Abstract
Stroke is defined as the acute onset of focal neurological disturbance arising due to a cerebrovascular cause, confirmed histopathologically or on imaging, where other causes have been excluded. Strokes may either be ischaemic (approximately 80% of cases) or haemorrhagic (20%). Although often thought of as a single disease, stroke represents the end stage of many different pathologies, each of which can result in cerebral ischaemia and/or haemorrhage. Therefore when investigating a stroke patient, investigations are performed to identify the underlying cause. Most cases of ischaemic stroke are caused by one of three pathologies: large vessel atherosclerotic disease (LVD), cerebral small vessel disease (SVD) or cardioembolism, although there are multiple rarer causes including cervical artery dissection. However, even with detailed investigation an underlying cause cannot be found in approximately a quarter of all ischaemic strokes. Haemorrhagic strokes are categorized according to the brain region they arise from; lobar or cortical haemorrhages are commonly caused by cerebral amyloid angiopathy, or an underlying structural lesion for example an arteriovenous malformation. Subcortical haemorrhages are usually associated with hypertension and believed to be often a manifestation of SVD.
This chapter will briefly outline the genetic basis of strokes in general, and highlight key examples of familial forms of stroke.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
24.1 Introduction
Stroke is defined as the acute onset of focal neurological disturbance arising due to a cerebrovascular cause, confirmed histopathologically or on imaging, where other causes have been excluded [1]. Strokes may either be ischaemic (approximately 80% of cases) or haemorrhagic (20%) [2]. Although often thought of as a single disease, stroke represents the end stage of many different pathologies, each of which can result in cerebral ischaemia and/or haemorrhage. Therefore when investigating a stroke patient, investigations are performed to identify the underlying cause. Most cases of ischaemic stroke are caused by one of three pathologies: large vessel atherosclerotic disease (LVD), cerebral small vessel disease (SVD) or cardioembolism, although there are multiple rarer causes including cervical artery dissection [3]. However, even with detailed investigation an underlying cause cannot be found in approximately a quarter of all ischaemic strokes. Haemorrhagic strokes are categorized according to the brain region they arise from; lobar or cortical haemorrhages are commonly caused by cerebral amyloid angiopathy, or an underlying structural lesion for example an arteriovenous malformation. Subcortical haemorrhages are usually associated with hypertension and believed to be often a manifestation of SVD.
This chapter will briefly outline the genetic basis of strokes in general, and highlight key examples of familial forms of stroke.
24.2 Genetics and Genomics of ‘Sporadic’ Stroke
The majority of strokes are apparently ‘sporadic’ , but considerable evidence demonstrates that genetic risk factors, likely interacting with environmental risk factors, are important even in these cases. Evidence from animals models [4], and also from studies in man of twins and affected sibling-pairs [5, 6], and epidemiological data of familial history of stroke [7] suggest that stroke is heritable. More recently this has been supported by complex trait analysis studies from genome-wide association study (GWAS) data [8]. Heritability is higher for younger onset cases [9].
GWAS in ischaemic stroke have identified a number of risk variants [9,10,11,12,13]. A sticking finding has been the subtype specificity of most loci reported to date, demonstrating that different subtypes of ischaemic stroke have different genetic architecture. GWAS studies have also identified loci for intracerebral haemorrhage [14].
24.3 Genetics of Familial Stroke
Stroke less commonly presents as a key feature of monogenic syndromes. Most monogenic forms of stroke also cause a specific stroke subtype (see Table 24.1 for ischaemic stroke and Table 24.2 for intracerebral haemorrhage, ICH) .
The most common monogenic form of stroke is Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) , which results from mutations in the NOTCH3 gene, and most frequently presents with migraine with aura and/or lacunar strokes, and can progress to dementia. Recently a number of other monogenic forms of small vessel disease have been reported which result in one of more of lacunar stroke, migraine, cognitive impairment, and cerebral microbleeds and ICH [15] (Table 24.3). These are not only important for the individual patient, but are also providing important insights into the pathophysiological mechanisms underlying not only monogenic SVD [16] but also sporadic SVD [17].
24.4 Diagnosing a Monogenic Cause of Stroke
Monogenic forms of stroke can either be part of a systemic disease, which presents with clinical features affecting multiple organs, or can present primarily with stroke. When stroke is part of a systemic disease, examples of which include Fabry disease and sickle cell disease, the diagnosis is often already known. In contrast, for diseases which present with stroke as the main manifestation, diagnosis and identification of an underlying single gene disorder can be challenging.
Most familial causes of stroke present in young or middle age and the diagnosis should be considered in a patient presenting with stroke at under 60 years, particularly when they have a family history of stroke. However, the majority of young onset strokes will not have an underlying single gene disorder, while increasing numbers of cases of stroke presenting at an older age (for example in the seventh decade for CADASIL) are being reported. Results of specific investigations may also highlight a likely monogenic cause. For example, involvement of the anterior temporal pole on MRI in CADASIL has been shown to be a useful marker of the disease [38].
In all cases it is important that investigations are performed to accurately subtype the stroke. This includes brain imaging with CT or MRI to differentiate an ischemic stroke from a haemorrhagic stroke. Ischemic strokes then require further investigation with imaging of the extra- and intra- cerebral arteries (with CT or MR angiography or ultrasound), investigation of the heart with ECG and echocardiography, and blood tests for lipids and other circulating disease markers. Small lacunar infarcts caused by SVD are frequently not visible on CT brain imaging, and in these cases MRI is important not only to confirm the infarct but also to look for other manifestations of SVD such as white matter hyperintensities and cerebral microbleeds.
If the initial images show an ICH , rather than an infarct, a different series of investigations are required. These can include repeat brain imaging when the blood has resolved to look for an underlying lesion (such as an arteriovenous malformation or a neoplasm), angiography to look for an underlying aneurysm or arterial malformation, and MRI with gradient echo sequences to look for cerebral microbleeds. Cerebral microbleeds characteristically occur in the cortex and grey-white matter junction in amyloid angiopathy, and in the basal ganglia and subcortical structures in hypertensive haemorrhage due to SVD.
If a monogenic cause is suspected, and once the underlying stroke subtype has been determined, appropriate tests can be performed to diagnose monogenic conditions causing that particular stroke subtype. In some cases, this may include a haematological or biochemical test as in sickle cell disease and Fabry disease respectively, while in other cases, and particularly for SVD, genotyping is required. Traditionally this has been performed on a gene-by-gene basis using Sanger sequencing or similar techniques. However, with the increasing availability of next generation sequencing techniques this is increasingly being performed using sequencing arrays which screen multiple genes at the same time. This is particularly useful, for example, for SVD where multiple genes can cause a similar phenotype.
