk [55].
Sustained cellular injury can also induce inflammation and stimulate carcinogenesis. Inflammatory and innate immune cells are recruited to sites of infection or inflammation, such that activated myeloid cells generate ROS and reactive nitrogen species (RNS), which facilitate tumor initiation. Thus, one mechanism by which chronic inflammation supports carcinogenesis is the generation of ROS and/or RNS in inflammatory tissue and subsequent DNA damage leading to oncogene activation and/or inactivation of tumor suppressor genes. Chronic exposure to ultraviolet (UV) B radiation can induce inflammatory tissue damage [56], tumor suppressor T-cells [57], and skin cancer [56]. Mutational changes in ras and p53 have also been observed with many lipid mediators resulting in the regulation of inflammation and cancer initiation and progression [58, 59]. The activation of ras oncogene and loss-of-p53 tumor suppressor gene function have been shown to support UVB-induced mouse skin carcinogenesis [60]. ROS-induced DNA damages, including DNA strand breaks, DNA-based modifications, and DNA cross-links, result in replication errors and genomic instability contributing to tumor initiation and progression [61, 62]. NO, another reactive species, has a role in inflammation-associated carcinogenesis by direct modification of DNA and inactivation of DNA repair enzymes [63]. 8-Oxo-7,8-dihydro-20-deoxyguanosine (8-oxo-dG), which is associated with oxidative and mutagenic DNA damage [64], is produced in association with H. pylori-induced gastric [65] and TNF-α-induced pulmonary carcinogenesis [66]. Peroxynitrite, formed by a reaction between NO radical and superoxide anion, causes DNA damage by forming 8-nitroguanine (8-NG) [67, 68], another biomarker of inflammation-associated cancers [69]. Thus, oxidative and nitrosative DNA damage products are associated with inflammation-driven carcinogenesis [70]. ROS and RNS can also induce lipid peroxidation resulting in other reactive species, such as manoldialdehyde and 4-hydroxynonenal (4-HNE), which are capable of forming DNA adducts [71]. Elevated intracellular ROS (e.g., superoxide anion, H2O2, and hydroxyl radicals) and RNS (e.g., peroxynitrite, NO, and S-nitrosothiols) also induce alterations in protein functions, including a perturbation of DNA-protein cross-links and posttranslational modification critical to maintaining cellular homeostasis. For example, NO has been shown to hyperphosphorylate and inactivate retinoblastoma protein, thereby increasing human colon cancer cell proliferation [72]. Moreover, in a mouse model of colitis, the hyperphosphorylation of retinoblastoma protein (Rb) was found to be reduced in the colons of iNOS-null mice as compared to wild-type littermates, suggesting that NO is involved in Rb hyperphosphorylation [72]. In the colon tissues of patients with ulcerative colitis, a positive correlation between iNOS levels and the phosphorylation of p53 as well as the activation of p53 transcriptional activity has been identified [73]. Thus, nitrosative stress also has a role in inflammation-associated carcinogenesis by activating activator protein-1(AP-1), a representative redox-sensitive transcription factor [74], which is involved in cell transformation and proliferation [75, 76].
Further, metabolic reprogramming from glycolysis to lipid metabolism regulates myeloid cell differentiation. For instance, IL-4-induced M2 macrophages rely on fatty acid oxidation (FAO) to proliferate, which is mediated through signal transducer and activator of transcription 6 (STAT6) and peroxisome proliferator-activated receptor gamma (PPARγ)- co-activator 1β (PGC1β) [77, 78]. Indeed, the uptake of triacylglycerols followed by lipolysis is critical for M2 activation [79] and tumor-infiltrating dendritic cells (DCs) develop high levels of intracellular triglycerides [80]. This increased lipid accumulation in DCs impairs their ability to process and present antigens and results in an inhibition of the induction of antigen-specific T-cells [81, 82]. Decreasing the lipid content by inhibiting fatty acid synthesis restores DC functions and may improve the efficacy of cancer vaccines [81]. Overall, it appears that myeloid cells can use the metabolic programs that support their survival and functional demands within their microenvironment. As such, an improved understanding of these metabolic pathways may support the development of novel therapeutic targets in cancer and other chronic inflammatory diseases [83, 84].
Tumor-infiltrating myeloid derived suppressor cells (MDSCs) use FAO as their primary source of adenosine triphosphate (ATP) [85, 86], such that pharmacologic inhibition of FAO blocks the immunosuppressive functions of MDSCs, delaying tumor growth in a T-cell-dependent fashion and enhancing therapeutic responses to low-dose chemotherapy and adoptive T-cell therapy [86]. The mechanisms by which the tumor microenvironment regulates the uptake of exogenous lipids and enhances the metabolic and functional reprogramming of tumor-associated MDSCs is associated with tumor-derived GFs (granulocyte-colony stimulating factor (G-CSF) and granulocyte/macrophage-colony stimulating factor (GM-CSF)) that can upregulate lipid transport receptors in tumor-infiltrating myeloid cells and increase lipid uptake in the tumor microenvironment [87]. This is associated with an enhanced oxidative metabolism and upregulated immunosuppression. Interestingly, human tumor-infiltrating and peripheral blood (PB) MDSCs also have increased levels of lipid transport proteins, supporting the development of more immunosuppressive MDSCs in vitro. Thus, tumor-derived cytokines facilitate myeloid cell lipid uptake, accumulation, and metabolism resulting in the induction of MDSC immunosuppressive functions. As such, lipids contribute to immunosuppressive myeloid cells (M2 macrophages, dendritic cells, and polymorphonuclear leukocyte (PMN) and monocytic (M)-MDSC) in cancer and chronic inflammatory pathologies [78,79,80,81, 83,84,85,86, 88]. Following infiltration of the tumor microenvironment, myeloid cells undergo metabolic reprogramming from glycolysis to FAO that is paralleled by the upregulation of the T-cell immunosuppressive mediators arginase I and iNOS [85, 86]. As such, metabolites in the tumor microenvironment regulate the immunometabolic induction of MDSCs. Tumor-derived inflammatory GFs including G-CSF and GM-CSF upregulate the expression of lipid transport receptors that facilitate the uptake of lipids in the tumor microenvironment, including FFAs and the triacylglycerol-carrying lipoproteins, very low-density lipoproteins (VLDL) and low-density lipoproteins (LDL) [89]. The uptake, accumulation, and oxidation [86] of these lipids activate and prolong the survival of immunosuppressive MDSCs [90]. Importantly, cancer-associated MDSCs also express lipid transporters, and therefore, human peripheral blood stem cells (PBSC), cultured in lipid-rich media, develop into highly inhibitory MDSCs [91]. Consistent with other myeloid cells, substantial lipid accumulation occurs with tumor-derived MDSCs [92, 93]. MDSCs with lipid overload have greater immunosuppressive effects on CD8+ T-cells, compared to MDSCs with normal lipid content. Lipid accumulation in tumor-derived MDSCs is linked to an increase in fatty acid uptake. This observation is supported by the study of Cao et al., which demonstrated an increased expression of fatty acid transport protein 4 (FATP4) in murine tumor-derived MDSCs [93]. The lipids in the MDSCs of tumor-bearing mice and cancer patients are oxidized, potentially by the oxidative activities of ROS and myeloperoxidase (MPO) [92, 94]. Inhibition of ROS and MPO can reduce the oxidation of lipids resulting in MDSCs with decreased immunosuppressive activity [92]. Studies such as these document an increase in lipids in the tumor microenvironment that are assimilated by MDSCs supporting previous reports of increased levels of serum triglycerides, LDL-cholesterol, and VLDL-cholesterol in cancer patients [95,96,97] and patients with chronic inflammatory diseases [95,96,97,98,99,100,101,102,103]. However, the origin of lipids in the tumor microenvironment is unclear, although it appears that lipids released from adipose tissue provide energy that supports tumor growth and invasion [104,105,106]. However, the mechanisms by which MDSCs mobilize lipids to support increasing FAO remain obscure. Adipocytes liberate fatty acids for FAO from lipids stored in lipid droplets by lipolysis, which is regulated by adipose triglyceride lipase, hormone-sensitive lipase, and lysosomal acid lipase (LAL) [107]. Recent studies showed that LAL-mediated lipolysis releases fatty acids supporting FAO in IL-4-induced M2 macrophages [79] and IL-15-driven memory T-cells [108]. Furthermore, lipids act as ligands for PPARs [109] that have a key role in the regulation of FAO [110]. In line with this, PPARγ and PPARδ, which are induced by STAT6, can regulate the alternative activation of macrophages [110].
8.3 Lipid Mediators Linking Inflammation and Cancer
8.3.1 Dietary PUFA Regulation of Myeloid Cell Functions
Diets rich in ω-6 PUFAs are proinflammatory, enhancing the expansion of myeloid cells [111] and MDSCs [112]. The increase in MDSCs is observed with both in vitro cultured murine bone marrow cells and in vivo in mice fed diets enriched in ω-6 PUFAs. In the latter studies, mice were given a linseed oil-based diet containing 45% of the shorter ω-3 PUFA, ALA, or a sunflower oil diet containing 45% of the ω-6 PUFA, LA. These studies suggested that the bioactivity of PUFAs occurred through Janus kinase-signal transducer and activator of transcription (JAK-STAT3) signaling, such that a JAK inhibitor reduced the bioactivity of PUFAs on MDSCs. Based on these and other studies, it was concluded that the inflammatory activity of PUFAs may be mediated, in part, by diet [113]. Thus, dietary fat contributes to tumor-associated inflammation that occurs, in part, through AA metabolism [114]. A Western-style diet increases the risk of tumorigenesis via myeloid recruitment, infiltration of tumors, and subsequent activation of TNF-a, PGE2, NF-kβ, and Wnt inflammatory pathways [115]. AA can make up to 40% of the fatty acid composition of cancer cell membranes [116]. The anti-tumorigenic effects of ω-3 PUFAs may be mediated in part by their anti-inflammatory effects [117].
