Abstract
The main objective of this work is to develop a static and spatial model of the human knee, based on mechanism theory, to provide orthopedic surgeons information that relates forces at the anterior cruciate ligament graft (ACL) with its fixing position. This fixing position must be defined at the preoperative planning phase of the ligament replacement surgery. The best position for the graft insertion is taken as the one where the force developed at the graft is similar to the forces seen in an intact ligament during the knee flexion movement. The methodology for the static model is based on reimplementing a pure kinematic knee model available in the literature. In particular, this kinematic model is redefined using Davies’ method to obtain a static model that yields the forces at ligaments and condyles. The current kinematic model is able to satisfactorily reproduce the passive movement of the knee. We believe that any theoretical improvement in modeling and simulation of the forces at ligaments and grafts is an important contribution to the preoperative planning and improve the medical decision making capacity.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
When anterior cruciate ligament (ACL) surgery is indicated, preoperative planning is a critical step in defining the procedure’s parameters. During this planning phase, surgeons must define the insertion position for the replacement graft that best matches the functionality of an intact ACL. Particularly complex cases arise when the ACL graft cannot be positioned in its natural insertion area, which may occur when the area is too small for a surgical procedure. In such cases, adjacent areas are chosen. However, if such insertion area is not satisfactorily chosen, the knee’s natural kinetostatics may be harmfully impacted.
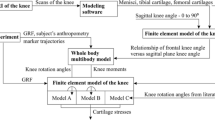
The objective of this work is to devise a spatial static model of the knee, based on mechanism design theory, on Davies’ Method [1, 2] and on a kinematic knee model previously proposed by [3, 4]. Our current model presents a tridimensional analysis of the forces arising in the knee’s anatomical elements, improving upon previously presented bidimensional static models [5,6,7] and also upon purely kinematic spatial models. The implementation of this methodology targets personalized knee models, in order to provide information for preoperative planning for ACL replacement procedures. In Part A of this work, the proposed method is presented, along with its validation and results. In Part B, a clinic application of the method is presented through simulation and validation of a case study.
2 Proposed Method
Knee models based on mechanism design theory focus on position kinematics. The forces in the model are not provided by these approaches, requiring additional analyses to be obtained. This is of special relevance due to the complexity of the problem, since the functionality of the ligaments vary depending on the knee’s flexion angle. Although dynamic models with more complex anatomical representations exist [8, 11,12,13], they are computationally demanding [3, 4], lose restriction functions of anatomical structures and produce results that are difficult to interpret by surgeons and prosthetists.
The kinetostatic modeling proposed in this work (Fig. 1b) aims at improving the kinematic model presented by [3, 4] through an additional static implementation of Davies’ method [1, 2]. Davies’ method provides an unique and systematized approach to the static analysis of the knee’s mechanical model, offering a solution for the calculation of the forces involved in each position. This enables an analysis with low computational demands of the function performed by each anatomical structure.
Particularly, the proposed static modeling (Fig. 1b) enables the analysis of the in situ force of the ACL (or graft) as a function of an external force applied on the knee. This analysis can provide valuable data for preoperative planning of ligament reconstruction. The method encompasses modeling and simulation of the experimental procedure implemented by [9] (Fig. 1a) to evaluate the in situ force on the ACL. The in situ force is the force acting upon the ligament (or graft) as the result of a load applied to the knee. The experimental in situ force serves as validation for the proposed model.
The experimental procedure consists in the application – with the aid of a robotic system – of a force \(F_x = 110\,\)N directed along the anterior tibial direction and a moment \(M_z\) around the lateral medial z axis, so that the articular structures are purely subject to the force \(F_x\). The force \(F_x\) and the moment \(M_z\), applied on the tibia, are transmitted through ligaments and condyles to the femur in the form of a reaction force \(F_{xr}\) and a reaction torque \(M_{zr}\), drawn in blue (Fig. 1a). These loads were applied to reproduce the drawer test (useful for clinical evaluation of the ACL), subjecting the ACL to tension.
(a) Experimental procedure [9], (b) Static modeling of the experimental procedure.
The proposed static modeling consists of five main steps: (a) Kinematic modeling and identification of the successive positions of anatomical elements, (b) topological characterization of the mechanism, (c) static characterization of the mechanism’s couplings, (d) formulation and solution of the static equations system and (e) results and validation.