In the remainder of the chapter we present a number of examples of monogenic forms of stroke. We particularly focus on SVD for a number of reasons. Firstly, this includes CADASIL, which is the most common monogenic form of stroke. Secondly because of the recent advances in this area and the identification of multiple non-CADASIL forms of familial SVD. Thirdly because monogenic forms of SVD represent the majority of cases of suspected familial stroke without other systemic disease, and fourthly because they illustrate a number of important features of monogenic stroke including gene-environment interactions and challenges in diagnosis. However, we have also covered sickle cell disease as a non-SVD example of monogenic stroke both because it represents a major problem in some parts of the world, and because identification of the disease and appropriate treatment can reduce the risk of stroke.
24.5 Sickle Cell Disease
Sickle cell disease (SCD) is an autosomal recessive haemoglobinopathy caused by a homozygous glutamic acid-valine substitution in the 6th position of the β-globin chain of haemoglobin. In the resulting haemoglobin (HbS), there are two normal α chains and two mutant β chains. As glutamic acid is a polar amino acid, while valine is non-polar and insoluble, the potential bonds formed by the globin chains are altered, resulting in impaired solubility of the resulting haemoglobin. HbS polymerizes to form fibres known as tactoids, which lead to the distortion of the red cell, which is rigid and dehydrated, and carries a sickle-shaped appearance. The sickled cells are also more adherent to vascular endothelium, thus promoting vessel occlusion [39].
SCD is prevalent in individuals of Sub-Saharan Africans and African Caribbean ancestry, and is also present in the Mediterranean, Middle East and India. Its distribution matches that of the endemic plasmodium falciparum malaria, which exerts a selection pressure, with the sickle gene in heterozygous form conferring protection from malaria [40].
SCD is clinically complex with a high degree of phenotypic heterogeneity, with patients experiencing a range of systemic effects from infancy. One of the key features is vaso-occlusion , which accounts for many systemic complications. Distorted red cells occlude blood vessels and lead to infarction, presenting as painful crises in the bones or joints of hands and feet, acute chest syndrome, pulmonary hypertension, renal papillae damage and stroke [41].
24.5.1 Stroke in SCD
Strokes are a common cause of morbidity and mortality in SCD, affecting up to 3.75% of patients [42]. In affected regions SCD is one of the most common cause of paediatric strokes between the ages of 2–9 years, although they can occur at any age [43]. Strokes in SCD may be ischaemic or haemorrhagic, with ischaemic strokes having a bimodal distribution, peaking around the first and third decade [44], and haemorrhagic strokes being less common, arising at a later age, after the second decade of life [42].
24.5.2 Ischaemic Strokes in SCD
Many strokes in SCD to arise due to narrowing of the major cerebral vessels, primarily the internal carotid artery (ICA) and proximal section of the middle cerebral artery (MCA), resulting in impaired perfusion of territories distal to the stenosis. Sickling of erythrocytes and anaemia results in hyperplasia of the intima of large vessels, a feature seen in 80% of SCD patients with stroke [45].
Beyond an arteriopathy, other mechanisms contribute to strokes in SCD. SCD patients are often in a hypercoagulable state at baseline, with raised levels of markers of coagulation and fibrinolysis, and reduced Protein C and S concentrations [46, 47]. During pain crises, there is also activation of platelets, and sickled erythrocytes may express phosphatidylserine which promotes the activation of prothrombin [48]. Chronic haemolysis of sickled cells depletes circulating nitric oxide, which is essential for maintaining vasomotor tone and preventing platelet aggregation, contributing to ischaemia via vasoconstriction [49]. Chronic hypoxia may also create a state of chronic inflammation, with high circulating proinflammatory cytokines promoting the interaction of sickled cells with the endothelium [50].
Less common causes of stroke are in SCD are underlying cardioembolism and cardiopathies, systolic dysfunction and atrial fibrillation. These are estimated to account for 24% of strokes in adult SCD patients [46]. Adults with SCD also have a high prevalence of posterior circulation aneurysms, and cerebral venous sinus thrombosis is a common event in adults with SCD—both of which may predispose the patient to strokes [51].
24.5.3 Haemorrhagic Strokes in SCD
Large intracerebral artery occlusion may also result in the formation of compensatory collateral subcortical vessels, giving a ‘puff of smoke’ appearance described as Moya-moya syndrome [52]. These can rupture, leading to ICH and this is often occurs young adulthood rather than childhood [53]. Aneurysms and arteriovenous malformations are common in SCD, and may also predispose individuals to haemorrhagic strokes [54, 55].
24.5.4 Clinically Silent Strokes in SCD
Clinically silent infarcts , and white matter hyperintensities on MRI, are more common than clinically overt strokes in SCD, occurring in more than 22% of patients [56]. These lesions are associated with cognitive impairment, and are also a recognised risk factor for overt strokes [57].
24.5.5 Risk Factors for Stroke in SCD
A number of clinical biomarkers can serve to predict an individual’s risk of developing silent, ischaemic or haemorrhagic strokes in SCD. (Table 24.3 and 24.4) One important tool is the use of transcranial Doppler ultrasonography (TCD) in predicting the risk of stroke in paediatric SCD patients—currently the most accurate prognostic tool available. Raised ICA or MCA flow velocities on TCD serve as a marker of focal stenosis, and can identify those at highest risk of first stroke [58].
24.5.6 Genetic Risk Factors for Stroke in SCD
As with other clinical phenomena in SCD, the occurrence and severity of stroke between patients can vary widely, and this is likely due to a combination of genetic and environmental risk factors interacting with the sickle cell genotype. Early studies have demonstrated a familial predisposition to cerebral vasculopathy in families with more than one child with SCD, showing that siblings of children with stroke or increased TCD blood flow velocities have an increased risk of stroke [59, 60].
The α-thalassaemia polymorphism, and concentratio n of foetal haemoglobin (HbF) are well-established modulators of stroke in SCD [61]. HbF decreases the stroke risk by inhibiting HbS polymerization, and genes such as BCL11A, HBS1L-MYB, β-globin genes and quantitative trait loci which affect HbF levels may contribute to this effect [62].
A number of studies have suggested other genes may influence the phenotype. A study of 80 candidate genes involved in vasoregulation, coagulation and other disease-associated processes utilised a Bayesian network approach to identify associations between genes and stroke in 1398 patients with SCD (92 with stroke) [63]. This study identified 31 SNPs in 12 genes as being associated with ischaemic stroke, with interaction between these genes and HbF as a possible mediating mechanism. Several of these genes were involved in the transforming growth factor-beta (TGFβ) pathway, a finding which was partially replicated in a subsequent candidate SNP study [61].