Recently, lipid accumulation in the adipose tissues of obese hosts have been shown to promote infiltrating macrophages with an M2 polarization shift, while M1 phenotype macrophages are observed in lean adipose tissue [118, 119]. Because dietary fish oil with LC-ω-3 PUFAs decreases PGE-2 production, LC-ω-3 PUFAs are considered anti-inflammatory. Such a diet also results in enhanced secretion of Th1-type cytokines and decreased major histocompatibility complex (MHC) II expression, lymphocyte proliferation, and NK cell activity. Consistent with these observations, the culture of human neutrophils with LC-ω-3 PUFAs has been reported to suppress superoxide production and phagocytosis [120]. Similarly, the incubation of murine peritoneal macrophages with EPA or DHA can inhibit MHC II expression [121]. In one study, human monocytes were cultured with EPA or DHA, resulting in a decreased frequency of human leukocyte antigens-DR or DP (HLA-DR or –DP) positive monocytes following addition of interferon gamma (IFN-γ) [122] and depressed antigen (Ag) presentation [123]. Similarly, adding fish oil to rodent diets can decrease superoxide and H2O2 secretion by macrophages [124]. Experiments comparing diets with safflower oil versus fish oil have been found to decrease peak plasma levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6 following lipopolysaccharide (LPS) injection [125]. However, super-pharmacologic doses were used in this study, contrasting with the majority of rodent studies which use dietary fish oil in which EPA plus DHA comprise up to 30% of the lipid fatty acids and up to 12% of dietary energy. The conclusions from these studies have been extended using lower levels of EPA or DHA (4.4% of total FAs or 1.7% of dietary energy), documenting that these levels have anti-inflammatory activities [126].
8.3.2 T-cell Immunoregulation and PUFA
Clearly, ω-6 PUFAs are proinflammatory [127] as they are metabolized to AA and subsequently by COX-/LOX- to inflammatory lipid mediators that include PGs and LTs [128]. These AA metabolites have tumor-promoting actions such that the COX downstream metabolite PGE-2 can enhance tumor growth by inducing tolerogenic DCs and Tregs. 5-LOX metabolites include the four series LTs that can also stimulate tumor growth and progression [129]. This contrasts with LC-ω-3 fatty acids that have alternative COX/LOX activities forming metabolites with alternative bioactivities, including the three series PGEs and five series LTs. AA metabolism results in the LOX products that can stimulate the expansion and differentiation of myeloid progenitor cells [111] including MDSCs. Similarly, tolerogenic DCs contribute to T-cell regulatory functions by inhibiting their activation. In steady-state conditions, tissue-resident, immature DCs internalize, process, and present tumor Ags. These DCs, identified as DC2s, are poorly immunogenic and do not secrete proinflammatory cytokines due to the expression of low levels of costimulatory molecules. Further, DC2s secrete immunosuppressive cytokines, including IL-10 and transforming growth factor – beta (TGF-β), which are critical to the induction of T-reg cell differentiation. The secretion of indoleamine 2,3-dioxygenase (IDO) secretion by DC2s can also contribute to immune tolerance [130]. Another myeloid cell population with a similar functional profile are alternatively activated macrophages (M2s) that differentiate from monocytes by IL-4 stimulation. M2s facilitate tumor angiogenesis, support tumor progression, invasion, and metastasis, and contribute to T-cell immunosuppression by secreting IL-10, facilitating Th2 cell differentiation. This provides a positive feedback cycle for differentiation of additional M2 macrophages, all of which can express programmed death-ligand 1 (PD-L1), further contributing to activated T-cell apoptosis [131].
Similar to M2 macrophages, MDSCs can infiltrate tumors, as well as circulate in the PB of tumor patients. MDSCs can be either of monocytic, PMN or immature in origin [132, 133]. In the blood of cancer patients, MDSCs lack lineage (LIN) markers for T and B lymphocytes (CD3 and CD19) and NK cells (CD56) and thus express an LIN−HLADR−CD11b+ phenotype [134, 135] that can be further subset based on CD14 expression (monocytic), CD15 expression (PMN), or expression of CD33+CD14−CD15− (immature) cells [132, 136]. A positive correlation between the frequency of MDSCs and tumor stage has been reported for numerous tumor pathologies [133]. The function of MDSC inhibition of T-cell activation occurs via arginase, iNOS, ROS, or RNS, as well as secretion of immunosuppressive cytokines [137]. MDSCs also deplete nutrients necessary for lymphocyte function (arginine), disrupt IL-2 receptor signaling, interfere with lymphocyte trafficking, promote activation of T-regs by CD40-CD40L ligation, and suppress CD3-zeta (ζ) expression and secrete IL-10 or TGF-β [138, 139]. While few studies have examined the response of MDSCs to dietary PUFA [140], myeloid cell and myeloid progenitor cell response to dietary ω-6 PUFA as extramedullary hematopoiesis and myeloplasia has been studied [140]. Chronic inflammation has a key role in the expansion and activation of MDSCs in both tumors and various inflammatory disorders. As discussed in this chapter, chronic inflammation is a hallmark of MDSC expansion and immunosuppression. Thus, ω-6 dietary associated inflammation [140] suggests a potential role for dietary PUFA in the regulation of MDSCs. A few studies have shown that dietary PUFA can promote the expansion of MDSCs via JAK-STAT3 signaling in mice [112]. Waight et al. [141] demonstrated that G-CSF and GM-CSF increased the generation of MDSCs by downregulating the expression of interferon regulatory factor 8 (IRF-8) in myeloid progenitors via signaling via the STAT3 and STAT5 pathways. Interestingly, Yan et al. [112] demonstrated that both the ω-3 and ω-6 PUFA treatments significantly enhanced the expansion of cultured bone marrow MDSCs, PMN-MDSCs. The proliferation of T-cells decreased in a dose-dependent manner in mice given a PUFA-containing diet. Murine diets containing PUFAs increased the percentage of PMN-MDSCs in both the bone marrow and the spleen. The administration of PUFAs also stimulated the immunosuppressive properties of MDSCs isolated from mouse spleens. PUFA diets also induced the activation of JAK-STAT3 signaling and the immunosuppression of T-cells was mediated by ROS. Finally, Yan et al. [112] revealed that a PUFA-enriched diet augmented the growth of CT26 and Lewis lung carcinoma in mice. In agreement with these results, given that PUFAs activate PGE-2 production through COX-2, it is interesting to note that PGE-2 can stimulate STAT3 signaling [142]. PGE-2 is a potent inducer of MDSC functions [143] and thus might stimulate their expansion. Indeed, inhibition of COX-2 with Celebrex has been shown to slow tumor growth and inhibit MDSC expansion and numbers [144].
T-regs, a T-cell-based immunosuppressive cell, are divided into two major populations. This includes a thymic origin cell [145] and one that is induced in the PB [146] by TGF-β. Under homeostatic conditions, T-regs can limit the induction of autoimmunity, inhibit tissue destruction, and ensure the maintenance of tolerance to self-antigens [147]. In the PB of cancer patients, the frequency of T-reg cells is increased compared to normal individuals [148]. In addition, tumor-infiltrating T-regs are increased in tumors of cancer patients [149]. T-regs are phenotypically described as CD4+ T-cells that co-express forkhead box P3 (Foxp3) and CD25 [148]. T-regs can also express checkpoint inhibitory molecules, including, but not limited to PD-1, lymphocyte activating gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM3), glucocorticoid-induced tumor necrosis factor receptor (GITR), and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), which can all suppress T-cell responses [148, 150]. T-regs also indirectly suppress effector T-cells by depleting local IL-2 levels, which are needed for the proliferation and survival of effector T-cells [151]. Indirect suppression is also associated with the secretion of immunosuppressive cytokines, such as IL-10 and TGF-β [152].
Lipid mediators derived mainly from dietary PUFAs can also contribute to the control of inflammation. These mediators, which are collectively called specialized pro-resolving lipid mediators (SPMs), include the families of compounds termed “resolvins,” “lipoxins,” “maresins,” and “protectins” [11]. The resolution of an inflammatory response by SPMs is characterized by lipid mediator “class switching,” in which cells downregulate enzymes responsible for the production of proinflammatory lipids, including prostaglandins and leukotrienes, while upregulating enzymes responsible for the production of SPMs [153]. SPMs exert pro-resolving and anti-inflammatory activities without suppressing immunity [153, 154]. This contrasts with corticosteroid and nonsteroidal anti-inflammatory drug (NSAID) anti-inflammatory therapies. SPM’s therapeutic potential is due to their activity in the nanomolar to picomolar range and secretion by many cells with minimal toxicities. LC-ω-3 PUFAs, which are precursors for SPMs, can inhibit T-cell proliferation and cytokine production [155]. Murine diets supplemented with fish oil or DHA have been reported to increase CD4+ T-cell proliferation in response to a Th2 stimulus and decrease Th2 cytokine production by CD8+ T-cells [156]. T-cells also express known SPM receptors, including ALX/FPR2 [157, 158], GPR32 [158], and BLT1 [159], which support their response to SPMs. Overall, SPMs show promise in regulating adaptive immune responses. Receptors for SPMs are found on DCs, T-cells, and B cells. Furthermore, SPM therapy has shown significant activity, including augmentation of pathogen clearance, resolution of inflammation, and the development of immune memory.
8.3.3 Dynamic Anti-inflammatory Activities of ω-3 PUFAs
One of the challenges with dietary studies using PUFA regulation of inflammation is that obesity is also associated with chronic low-grade inflammation and increased levels of FFA, proinflammatory cytokines, hormones, and circulating myeloid cells [160, 161]. Adipocytes can secrete metabolites that either promote or resolve an inflammatory response [162]. Thus, adipose cell hypertrophy and hyperplasia increases oxygen consumption, which can result in hypoxia [163], activation of cellular stress and inflammation in association with proinflammatory cytokine secretion [163]. Adipocyte hyperplasia also results in myeloid infiltration of adipose tissue, notably surrounding both dead and dying adipocytes, and a phenotypic shift of adipose tissue macrophages, that can release proinflammatory cytokines that induce ROS and activate inflammatory signaling pathways [164]. Obesity can also contribute to changes in the tumor microenvironment by increasing inflammatory cell infiltration and the presence of FFAs [165]. High levels of proinflammatory adipokines have also been shown to contribute to tumor infiltration of inflammatory cells within the microenvironment [166, 167] through autocrine and paracrine activation of signaling pathways including NF-κβ [168], STAT3, and extracellular regulated kinase (ERK)1/2, all of which stimulate tumor cell proliferation and inhibit apoptosis [169].