2.1 Step a: Kinematic Modeling and Identification of the Successive Positions of Anatomical Elements
The kinematic model of the knee as proposed by [3, 4] is presented in Fig. 2a. This model, composed by two rigid links connected via spherical joints, has been capable of satisfactorily reproducing the knee’s passive motion. As Fig. 2a presents a schematic (topological) drawing of the model, an equivalent model with a more anatomically representative geometry is adopted in this work – as shown in Fig. 2b. Figure 2c represents the human knee’s anatomy, where PCL = posterior cruciate ligament, MCL = medial collateral ligament, and LCL = lateral collateral ligament.
The kinematic model has 1 spatial degree of freedom (DOF), that is, for each imposed flexion angle, the position and orientation of the upper platform in respect to the lower one can be unequivocally determined. In this model, the tibia is represented by the lower platform, with its anatomical center located at \(S_{t}\). The femur is in turn represented by the upper platform, with the anatomical center at \(S_{f}\). The identification of the ligaments and condyles adopted in Fig. 2a and b are detailed as follows:
-
\(A_{1}\), \(A_{2}\) and \(A_{3}\): spatial positions of the ACL, PCL and MCL tibial insertion points, respectively.
-
\(B_{1}\), \(B_{2}\) and \(B_{3}\): spatial positions of the ACL, PCL and MCL femoral insertion points, respectively.
-
\(A_{4}\) and \(A_{5}\): spatial positions of the medial and lateral tibial condyles’ centroids, respectively.
-
\(B_{4}\) and \(A_{5}\): spatial positions of the medial and lateral femoral condyles’ centroids, respectively.
-
L1, L2 and L3: ACL, PCL and MCL lengths, respectively.
-
\(L_{4}\) and \(L_{5}\): distances between the medial and lateral condyles’ centroids, respectively.
Spherical joints were modeled on coordinates \(A_{i}\) and \(B_{i}\), (\(i=1,\ldots ,5\)), while \(L_{i}\), (\(i=1,\ldots ,5\)) were modeled as rigid links. The positions \(A_{i}\) and \(B_{i}\) and the lengths \(L_{i}\) are known as geometric parameters, or GP.
Motion data from \(S_{f}\) on \(S_{t}\), the lengths \(L_{i}\) and the initial positions for \(A_{i}\) and \(B_{i}\) (when the flexion angle \(\alpha = 0^{\circ }\)) are obtained from [3, 4]. In this current work, the motion of each anatomical element \(A_{i}\) and \(B_{i}\), during the displacement from \(S_{f}\) on \(S_{t}\), was obtained via inverse kinematics.
Since this modeling process employs the Davies’ method, from now on the GP \(A_{i}\) and \(B_{i}\) will be represented by an appropriate notation. Thus: \(A_{i}\) = \(\mathbf {S}_{0A_{i}}\) and \(B_{i}\) = \(\mathbf {S}_{0B_{i}}\), (\(i=1,\ldots ,5\)).
Regarding the experimental procedure (Fig. 1a), the knee’s motion occurs with a fixed femur. Therefore, the kinematics inversion presented in (Eq. 1) – formulated in terms of screw theory – is adopted, allowing one to obtain \(\mathbf {S}_{0A_{i}}\) on \(S_{f}\),
where:
-
\(^{f}\mathbf {S}_{0A_{i}}\) is the point \(\mathbf {S}_{0A_{i}}\) measured in relation to \(S_{f}\);
-
\(^{B}R_{A}=[^{A}R_{B}]^{-1}\) is the matrix describing the rotation of \(S_{t}\) in relation to \(S_{f}\);
-
p is the vector describing the position (x, y, z) of \(S_{t}\) in relation to \(S_{f}\).
Similarly, the positions \(\mathbf {S}_{0B_{i}}\) are fixed and measured in relation to \(S_{f}\) (Eq. 2), and therefore \(\mathbf {S}_{0B_{i}}\) can be directly obtained from the GP in [3].
2.2 Step b: Topological Characterization of the Mechanism
In this step, the mechanism’s coupling network and coupling graph are established [2].
The coupling network is a representation of the proposed mechanical model’s topology. In the coupling network, the mechanism’s couplings are represented by vertices labeled by letters \(A_{i}\) and \(B_{i}\), \((i=1,\ldots ,5)\). Each body in the coupling network represents a link in the mechanism, and is labeled by a number.
In the coupling graph, each body in the coupling network is represented by a node and each coupling is represented by a edge, as shown in Fig. 3b, where the edges of \(G_{C}\) are oriented from smaller to larger nodes.
In order to determine the cuts in the \(G_{C}\) graph, it must be considered that each one of the \(k=6\) cuts must split the \(G_{C}\) graph into 2 different graphs [2]. Each k-th cut is thus represented with a dashed red line in (Fig. 3a).