The use of MRI to phenotype stroke subtypes in SCD has also contributed to the discovery of genetic risk factors. In a study of 230 MRI-phenotyped SCA children, 104 SNPs were studied in 65 candidate vascular genes, and demonstrated that SNPs in IL4R, TNFα, and ADRB2 genes were associated with increased risk of large vessel strokes, while VCAM1 and LDLR genes were associated with increased (VCAM1) or decreased (LDLR) small vessel stroke risk [64]. Another variant in VCAM1 was previously also identified as protective against high TCD flow velocity and thus stroke risk in SCD [65].
Other studies have suggested a potential role for the immune system in the development of stroke in patients with SCD with HLA DPB1 being associated with stroke risk [66].
Genome-wide approaches have been limited in the study of stroke in SCD, and to date have not validated findings in candidate gene or SNP studies [67]. The reader is also directed to a recent comprehensive review of the genetics of SCA-associated cardiovascular disease [68].
24.5.7 Management of Strokes in SCD
Although studies in the acute management of stroke in SCD are limited, there is no clear evidence against the use of thrombolysis in adults with SCD [46]. Supplemental oxygen in the acute setting can also help to maintain blood oxygen saturation at ≥95%, preventing further sickling and blood hyperviscosity [69]. Beyond standard stroke care, exchange blood transfusions are recognised as the standard of care for the primary and secondary prevention of strokes (excluding silent infarcts) [70].
Children with SCD who are at risk of stroke, as identified by high cerebral blood flow velocity on TCD, may have their absolute risk of first stroke being reduced by 9%, and relative risk lowered by 92%, by lowering the proportion of HbS to <30% with exchange transfusion [71]. Cessation of transfusion therapy may result in patients reverting to previous risk status [72]. In SCD children with a previous stroke, the risk of recurrent stroke is as high as 90%, and can be lowered to below 10% by regular exchange blood transfusion [70].
Reducing the proportion of HbS to can improve oxygen saturation through normal red blood cells, reducing further vaso-occlusion, improving tissue perfusion and preventing further ischaemic damage caused by the stroke [46]. A long-term exchange transfusion programme to reduce the proportion of HbS to <20% is thus recommended for children with either prior silent cerebral infarcts or raised TCD velocities [70]. Top-up blood transfusions are not recommended for the acute treatment of stroke as an increased blood viscosity may worsen stroke or painful crises [70].
24.6 Monogenic Forms of Small Vessel Disease
24.6.1 CADASIL
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is the most common monogenic cause of SVD. CADASIL is caused by cysteine-changing mutations in exons 2–24 of the NOTCH3 gene, which encode the extracellular portion of the Notch 3 protein, a transmembrane receptor [18]. CADASIL is estimated to affect 2–4 per 100,000 population in the UK [73, 74]. Disease-causing NOTCH3 mutations were found in 0.5% of 1000 apparently sporadic young-onset (≤70 years) MRI-defined SVD stroke patients, with this figure rising to 1.5% when considering only patients with confluent white matter hyperintensities on MRI [75].
The clinical features of CADASIL are exclusively neurological. Migraine, usually with aura, is most commonly the earliest feature of disease, with onset usually in the 1920s or 1930s. Subcortical ischaemic lacunar strokes may occur, with an average age of onset of 47 years, and progressive subcortical cognitive impairment can occur in middle age leading to vascular dementia [76]. Depression is common and may precede other symptoms. Less common presentations of CADASIL include an acute reversible encephalopathy or ‘coma’ episode, which is often misdiagnosed as an acute encephalitis [77].
24.6.1.1 Phenotypic Variation and the Importance of Gene-Gene and Gene-Environment Interactions
Disease severity can vary widely between individuals, both between and within families. Almost all of CADASIL mutations result in the loss or gain of a cysteine amino acid in one of the epidermal growth factor (EGF) repeats in the extra-cellular portion of the Notch 3 protein [78]. Studies have shown no relationship between phenotype and mutation sites [79]. Why the phenotype varies so much between individuals is not well understood but it is thought that both gene-gene and gene-environment interaction s are important. Family studies have shown a significant heritability for MRI determined white matter legion volume suggesting additional genes are important in determining phenotypic severity [80]. Conventional cardiovascular risk factors also seem to influence phenotypes. For example, CADASIL carriers who smoke on average develop stroke 10 years earlier [79]. Hypertension also seems to be important with the rate of progression of brain atrophy on MRI related to the level of blood pressure [81], and hypertension related to risk of stroke [77].
24.6.1.2 Diagnosis of CADASIL
CADASIL should be considered in all younger onset cases of lacunar stroke where there are white matter changes such as white matter hyperintensities on the MRI scan. There may be additional clues in the history including migraine with an aura which can be prolonged and confusional. There is usually a family history of clinical features of CADASIL but this is not always immediately clear. Vascular dementia in a relative can frequently be diagnosed as Alzheimer’s disease, while particularly in the past CADASIL has been misdiagnosed as multiple sclerosis, so one should always be aware of this diagnosis in the family history. Furthermore, because CADASIL can present in middle age there may be no family history if the parents died relatively young.
As with diagnosis of many forms of SVD, careful assessment of the MRI is crucial. In the case of CADASIL this can reveal features which are almost diagnostic. For example, involvement of the anterior temporal pole has shown to be 90% sensitive and 90% specific [38]. Other features on MRI include confluent involvement of the external capsule, and involvement of the corpus callosum (a structure which is not usually involved in the sporadic small vessel disease although it is frequently involved in multiple sclerosis) [82].
The next step is genetic testing to confirm the diagnosis. Mutations tend to cluster in certain exons particularly exon 4 [78] and this initially led to limited screening to reduce cost and exclude the majority of cases. However, this approach will miss a significant number of cases and many labs now screen all of exons 2–24 in which mutations can occur. Although CADASIL only produces clinical features in the brain, arteries throughout the body are affected by the pathological process. This has led to the use of skin biopsy to detect the characteristic granular osmiophilic material (GOM) which can be seen on Electron Microscopy. However, with the wider availability of genetic testing this is less frequently performed.
Appropriate genetic counselling should be given before genetic testing and we use the Huntingdon’s disease protocol. It’s important to remember that in an individual with a family history with CADASIL an MRI is essentially a genetic test. If it shows characteristic changes it indicates the individual is a carrier of the mutation.