Further, LC-ω-3 PUFAs are metabolized into anti-inflammatory, bioactive SPMs, which can reduce inflammation [170]. These observations suggest that the resolving phase of inflammation is not passive but is actively downregulated by endogenous anti-inflammatory mediators [171]. This contrasts with ω-6 PUFA metabolites, including PGD-2, LTD-4, LTC-4, and LTE-4, which are inflammatory. Although AA is a precursor to LTs, its metabolite PGE-2 can also regulate macrophage and lymphocyte functions. Thus, dietary consumption of the ω-6 PUFA LA, as the precursor of AA, is causally linked to allergic diseases and provides a potential treatment strategy using LC-ω-3 PUFAs [172].
8.4 Dietary Lipid Regulation of Murine and Human Tumor Initiation, Progression, and Metastasis
8.4.1 Dietary LC-ω-3 PUFA Controls of Murine Tumor Growth
Clinically, associations have been reported between PUFA consumption/composition and inflammation, although co-variable includes genetic susceptibility, tissue microenvironments, stress, obesity, age, caloric intake, and dietary duration. Murine models have also suggested mechanisms whereby PUFA composition can regulate tumor initiation and progression. These studies provide insight into various pathologic conditions including infections, autoimmune, inflammatory, neoplastic, and obesity conditions and a relationship with neutrophilia, splenomegaly, and multifocal, hepatic extramedullary myelopoiesis (i.e., the formation of myeloid tissue outside of the bone marrow) [173, 174]. Such inflammatory pathologies, which are associated with tumor initiation, are controlled by multiple risk factors, including hormones, obesity, diet, and age. However, following tumor initiation, inflammation is also regulated by tumor GF and chemokine secretion, as well as, additional risk factors. Thus, within the tumor microenvironment, crosstalk occurs between the immune response and inflammation, including EMM and tumor-secreted GFs.
Dietary PUFA regulation of tumor progression and metastasis has been studied in transplanted syngeneic and xenograft tumor models. In one xenograft model using MDA-MB-435 tumor cells, athymic nude mice were inoculated with tumor cells following the placing of recipients on either LA, EPA, or DHA diets. These studies revealed a significant delay in tumor growth and reduced metastases in mice fed an EPA or DHA diet, including reduced AA levels in tumor membrane phospholipids [175]. The results from one of our studies in a syngeneic tumor model, with two groups of mice receiving pair-fed isocaloric and isolipidic liquid diets documented the impact of PUFA composition on tumor growth [176]. Ten weeks following initiation of the diets, groups of mice received orthotopic injections of 4T1 mammary tumor cells. The results showed that mice consuming a LC-ω-3 PUFA diet had a significant delay in tumor initiation, slower growing tumors, and prolonged survival compared to mice given an ω-6 PUFA diet [176]. Interestingly, when mice were autopsied 35 days post-orthotopic injection, the hosts consuming the ω-6 based diets had a significantly greater number and frequency of pulmonary, hepatic, renal, cardiac, and bone marrow metastases. These results suggested that dietary PUFA composition is not only critical to tumor initiation but also modulates tumor growth and the extent of metastasis and distribution of localization metastatic sites.
As part of this study, tumors were collected 35 days after tumor injection, frozen in OCT and immunohistochemistry (IHC) performed to evaluate the frequency of proliferating cells using antibodies to KI67 (Fig. 8.2a) infiltration by macrophages, by staining with F4/80 (Fig. 8.2b) and infiltration by granulocytes and MDSCs, by staining with antibodies to neutrophil elastase (Fig. 8.2c). In every instance, the tumors in mice receiving an ω-6 based diet had a significant increase in proliferating tumor cells and infiltrating macrophages and granulocytes, as opposed to tumors from mice on an LC-ω-3 PUFA diet as described above. It is noted that there was a slight but significant difference in tumor sizes at this time, with a median tumor volume of 888.5 ± 115.2 mm3 for the tumors from the ω-6 tumor-bearing mice and 446.3 ± 52.3 mm3 in the ω-3 diets. This documents the effect of an ω-6 PUFA diet on innate inflammatory cell infiltration of 4T1 tumors and on tumor cell proliferation. Further, in murine studies of neoadjuvant therapy where treatment with EPA and DHA preceded surgery, the number of pulmonary metastases was significantly decreased compared to mice on an LA diet [177]. Similar results focused on immune-augmentation and therapeutic activities have been documented in R3230RC and MCF-7 breast adenocarcinoma tumor studies [178, 179], which included a reduced number of MDSCs [180]. In addition to a neoadjuvant activity, LC-ω-3 PUFA diets have therapeutic potential. In a tumor survival study, mice were switched from an 8% corn oil (1% ALA) diet to an 8% canola oil (10% ALA) diet, when the average primary tumor volume became 60 mm3. This ω-3 PUFA canola oil diet therapy significantly reduced tumor growth as compared to an ω-6 PUFA corn oil diet [181]. Based on these and other rodent studies, it appears that LC-ω-3 PUFA dietary intervention may be used with therapeutic intent [182].
Mice were fed omega-3 and omega-6 diets for 10 weeks prior to injection of 5000 4T1 cells in an abdominal mammary gland. Tumors were collected after 35 days from tumor injection, and IHC was performed for the evaluation of proliferating cells by Ki67 (a), intra-tumor macrophages by (b) F4/80, and neutrophil infiltration by staining with neutrophil elastase (c). (Images were taken at 400× magnification)
Murine studies using LC-ω-3 PUFA and autochthonous chemically induced mammary tumors have confirmed and extended these observations using transplanted tumors. In an autochthonous 7, 12-dimethylbenz (α) anthracene (DMBA)-induced mammary tumor model, mice on a fish oil diet had a significantly reduced tumor incidence, slower tumor growth, and a reduced numbers of metastasis [183, 184]. The LC-ω-3 PUFA diet affected tumor induction and growth, which correlated with reduced AA serum levels, suppressed tumor cell proliferation, protection against DNA single-strand breaks, and an increase in tumor cell apoptosis [184,185,186]. Similarly, a comparison of tumorigenic using a tumor model with N-methyl-N-nitrosourea (MNU)-induced rat mammary tumors and diets with differing fat composition were compared, including an SFA diet, a monounsaturated fat (MUFA) diet, an ω-6 PUFA diet, or diets with different ratios of ω-6: ω-3 PUFA. It was found that the diet incorporating a 1:1 ratio of ω-6:ω-3 PUFA could prevent mammary tumor development. Studies into causal relationships revealed that this diet group had decreased levels of COX-2 and 5-LOX transcription levels in mammary tissues and PPAR-γ levels [187]. Together, these and other studies support a role for LC-ω-3 PUFAs in regulating the metabolic inflammatory tumor microenvironment by upregulating PPAR-γ [186, 187]. Consistent with these studies, when dietary LC-ω-3 PUFA content is increased to an ω-6:ω-3 ratio of 1:14.6, as compared to a control diet of 1:0.7, a 60% decrease in tumor growth was observed [188]. Similar studies, using a therapy model with transplanted, orthotopic 4T1 mammary tumors, in which a 5% fish oil diet was initiated when the hosts had developed primary tumors that were 8–10 mm3 in diameter, resulted in significantly reduced growth and metastases that had a correlation with decreased tumor cell proliferation [189]. A similar therapy study using C3(1) Tag mice revealed that a switch to a fish oil concentrate from a corn oil diet slowed prostate tumorigenesis and progression in association with lower estradiol, testosterone, and androgen receptor levels [190]. The ability of LC-ω-3 PUFAs to downregulate inflammatory mediators and increase tumor cell apoptosis supports the importance of its regulation in the tumor microenvironment. In vivo studies have studied the effect of dietary LC-ω-3 PUFA on inflammatory cells in animal models of both LPS inflammatory disease and tumor-induced inflammation. However, the majority of murine models have used diets that were neither isocaloric nor pair-fed, raising the question of obesity versus a role for dietary composition? Since obesity itself is inflammatory, clarifying the effects of obesity-associated inflammation, as opposed to dietary regulation of inflammation, is critical to determining the regulatory activity of dietary components. Thus, using animal models, with an isocaloric, isolipidic liquid diet that allows pair feeding and controlled dietary caloric intake, is needed to assess the impact on host weight and adipose changes, as well as dissociate effects between obesity and dietary composition.
Epidemiologic studies support the role for NSAIDs, including aspirin in reducing the incidence of cancer and can prolong survival if administered postdiagnosis [114]. Initial studies focused on colorectal cancers; however, low-dose aspirin has also demonstrated antitumor activity for other tumors, including lung, breast, prostate, and metastatic cancers [191, 192]. In addition, low-dose aspirin has been found to improve survival and provide chemopreventive benefits in combination with cytotoxic therapy and/or surgical resection [193, 194]. Many of these studies are derived from patients receiving low-dose aspirin for cardio-prevention, in which 20–30% have been suggested to obtain benefit with a decrease in cancer incidence [195]. However, studies also show that neither non-aspirin NSAIDs nor acetaminophen is associated with a reduced risk of cancer or chemoprevention [196, 197]. While the known anti-inflammatory activity of aspirin offers a rational mechanism of action, the unique antitumor mechanisms of aspirin compared with other NSAIDs is confounding. It is noted that the use of low-dose aspirin in cancer patients is limited by gastrointestinal bleeding and hemorrhagic stroke [198].
Investigations into anti-inflammatory mechanisms in cancer patients have focused on the downregulation of proinflammatory mediators, including cytokines, eicosanoids, and enzymes [114]. COX-1 and COX-2 are key targets of aspirin and are involved in the biosynthesis of proinflammatory lipid autacoids, including prostaglandins. Aspirin’s anticancer activity has been suggested to be associated with the irreversible acetylation of cyclooxygenases that are overexpressed in cancer patients [199, 200]. In contrast to other NSAIDs that reversibly block COX enzymes, aspirin qualitatively alters enzymatic substrate specificity and activity of COX. A unique function of aspirin-acetylated COX is the metabolism of aspirin-triggered (AT) SPMs, including AT-lipoxin A4 (AT-LXA4) and AT-resolvins D1 (AT-RvD1) and D3 (AT-RvD3) [201,202,203]. Other NSAIDs do not trigger endogenous SPM production [204]. Thus, SPMs, such as resolvins and lipoxins, promote the resolution of inflammation by stimulating phagocytosis of cellular debris and counter-regulating proinflammatory cytokines without being immunosuppressive [204, 205].