2.3 Step c: Static Characterization of the Mechanism’s Couplings
In this step the external loads must be determined, modeled and internalized, gathering all coupling’s characteristics required in the formation of the wrenches and in the construction of the action graph \(G_{A}\). This step thus comprises 4 sub-steps, detailed as follows:
Sub-step c.1: Modeling the load conditions. In this step, the external loads applied on the knee are modeled based upon the experimental procedure performed in [9] – shown in (Fig. 1a). For such modeling, the cut-set law defined by [1, 2] is employed. The experimental procedure consists in exerting a force \(F_x = 110\,\)N on the knee specimen – with the aid of a robotic system – directed along the anterior tibial direction, and a moment \(M_z\) around the lateral medial z axis. The force \(F_x\) and the moment \(M_z\) are applied on the tibia, while the femur remains fixed. The moment \(M_z\) locks the flexion motion imposed by the force \(F_x\). The force \(F_{x}\) and the moment \(M_{z}\), applied on the tibia, are transmitted through ligaments and condyles to the femur in the form of a reaction force \(F_{xr}\) and a reaction torque \(M_{zr}\), drawn in blue in (Fig. 1a). These loads are applied for various flexion angles (\(0^\circ \), \(15^\circ \), \(30^\circ \), \(60^\circ \) and \(90^\circ \)), also via a robotic system. The robot’s Universal Force Sensor (UFS), attached to the tibia, provides an indirect measure of the in situ forces of the ACL through the inverse Jacobian [10], defining a vector of forces and moments for the anatomical coordinate system of the femur, labeled \(^{Anat}F\) (Eq. 3).
Sub-step c.2: Internalization of external loads. Here, the mechanism must be restricted in order to prevent any motion [1, 2]. The reactions – or active actions – \(F_{xr}\) and \(M_{zr}\), must be internalized in the couplings as passive actions \(R_{Aix}\), \(R_{Aiy}\), \(R_{Aiz}\), \(R_{Bix}\), \(R_{Biy}\) e \(R_{Biz}\), \((i=1,\ldots ,5)\) (Fig. 4a).
In order to form the wrenches, three unit direction vectors \(\mathbf {S}\), subscripted x, y and z, are attached to each spherical pair \(A_{i}\) and \(B_{i}\) of the mechanism (Fig. 4a).
Sub-step c.3: Representation of the action graph \(G_A\) . The action graph \(G_A\) is created [2] (Fig. 4b), in which: each vertex represents a link in the mechanism; 30 dark edges represent the passive actions, 2 red edges represent the active actions \(F_{x}\) and \(M_{z}\), and \(k=6\) cuts from the coupling network (Fig. 3b) are substituted as red dashed lines. The number of variables C in the system corresponds to the total amount of edges in the action graph \(G_A\) (C = 32).
Sub-step c.4: \(\$\) wrench construction. The results of the passive actions and of \(F_x\) are pure force states, therefore, its wrenches are (Eq. 5):
Furthermore, \(M_z\) is a pure torque state (\(\mathbf {T}\)), equivalent to the wrench in (Eq. 6).
Considering (Eqs. 1, 2, 4, 5 and 6), the following wrenches are obtained for each active load and for each coupling in the proposed static model:
where \(^{f}\mathbf {S}_{0Fx}\) represents the point where the force \(F_x\) is applied on the tibia, measured in relation to \(S_f\).
2.4 Step d: Formulation and Solution of the Static Equations System
For a restricted chain with internalized actions, with k cuts in the \(\lambda \) space, one can write \(\lambda k\) equations, expressing conditions that must be satisfied by C variables. In the following, the steps required to formulate the static equations system defined by Davies’ cut-set law are carried out. The solution to the system yields the forces actuating on each link of the proposed static model.