24.6.1.3 Management of CADASIL
While there is no specific treatment available for CADASIL, aggressive control of conventional cardiovascular risk factors is essential. A study of 200 patients with CADASIL showed that those with poorly controlled hypertension, or those who had a history of smoking, had an increased risk of stroke [77]. We advise our patients not to smoke, to maintain optimal weight and to exercise regularly. We would recommend avoiding the combined oral contraceptive pill, certainly from age 30 upwards. If cholesterol is elevated we often treat with statin therapy although there is no evidence supporting this approach in CADASIL itself, as opposed to more generally in sporadic stroke prevention. We give aspirin or clopidogrel to patients who have suffered ischaemic stroke, and to carriers over the age of 40, but avoid dual antiplatelet therapy and anticoagulants due to a generally increased risk of ICH with these treatments in SVD [83].
Migraines in CADASIL tend to be more complicated than those seen in the general population. Patients with CADASIL are more likely to have atypical migraine auras such as dysphasia and confusion, and can have more prolonged auras [76, 77]. There have been few studies on the management of migraine specifically in CADASIL although drugs used for management of migraine in the general population appear to be similarly effective in CADASIL [84,85,86,87]. Although triptans carry a theoretical risk of exacerbating ischaemia in patients with vasculopathy [88], retrospective data from a group of 300 patients with CADASIL suggests that triptans are safe to use and helpful in treating migraines in CADASIL [76].
Depression is frequent in CADASIL, as it is in other forms of SVD. Contributing factors include the stress of a monogenic disease diagnosis, as well as of complications such as stroke. However, there is also a biological reason with white matter lesions thought to disrupt cortical-subcortical pathways involved in mood regulation. It is important to be aware of the diagnosis and treat it with cognitive therapy/counselling and anti-depressants as this can be associated with a markedly improved quality of life.
24.6.1.4 Clinical Case 1
A 54-year-old right-handed female presented with sudden onset of double vision and vomiting, and abnormal eye movements.
She had a history of migraine with aura from the age of 15, experiencing visual changes and numbness in her arm. She did not have any past medical history of depression, seizures, or encephalopathy. She was not hypertensive, and had only smoked briefly as a teenager.
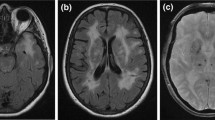
She had an MRI scan which showed an acute infarct in the midbrain with high signal on diffusion-weighted imaging (Fig. 24.1, 1-4), as well as extensive white matter changes with prominent involvement of the anterior temporal poles. (Fig. 24.1, 1-2, 1-3).
(inset 1-1). Pedigree of clinical case 1. Although II.1, II.2 and III.1 had neurological symptoms, there was no clear history of stroke or dementia in the family. (1-2) MR T2-weighted imaging showing white matter intensities involving the anterior temporal poles (arrowed) and (1-3) external capsules. (1-4) Midbrain lesion shown on MR diffusion-weighted imaging, and (1-5) apparent diffusion coefficient imaging (arrowheads). (Copyright Hugh Markus)
Her mother was alive at age 83, with a history of migraines and bipolar disease . Her father died at age 72, having had possible complex partial seizures. Her identical twin sister had a history of migraine with aura. Despite the absence of a clear family history of stroke or dementia, the classical involvement of the anterior temporal poles and possible family history led to suspicion of CADASIL (Fig. 24.1). Genetic testing confirmed a p.Arg90Cys mutation on exon 3 of the NOTCH3 gene.
24.6.1.5 Clinical Case 2
A 58-year-old female teacher presented with a confusional episode typical of CADASIL encephalopathy. While teaching she experienced the beginning of what she thought was a migraine with visual disturbance. She was aware of a colleague saying something but could not remember what happened next. She was found to be conscious but poorly responsive to commands.
She was taken to hospital where she suffered four generalised seizures and was treated for encephalitis with acyclovir and antibiotics, as well as anti-epileptics. She continued to experience fluctuating confusion associated with visual hallucinations for the next eight days before regaining full consciousness. She had a past medical history of migraine with visual and sensory aura from the age of 28.
Although there were no known strokes in the family, she had a family history of dementia, with her father being diagnosed of ‘Alzheimer’s disease’ at age 55 and dying at age 63. Her father’s identical twin had no strokes or dementia but had not had an MRI scan prior to death also at age 55, and his sister had a diagnosis of ‘probable dementia ’ (Fig. 24.2).
MR imaging of her brain showed confluent T2 hyperintensities in the white matter involving the anterior temporal poles and external capsules. (Fig. 24.2, 2-2, 2-3) A lumbar puncture performed at the time of her first admission was normal and negative for oligoclonal bands. Genetic screening for NOTCH3 mutations revealed a p.Arg151Cys mutation in Exon 4.
This patient’s prolonged confusion episode is classical of a CADASIL ‘coma’ or encephalopathic episode. This is a feature of CADASIL that often follows a typical migraine aura, and can last up to 14 days before resolving completely [89].
24.7 Recently Described Monogenic Forms of SVD Stroke
24.7.1 CARASIL and HTRA1-Related Autosomal Dominant SVD
Cerebral Autosomal Recessive Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CARASIL) is caused by homozygous mutations in the HTRA1 gene [19]. CARASIL was initially described in a few families in consanguineous Japanese and Chinese populations [19, 90], and subsequently in a consanguineous European family, and a patient with compound heterozygous HTRA1 mutations [91].
Patients with CARASIL have been described as having a more rapid progression of the neurological features seen in CADASIL. A distinguishing feature of CARASIL is the presence of non-neurological features such as young-onset alopecia and degenerative spinal disc disease. On imaging, these patients may have characteristic arc-shaped hyperintensities extending from the pons to the middle cerebellar peduncles [92]. CARASIL patients have not been found to have the classical imaging feature of anterior temporal pole involvement seen in CADASIL [93].
More recently, whole exome sequencing in 201 Caucasian patients with suspected familial SVD and no NOTCH3 mutations identified heterozygous missense HTRA1 mutations in 10 cases, and segregation of the mutation with disease was also demonstrated in one pedigree. These patients had a later age at onset of disease, and did not report any extra-neurological features seen in CARASIL [20]. A similar study in 113 suspected familial SVD patients with no known genetic cause in Japan also identified four heterozygous missense HTRA1 mutations in six cases [21]. An example of a patient of HTRA1-associated autosomal dominant SVD is described in clinical case 3.
The HTRA1 gene encodes for high temperature requirement serine protease A1 (HtrA1), a homotrimeric serine protease which switches off the TGFβ pathway. This role of this pathway in blood vessel formation and vasoreactivity, as well as vessel and organ fibrosis in disease has been well described [94]. The impact of disease-causing HTRA1 mutations are, however, poorly understood. While most mutations impair protease activity [20], others do not cause a loss-of-function but have been predicted to impact trimer formation and activation [21].
