Abstract
Objective: Computed tomography (CT) of the brain can allow rapid assessment of intracranial pathology after traumatic brain injury (TBI). Frequently in paediatric TBI, CT imaging can fail to display the classical features of severe brain injury with raised intracranial pressure. The objective of this study was to determine early CT brain features that influence intracranial or systemic physiological trends following paediatric TBI.
Materials and methods: Thirty-three patients (mean age, 10 years; range, 0.5–16) admitted between 2002 and 2015 were used for the current analysis. Presence of petechial haemorrhages, basal cistern compression, subarachnoid blood, midline shift and extra-axial masses on the initial trauma CT head were assessed. ICP and arterial blood pressure (ABP) were then monitored continuously with an intraparenchymal microtransducer and an indwelling arterial line. Pressure monitors were connected to bedside computers running ICM+ software. Pressure reactivity was determined as the moving correlation between 30, 10-s averages of ABP and ICP (PRx). The mean ICP, ABP, cerebral perfusion pressure (CPP; ABP minus ICP) and PRx were calculated for the whole monitoring period for each patient.
Results: The presence of subarachnoid blood was related to higher ICP, higher ABP and a trend toward higher PRx. Smaller basal cisterns were related to increased ICP (R = −0.42, p = 0.02), impaired PRx (R = −0.5, p = 0.003). The presence of an extra-axial mass was associated with deranged PRx (−0.02 vs. 0.41, p = 0.003) and a trend toward higher ICP (14 vs. 40, p = 0.07). Interestingly the degree of midline shift was not related to ICP or PRx.
Conclusions: The size of the basal cisterns, the presence of subarachnoid blood or an extra-axial mass are all related to disturbed ICP and pressure reactivity in this paediatric TBI cohort. Patients with these features are ideal candidates for invasive multimodal monitoring.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Traumatic brain injury (TBI) is a major contributor to mortality and morbidity worldwide, particularly in children [1]. The management of paediatric brain injury aims to attenuate evolving secondary brain injury that follows the initial mechanical insult. Hypotension, hypoxia, hypoglycaemia, sustained increased intracranial pressure (ICP), seizures and infections are avoided with the aim of maintaining adequate cerebral perfusion and preventing herniation syndromes [2]. A complex mix of these pathologies can lead to increases in ICP and may compromise brain perfusion by reducing cerebral perfusion pressure. Uncontrolled ICP is widely accepted to be closely linked with disability, poor neurological outcome and decreased survival in paediatric TBI patients [3]; as such, effectively monitoring and managing ICP has become a cornerstone of treatment for severe paediatric TBI [3].
Nevertheless, when to institute ICP monitoring in paediatric TBI is not well defined. The evidence base for an internationally accepted published recommendation for ICP monitoring in children with severe TBI was only sufficient to state that ICP monitoring was appropriate, and only at the level of an option. Not enough support exists for a standard or guideline [3].
In clinical practice, the implementation of ICP monitoring is usually dependent on the severity of the injury according to the level of consciousness as measured with the Glasgow Coma Scale (GCS). However, the increased use of early sedation, intubation and ventilation in more severe patients has decreased the value of the full GCS for purposes of classification [3, 4]. As such, a greater reliance has been placed on morphological criteria based on computed tomographic (CT) or magnetic resonance imaging (MRI) investigations.
Conventional classification of TBI with CT findings differentiates between focal and diffuse injuries [5]. Marshall et al. [6], after analysis of the Traumatic Coma Data Bank, proposed a CT classification for grouping patients with TBI according to multiple CT characteristics. This CT classification identifies six different groups of patients with TBI, based on the type and severity of several abnormalities on the CT scan.
It differentiates between patients with and without mass lesions and permits further discrimination of patients with diffuse injuries into four categories, taking into account signs of raised ICP. Since its introduction, this CT classification has become widely accepted for descriptive purposes and is increasingly being used as major predictor of outcome in TBI. Various studies have confirmed the predictive value of the CT classification [7]. Of these signs, the basal cisterns of the midbrain are arguably the most commonly used measure of the degree of brain swelling, being an indicator of the available perimesencephalic space at the tentorial incisura. Often the assumption is made that if the cisterns are not effaced, ICP is unlikely to be significantly elevated, and ICP monitoring may not be required [8]. Problems arise in paediatric TBI, where initial CT scans fails to demonstrate the same devastating levels of injury found in adult patients with similar ICP trends and mechanism of injury [9].
Here in we analyse the patterns of CT abnormality that are linked to deranged patterns of multi-modality monitoring in a group of paediatric TBI patients. In particular, we have focused on the patency of the basal cisterns and depth of mass lesions to give critical volumes that would encourage surgical intervention.