The aspirin-acetylated COX facilitates the biosynthesis of aspirin-triggered SPMs from ω-3 PUFA substrates, including EPA and DHA [206]. While AT-resolvins exhibit potent anti-inflammatory activity characteristic of native resolvins, the AT forms resist rapid inactivation and have longer half-lives [204]. AT-SPMs are increased in patients who respond to the anti-inflammatory activity of aspirin compared with those that do not respond to aspirin [207]. AT-lipoxins also modulate tumor-associated macrophages and reduce bone cancer pain [208, 209]. Further, SPMs, such as resolvins, enhance cytotoxic cancer therapy by promoting the clearance of therapy-generated apoptotic tumor cells by macrophages [210]. AT-resolvins and AT-lipoxins have been shown to be critical for the anticancer activity of low-dose aspirin by resolving tumor-promoting inflammation in mice [211].
8.4.2 Dietary LC-ω-3 PUFAs and Improved Cancer Patient Outcomes
The tumor microenvironment includes, in addition to tumor cells, extracellular matrix, endothelial cells, stromal cells, fibroblasts, adipocytes, and critically infiltrating inflammatory cells (M2 macrophages and MDSCs) as well as suppressive and effector lymphocytes, all of which have a role in regulating tumor progression and metastasis. The infiltrating lymphocytes, particularly mature CD8 T-cells, serve as mediators of antitumor activities [212, 213]. In cancer patients, the infiltration of lymphocytes provides an independent, positive prognostic factor as assessed by IHC staining [214]. Studies into the type of infiltrating immune cells (e.g., CD3+, CD8+, and FOXP3+ T-lymphocytes) and the density or location of infiltrating T-cells also provide prognostic correlations with positive (or negative) outcomes in patients with colorectal cancer (CRC) [215,216,217,218,219,220,221,222], ovarian cancer, and breast cancer [223,224,225,226]. A meta-analysis of these clinical studies assessed the impact of tumor-infiltrating leukocytes on outcomes, including one incorporating 30 studies with 2988 patients [227]. This analysis examined associations between survival and inflammatory cell (N = 12) and T-lymphocyte subset infiltration (N = 18) studies. Pooled analyses documented that a generalized tumor inflammatory infiltrate was associated with significantly improved cancer-specific survival (CS), overall survival (OS), and disease-free survival (DFS). Stratification by cellular location and T-lymphocyte subset suggested that in the tumor microenvironment, CD3+, CD8+, and FoxP3+ cellular infiltrates were not significant prognostic markers for OS or CS. In contrast, a high frequency of infiltrating CD8+ but not CD3+ or FoxP3+ T-cells was predictive of an increased OS. Furthermore, a high frequency of tumor-infiltrating CD3+ T-cells, at the invasive tumor border, was associated with improved OS and DFS [227].
Consistent with the effects of LC-ω-3 PUFAs on tumor-infiltrating leukocytes is an inverse relationship between dietary consumption of LC-ω-3 PUFAs and the probability of developing CRC, as found in case-control studies by Murff et al. [228] and Habermann et al. [229]. However, the benefits were limited such that, in one study [228], an increased LC-ω-3 PUFA intake was associated with a reduced risk of CRCs in women. In a second trial [230], an inverse relationship was observed between low DHA intake and an increased risk of CRC in patients with genetic variants that resulted in higher proinflammatory mediators. Recently, a relationship between LC-ω-3 PUFA consumption and survival was observed in a retrospective analysis of the CALGB 89803 randomized trial of adjuvant chemotherapy for completely resected stage III CRC (n = 1264) [230]. Patients in the highest quartile of LC-ω-3 PUFA dietary intake had a significantly increased DFS compared with the patients in the lowest quartile. Notably, this relationship appeared to be highest for patients with high CRC COX-2 expression [230]. Further, clinical studies have also examined adjuvant therapy with LC-ω-3 PUFA [231] such that higher consumption of LC-ω-3FA was associated with improved OS in patients with established CRC using two independent cohort studies [230, 232].
The EMT study is the only reported randomized trial of purified LC-ω-3 PUFA treatment in patients with metastatic CRC [233]. This is a phase II double-blind, randomized, placebo-controlled trial of EPA, in the FFA form, 2 g daily before surgery in patients (n = 88) undergoing liver resection for CRC liver metastases. In the first 18 months after resection, EPA-treated individuals obtained an OS and DFS benefit compared to the placebo cohort [233]. This preliminary observation from a “window” trial of limited LC-ω-3 PUFA use, prior to metastasis surgery, resulted in a phase III randomized trial of EPA (4 g daily in the ethyl ester form) in patients undergoing liver resection for CRC liver metastasis (the EMT2 trial). In this trial, subjects are randomized to EPA or placebo at least 2 weeks before surgery and continue the drug long term, with progression-free survival (PFS) as the primary endpoint and OS as the key secondary endpoint (ClinicalTrials.gov; NCT03428477).
The possible beneficial effects of ω-3 PUFAs in CRC incidence was first suggested in 1997 in West Coast fishermen [234]. Two years later, it was pointed out that several of the known risk factors for some cancers, including colon cancer, may be reduced by dietary ω-3 PUFAs supplementation, and implementation of clinical chemoprevention trials was encouraged [235]. Based on these observations and other studies, two randomized trials of LC-ω-3 PUFA supplementation are underway that have secondary CRC endpoints. These include the ASCEND (A Study of Cardiovascular Events in Diabetes) trial (NCT00135226), which is a 2 × 2 factorial study of long-term (median 7.5 years) LC-ω-3 PUFA (840 mg EPA/DHA ethyl ester daily) and aspirin (100 mg daily) treatment for the prevention of cardiovascular and cerebrovascular events in patients with diabetes (n = 15,480). In this study, cancer outcomes were a secondary endpoint, with the ability to continue with posttrial follow-up. In the ASCEND trial which tested ω-3 supplementation (at a dose of 1 g per day) in adults with diabetes in the United Kingdom provided generally null results. In contrast, the VITamin D and OmegA-3 Trial (VITAL) study (NCT01169259), which was a 2 × 2 factorial study of the same dose and formulation of LC-ω-3 PUFA (also 840 mg EPA/DHA ethyl ester) and vitamin D3 in 25,871 US male and female participants had different outcomes. During the overall treatment period of 5.3 years, there was a statistically nonsignificant 17% reduction in cancer death, with a hazard ratio of 0.83 [236, 237]. However, the protocol planned to account for a latency period with some analyses that excluded early follow-up. In an analysis that excluded the first 2 years of follow-up, there was a reduction in cancer deaths that was statistically significant, a 25% reduction, as well as a nonsignificant 6% reduction in cancer incidence with vitamin D. A beneficial association between higher consumption of LC-ω-3FA and a lower incidence of CRC was reported to be restricted to a subset of tumors with microsatellite instability (MSI) [238]. MSI occurs in 15% of CRC patients and is caused by a loss of DNA mismatch repair (MMR) activity [239]. Consistent with the anti-inflammatory activities of LC-ω-3FA, data support the critical role of inflammation and dysregulated anti-tumor immune response in the development of MSI tumors [240]. It should be noted that immune checkpoint inhibitor therapy has been shown to be more effective for treating cancers with MSI [241, 242]. This suggests that increased dietary consumption of LC-ω-3FA after diagnosis may benefit patients with MSI tumors [243].
As supported by our transplantable tumor studies as discussed above and shown in Fig. 8.2, IHC analyses of infiltrating lymphocytes, particularly CD3+ T-lymphocytes in primary tumors, provides a biomarker that predicts improved clinical outcomes [244,245,246]. Furthermore, basic histological quantification of T-lymphocyte density, cytotoxicity, and a memory phenotype, by CD3+, CD8+, and CD45RO+ markers, respectively, demonstrated that an increase in T-lymphocyte infiltration is associated with significant improvements in DFS and OS [217, 245, 247]. In CRC, identifying the location of infiltrating cytotoxic T lymphocytes (CTLs), assessed as CD3+CD8+ T-cells within the center (CT) and invading margin (IM) of the primary tumor, predicts clinical outcomes [217]. The quantification of the density, phenotype, and location (CT or IM) of infiltrating CTL provides an immunoscore [248,249,250]. Indeed, the significance of the CD3+ cell infiltration analysis surpasses a diagnosis of tumor stage, lymph node, and metastatic invasion, sub-setting patients into five categories based on the location in the tumor (CT and IM) of CD3+ and CD8+ T-cells [251, 252].
In association with immunoregulatory properties, a patient’s lifestyle, preceding and following diagnosis and therapeutic interventions, is associated with controlling cancer initiation, progression [253], and responses to therapeutic interventions [254]. Specifically, patients who consume a high-fat diet (saturated fat, or ω-6 PUFAs) frequently exhibit neutrophilia that can facilitate tumor initiation, progression, and result in poor outcomes [255, 256]. Conversely, diets that contain a high LC-ω-3 PUFA content have been associated with decreased inflammation, lower EMM, and improved outcomes [173]. The improved clinical outcomes were initially suggested by epidemiological studies into the incidence and progression of breast cancer in American women of Japanese descent, as compared to Japanese women living in Japan. The results from one study indicated a significantly higher breast cancer incidence in American women of Japanese descent compared to Japanese women in Japan [257]. This observation is supported and extended by studies with female children from Japanese immigrants to America, but not the immigrants themselves, who had breast cancer rates similar to the general American population [258]. In the 1990s, dietary components were found to be implicated in these different incidences [259]. These correlative epidemiologic studies are supported by rodent studies, which demonstrated that LC-ω-3 PUFAs can reduce proinflammatory cytokines, inflammation, and cancer development [260].
Case-control studies have also shown an inverse relationship between dietary ω-6 and LC-ω-3 PUFAs ratio and the incidence of breast cancer, supporting their dietary importance [261]. An epidemiological study of 56,007 French women over 8 years revealed that the risk of breast cancer was unrelated to dietary PUFA consumption. Rather, a significant risk was associated with the ratio of dietary ω-6 versus LC-ω-3 PUFAs, which was inversely related to LC-ω-3 PUFA levels in women with the highest intake of ω-6 PUFAs, indicating interactions with PUFA consumption [262]. Subsequent studies revealed a decreased risk of developing breast cancer with dietary LC-ω-3 PUFA in a case-controlled, population-based study [263] that showed a reduction in all-cause mortality that was reduced 16–34% in women consuming high levels of LC-ω-3 PUFAs [264]. Indeed, during the last 20 years, data has accumulated supporting the observation that high ω-6 PUFA dietary consumption is proinflammatory, likely involving COX-2 secretion and NFκβ activation, resulting in an increased incidence of cancer and all-cause mortality. In contrast, consumption of high levels of LC-ω-3 PUFA were found to be protective against neoplasia, including a decreased incidence of cancer associated, all-cause mortality [265]. Indeed, in a meta-analysis of 11 independent prospective studies, it was observed that a decrease in the dietary ω-6: LC-ω-3 PUFA ratio significantly lowered the risk of breast cancer [266]. However, some studies have shown no association between heightened ω-6: LC-ω-3 PUFA ratios in the diet and breast cancer development.