24.7.2 Clinical Case 3
A 45-year-old female presented with a one-year history of migrainous aura without headache. These were stereotyped episodes of left-sided sensory symptoms, characterised by a sensation of flowing water down the left side of her body. She felt that her left hand was clumsy, although she was still able to mobilise with some clumsiness. She denied any speech disturbance or associated headache. These symptoms would last a week, after which she recovered completely.
She had a past history of depression.
She underwent neuropsychological assessment and was found to have impaired attention, information processing skills and some executive function difficulties—features commonly seen in small vessel disease-related cognitive impairment. An MRI of her brain showed widespread hyperintensities in the periventricular white matter and central pons. (Fig. 24.3, 3-2, 3-3).
(inset 3-1) Pedigree of clinical case 3, showing a clear family history of early-onset strokes in II.1, II.2 and II.3. Insets 3-2 and 3-3 MR T2-weighted imaging showing confluent white matter hyperintensities not dissimilar to those seen in sporadic cerebral small vessel disease, or CADASIL. (Copyright Hugh Markus)
She had a strong family history of strokes. Her father suffered recurrent strokes from 58, and was also found to have cognitive impairment. Her paternal aunt and uncle both died of strokes in their early 1960s. (Fig. 24.3, 3-1) A screen of exons 2, 3, 4, 5, 6, 8, 11, 18, 19 and 22 of the NOTCH3 gene was performed and no cysteine-changing mutations were found. Whole genome sequencing was performed, and a screen of the HTRA1 gene showed a heterozygous c.854C>T (p.P285L) mutation. This mutation had previously been identified in patients with both autosomal dominant [21] and autosomal recessive (CARASIL) [95] forms of familial SVD. Decreased protease activity was also demonstrated in cellular assays of this mutation [21].
24.8 Haemorrhagic Strokes
24.8.1 COL4A1/A2-Related SVD: Subcortical Haemorrhages, Infarcts and Aneurysms
Mutations in the COL4A1/2 genes have been recently recognised a cause of lacunar stroke as well as subcortical ICH; i.e. they can cause both ischaemic and haemorrhagic stroke even within the same family. The COL4A1/A2 genes encode Type IV collagen α1 or α2 chains, which are the most abundant type of collagen in humans. COL4A1/A2-related SVD encompasses a broad spectrum of symptoms ranging from porencephaly in infants to adult-onset subcortical ischaemic and haemorrhagic strokes. These were previously described as specific paediatric syndromes, but have now been recognised as being attributable to mutations in the same genes [96, 97].
Patients with COL4A1/A2-related SVD can develop subcortical ICH, ischaemic lacunar infarcts, seizures, cognitive impairment and dementia. They may also have systemic involvement in the form of renal agenesis, nephropathy, visual loss and muscle cramps [98].
Type IV collagen is an integral component of basement membranes. As the most abundant form of collagen in the extracellular matrix, it lends tensile strength, helps to maintain vascular tone and also contributes to endothelial cell function.
The majority of pathogenic mutations in COL4A1 or COL4A2 are missense mutations which substitute a highly conserved glycine residue in the Gly-X-Y repeat region which aid the formation of tropocollagen. The resulting altered three dimensional confirmation of collagen impairs its ability to form heterotrimers in the vascular basement membrane, contributing to vessel wall fragility [99]. There may be some element of genotype-phenotype correlation, as mutations in the CB3[IV] fragment of COL4A1 have been found to be associated with Hereditary Angiopathy with Nephropathy, Aneurysms and muscle Cramps (HANAC) syndrome [100].
Recently common variants in the COL4A2 have been found to be risk factors for sporadic SVD [101].
24.8.2 Clinical Case 4
A 14-year-old boy presented with a subcortical ICH He was tested for and diagnosed with a p.Gly755Arg mutation in exon 30 of the COL4A1 gene. Following this diagnosis, other family members were tested and his 46-year-old mother was found to have same mutation.
Despite the proband’s early onset of disease, his mother was relatively asymptomatic and had not had any strokes. She had a history of migraines with aura (visual and/or sensory) from the age of 39. She had no history of depression.
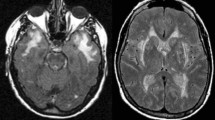
On MR imaging of the brain, she was found to have marked white matter hyperintensities and lacunar infarcts, (Fig. 24.4, 4-2, 4-3) and an aneurysm of the left internal carotid artery in the region of the carotid ophthalmic artery. There was also evidence of microbleeds on gradient echo MRI. (Fig. 24.4, 4-4, 4-5).
(inset 4-1). Pedigree of Clinical Case 4 illustrating a wide variability of phenotypes among mutation carriers. While the proband had a paediatric onset of strokes, II.2 had MRI features of SVD in the fourth decade of life, but no strokes, and II.3 was born without a kidney—a feature previously reported in COL4A1-associated SVD cases. (4-2, 4-3) T2-weighted FLAIR MR images of II.2 showing confluent white matter hyperintensities and silent lacunar infarcts. Insets 4-4 and 4-5: Gradient-echo MR images of II.2 showing haemosiderin deposits and microbleeds. (Copyright Hugh Markus)
Her mother died at the age of 73, having had ‘facial palsy’ at the age of 47, and an episode of self-resolving hemiparesis in her teenage years. Brain imaging showed that she had had a number of strokes and a diagnosis of multiple sclerosis was considered at one point. Her father was 74 and had only a history of cataracts. She had a brother who was born with only one kidney. (Fig. 24.4, 4-1).
24.9 Hereditary Cerebral Amyloid Angiopathy: Lobar or Cortical Haemorrhages
Cerebral amyloid angiopathy (CAA) refers to a small artery vasculopathy which involves the deposition of amyloid fibrils in the small and medium blood vessel walls, and also in the capillaries of the brain parenchyma and leptomeninges [32]. These depositions are altered proteins which have adopted a β-pleated sheet conformation. CAA is most classically characterised by large lobar haemorrhages, but can also cause transient behavioural changes, seizures and cognitive impairment. CAA is definitively diagnosed by brain biopsy or post-mortem histopathological analysis, but the likelihood of CAA can also be determined based on the clinical syndrome and imaging, as described by the modified Boston criteria [102].
CAA most often occurs sporadically in the elderly population, with the deposition of amyloid beta (Aβ) protein in the walls of blood vessels, in association with parenchymal Aβ plaques in Alzheimer’s disease [103]. In addition to lobar haemorrhages, CAA patients often have a distinctive distribution of classical SVD features, such as white matter hyperintensities with a predominant posterior distribution, and cerebral microbleeds in the lobar regions as visualised on gradient-echo MR imaging [104]. They may also have cortical superficial siderosis, which is a marker used for radiologically diagnosing CAA according to the modified Boston criteria [102].