Methods
Patients
The data in this study were collected retrospectively from data records of paediatric severe traumatic brain injury patients admitted to Addenbrookes Hospital Paediatric Intensive Care Unit (PICU) between January 2002 and December 2015. The insertion of an intracranial monitoring device is part of routine clinical practice and, as such, did not require ethical approval. The data are routinely collected for clinical purposes and guide the management of patients. The analysis of data within this study for the purposes of service evaluation was approved by the Cambridge University Hospital NHS Trust, Audit and Service Evaluation Department (Ref. 2143) and did not require ethical approval or patient consent.
Patients were included in this cohort if they had a clinical need for ICP monitoring. Patients were treated according to current paediatric traumatic brain injury guidelines [2], which aim to keep the ICP below 20 mmHg through a stepwise regime including: positioning, sedation, paralysis, osmotic agents, ventriculostomy and induced hypothermia.
Data Acquisition and Analysis
Arterial blood pressure (ABP) was measured and the radial or femoral artery zeroed at the level of heart (Baxter Healthcare, Newbury Park, CA, USA; Sidcup, UK). ICP was measured with an intraparenchymal microsensor in the frontal cortex (Codman ICP Micro- Sensor; Codman & Shurtleff, Raynham, MA, USA). ICP and ABP data were collected at 100 Hz with an analogue-to-digital converter (DT9801; Data Translation, Marlboro, MA, USA) coupled with a laptop computer running ICM+ software (University of Cambridge, Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus). Pressure reactivity index was calculated as the moving correlation between 10 s averages of ICP and ABP. The patient’s ICP, PRx, mean arterial pressure (MAP) and CPP were then averaged over the first 72 h of their intensive care unit stay.
Basal Cistern Measurements
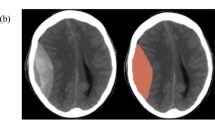
Independently, two investigators (A.Y., R.P.), not involved with data collection and blinded to the patient’s condition, measured the basal cisterns on from the initial admission CT head. All CT scans were performed with a basic non-contrast protocol using a 16-slice scanner (Somatom Sensation 16 scanner; Siemens Healthcare, Erlangen, Germany). Each observer scrolled through the CT head slices to visualise the specific imaging cut demonstrating a visual estimation of the greatest width. The image window was configured to the ‘brain’ view which yielded the basal cisterns in more detail (Hounsfield unit range, 25–40). The image was then magnified and the cisterns were measured using electronic callipers, with a digital viewer (Centricity PACS; General Electric Healthcare, Little Chalfont, UK) (Fig. 1).
Statistical Analysis
Mean ICP, PRx, CPP and MAP over the first 3 days of monitoring were calculated. The influence of the presence or absence of pre-hospital factors (hypotension, hypoxia, >1 unreactive pupil. Motor score 3 or less) on subsequent ICP and PRx were tested using a non-parametric Wilcoxon test. All data manipulations and analysis were performed on R language and software environment for statistical computation (version 2.12.1) [10].
Results
The median age was 12 (range, 0.5–15). Of the 39 patients, 36% were female. 23% of patients presented with a low GCS (Motor score 1–4). 5% of patients had bilateral fixed pupils and 13% with a unilateral fixed pupil. Mortality occurred in 18% of patients.
The median ICP was 15.2 (IQR, 12.7–19.2) with a median PRx −0.02 (IQR, −0.18 to 0.14). Baseline CT features included subarachnoid haemorrhage in 31% of patients and midline shift in 63% of patients. Forty-four percent were observed to have a focal lesion in the form of a contusion of an extra-axial haematoma. Fifty-six percent of patients suffered diffuse axonal injury (DAI). Taking a threshold of <2 mm as “compressed basal cisterns”, it was observed that on days 1 and 2 of monitoring there was no significant correlation between compression of the basal cisterns and ICP. Only on day 3 of monitoring was there a significant difference in ICP between open basal cisterns (16.2 mmHg) compared to those that were compressed (20.1 mmHg; p = 0.004; Fig. 1). With regards to PRx, compressed basal cisterns trended towards impaired PRx; however, this never reach significance. Interestingly, evidence of subarachnoid haemorrhage on CT was associated with raised ICP on days 2 (20.3 mmHg) and 3 (19.8 mmHg) compared to those who did not (14.2 mmHg and 14.9 mmHg) on days 2 and 3 respectively (Fig. 2). Finally, features of midline shift were not associated with deranged ICP or PRx (p = 0.62; Fig. 3).