Recent studies have investigated the underlying mechanisms of this observation and its relationship to innate and acquired immune cell infiltration of the tumor microenvironment. The regulatory activity of LC-ω-3 PUFA on macrophage functions has also been documented with the use of antagonists to G protein-coupled receptor (GPR120), which is expressed by some myeloid cell populations and acts as a PUFA receptor [267]. This is supportive of a role for LC-ω-3 PUFA mediation and anti-inflammatory effects via this receptor. However, PPAR-γ also acts as a receptor for PUFAs and the regulatory mechanisms of LC-ω-3 and ω-6 PUFA on obesity [268], postmenopausal breast cancer [269], and microenvironmental inflammation [270], suggesting a need for additional studies. Further, PUFAs contribute to the regulation of bone marrow (BM) and EMH at sites such as the spleen [271, 272] and may also expand the frequency of MDSCs [112].
Unfortunately, LC-ω-3 PUFA dietary supplements can lead to various toxicities. Despite the therapeutic benefits discussed herein, there are potential risks associated with high doses. The primary adverse effects are altered platelet function. The presence of EPA and DHA leads to the production of TX A3, which is a less potent platelet activator than TX A2. Supplementation of EPA and DHA, therefore, can affect platelet activation because of the different eicosanoids produced, resulting in an antithrombotic effect that can impact blood coagulation and wound healing [273]. The impact depends on the amount and the duration of LC-ω-3 PUFA supplementation. When given in combination with other medications, such as aspirin or warfarin LC-ω-3 PUFA interactions may exacerbate adverse effects that can occur with LC-ω-3 PUFA supplementation alone [274,275,276,277,278]. LC-ω-3 PUFAs supplementation is contraindicated during antiplatelet and anticoagulant treatment because of the additive effect on bleeding times when administered together [279].
8.5 Future Trends or Directions
Dietary PUFA consumption may not only affect inflammation and the incidence and progression of neoplasia, but may also support responses to therapeutic interventions in cancer patients via the regulation of inflammation. In general, increased dietary ω-6 PUFA consumption is associated with a heightened risk of cancer that is suggested to be due to a proinflammatory tumor microenvironment. In contrast, an LC-ω-3 PUFA diet has potential protective effect to suppress ω-6 PUFA-associated inflammation. Nutritional recommendations are that individuals should decrease dietary ω-6 PUFA intake and increase LC-ω-3 PUFA consumption with an intake of at least 500 mg/day of LC-ω-3 PUFA [280]. PPAR-γ and GPR120 agonists also have potential for use as chemopreventive drugs, although their use may, perhaps, be better targeted toward either high-risk individuals or as part of therapeutic interventions. Both of these are receptors for LC-ω-3 PUFA [281, 282]. Regardless, we need to further study both pharmacophores and dietary regulation of PUFAs as protective and therapeutic strategies for cancer and their association with leukocyte infiltration of tumors.
Research on the role of LC-ω-3 PUFAs and SPMs on inflammation and cancer is increasing and suggests a positive role for use as an adjuvant in cancer therapy. Increased efforts are needed using high-quality randomized control trials to establish their mechanisms of action, the optimal timing for supplementation, dosage, product source, method of extraction, preparation, and quantification to obtain efficacy, which will optimize their clinical use for cancer prevention and therapy. These future trials should address these questions as well as the impact on the tumor microenvironment, specifically infiltrating cellular subtypes. We also stress the need for translational/preclinical studies that utilize isocaloric and isolipidic pair-fed diets to segregate the regulation of immunity and inflammation by obesity versus dietary PUFA. Further, care must be taken to differentiate between activity on tumor growth as opposed to metastasis, as these biologic parameters are interrelated such that, larger tumors typically have more metastases. In our experience, ω-6 PUFA diets impact not only primary tumor growth but also the extent and critically sites of metastasis, all of which are typically unstudied but highly relevant since metastasis is frequently the ultimate cause of patient mortality.
References
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71:2455–2465
Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, Rio MC (2005) Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res 65:10862–10871
Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C (2013) Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res 73:6080–6093
Zeyda M, Stulnig TM (2007) Adipose tissue macrophages. Immunol Lett 112:61–67
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808
Sartipy P, Loskutoff DJ (2003) Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 100:7265–7270
Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS (2009) Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150:2161–2168
Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, Kim-Kaneyama JR, Sato F, Bando M, Yagi S, Soeki T, Hayashi T, Imoto I, Sakaue H, Shimabukuro M, Sata M (2016) Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv 2:e1501332
Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 68:280–289
Serhan CN (2007) Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25:101–137
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361
Mocellin MC, Camargo CQ, Nunes EA, Fiates GM, Trindade EB (2016) A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr 35:359–369
Badwey JA, Curnutte JT, Robinson JM, Berde CB, Karnovsky MJ, Karnovsky ML (1984) Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem 259:7870–7877
Bates EJ, Ferrante A, Smithers L, Poulos A, Robinson BS (1995) Effect of fatty acid structure on neutrophil adhesion, degranulation and damage to endothelial cells. Atherosclerosis 116:247–259
Soyland E, Nenseter MS, Braathen L, Drevon CA (1993) Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Investig 23:112–121
Santoli D, Phillips PD, Colt TL, Zurier RB (1990) Suppression of interleukin 2-dependent human T cell growth in vitro by prostaglandin E (PGE) and their precursor fatty acids. Evidence for a PGE-independent mechanism of inhibition by the fatty acids. J Clin Invest 85:424–432
Kelly JP, Parker CW (1979) Effects of arachidonic acid and other unsaturated fatty acids on mitogenesis in human lymphocytes. J Immunol 122:1556–1562
Calder PC (1998) Dietary fatty acids and the immune system. Nutr Rev 56:S70–S83
Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC et al (1989) The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 320:265–271
German JB, Lokesh B, Kinsella JE (1988) The effect of dietary fish oils on eicosanoid biosynthesis in peritoneal macrophages is influenced by both dietary N-6 polyunsaturated fats and total dietary fat. Prostaglandins Leukot Essent Fatty Acids 34:37–45
Siriwardhana N, Kalupahana NS, Fletcher S, Xin W, Claycombe KJ, Quignard-Boulange A, Zhao L, Saxton AM (2012) Moustaid-Moussa N: n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-kappaB-dependent mechanisms. J Nutr Biochem 23:1661–1667
Babu US, Bunning VK, Wiesenfeld P, Raybourne RB, O’Donnell M (1997) Effect of dietary flaxseed on fatty acid composition, superoxide, nitric oxide generation and antilisterial activity of peritoneal macrophages from female Sprague-Dawley rats. Life Sci 60:545–554
Turek JJ, Schoenlein IA, Bottoms GD (1991) The effect of dietary n-3 and n-6 fatty acids on tumor necrosis factor-alpha production and leucine aminopeptidase levels in rat peritoneal macrophages. Prostaglandins Leukot Essent Fatty Acids 43:141–149
Jeffery NM, Newsholme EA, Calder PC (1997) Level of polyunsaturated fatty acids and the n-6 to n-3 polyunsaturated fatty acid ratio in the rat diet alter serum lipid levels and lymphocyte functions. Prostaglandins Leukot Essent Fatty Acids 57:149–160
Turchini GM, Nichols PD, Barrow C, Sinclair AJ (2012) Jumping on the omega-3 bandwagon: distinguishing the role of long-chain and short-chain omega-3 fatty acids. Crit Rev Food Sci Nutr 52:795–803
Simopoulos AP (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505
Wild GE, Drozdowski L, Tartaglia C, Clandinin MT, Thomson AB (2007) Nutritional modulation of the inflammatory response in inflammatory bowel disease--from the molecular to the integrative to the clinical. World J Gastroenterol 13:1–7
James M, Proudman S, Cleland L (2010) Fish oil and rheumatoid arthritis: past, present and future. Proc Nutr Soc 69:316–323
Pasqualini R, Arap W, McDonald DM (2002) Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med 8:563–571
Minami Y, Sasaki T, Kawabe J I, Ohsaki Y (2013) Accessory cells in tumor angiogenesis—tumor–associated pericytes. In: Research directions in tumor angiogenesis. (London: InTechOpen Limited), London, pp 73–88
Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160:985–1000
O’Keeffe MB, Devlin AH, Burns AJ, Gardiner TA, Logan ID, Hirst DG, McKeown SR (2008) Investigation of pericytes, hypoxia, and vascularity in bladder tumors: association with clinical outcomes. Oncol Res 17:93–101
Stefansson IM, Salvesen HB, Akslen LA (2006) Vascular proliferation is important for clinical progress of endometrial cancer. Cancer Res 66:3303–3309
Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, Tachibana T, Imamura M (2005) Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology 69:159–166
Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, Semb H (2006) Pericytes limit tumor cell metastasis. J Clin Invest 116:642–651
Turner EC, Mulvaney EP, Reid HM, Kinsella BT (2011) Interaction of the human prostacyclin receptor with the PDZ adapter protein PDZK1: role in endothelial cell migration and angiogenesis. Mol Biol Cell 22:2664–2679
Zhu W, Saddar S, Seetharam D, Chambliss KL, Longoria C, Silver DL, Yuhanna IS, Shaul PW, Mineo C (2008) The scavenger receptor class B type I adaptor protein PDZK1 maintains endothelial monolayer integrity. Circ Res 102:480–487
Honn KV, Cicone B, Skoff A (1981) Prostacyclin: a potent antimetastatic agent. Science 212:1270–1272
Minami Y, Sasaki T, Bochimoto H, Kawabe J, Endo S, Hira Y, Watanabe T, Okumura S, Hasebe N, Ohsaki Y (2015) Prostaglandin I2 analog suppresses lung metastasis by recruiting pericytes in tumor angiogenesis. Int J Oncol 46:548–554
DeCaterina R, Giannessi D, Mazzone A, Bernini W, Lazzerini G, Maffei S, Cerri M, Salvatore L, Weksler B (1990) Vascular prostacyclin is increased in patients ingesting omega-3 polyunsaturated fatty acids before coronary artery bypass graft surgery. Circulation 82:428–438
Malyguine A, Umansky V, Shurin MR (2013) Role of the immunological environment in cancer initiation, development and progression. In: Shurin MR, Umansky V, Malyguine A (eds) The tumor immunoenvironment. Springer, Dordrecht, pp 1–12
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Mueller MM, Fusenig NE (2004) Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4:839–849
Hussain SP, Harris CC (2007) Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 121:2373–2380
Jackson L, Evers BM (2006) Chronic inflammation and pathogenesis of GI and pancreatic cancers. Cancer Treat Res 130:39–65