CAA may also occur as a familial disease. Several large families worldwide have been identified as having a hereditary form of CAA, and affected individuals tend to have an earlier onset of symptoms than in sporadic CAA. The gene most commonly affected is the amyloid precursor protein (APP), which encodes the amyloid beta protein, and thus CAA arising due to APP mutations may also co-occur with familial Alzheimer’s disease.
24.10 Summary and Conclusions
Stroke represents a collection of different aetiologies which lead to a similar clinical syndrome. Most strokes are multifactorial, most commonly occurring in the elderly, although considerable evidence has shown these have a genetic predisposition and GWAS studies are unravelling the specific genetic risk factors.
Stroke less commonly presents as a monogenic disease, where a single gene mutation results in a syndrome which includes early-onset stroke as a key clinical feature. The most common of these is CADASIL, a familial form of SVD which shares many features with sporadic SVD. In recent years, other causative genes have also been identified, such as COL4A1/A2 and HTRA1 which may have a higher prevalence than previously thought.
References
Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the twenty-first century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. https://doi.org/10.1161/STR.0b013e318296aeca.
Flossmann E, Schulz U, Rothwell P. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35:212–27. https://doi.org/10.1161/01.STR.0000107187.84390.AA.
Meschia JF. Ischaemic stroke: one or several complex genetic disorders? Lancet Neurol. 2003;2:459.
Rubattu S, Volpe M, Kreutz R, et al. Chromosomal mapping of quantitative trait loci contributing to stroke in a rat model of complex human disease. Nat Genet. 1996;13:429–34. https://doi.org/10.1038/ng0896-429.
Bak S, Gaist D, Sindrup SH, et al. Genetic liability in stroke: a long-term follow-up study of Danish twins. Stroke. 2002;33:769–74.
Brass LM, Isaacsohn JL, Merikangas KR, Robinette CD. A study of twins and stroke. Stroke. 1992;23:221–3.
Kiely DK, P A W, L A C, et al. Familial aggregation of stroke. The Framingham Study. Stroke. 1993;24:1366–71. https://doi.org/10.1161/01.STR.24.9.1366.
Bevan S, Traylor M, Adib-Samii P, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–7. https://doi.org/10.1161/STROKEAHA.112.665760.
Traylor M, Malik R, Nalls MA, et al. Genetic variation at 16q24.2 is associated with small vessel stroke. Ann Neurol. 2016;81(3):383–94. https://doi.org/10.1002/ana.24840.
Bellenguez C, Bevan S, Gschwendtner A, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–33. https://doi.org/10.1038/ng.1081.
Traylor M, Farrall M, Holliday EG, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–62. https://doi.org/10.1016/S1474-4422(12)70234-X.
Neurology Working Group of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium, Stroke Genetics Network (SiGN), International Stroke Genetics Consortium (ISGC). Identification of additional risk loci for stroke and small vessel disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2016;15:695–707. https://doi.org/10.1016/S1474-4422(16)00102-2.
Traylor M, Mäkelä K-M, Kilarski LL, et al. A novel MMP12 locus is associated with large artery atherosclerotic stroke using a genome-wide age-at-onset informed approach. PLoS Genet. 2014;10:e1004469. https://doi.org/10.1371/journal.pgen.1004469.
Woo D, Falcone GJ, Devan WJ, et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. 2014;94:511–21. https://doi.org/10.1016/j.ajhg.2014.02.012.
Tan RYY, Markus HS. Monogenic causes of stroke: now and the future. J Neurol. 2015;262(12):2601–16. https://doi.org/10.1007/s00415-015-7794-4.
Joutel A, Haddad I, Ratelade J, Nelson MT. Perturbations of the cerebrovascular matrisome: a convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab. 2016;36:143–57. https://doi.org/10.1038/jcbfm.2015.62.
Tan RYY, Traylor M, Rutten-Jacobs L, Markus HS. New insights into mechanisms of small vessel disease stroke from genetics. Clin Sci. 2017;131(7):515–31.
Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–10. https://doi.org/10.1038/383707a0.
Fukutake T. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): from discovery to gene identification. J Stroke Cerebrovasc Dis. 2011;20:85–93. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.11.008.
Verdura E, Hervé D, Scharrer E, et al. Heterozygous HTRA1 mutations are associated with autosomal dominant cerebral small vessel disease. Brain. 2015;138(Pt 8):2347–58. https://doi.org/10.1093/brain/awv155.
Nozaki H, Kato T, Nihonmatsu M, et al. Distinct molecular mechanisms of HTRA1 mutants in manifesting heterozygotes with CARASIL. Neurology. 2016;86:1964–74. https://doi.org/10.1212/WNL.0000000000002694.
Bugiani M, Kevelam SH, Bakels HS, et al. Cathepsin A-related arteriopathy with strokes and leukoencephalopathy (CARASAL). Neurology. 2016;87(17):1777–86. https://doi.org/10.1212/WNL.0000000000003251.
DiFrancesco JC, Novara F, Zuffardi O, et al. TREX1 C-terminal frameshift mutations in the systemic variant of retinal vasculopathy with cerebral leukodystrophy. Neurol Sci. 2014;36(2):323–30. https://doi.org/10.1007/s10072-014-1944-9.
French CR, Seshadri S, Destefano AL, et al. Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J Clin Invest. 2014;124:4877–81. https://doi.org/10.1172/JCI75109.
Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370:911–20. https://doi.org/10.1056/NEJMoa1307361.
Jenkinson EM, Rodero MP, Kasher PR, et al. Mutations in SNORD118 cause the cerebral microangiopathy leukoencephalopathy with calcifications and cysts. Nat Genet. 2016;48(10):1185–92. https://doi.org/10.1038/ng.3661.
Mitsias P, Levine SR. Cerebrovascular complications of Fabry’s disease. Ann Neurol. 1996;40:8–17. https://doi.org/10.1002/ana.410400105.
Chassaing N, Martin L, Calvas P, et al. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881–92. https://doi.org/10.1136/jmg.2004.030171.
Gutmann DH, Ferner RE, Listernick RH, et al. Neurofibromatosis type 1. Nat Rev. Dis Prim. 2017;3:17004. https://doi.org/10.1038/nrdp.2017.4.
Buoni S, Molinelli M, Mariottini A, et al. Homocystinuria with transverse sinus thrombosis. J Child Neurol. 2001;16:688–90. https://doi.org/10.1177/088307380101600913.