Discussion
In this study, we investigated the relationship between key features of traumatic brain injury on CT and subsequent intracranial physiology after severe traumatic brain injury in children. Children with evidence of severe injury on scan went on to develop higher intracranial pressure. Particularly, evidence of subarachnoid haemorrhage on CT was related to raised ICP but not deranged PRx. Compression of the basal cisterns was linked to increased ICP in these patients; however, unlike adults, complete obliteration of the cisterns was rare. Raised ICP, particularly in days 1 and 2 after injury, was present regardless of the patency of the basal cisterns. This finding replicates that of Kouvarellis et al. [9], who concluded that children with severe TBI frequently may have open basal cisterns on head CT despite increased ICP. Open cisterns should not discourage ICP monitoring.
The relationship between subarachnoid haemorrhage and raised ICP is interesting. It could simply be related to the degree of injury; i.e. a severe injury will be more likely to induced haemorrhage and, as such, raise ICP. However, there is a suspicion that even a subtle occlusion of cerebrospinal fluid drainage could lead to minimal rises in ICP [11].
The lack of correlation between midline shift and ICP or PRx is most likely associated with the fact that extra-axial haematomas that were causing severe shift were evacuated in a timely fashion. Those that were not evacuated were done so on clinical grounds and, as a result, justifiable based on the lack of ICP/PRx derangement.
Implications
Early prediction of raised ICP has the potential to facilitate the management of TBI patients by alerting the clinicians to patients which require more intensive monitoring or perhaps a lower threshold for ICP treatment. However, prediction remains difficult and is usually confined to analysis of high-frequency ICP waveforms. Thus, the current preliminary findings, if confirmed, could enhance existing ICP prediction tools. In addition, the current data potentially indicate that enhanced pre-hospital care may aid in avoiding secondary insults occurring up to 72 h after admission to hospital. Furthermore, the finding of a relationship between subarachnoid haemorrhage and subsequent ICP highlights the need to determine individualised paediatric scoring systems in the analyses between physiological variables and patient outcome.
Limitations
As this current analysis is from quite a small dataset, we cannot exclude that more subtle relationships between CT variables and ICP or PRx may emerge with a larger dataset [12].
Conclusion
After severe paediatric TBI, those patients with subarachnoid haemorrhage and compression of the basal cisterns go on to develop higher ICP. It is unusual to have obliterated basal cisterns in children even with deranged ICP.
References
Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA; 2010.
Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr Crit Care Med. 2012;13(Suppl):S1–82.
Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, Warden CW, Wright DW, American Association for Surgery of Trauma, Child Neurology Society, International Society for Pediatric Neurosurgery, International Trauma Anesthesia and Critical Care Society, Society of Critical Care Medicine, World Federation of Pediatric Intensive and Critical Care Societies. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S65–7.
Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury. 3rd edition. J Neurotrauma. 2007;24(Suppl 1):S37–44.
Chesnut RM. Computed tomography of the brain: a guide to understanding and interpreting normal and abnormal images in the critically ill patient. Crit Care Nurs Q. 1994;17(1):33–50.5.
Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92.
Jagannathan J, Okonkwo DO, Yeoh HK, Dumont AS, Saulle D, Haizlip J, Barth JT, Jane JA Sr, Jane JA Jr. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2(4):240–9.
Teasdale E, Cardoso E, Galbraith S, Teasdale G. CT scan in severe diffuse head injury: physiological and clinical correlations. J Neurol Neurosurg Psychiatry. 1984;47(6):600–3.
Kouvarellis AJ, Rohlwink UK, Sood V, Van Breda D, Gowen MJ, Figaji AA. The relationship between basal cisterns on CT and time-linked intracranial pressure in paediatric head injury. Childs Nerv Syst. 2011;27(7):1139–44. https://doi.org/10.1007/s00381-011-1464-3. Epub 2011 May 3
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. http://www.r-project.org/.
Noraky J, Verghese GC, Searls DE, Lioutas VA, Sonni S, Thomas A, Heldt T. Noninvasive intracranial pressure determination in patients with subarachnoid hemorrhage. Acta Neurochir Suppl. 2016;122:65–8. https://doi.org/10.1007/978-3-319-22533-3_13.
Young AM, Guilfoyle MR, Fernandes H, Garnett MR, Agrawal S, Hutchinson PJ. The application of adult traumatic brain injury models in a pediatric cohort. J Neurosurg Pediatr. 2016;18(5):558–64.
Conflicts of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Young, A.M.H. et al. (2018). Computed Tomography Indicators of Deranged Intracranial Physiology in Paediatric Traumatic Brain Injury. In: Heldt, T. (eds) Intracranial Pressure & Neuromonitoring XVI. Acta Neurochirurgica Supplement, vol 126. Springer, Cham. https://doi.org/10.1007/978-3-319-65798-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-65798-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65797-4
Online ISBN: 978-3-319-65798-1
eBook Packages: MedicineMedicine (R0)