Schottenfeld D, Beebe-Dimmer J (2006) Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin 56:69–83
Itzkowitz SH, Yio X (2004) Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 287:G7–17
Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB (2004) The role of inflammation in the pathogenesis of prostate cancer. J Urol 172:S6–11; discussion S-2
O’Byrne KJ, Dalgleish AG (2001) Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 85:473–483
Whitcomb DC (2004) Inflammation and cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 287:G315–G319
Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J, Okazaki K, Seki T (2007) Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 46:48–57
Philpott M, Ferguson LR (2004) Immunonutrition and cancer. Mutat Res 551:29–42
Herszenyi L, Miheller P, Tulassay Z (2007) Carcinogenesis in inflammatory bowel disease. Dig Dis 25:267–269
Seril DN, Liao J, Yang GY, Yang CS (2003) Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 24:353–362
Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J (2000) Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther 14:145–153
Halliday GM (2005) Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res 571:107–120
Thorn RM, Fisher MS, Kripke ML (1981) Further characterization of immunological unresponsiveness induced in mice by ultraviolet radiation. II. Studies on the origin and activity of ultraviolet-induced suppressor lymphocytes. Transplantation 31:129–133
Rajalingam K, Schreck R, Rapp UR, Albert S (1773) Ras oncogenes and their downstream targets. Biochim Biophys Acta 2007:1177–1195
Strano S, Dell’Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G (2007) Mutant p53: an oncogenic transcription factor. Oncogene 26:2212–2219
Hattori Y, Nishigori C, Tanaka T, Uchida K, Nikaido O, Osawa T, Hiai H, Imamura S, Toyokuni S (1996) 8-hydroxy-2′-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol 107:733–737
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214
Marnett LJ (2000) Oxyradicals and DNA damage. Carcinogenesis 21:361–370
Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ (2000) Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 60:184–190
Hoki Y, Hiraku Y, Ma N, Murata M, Matsumine A, Nagahama M, Shintani K, Uchida A, Kawanishi S (2007) iNOS-dependent DNA damage in patients with malignant fibrous histiocytoma in relation to prognosis. Cancer Sci 98:163–168
Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA Jr, Wilson KT (2004) Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res 64:8521–8525
Babbar N, Casero RA Jr (2006) Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res 66:11125–11130
Ohshima H, Sawa T, Akaike T (2006) 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal 8:1033–1045
Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H (1995) Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis 16:2045–2050
Kawanishi S, Hiraku Y (2006) Oxidative and nitrative DNA damage as biomarker for carcinogenesis with special reference to inflammation. Antioxid Redox Signal 8:1047–1058
Pinlaor S, Sripa B, Ma N, Hiraku Y, Yongvanit P, Wongkham S, Pairojkul C, Bhudhisawasdi V, Oikawa S, Murata M, Semba R, Kawanishi S (2005) Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J Gastroenterol 11:4644–4649
Bartsch H, Nair J (2005) Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat Res 591:34–44
Ying L, Hofseth AB, Browning DD, Nagarkatti M, Nagarkatti PS, Hofseth LJ (2007) Nitric oxide inactivates the retinoblastoma pathway in chronic inflammation. Cancer Res 67:9286–9293
Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP, Quezado MM, Zurer I, Rotter V, Wink DA, Appella E, Harris CC (2003) Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A 100:143–148
Kroncke KD (2003) Nitrosative stress and transcription. Biol Chem 384:1365–1377
Cerutti PA, Trump BF (1991) Inflammation and oxidative stress in carcinogenesis. Cancer cells (Cold Spring Harbor, NY: 1989) 3:1–7
Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136
Odegaard JI, Chawla A (2011) Alternative macrophage activation and metabolism. Annu Rev Pathol 6:275–297
Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A (2006) Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 4:13–24
Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill CM, Yan C, Du H, Abumrad NA, Urban JF Jr, Artyomov MN, Pearce EL, Pearce EJ (2014) Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15:846–855
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH (2015) ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161:1527–1538
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI (2010) Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16:880–886
Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, Kagan VE, Gabrilovich DI (2014) Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol 192:2920–2931
Al-Khami AA, Rodriguez PC, Ochoa AC (2017) Energy metabolic pathways control the fate and function of myeloid immune cells. J Leukoc Biol 102:369–380
Buck MD, Sowell RT, Kaech SM, Pearce EL (2017) Metabolic instruction of immunity. Cell 169:570–586
Al-Khami AA, Rodriguez PC, Ochoa AC (2016) Metabolic reprogramming of myeloid-derived suppressor cells (MDSC) in cancer. Onco Targets Ther 5:e1200771
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, Rodriguez PC, Ochoa AC (2015) Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res 3:1236–1247
Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC (2017) Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Onco Targets Ther 6:e1344804
Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI (2016) Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 1(2):aaf8943
Sieow JL, Gun SY, Wong SC (2018) The sweet surrender: how myeloid cell metabolic plasticity shapes the tumor microenvironment. Front Cell Dev Biol 6:168
Yan D, Adeshakin AO, Xu M, Afolabi LO, Zhang G, Chen YH, Wan X (2019) Lipid metabolic pathways confer the immunosuppressive function of myeloid-derived suppressor cells in tumor. Front Immunol 10:1399
Consonni FM, Porta C, Marino A, Pandolfo C, Mola S, Bleve A, Sica A (2019) Myeloid-derived suppressor cells: ductile targets in disease. Front Immunol 10:949
Veglia F, Tyurin V, Kagan V, Gabrilovich D (2015) Abstract 467: Oxidized lipids contribute to the suppression function of myeloid derived suppressor cells in cancer. Cancer Res 75:467
Cao W, Gabrilovich D (2011) Abstract 3649: Contribution of fatty acid accumulation to myeloid-derived suppressor cell function in cancer. Cancer Res 71:3649
Veglia F, Tyurin VA, Kagan VE, Gabrilovich D (2018) Abstract 5133: Lipids and suppressive functions of MDSC in cancer. Cancer Res 78:5133
Laisupasin P, Thompat W, Sukarayodhin S, Sornprom A, Sudjaroen Y (2013) Comparison of serum lipid profiles between normal controls and breast cancer patients. J Lab Phys 5:38–41
Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A (2007) Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem 40:1129–1134
Fiorenza AM, Branchi A, Sommariva D (2000) Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res 30:141–145
Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M (2013) Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol 14:1045–1053
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186:3299–3303
Robblee MM, Kim CC, Porter Abate J, Valdearcos M, Sandlund KL, Shenoy MK, Volmer R, Iwawaki T, Koliwad SK (2016) Saturated fatty acids engage an IRE1alpha-dependent pathway to activate the NLRP3 inflammasome in myeloid cells. Cell Rep 14:2611–2623
Hale JS, Otvos B, Sinyuk M, Alvarado AG, Hitomi M, Stoltz K, Wu Q, Flavahan W, Levison B, Johansen ML, Schmitt D, Neltner JM, Huang P, Ren B, Sloan AE, Silverstein RL, Gladson CL, DiDonato JA, Brown JM, McIntyre T, Hazen SL, Horbinski C, Rich JN, Lathia JD (2014) Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells 32:1746–1758
Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK (2016) Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov 6:852–869
Worm SW, Kamara DA, Reiss P, Kirk O, El-Sadr W, Fux C, Fontas E, Phillips A, D’Arminio Monforte A, De Wit S, Petoumenos K, Friis-Mller N, Mercie P, Lundgren JD, Sabin C (2011) Elevated triglycerides and risk of myocardial infarction in HIV-positive persons. AIDS 25:1497–1504
Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T, Holst J, Saunders DN, Hoy AJ (2017) Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 5:1
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17:1498–1503
Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA (2017) Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541:41–45
Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F (2012) FAT SIGNALS – lipases and lipolysis in lipid metabolism and signaling. Cell Metab 15:279–291
O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, Hsu FF, Birnbaum MJ, Pearce EJ, Pearce EL (2014) Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41:75–88
Varga T, Czimmerer Z, Nagy L (1812) PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011:1007–1022
Chawla A (2010) Control of macrophage activation and function by PPARs. Circ Res 106:1559–1569
Greene ER, Huang S, Serhan CN, Panigrahy D (2011) Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat 96:27–36
Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J (2013) Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol 43:2943–2955
Clements VK, Long T, Long R, Figley C, Smith DMC, Ostrand-Rosenberg S (2018) Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol 103:395–407
Wang D, Dubois RN (2010) Eicosanoids and cancer. Nat Rev Cancer 10:181–193
Kim IW, Myung SJ, Do MY, Ryu YM, Kim MJ, Do EJ, Park S, Yoon SM, Ye BD, Byeon JS, Yang SK, Kim JH (2010) Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis. J Gastroenterol Hepatol 25:1785–1794
Wallace JM (2002) Nutritional and botanical modulation of the inflammatory cascade – eicosanoids, cyclooxygenases, and lipoxygenases – as an adjunct in cancer therapy. Integr Cancer Ther 1:7–37; discussion
Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan YY, Yang P, Newman RA, Kang JX, McMurray DN, Chapkin RS (2008) Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res 68:3985–3991
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184
Morris DL, Singer K, Lumeng CN (2011) Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care 14:341–346
Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N, Suzuki N, Ito I (1999) Effect of intravenous omega-6 and omega-3 fat emulsions on nitrogen retention and protein kinetics in burned rats. Nutrition 15:135–139
Khair-el-Din TA, Sicher SC, Vazquez MA, Wright WJ, Lu CY (1995) Docosahexaenoic acid, a major constituent of fetal serum and fish oil diets, inhibits IFN gamma-induced Ia-expression by murine macrophages in vitro. J Immunol 154:1296–1306
Hughes DA, Southon S (1996) Pinder AC: (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J Nutr 126:603–610
Hughes DA, Pinder AC (1997) N-3 polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes and inhibit antigen-presentation in vitro. Clin Exp Immunol 110:516–523
Hubbard NE, Somers SD, Erickson KL (1991) Effect of dietary fish oil on development and selected functions of murine inflammatory macrophages. J Leukoc Biol 49:592–598
Sadeghi S, Wallace FA, Calder PC (1999) Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 96:404–410
Peterson LD, Thies F, Sanderson P, Newsholme EA, Calder PC (1998) Low levels of eicosapentaenoic and docosahexaenoic acids mimic the effects of fish oil upon rat lymphocytes. Life Sci 62:2209–2217
