Abstract
Objective: Brain tissue oxygenation (pbtO2) monitoring with microprobes is increasingly used as an important parameter in addition to intracranial pressure in acutely brain-injured patients. Data on accuracy and long-term drift after use are scarce. We investigated room air readings of used pbtO2 probes for their relationship with the duration of monitoring, geographic location of the center, and manufacturer type.
Methods: After finishing clinically indicated monitoring in patients, pbtO2 probes used in two centers in Berlin and Munich were explanted and cleaned to avoid blood contamination. Immediately afterward, room air readings of partial oxygen pressure (pairO2) from 44 Licox® and 10 Raumedic® pbtO2 probes were recorded. Assumed height above sea level was 42 m for Berlin and 485 m for Munich; this resulted in assumed theoretical pairO2 readings of 157.8 mmHg in Berlin and 149.9 mmHg in Munich.
Results: Licox® probes in Berlin showed a mean pairO2 of 160.5 (SD 14.4) mmHg and of 147.8 (11.9) mmHg in Munich. Raumedic® probes in Berlin showed a mean pairO2 of 170.5 (12.2) mmHg and the single Raumedic® probe used in Munich 155 mmHg. No significant drift was found over time for probes with up to 14 days of monitoring. Prolonged use of up to 20 days showed a clinically negligible drift of 1.2 mmHg per day of use for Licox® probes.
Mean absolute deviation for pairO2 from expected values was 6.4% for Licox® and 9.7% for Raumedic® probes.
Conclusion: Room air partial oxygen pressure pairO2 may be utilized to assess the proper function of a pbtO2 probe. It provides a tool for quality control which is easy to implement. Probe readings are stable in the clinically relevant range, even after prolonged use.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Monitoring brain tissue oxygenation (pbtO2) with intraparenchymal microprobes is an emerging tool and recommended in guidelines after traumatic brain injury and aneurysmal subarachnoid hemorrhage [1, 2]. A commonly proposed threshold is 20 mmHg: values below this margin are associated with a worse outcome [3]. As monitoring of critically injured patients may be required for days or even 1 or 2 weeks, it is crucial to know how accurate the readings of pbtO2 probes are over time and whether long-term drift is present. Currently, bench testing is available only for probes in mint condition and an assessment of pbtO2 devices after in vivo use is lacking [4, 5].
On earth, ambient dry air provides a stable environment with an oxygen fraction of 20.95% and a partial pressure of 159.21 mmHg at sea level. Therefore, a simple and ubiquitously available method of assessing the accuracy of a pbtO2 probe may be to use plain room air as a reference. Therefore, we investigated room air readings of used pbtO2 probes for their relationship with duration of monitoring, geographic location of the center, and manufacturer type.
Methods
Indications for pbtO2 monitoring were based on clinical considerations and were not part of the study. We investigated equipment from both vendors currently on the market; in particular, Licox CC1.SB probes (Integra Neuroscience, Saint Priest, France) and Neurovent PTO probes (Raumedic AG, Münchberg, Germany). We gathered data from probes used in two centers, located in Berlin and Munich, Germany. After finishing clinically indicated monitoring, pbtO2 probes were removed, superficially cleaned with a pad to prevent blood contamination and inspected for obvious mechanical damage. Immediately afterward, room air readings of partial oxygen pressure (pairO2) were recorded until a stable reading was achieved. As no patient data were used in this setup, the need for informed consent was waived.
Physical Laws and Considerations
Average standard atmospheric pressure at sea level is 1,013.25 kPa, equivalent to 760 mmHg (29.92 in), with a typical range between 670 mmHg (26.5 in) and 800 mmHg (31.5 in) on a mercury column barometer. Introduced by daily temperature fluctuations, atmospheric pressure shows a semicircadian rhythm. The amplitude of these fluctuations is dependent on latitude, with about 5 kPa (3.75 mmHg) at the equator, and 0.5–1 kPa (0.38–0.75 mmHg) at continental climate zones.
Simplified, atmospheric pressure decreases with altitude by:
pheight~p0 ∗ exp (−height/h0),
with h0 = 8435m. For low altitudes, this equals approximately about 1.2 kPa (0.9 mmHg) for every 100 m.
Assumed elevation above sea level was 42 m for Berlin, Germany, and 485 m for Munich, Germany. Using the stable oxygen fraction of 20.95%, this resulted in assumed theoretical pairO2 readings of 157.8 mmHg in Berlin and 149.9 mmHg in Munich [6].
Statistical Analysis
Data analysis was performed using R 3.3.1, R foundation for Statistical Computing, Vienna, Austria. The duration of previous use was plotted against the room air reading. Multivariate analysis was performed with linear regression using room air reading as a dependent variable, duration of use as an independent linear predictor, and probe type and center location as cofactors.
Results
Room air readings of pairO2 from 44 Licox® and 10 Raumedic® pbtO2 probes were available for analysis. One probe from the Munich center showed a pairO2 reading of 334 mmHg, more than 10 standard deviations off from the mean of all other probes. Although no mechanical damage was noted, this single probe was considered defective and excluded from analysis.