Sub-step d.1: Defining the unitary actions matrix. Davies’ cut-set law [2] defines that the algebraic sum of the \(\$\) wrenches belonging to a same cut is zero. To apply this law, one must first construct the Unit Network Action Matrix \(\hat{[A_{N}]}_{\lambda .k\times {C}}\). The rows in this matrix correspond to the \(\$\) wrenches (edges in \(G_A\)) intercepted by the k cuts, while the columns respectively represent the normalized wrenches \(\hat{\$}_{A_{1}}\), \(\hat{\$}_{A_{2}}\), \(\hat{\$}_{A_{3}}\), \(\hat{\$}_{A_{4}}\), \(\hat{\$}_{A_{5}}\), \(\hat{\$}_{B_{1}}\), \(\hat{\$}_{B_{2}}\), \(\hat{\$}_{B_{3}}\), \(\hat{\$}_{B_{4}}\), \(\hat{\$}_{B_{5}}\), \(\hat{\$}_{M_{z}}\), \(\hat{\$}_{F_{X}}\), where it must be considered that: \(\$\hat{A}_{i} = [ \$ \hat{A}_{ix}; \$\hat{A}_{iy}, \$\hat{A}_{iz}]_{6\times 3}\); \(\$\hat{B}_{i} = [ \$ \hat{B}_{ix}, \$\hat{B}_{iy}, \$\hat{B}_{iz}]_{6\times 3}\), (\(i=1,..,5\)); \(\$ M_{z}=[\$M_{z}]_{6\times 1}\); \(\$ F_{x}=[\$F_{x}]_{6\times 1}\). The vector representing the magnitudes of the wrenches is known as the magnitude action vector \(\left\{ \mathbf {\Psi }\right\} _{C\times {1}}\). To define this vector, it must be considered that: \(R_{Ai}=[R_{A_{ix}};R_{A_{iy}};R_{A_{iz}}]_{1 \times 3}^{T}\); \(R_{Bi}=[R_{B_{ix}};R_{B_{iy}};R_{B_{iz}}]_{1 \times 3}^{T}\), (\(i=1,..,5\)); \(M_{z}=[M_{z}]_{1 \times 1}\); \(F_{x}=[F_{x}]_{1 \times 1}\).
Sub-step d.2: Construction and solution of the static equations system. Applying the cut-set law [2] to the static model, the following system is obtained:
The consistency of the equation system depends on the rank a of the matrix \([\hat{A}_{N}]_{36\times {32}}\), where the rank a corresponds to the amount of linearly independent lines (that is, equations).
By reducing the matrix \([\hat{A}_{N}]_{36\times {32}}\) to its echelon form, the matrix \([\hat{A}_{N_{ESC}}]_{31\times {32}}\) is obtained, with a corresponding rank a = 31 of linearly independent lines. The cut-set law in the echelon form is presented in Eq. 9:
Since \(C=32\) and a = 31, the number of independent variables is \(C_N=1\), and therefore 1 variable must be imposed in order to enable the computation of a solution for the system. Independent variables are labeled with the sub-index P, and dependent variables are sub-indexed with S:
The last step is isolating the vector of unknowns \(\left\{ \varPsi _{S}\right\} _{31\times 1}=[R_{A_{1}} \; R_{A_{2}} \; R_{A_{3}} \; R_{A_{4}} \; R_{A_{5}} \; R_{B_{1}} \; R_{B_{2}} \; R_{B_{3}} \; R_{B_{4}} \; R_{B_{5}}\; M_{z}]^{T}\), which yields the static solution
By attributing a value to the primary static variable \(\mathbf {\Psi }_{P}=\mathbf {F}_{x}\), it is possible to obtain the static solution \(\left\{ \mathbf {\Psi }_{S}\right\} _{31\times 1}\) for Eq. 11.
3 Results and Validation
Results: The static solution \(\left\{ \varPsi _{S}\right\} _{31\times 1}=[R_{A_{1}} \; R_{A_{2}} \; R_{A_{3}} \; R_{A_{4}} \; R_{A_{5}} \; R_{B_{1}} \; R_{B_{2}}\; R_{B_{3}} \; R_{B_{4}} \; R_{B_{5}}\; M_{z}]^{T}\) is obtained by imposing \(\mathbf {\Psi }_{P}=\mathbf {F}_{x}\) = 110 N (Eq. 11) over the entire flexion trajectory of the knee. The in situ forces on the ACL (\(F_{ACL}\)), PCL (\(F_{PCL}\)) and MCL (\(F_{MCL}\)) are computed via the norm of the actions occurring on the couplings \(A_{1}\), \(A_{2}\) and \(A_{3}\) respectively, and are represented in Fig. 5 as FLCA, FLCP and FLCM. The compression forces on the medial and lateral condyles (MC and LC, respectively), are calculated via the norm of the forces occurring in the couplings \(A_{4}\) and \(A_{5}\) respectively, and are represented in Fig. 5 as FCM and FCL.
Validation: In order to validate the results, the in situ forces on the ACL obtained through the static model are compared with the results obtained in the experimental procedure proposed by [9]. The comparison between both results is shown in Fig. 6. Experimental data are represented by a continuous black line, with vertical bars representing the standard deviation. Data obtained from the proposed static model are represented with a dotted blue line, which finds itself inside the standard deviation specified by the experimental data.