Ghosh S, Novak EM, Innis SM (2007) Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 alpha-linolenic acid ratios in normal, fat-fed pigs. Am J Physiol Heart Circ Physiol 293:H2919–H2927
Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C (2012) Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab 2012:539426
Naveena B, Janakiram AM, Lang ML, Rao CV (2015) Immune modulation by agents used in the prevention and treatment of colon and pancreatic cancers. In: Rezaei N (eds) Cancer immunology. Springer, Berlin, Heidelberg, pp 249–275
Fitzgerald-Bocarsly P, Dai J, Singh S (2008) Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 19:3–19
Szebeni GJ, Vizler C, Kitajka K, Puskas LG (2017) Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediat Inflamm 2017:9294018
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13:739–752
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58:49–59
Ostrand-Rosenberg S, Fenselau C (2018) Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol 200:422–431
Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2010) HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207:2439–2453
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150
Anani W, Shurin MR (2017) Targeting myeloid-derived suppressor cells in cancer. Adv Exp Med Biol 1036:105–128
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22:275–281
Appleby LJ, Nausch N, Heard F, Erskine L, Bourke CD, Midzi N, Mduluza T, Allen JE, Mutapi F (2015) Down regulation of the TCR complex CD3zeta-chain on CD3+ T cells: a potential mechanism for helminth-mediated immune modulation. Front Immunol 6:51
Salminen A, Kauppinen A, Kaarniranta K (2019) AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): impact on cancer and aging. J Mol Med (Berl) 97:1049–1064
Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI (2013) Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 123:4464–4478
Lin A, Wang G, Zhao H, Zhang Y, Han Q, Zhang C, Tian Z, Zhang J (2016) TLR4 signaling promotes a COX-2/PGE2/STAT3 positive feedback loop in hepatocellular carcinoma (HCC) cells. Onco Targets Ther 5:e1074376
Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P (2012) PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Investig 41:635–657
Abe F, Donkor M, Scholar E, Younos I, Dafferner A, Westphal S, Hoke T, Talmadge J (2009) Chemoprevention by cyclooxygenase-2 inhibition in FVB transgenic mice for Her2/neu induced mammary cancer is associated with reduced myeloid derived suppressor cells. Cancer Prev Res (Phila Pa) 7: 140–151
Levine AG, Hemmers S, Baptista AP, Schizas M, Faire MB, Moltedo B, Konopacki C, Schmidt-Supprian M, Germain RN, Treuting PM, Rudensky AY (2017) Suppression of lethal autoimmunity by regulatory T cells with a single TCR specificity. J Exp Med 214:609–622
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A (2006) Fazekas de St Groth B: expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:1693–1700
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711
Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM (2012) Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother 35:702–710
Chao JL, Savage PA (2018) Unlocking the complexities of tumor-associated regulatory T-cells. J Immunol 200:415–421
Spellman A, Tang SC (2016) Immunotherapy for breast cancer: past, present, and future. Cancer Metastasis Rev 35:525–546
Seledtsov VI, Goncharov AG, Seledtsova GV (2015) Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum Vaccin Immunother 11:851–869
Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr, Muller W, Rudensky AY (2008) Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28:546–558
Basil MC, Levy BD (2016) Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16:51–67
Serhan CN, Chiang N, Dalli J (2015) The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol 27:200–215
Costabile M, Hii CS, Robinson BS, Rathjen DA, Pitt M, Easton C, Miller RC, Poulos A, Murray AW, Ferrante A (2001) A novel long chain polyunsaturated fatty acid, beta-Oxa 21:3n-3, inhibits T lymphocyte proliferation, cytokine production, delayed-type hypersensitivity, and carrageenan-induced paw reaction and selectively targets intracellular signals. J Immunol 167:3980–3987
Arrington JL, Chapkin RS, Switzer KC, Morris JS, McMurray DN (2001) Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin Exp Immunol 125:499–507
Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN (2003) Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol 170:6266–6272
Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN (2016) Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med 8:353ra111
Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD (2003) Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol 4:982–990
Ahima RS (2011) Digging deeper into obesity. J Clin Invest 121:2076–2079
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (1820) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012:940–948
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–139
de Luca C, Olefsky JM (2008) Inflammation and insulin resistance. FEBS Lett 582:97–105
Correa LH, Correa R, Farinasso CM, de Sant’Ana Dourado LP, Magalhaes KG (2017) Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: new implications in cancer progression. Front Immunol 8:1129
Roberts DL, Dive C, Renehan AG (2010) Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 61:301–316
Trayhurn P, Wood IS (2004) Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355
Vona-Davis L, Rose DP (2013) The obesity-inflammation-eicosanoid axis in breast cancer. J Mammary Gland Biol Neoplasia 18:291–307
Ben-Neriah Y, Karin M (2011) Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 12:715–723
Honma S, Shimodaira K, Shimizu Y, Tsuchiya N, Saito H, Yanaihara T, Okai T (2002) The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr J 49:371–377
Norling LV, Serhan CN (2010) Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J Intern Med 268:15–24
Gilroy DW, Lawrence T, Perretti M, Rossi AG (2004) Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 3:401–416
Rueter K, Haynes A, Prescott SL (2015) Developing primary intervention strategies to prevent allergic disease. Curr Allergy Asthma Rep 15:40
Khadge S, Sharp JG, Thiele GM, McGuire TR, Klassen LW, Duryee MJ, Britton HC, Dafferner AJ, Beck J, Black PN, DiRusso CC, Talmadge J (2018) Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J Nutr Biochem 52:92–102
Jackson JD, Yan Y, Brunda MJ, Kelsey LS, Talmadge JE (1995) Interleukin-12 enhances peripheral hematopoiesis in vivo. Blood 85:2371–2376
Rose DP, Connolly JM, Rayburn J, Coleman M (1995) Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst 87:587–592
Khadge S, Thiele GM, Sharp JG, McGuire TR, Klassen LW, Black PN, DiRusso CC, Cook L, Talmadge JE (2018) Long-chain omega-3 polyunsaturated fatty acids decrease mammary tumor growth, multiorgan metastasis and enhance survival. Clin Exp Metastasis 35:797–818
Rose DP, Connolly JM, Coleman M (1996) Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res 2:1751–1756
Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N (2010) Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun 402:602–607
Gonzalez MJ, Schemmel RA, Gray JI, Dugan L Jr, Sheffield LG, Welsch CW (1991) Effect of dietary fat on growth of MCF-7 and MDA-MB231 human breast carcinomas in athymic nude mice: relationship between carcinoma growth and lipid peroxidation product levels. Carcinogenesis 12:1231–1235
Talmadge J (2007) Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res 13:5243–5248
Hardman WE (2007) Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr Cancer 57:177–183
Sleeman JP (2018) Dietary regulation of metastasis. Clin Exp Metastasis 35:713–714
Manna S, Janarthan M, Ghosh B, Rana B, Rana A, Chatterjee M (2010) Fish oil regulates cell proliferation, protect DNA damages and decrease HER-2/neu and c-Myc protein expression in rat mammary carcinogenesis. Clin Nutr 29:531–537
Noguchi M, Minami M, Yagasaki R, Kinoshita K, Earashi M, Kitagawa H, Taniya T, Miyazaki I (1997) Chemoprevention of DMBA-induced mammary carcinogenesis in rats by low-dose EPA and DHA. Br J Cancer 75:348–353
Manna S, Chakraborty T, Ghosh B, Chatterjee M, Panda A, Srivastava S, Rana A, Chatterjee M (2008) Dietary fish oil associated with increased apoptosis and modulated expression of Bax and Bcl-2 during 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis in rats. Prostaglandins Leukot Essent Fatty Acids 79:5–14
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Wei N, Wang B, Zhang QY, Mi MT, Zhu JD, Yu XP, Yuan JL, Chen K, Wang J, Chang H (2008) Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr Cancer 60:810–825
Jiang W, Zhu Z, McGinley JN, El Bayoumy K, Manni A, Thompson HJ (2012) Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res 72:3795–3806
Xue M, Wang Q, Zhao J, Dong L, Ge Y, Hou L, Liu Y, Zheng Z (2014) Docosahexaenoic acid inhibited the Wnt/beta-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J Nutr Biochem 25:104–110
Akinsete JA, Ion G, Witte TR, Hardman WE (2012) Consumption of high omega-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice. Carcinogenesis 33:140–148
Arber N, DuBois RN (1999) Nonsteroidal anti-inflammatory drugs and prevention of colorectal cancer. Curr Gastroenterol Rep 1:441–448
Hudis CA, Subbaramaiah K, Morris PG, Dannenberg AJ (2012) Breast cancer risk reduction: no pain, no gain? J Clin Oncol 30:3436–3438
Restivo A, Cocco IM, Casula G, Scintu F, Cabras F, Scartozzi M, Zorcolo L (2015) Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer 113:1133–1139
Fontaine E, McShane J, Page R, Shackcloth M, Mediratta N, Carr M, Soorae A, Poullis M (2010) Aspirin and non-small cell lung cancer resections: effect on long-term survival. Eur J Cardiothorac Surg 38:21–26
Umar A, Steele VE, Menter DG, Hawk ET (2016) Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin Oncol 43:65–77
Salinas CA, Kwon EM, FitzGerald LM, Feng Z, Nelson PS, Ostrander EA, Peters U, Stanford JL (2010) Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol 172:578–590
Bardia A, Ebbert JO, Vierkant RA, Limburg PJ, Anderson K, Wang AH, Olson JE, Vachon CM, Cerhan JR (2007) Association of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs with cancer incidence and mortality. J Natl Cancer Inst 99:881–889
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376:1741–1750
Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235
Kalgutkar AS, Crews BC, Rowlinson SW, Garner C, Seibert K, Marnett LJ (1998) Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science 280:1268–1270
Claria J, Serhan CN (1995) Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A 92:9475–9479
Claria J, Lee MH, Serhan CN (1996) Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med 2:583–596
Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN (2013) Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol 20:188–201
Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101
Serhan CN, Levy BD (2018) Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 128:2657–2669
Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196:1025–1037
Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, Gilroy DW (2010) Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A 107:8842–8847