Licox® probes in Berlin showed a mean pairO2 of 160.5 (SD 14.4) mmHg and of 147.8 (11.9) mmHg in Munich. Raumedic® probes in Berlin showed a mean pairO2 of 170.5 (12.2) mmHg and a single Raumedic® probe used in Munich displayed 155 mmHg.
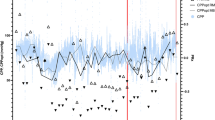
Licox® probes showed an increase of 1.28 mmHg (p < 0.001) per day of use when all data with up to 20 days of forgone monitoring time were considered. No significant trend was found if readings from probes with up to 14 days of previous use were examined. We found no significant increase per day of previous use for Raumedic® probes. Mean absolute deviation for pairO2 from expected values was 6.4% for Licox® and 9.7% for Raumedic® probes. Figure 1 shows the relationship among time, location of use, and type of probe with the acquired room air readings.
Room air readings (pairO2) of brain tissue oxygenation probes from two manufacturers plotted versus days of previous use. Licox® probes are indicated by circles, Raumedic® probes by squares. Dotted and dashed lines indicate the theoretical readings in two centers, located in Berlin and Munich, Germany, respectively
Discussion
Our findings show that room air may be utilized as a verification tool of proper function of a pbtO2 probe. Differences in geographic location and altitude are reflected accurately by room air readings of pbtO2 probes. For use of up to 14 days, readings of used probes remained stable. Even with prolonged utilization beyond that time period, the average drift of 1.2% per day of use translates to lower values than differences in measurement to be expected in vital brain tissue [7, 8]. Therefore, we consider probe drift per day of use to be clinically negligible.
The knowledge derived may help a clinician to decide whether previous readings obtained during clinical monitoring had been accurate. In the case of challenged low (or high) readings during previous clinical use, a finding compliant with proper probe function may trigger implantation of a new probe. Unfortunately, our findings do not represent an online function test for probes under consideration. The fragile nature of Licox® probes prohibits reinsertion after room air testing. In theory, this may be possible for the more rigid Raumedic® probes, but is strongly disadvised owing to potential breaches of sterility.
The main limitation of our study is that we did not the measure actual pairO2 with a dedicated and calibrated device. Rather, we relied on the theoretical calculated pairO2 value for the environment of the respective location. Daily fluctuations, in addition to high or low barometric pressures may subtly alter theoretical values of pairO2. Furthermore, it was assumed that it remained stable when measured indoors in the ICU with active air conditioning. Although both manufacturers perform adjustment of displayed pO2 readings according to surrounding tissue temperature, the use of probes at room temperature is not at a level occurring in the living human body and outside of the manufacturers’ specifications. Additionally, the cleansing process of the probes was not standardized and remnants of blood and tissue after clinical use may have contributed to unaccounted confounding. However, despite these limitations, the robustness of findings serve as important proof-of-principle of the validity of our analysis.
Conclusion
Room air partial oxygen pressure pairO2 may be utilized to assess the proper function of a pbtO2 probe. When the local altitude above sea level is considered, it provides a tool for quality control which is easy to implement. Probe readings are stable within the clinically relevant range, even after prolonged use.
References
Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. X. Brain oxygen monitoring and thresholds. J Neurotrauma. 2007;24(Suppl 1):S65–70.
Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15:211–40.
Ngwenya LB, Burke JF, Manley GT. Brain tissue oxygen monitoring and the intersection of brain and lung: a comprehensive review. Respir Care. 2016;61:1232–44.
Purins K, Enblad P, Sandhagen B, Lewén A. Brain tissue oxygen monitoring: a study of in vitro accuracy and stability of Neurovent-PTO and Licox sensors. Acta Neurochir. 2010;152:681–8.
Stewart C, Haitsma I, Zador Z, Hemphill JC, Morabito D, Manley G, Rosenthal G. The new Licox combined brain tissue oxygen and brain temperature monitor: assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery. 2008;63:1159–1164; discussion 1164–1165.
Atmospheric pressure. Wikipedia. https://en.wikipedia.org/wiki/Atmospheric_pressure. Accessed 15 November 2016.
Ponce LL, Pillai S, Cruz J, Li X, Julia H, Gopinath S, Robertson CS. Position of probe determines prognostic information of brain tissue PO2 in severe traumatic brain injury. Neurosurgery. 2012;70:1492–1502; discussion 1502–1503.
Hawryluk GWJ, Phan N, Ferguson AR, Morabito D, Derugin N, Stewart CL, Knudson MM, Manley G, Rosenthal G. Brain tissue oxygen tension and its response to physiological manipulations: influence of distance from injury site in a swine model of traumatic brain injury. J Neurosurg. 2016;125:1217–28.
Conflicts of interest statement
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Wolf, S., Schürer, L., Engel, D.C. (2018). Room Air Readings of Brain Tissue Oxygenation Probes. In: Heldt, T. (eds) Intracranial Pressure & Neuromonitoring XVI. Acta Neurochirurgica Supplement, vol 126. Springer, Cham. https://doi.org/10.1007/978-3-319-65798-1_40
Download citation
DOI: https://doi.org/10.1007/978-3-319-65798-1_40
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65797-4
Online ISBN: 978-3-319-65798-1
eBook Packages: MedicineMedicine (R0)