Renard D, Miné M, Pipiras E, et al. Cerebral small-vessel disease associated with COL4A1 and COL4A2 gene duplications. Neurology. 2014;83:1029–31. https://doi.org/10.1212/WNL.0000000000000769.
Revesz T, Holton JL, Lashley T, et al. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–30. https://doi.org/10.1007/s00401-009-0501-8.
Vidal R, Frangione B, Rostagno A, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–81. https://doi.org/10.1038/21637.
Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17:860–71. https://doi.org/10.1038/ejhg.2009.35.
Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev. Nephrol. 2015;11:589–98. https://doi.org/10.1038/nrneph.2015.128.
Fischer A, Zalvide J, Faurobert E, et al. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med. 2013;19:302–8. https://doi.org/10.1016/j.molmed.2013.02.004.
Weitz NA, Lauren CT, Behr GG, et al. Clinical spectrum of capillary malformation-arteriovenous malformation syndrome presenting to a pediatric dermatology practice: a retrospective study. Pediatr Dermatol. 2015;32:76–84. https://doi.org/10.1111/pde.12384.
O’Sullivan M, Jarosz JM, Martin RJ, et al. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56:628–34.
Bellingham AJ. The sickling process in relation to clinical manifestations. J Clin Pathol. 1974;8:23–5.
Allison AC. Protection afforded by sickle-cell trait against subtertian malarial infection. BMJ. 1954;1:290–4.
Bender M, Douthitt Seibel G. Sickle cell disease. 1993. https://www.ncbi.nlm.nih.gov/books/NBK1377/.
Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–94.
Gemmete JJ, Davagnanam I, Toma AK, et al. Arterial ischemic stroke in children. Neuroimaging Clin N Am. 2013;23:781–98. https://doi.org/10.1016/j.nic.2013.03.019.
Strouse JJ, Jordan LC, Lanzkron S, Casella JF. The excess burden of stroke in hospitalized adults with sickle cell disease. Am J Hematol. 2009;84:548–52. https://doi.org/10.1002/ajh.21476.
Kirkham FJ. Therapy Insight: stroke risk and its management in patients with sickle cell disease. Nat Clin Pract Neurol. 2007;3:264–78. https://doi.org/10.1038/ncpneuro0495.
Lawrence C, Webb J. Sickle cell disease and stroke: diagnosis and management. Curr Neurol Neurosci Rep. 2016;16:27. https://doi.org/10.1007/s11910-016-0622-0.
Schnog JB, Mac Gillavry MR, van Zanten AP, et al. Protein C and S and inflammation in sickle cell disease. Am J Hematol. 2004;76:26–32. https://doi.org/10.1002/ajh.20052.
Yasin Z, Witting S, Palascak MB, et al. Phosphatidylserine externalization in sickle red blood cells: associations with cell age, density, and hemoglobin F. Blood. 2003;102:365–70. https://doi.org/10.1182/blood-2002-11-3416.
Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5:501–12. https://doi.org/10.1016/S1474-4422(06)70469-0.
Francis RB, Haywood LJ. Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J Natl Med Assoc. 1992;84:611–5.
Hines PC, McKnight TP, Seto W, et al. Central nervous system events in children with sickle cell disease presenting acutely with headache. J Pediatr. 2011;159:472–8. https://doi.org/10.1016/j.jpeds.2011.02.009.
Seeler RA, Royal JE, Powe L, Goldberg HR. Moyamoya in children with sickle cell anemia and cerebrovascular occlusion. J Pediatr. 1978;93:808–10.
Powars D, Adams RJ, Nichols FT, et al. Delayed intracranial hemorrhage following cerebral infarction in sickle cell anemia. J Assoc Acad Minor Phys. 1990;1:79–82.
Powars D, Wilson B, Imbus C, et al. The natural history of stroke in sickle cell disease. Am J Med. 1978;65:461–71.
Oyesiku NM, Barrow DL, Eckman JR, et al. Intracranial aneurysms in sickle-cell anemia: clinical features and pathogenesis. J Neurosurg. 1991;75:356–63. https://doi.org/10.3171/jns.1991.75.3.0356.
Armstrong FD, Thompson RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle Cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics. 1996;97:864–70.
Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the cooperative study of sickle cell disease. J Pediatr. 2001;139:385–90. https://doi.org/10.1067/mpd.2001.117580.
Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–10. https://doi.org/10.1056/NEJM199202273260905.
Driscoll MC, Hurlet A, Styles L, et al. Stroke risk in siblings with sickle cell anemia. Blood. 2003;101:2401–4.
Sampaio Silva G, Vicari P, Figueiredo MS, et al. Transcranial doppler in adult patients with sickle cell disease. Cerebrovasc Dis. 2006;21:38–41. https://doi.org/10.1159/000089592.
Flanagan JM, Frohlich DM, Howard TA, et al. Genetic predictors for stroke in children with sickle cell anemia. Blood. 2011;117:6681–4. https://doi.org/10.1182/blood-2011-01-332205.
Lettre G, Sankaran VG, Bezerra MAC, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and -globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci. 2008;105:11869–74. https://doi.org/10.1073/pnas.0804799105.
Hoppe C, Klitz W, Cheng S, et al. Gene interactions and stroke risk in children with sickle cell anemia. Blood. 2004;103:2391–6. https://doi.org/10.1182/blood-2003-09-3015.
Sebastiani P, Ramoni MF, Nolan V, et al. Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia. Nat Genet. 2005;37:435–40. https://doi.org/10.1038/ng1533.
JGT VI, Tang DC, Savage SA, et al. Variants in the VCAM1 gene and risk for symptomatic stroke in sickle cell disease. Blood. 2002;100:4303–9. https://doi.org/10.1182/blood-2001-12-0306.
Hoppe C, Klitz W, Noble J, et al. Distinct HLA associations by stroke subtype in children with sickle cell anemia. Blood. 2003;101:2865–9. https://doi.org/10.1182/blood-2002-09-2791.
Flanagan JM, Sheehan V, Linder H, et al. Genetic mapping and exome sequencing identify 2 mutations associated with stroke protection in pediatric patients with sickle cell anemia. Blood. 2013;121:3237–45. https://doi.org/10.1182/blood-2012-10-464156.
Geard A, Pule GD, Chelo D, et al. Genetics of sickle cell-associated cardiovascular disease: an expert review with lessons learned in Africa. Omi A J Integr Biol. 2016;20:581–92. https://doi.org/10.1089/omi.2016.0125.
Johnson CS. Arterial blood pressure and hyperviscosity in sickle cell disease. Hematol Oncol Clin North Am. 2005;19:827–37. https://doi.org/10.1016/j.hoc.2005.08.006.