4 Conclusions
A kinetostatic modeling of the knee was presented, which enables the determination of the in situ forces of the ligaments and the compression forces on the condyles when subjected to an external load. This model emerges from the reimplementation of the statics on an existing kinematic model [3, 4], capable of adequately reproducing the knee’s passive motion. Considering the satisfactory validation of the obtained results, the present model may contribute in supporting preoperative planning for human knee operation procedures, as well as in ACL substitution procedures. Also, the implementation of the present model allowed to improve previously presented results, in which the two-dimensional static modeling [5,6,7] had limitations in some aspects. Based on the results, the proposed work stands as an evidence that modeling based upon mechanism theory, screw theory and Davies’ method enables simulations with results similar to physiological ones, and can be considered a validated tool for the modeling of biomechanical systems.
References
Davies, T.: Circuit actions attributable to active couplings. Mech. Mach. Theor. 30(7), 1001–1012 (1995)
Davies, T.: Freedom and constraint in coupling networks. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 220(7), 989–1010 (2006)
Parenti-Castelli, V., Sancisi, N.: Synthesis of spatial mechanisms to model human joints. In: 21st Century Kinematics, pp. 49–84. Springer, Heidelberg (2013)
Sancisi, N., Parenti-Castelli, V.: A 1 DoF parallel spherical wrist for the modelling of the knee passive motion. Mech. Mach. Theor. 45(3), 658–665 (2010)
Ponce, D., Martins, D., de Mello-Roesler, C.R., Teixeira-Pinto, O., Fancello, E.A.: Relevance of the hyperelastic behavior of cruciate ligaments in the modeling of the human knee joint in sagittal plane, Revista Facultad de Ingeniera Universidad de Antioquia, vol. 76, pp. 123–133 (2015)
Ponce, D., Martins, D., Roesler, C.R.M., Rosa, F., More, A.: Modeling of human knee joint in sagittal plane considering elastic behavior of cruciate ligaments. In: 22nd International Congress of Mechanical Engineering - COBEM 2013, Ribeirao Preto, Sao Paulo. Proceedings of the 22nd International Congress of Mechanical Engineering [S.l.:s.n.] (2013)
Ponce, D., de Mello-Roesler, C.R., Martins, D.: A human knee joint model based on screw theory and its relevance for preoperative planning. In: Mecanica Computacional. Computational Modeling in Bioengineering and Biomedical Systems (B), vol. XXXI, no. 24, pp. 3847–3871 (2013)
Olanlokun, K., Wills, D.: A spatial model of the knee for the preoperative planning of knee surgery. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 216(1), 63 (2002). Sage Publications
Woo, S., Fox, R., Sakane, M., Livesay, G., Rudy, T., Fu, F.: Biomechanics of the ACL: measurements of in situ force in the ACL and knee kinematics. The Knee 5(4), 267–288 (1998). Elsevier
Fujie, H., Livesay, G.A., Fujita, M., Woo, S.L.: Forces and moments in six-DOF at the human knee joint: mathematical description for control. J. Biomech. 29(12), 1577–1585 (1996). Elsevier
Andriacchi, T., Mikosz, R., Hampton, S., Galante, J.: Model studies of the stiffness characteristics of the human knee joint. J. Biomech. 16(1), 23–29 (1983). Elsevier
Abdel-Rahman, E., Hefzy, M.S.: A two-dimensional dynamic anatomical model of the human knee joint. J. Biomech. Eng. 115(4A), 357–365 (1993). American Society of Mechanical Engineers
Wismans, J., Veldpaus, F., Janssen, J., Huson, A., Struben, P.: A three-dimensional mathematical model of the knee-joint. J. Biomech. 13(8), 677–685 (1980). Elsevier
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Ponce, D., Mejia, L., Ponce, E., Martins, D., Roesler, C.R.M., Golin, J.F. (2018). Kinetostatic Model of the Human Knee for Preoperative Planning: Part A. In: Carvalho, J., Martins, D., Simoni, R., Simas, H. (eds) Multibody Mechatronic Systems. MuSMe 2017. Mechanisms and Machine Science, vol 54. Springer, Cham. https://doi.org/10.1007/978-3-319-67567-1_42
Download citation
DOI: https://doi.org/10.1007/978-3-319-67567-1_42
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67566-4
Online ISBN: 978-3-319-67567-1
eBook Packages: EngineeringEngineering (R0)