Simoes RL, De-Brito NM, Cunha-Costa H, Morandi V, Fierro IM, Roitt IM, Barja-Fidalgo C (2017) Lipoxin A4 selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int J Cancer 140:346–357
Hu S, Mao-Ying QL, Wang J, Wang ZF, Mi WL, Wang XW, Jiang JW, Huang YL, Wu GC, Wang YQ (2012) Lipoxins and aspirin-triggered lipoxin alleviate bone cancer pain in association with suppressing expression of spinal proinflammatory cytokines. J Neuroinflammation 9:278
Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, Lehner KA, Bielenberg DR, Schmidt B, Dalli J, Greene ER, Gus-Brautbar Y, Piwowarski J, Mammoto T, Zurakowski D, Perretti M, Sukhatme VP, Kaipainen A, Kieran MW, Huang S, Panigrahy D (2018) Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med 215:115–140
Gilligan MM, Gartung A, Sulciner ML, Norris PC, Sukhatme VP, Bielenberg DR, Huang S, Kieran MW, Serhan CN, Panigrahy D (2019) Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc Natl Acad Sci U S A 116:6292–6297
Liotta LA, Kohn EC (2001) The microenvironment of the tumour-host interface. Nature 411:375–379
Li H, Fan X, Houghton J (2007) Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 101:805–815
Jass JR (1986) Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol 39:585–589
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S (2004) Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer 91:1711–1717
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ (2009) Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 137:1270–1279
Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, Tassone P, Francini G, Tagliaferri P (2010) Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 33:435–441
Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB (2010) Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol 11:19
Chew A, Salama P, Robbshaw A, Klopcic B, Zeps N, Platell C, Lawrance IC (2011) SPARC, FOXP3, CD8 and CD45 correlation with disease recurrence and long-term disease-free survival in colorectal cancer. PLoS One 6:e22047
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 102:18538–18543
Tomsova M, Melichar B, Sedlakova I, Steiner I (2008) Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 108:415–420
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO (2012) CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 14:R48
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C (2014) Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 110:1595–1605
Murff HJ, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, Milne GL, Ness RM, Zheng W (2012) Dietary intake of PUFAs and colorectal polyp risk. Am J Clin Nutr 95:703–712
Habermann N, Ulrich CM, Lundgreen A, Makar KW, Poole EM, Caan B, Kulmacz R, Whitton J, Galbraith R, Potter JD, Slattery ML (2013) PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: interaction with fatty acids in colon cancer and rectal cancer. Genes Nutr 8:115–126
Van Blarigan EL, Fuchs CS, Niedzwiecki D, Ye X, Zhang S, Song M, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Atienza D, Messino M, Kindler H, Venook A, Ogino S, Giovannucci EL, Meyerhardt JA (2018) Marine omega-3 polyunsaturated fatty acid and fish intake after colon cancer diagnosis and survival: CALGB 89803 (Alliance). Cancer Epidemiol Biomark Prev 27:438–445
Mazurak VC (2016) n-3 polyunsaturated fatty acid supplementation during cancer chemotherapy. J Nutr Intermed Metab 5:107–116
Song M, Zhang X, Meyerhardt JA, Giovannucci EL, Ogino S, Fuchs CS, Chan AT (2017) Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 66:1790–1796
Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A, Loadman PM, Toogood GJ, Hull MA (2014) Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 63:1760–1768
Schloss I, Kidd MS, Tichelaar HY, Young GO, O’Keefe SJ (1997) Dietary factors associated with a low risk of colon cancer in coloured west coast fishermen. S Afr Med J 87:152–158
Rose DP, Connolly JM (1999) Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther 83:217–244
Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE (2012) The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 33:159–171
Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, Group VR (2019) Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 380:23–32
Song M, Nishihara R, Wu K, Qian ZR, Kim SA, Sukawa Y, Mima K, Inamura K, Masuda A, Yang J, Fuchs CS, Giovannucci EL, Ogino S, Chan AT (2015) Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst 107
Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138:2073–87.e3
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5:43–51
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520
Song M, Ou FS, Zemla TJ, Hull MA, Shi Q, Limburg PJ, Alberts SR, Sinicrope FA, Giovannucci EL, Van Blarigan EL, Meyerhardt JA, Chan AT (2019) Marine omega-3 fatty acid intake and survival of stage III colon cancer according to tumor molecular markers in NCCTG Phase III trial N0147 (Alliance). Int J Cancer 145:380–389
Angell H, Galon J (2013) From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 25:261–267
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12:298–306
Galon J, Angell HK, Bedognetti D, Marincola FM (2013) The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39:11–26
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D’Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pages F (2014) Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 232:199–209
Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O’Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D’Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA (2012) Cancer classification using the Immunoscore: a worldwide task force. J Transl Med 10:205
Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA (2012) The immune score as a new possible approach for the classification of cancer. J Transl Med 10:1
Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J (2011) Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 29:610–618
Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J (2009) In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 27:5944–5951
Zanoaga O, Jurj A, Raduly L, Cojocneanu-Petric R, Fuentes-Mattei E, Wu O, Braicu C, Gherman CD, Berindan-Neagoe I (2018) Implications of dietary omega-3 and omega-6 polyunsaturated fatty acids in breast cancer. Exp Ther Med 15:1167–1176
Chagas TR, Borges DS, de Oliveira PF, Mocellin MC, Barbosa AM, Camargo CQ, Del Moral JAG, Poli A, Calder PC, Trindade E, Nunes EA (2017) Oral fish oil positively influences nutritional-inflammatory risk in patients with haematological malignancies during chemotherapy with an impact on long-term survival: a randomised clinical trial. J Hum Nutr Diet 30:681–692
do Carmo LS, Rogero MM, Paredes-Gamero EJ, Nogueira-Pedro A, Xavier JG, Cortez M, Borges MC, Fujii TM, Borelli P, Fock RA (2013) A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp Biol Med (Maywood) 238:375–384
Rosales C (2018) Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol 9:113
Berg JW (1975) Can nutrition explain the pattern of international epidemiology of hormone-dependent cancers? Cancer Res 35:3345–3350
Tominaga S (1985) Cancer incidence in Japanese in Japan, Hawaii, and western United States. Natl Cancer Inst Monogr 69:83–92
Goodstine SL, Zheng T, Holford TR, Ward BA, Carter D, Owens PH, Mayne ST (2003) Dietary (n-3)/(n-6) fatty acid ratio: possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women. J Nutr 133:1409–1414
Calder PC (1997) N-3 polyunsaturated fatty acids and immune cell function. Adv Enzym Regul 37:197–237
Simonsen N, van’t Veer P, Strain JJ, Martin-Moreno JM, Huttunen JK, Navajas JF, Martin BC, Thamm M, Kardinaal AF, Kok FJ, Kohlmeier L (1998) Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. Am J Epidemiol 147:342–352
Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Benichou J, Clavel-Chapelon F (2009) Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer 124:924–931
Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES (2009) Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: a case-control study. BMC Cancer 9:216
Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, Ahn J, Chen Y, Ahsan H, Terry MB, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD (2015) Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: a population-based follow-up study on Long Island, New York. Cancer 121:2244–2252
Bagga D, Anders KH, Wang HJ, Glaspy JA (2002) Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer 42:180–185
Yang B, Ren XL, Fu YQ, Gao JL, Li D (2014) Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer 14:105
Im DS (2016) Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol 785:36–43
Bjursell M, Xu X, Admyre T, Bottcher G, Lundin S, Nilsson R, Stone VM, Morgan NG, Lam YY, Storlien LH, Linden D, Smith DM, Bohlooly YM, Oscarsson J (2014) The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS One 9:e114942
Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ, Sears DD, Olefsky JM, Ellies LG (2015) Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene 34:3504–3513
Calder PC (1851) Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015:469–484
Xia S, Li XP, Cheng L, Han MT, Zhang MM, Shao QX, Xu HX, Qi L (2015) Fish oil-rich diet promotes hematopoiesis and alters hematopoietic niche. Endocrinology 156:2821–2830
Schumann T, Adhikary T, Wortmann A, Finkernagel F, Lieber S, Schnitzer E, Legrand N, Schober Y, Nockher WA, Toth PM, Diederich WE, Nist A, Stiewe T, Wagner U, Reinartz S, Muller-Brusselbach S, Muller R (2015) Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget 6:13416–13433
Wensing AG, Mensink RP, Hornstra G (1999) Effects of dietary n-3 polyunsaturated fatty acids from plant and marine origin on platelet aggregation in healthy elderly subjects. Br J Nutr 82:183–191
Gross BW, Gillio M, Rinehart CD, Lynch CA, Rogers FB (2017) Omega-3 fatty acid supplementation and warfarin: a lethal combination in traumatic brain injury. J Trauma Nurs 24:15–18
Buckley MS, Goff AD, Knapp WE (2004) Fish oil interaction with warfarin. Ann Pharmacother 38:50–52
Jalili M, Dehpour AR (2007) Extremely prolonged INR associated with warfarin in combination with both trazodone and omega-3 fatty acids. Arch Med Res 38:901–904
McClaskey EM, Michalets EL (2007) Subdural hematoma after a fall in an elderly patient taking high-dose omega-3 fatty acids with warfarin and aspirin: case report and review of the literature. Pharmacotherapy 27:152–160
Stanger MJ, Thompson LA, Young AJ, Lieberman HR (2012) Anticoagulant activity of select dietary supplements. Nutr Rev 70:107–117
Harris WS, Silveira S, Dujovne CA (1990) The combined effects of N-3 fatty acids and aspirin on hemostatic parameters in man. Thromb Res 57:517–526
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD (2010) Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121:586–613
Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11:90–94
Edwards IJ, O’Flaherty JT (2008) Omega-3 fatty acids and PPARgamma in cancer. PPAR Res 2008:358052
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khadge, S., Sharp, J.G., Thiele, G.M., McGuire, T.R., Talmadge, J.E. (2020). Fatty Acid Mediators in the Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1259. Springer, Cham. https://doi.org/10.1007/978-3-030-43093-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-43093-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43092-4
Online ISBN: 978-3-030-43093-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)