Estcourt LJ, Fortin PM, Hopewell S, et al. Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease. Cochrane Database Syst Rev. 2017;11:CD003146.
Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. N Engl J Med. 1998;339:5–11. https://doi.org/10.1056/NEJM199807023390102.
Investigators TOPSP in SCA (STOP 2) T. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–78. https://doi.org/10.1056/NEJMoa050460.
Razvi SSM, Davidson R, Bone I, Muir KW. The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland. J Neurol Neurosurg Psychiatry. 2005;76:739–41. https://doi.org/10.1136/jnnp.2004.051847.
Narayan SK, Gorman G, Kalaria RN, et al. The minimum prevalence of CADASIL in northeast England. Neurology. 2012;78:1025–7. https://doi.org/10.1212/WNL.0b013e31824d586c.
Kilarski LL, Rutten-Jacobs LCA, Bevan S, et al. Prevalence of CADASIL and Fabry disease in a Cohort of MRI defined younger onset lacunar stroke. PLoS One. 2015;10:e0136352. https://doi.org/10.1371/journal.pone.0136352.
Tan RYY, Markus HS. CADASIL: Migraine, Encephalopathy, Stroke and Their Inter-Relationships. PLoS One. 2016;11:e0157613. https://doi.org/10.1371/journal.pone.0157613.
Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41(4):630. https://doi.org/10.1161/STROKEAHA.109.568402.
Joutel A, Vahedi K, Corpechot C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–5. https://doi.org/10.1016/S0140-6736(97)08083-5.
Singhal S, Bevan S, Barrick T, et al. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain. 2004;127:2031–8. https://doi.org/10.1093/brain/awh223.
Opherk C, Peters N, Holtmannspötter M, et al. Heritability of MRI lesion volume in CADASIL: evidence for genetic modifiers. Stroke. 2006;37:2684–9. https://doi.org/10.1161/01.STR.0000245084.35575.66.
Peters N, Holtmannspotter M, Opherk C, et al. Brain volume changes in CADASIL: a serial MRI study in pure subcortical ischemic vascular disease. Neurology. 2006;66:1517–22. https://doi.org/10.1212/01.wnl.0000216271.96364.50.
Singhal S, Rich P, Markus HS. The spatial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. AJNR Am J Neuroradiol. 2005;26:2481–7.
Investigators TS. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–25. https://doi.org/10.1056/NEJMoa1204133.
Donnini I, Nannucci S, Valenti R, et al. Acetazolamide for the prophylaxis of migraine in CADASIL: a preliminary experience. J Headache Pain. 2012;13:299–302. https://doi.org/10.1007/s10194-012-0426-9.
Forteza AM, Brozman B, Rabinstein AA, et al. Acetazolamide for the treatment of migraine with aura in CADASIL. Neurology. 2001;57:2144–5. https://doi.org/10.1212/WNL.57.11.2144.
Weller M, Dichgans J, Klockgether T. Acetazolamide-responsive migraine in CADASIL. Neurology. 1998;50:1505. https://doi.org/10.1212/WNL.50.5.1505.
Martikainen MH, Roine S. Rapid improvement of a complex migrainous episode with sodium valproate in a patient with CADASIL. J Headache Pain. 2012;13:95–7. https://doi.org/10.1007/s10194-011-0400-y.
MHRA. Imigran 100 mg tablets (sumatriptan succinate) patient information leaflet. In: Medical information and production details. 2013. http://www.medicines.org.uk/emc/medicine/749. Accessed 27 Sept 2015.
Schon F, Martin RJ, Prevett M, et al. “CADASIL coma”: an underdiagnosed acute encephalopathy. J Neurol Neurosurg Psychiatry. 2003;74:249–52. https://doi.org/10.1136/jnnp.74.2.249.
Zheng DM, FF X, Gao Y, et al. A Chinese pedigree of cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): clinical and radiological features. J Clin Neurosci. 2009;16:847–9. https://doi.org/10.1016/j.jocn.2008.08.031.
Mendioroz M, Fernández-Cadenas I, Del Río-Espinola A, et al. A missense HTRA1 mutation expands CARASIL syndrome to the Caucasian population. Neurology. 2010;75:2033–5. https://doi.org/10.1212/WNL.0b013e3181ff96ac.
Nozaki H, Sekine Y, Fukutake T, et al. Characteristic features and progression of abnormalities on MRI for CARASIL. Neurology. 2015;85:459–63. https://doi.org/10.1212/WNL.0000000000001803.
Yanagawa S, Ito N, Arima K, Ikeda S -i S. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy. Neurology. 2002;58:817–20. https://doi.org/10.1212/WNL.58.5.817.
Oka C, Tsujimoto R, Kajikawa M, et al. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development. 2004;131:1041–53. https://doi.org/10.1242/dev.00999.
Chen Y, He Z, Meng S, et al. A novel mutation of the high-temperature requirement A serine peptidase 1 (HTRA1) gene in a Chinese family with cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL). J Int Med Res. 2013;41:1445–55. https://doi.org/10.1177/0300060513480926.
Gunda B, Mine M, Kovács T, et al. COL4A2 mutation causing adult onset recurrent intracerebral hemorrhage and leukoencephalopathy. J Neurol. 2014;261(3):500. https://doi.org/10.1007/s00415-013-7224-4.
Breedveld G, de Coo IF, Lequin MH, et al. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–5. https://doi.org/10.1136/jmg.2005.035584.
Alamowitch S, Plaisier E, Favrole P, et al. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. 2009;73:1873–82. https://doi.org/10.1212/WNL.0b013e3181c3fd12.
Jeanne M, Labelle-Dumais C, Jorgensen J, et al. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90:91–101. https://doi.org/10.1016/j.ajhg.2011.11.022.
Plaisier E, Gribouval O, Alamowitch S, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–95. https://doi.org/10.1056/NEJMoa071906.
Rannikmäe K, Davies G, Thomson PA, et al. Common variation in COL4A1/COL4A2 is associated with sporadic cerebral small vessel disease. Neurology. 2015;84(9):918–26. https://doi.org/10.1212/WNL.0000000000001309.
Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–50. https://doi.org/10.1212/WNL.0b013e3181dad605.
Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–24.
Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014;83:794–800. https://doi.org/10.1212/WNL.0000000000000732.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Tan, R.Y.Y., Markus, H.S. (2018). Genetics and Genomics of Stroke. In: Kumar, D., Elliott, P. (eds) Cardiovascular Genetics and Genomics. Springer, Cham. https://doi.org/10.1007/978-3-319-66114-8_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-66114-8_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66112-4
Online ISBN: 978-3-319-66114-8
eBook Packages: MedicineMedicine (R0)